Abstract
In recent years, advances in ocular imaging, drug delivery, and ophthalmic surgery have allowed for better visualization and access to the suprachoroidal space. Although previously considered as only a potential space, the suprachoroidal space may serve as a route for drug delivery to the posterior pole, an egress for glaucoma drainage devices, a location for temporary buckling, and a site for prosthesis implantation. Drugs delivered to the suprachoroidal space may achieve higher concentrations in the retina while minimizing exposure to anterior segment tissues, potentially reducing risks of glaucoma or cataracts. Finally, advanced multimodal imaging now allows not only a better understanding of the physiology of the suprachoroid, but also in vivo monitoring of pathologies and drug delivery to the suprachoroidal space. Here, we discuss the newest developments in the medical and surgical applications of this space with potential.
Introduction:
Until recently, the suprachoroidal space (SCS) has been mainly considered as a potential space between the choroid and sclera that only becomes visible or accessible in pathologic conditions. Recent advancements in ocular imaging, however, have enabled better visualization of this space and is providing new insights into its role in health and disease.1 At the same time, novel instrumentation provides precise access to the SCS, and has opened up new pathways for targeted medical and surgical therapies.
Anatomy of the SCS:
Under normal conditions, the posterior border of the choroid is defined by the lamina fusca (meaning “dark layer”), which consists of a gradual transition from the loose lamellar fibers of the choroidal stroma to the more compact architecture of the sclera.2 This layer in normal individuals has a thickness of approximately 35μm and plays a role in controlling intraocular pressure by regulating the flow of choroidal proteins through the sclera forming the uveoscleral outflow tract.3 Under physiological conditions, the lamellae are in close apposition to each other. But in pathologic states, serous fluid or blood can accumulate in this layer, resulting in suprachoroidal effusion or hemorrhage.4 The anterior border of the SCS is the scleral spur, while it is bound by the optic nerve posteriorly. Choroid and sclera are tightly bound in these spots; thus, fluid accumulation in the SCS cannot spread past these boundaries.5
Imaging the SCS:
While optical coherence tomography (OCT) technology was still in its infancy, visualization of the choroid and SCS was limited by poor signal penetration past the retinal pigment epithelium (RPE). The SCS could only be visualized on B-scan ultrasonography in pathologic conditions such as posterior scleritis, where suprachoroidal fluid accumulates in a T-shaped pattern along the posterior pole and optic nerve. With the increasing use of enhanced depth imaging (EDI)-OCT, which takes advantage of the greater depth of field of the inverted image obtained when placing a spectral domain OCT device closer to the eye,6 as well as swept-source OCT, which employs longer wavelength light sources and deeper signal penetration, the choroid and SCS became more readily visualized.7
The SCS appears as a hyporeflective band between the outer border of the choroid stroma and sclera on EDI-OCT and swept-source OCT scans and is variably seen in normal individuals (Figure 1). Since visualization of the SCS depends on the light penetration through the choroid, it is more readily seen in older subjects and more myopic eyes where the choroid is thinner.4 SCS visibility is also impacted by uveal pigmentation, and may be more difficult to delineate in subjects with darker uvea such as Asians and African-Americans.8 In eyes with age-related macular degeneration (AMD), the SCS can be seen in 50% of eyes with non-exudative AMD and 20% of eyes with exudative AMD.9 This may be due to the choroidal thinning that is associated with eyes with geographic atrophy,10 or the fact that macular fluid, especially the fluid in the subretinal space, also impairs visualization of the choroid-scleral junction (Figure 2).11 It is unclear why the SCS appears as a hyporeflective layer in healthy eyes, although the presence of physiologic suprachoroidal fluid has been postulated. In eyes where the SCS is visible, the thickness of the SCS correlates with more hyperopic refractive error, suggesting that healthy eyes may form a spectrum with eyes with abnormally short axial length, such as eyes affected by nanophthalmos where impairment of the normal egress of choroidal proteins through the more compact sclera results in the osmotic accumulation of suprachoroidal fluid.
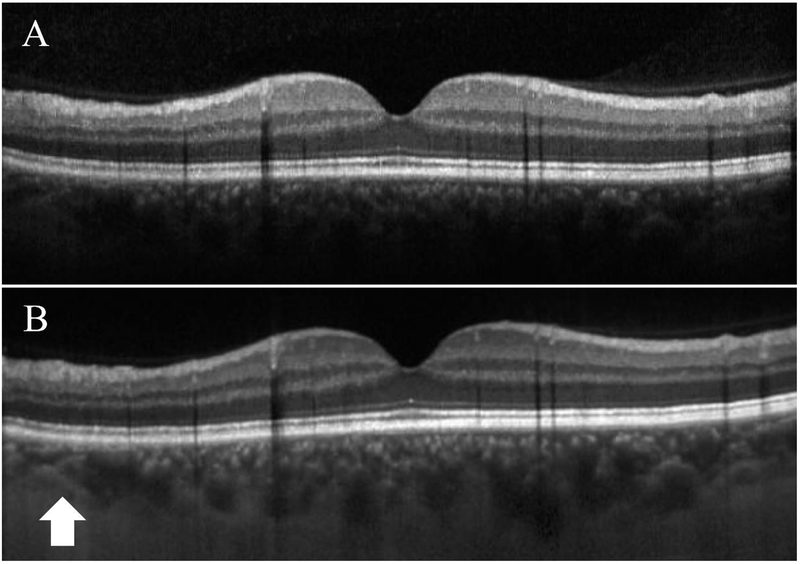
Figure 1.
Standard spectral domain (A) and enhanced-depth imaging (B) OCTs in a 65-year-old patient with normal macular anatomy. The choroid-scleral junction (arrow) is well visualized on EDI-OCT, but there is no clear suprachoroidal layer.
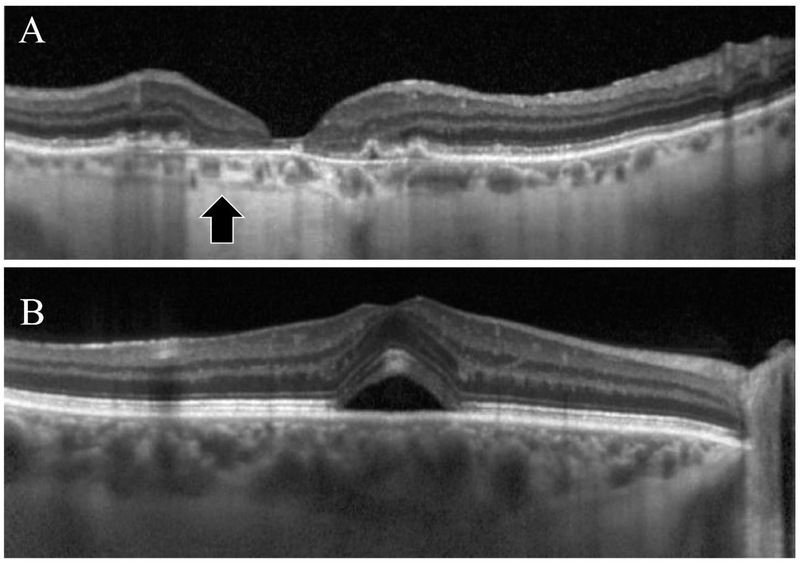
Figure 2.
Enhanced-depth imaging (EDI) OCT images showing the hyporeflective layer representing suprachoroidal space (arrow) in a patient with non-exudative age-related macular degeneration and geographic atrophy (A) and one with central serous chorioretinopathy and subretinal fluid (B). Note the thinner choroid and increased signal penetration allow better visualization of the suprachoroidal space in (A), and the impaired visualization of the space in (B).
Accessing the SCS:
Several new technologies have been developed to allow access to the suprachoroidal space, providing a novel route for both drug delivery and surgical intervention. Transcleral cannulation of the SCS can be achieved using a microcatheter that has been FDA-cleared (iTrack®, iScience Interventional, Inc.). The procedure is performed in the operating room under general or monitored anesthesia. After a full-thickness scleral incision is made near the pars plana, the catheter is passed through the SCS and threaded towards the target tissue under the guidance of an optical fiber to light the catheter tip.3 An important advantage of the microcatheter system is the ability to precisely deliver a drug to or viscoelastic substance to a specific location, such as the macula or the region of a retinal break. However, the technique has a steep learning curve and carries the risk of iatrogenic choroidal hemorrhage.
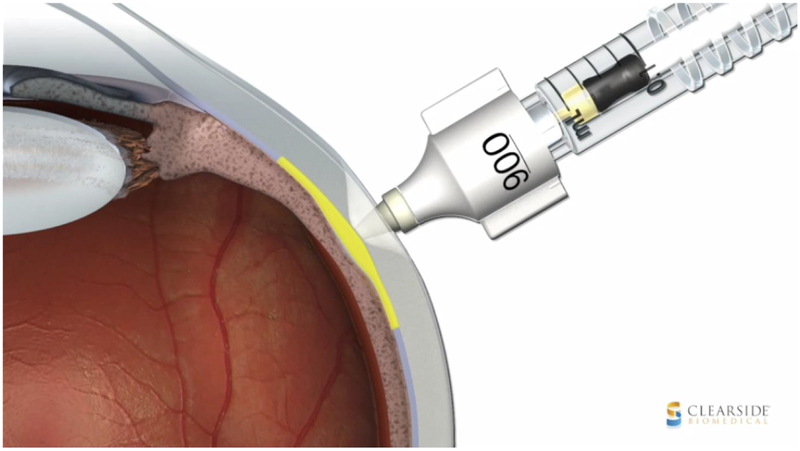
Microneedles have also been developed which allow for in-office injection into the SCS using techniques similar to those used for intravitreal injections. A hollow-bore 750 um-long microneedle (Clearside Biomedical, Inc.) inserted at the pars plana has been shown to be safe and effective for drug delivery in several clinical trials (Figure 3).3,5
Figure 3.
Schematic diagram of a hollow-bore 750um-long microneedle used for injection into the suprachoroidal space (image courtesy of Clearside Biomedical, Inc.)
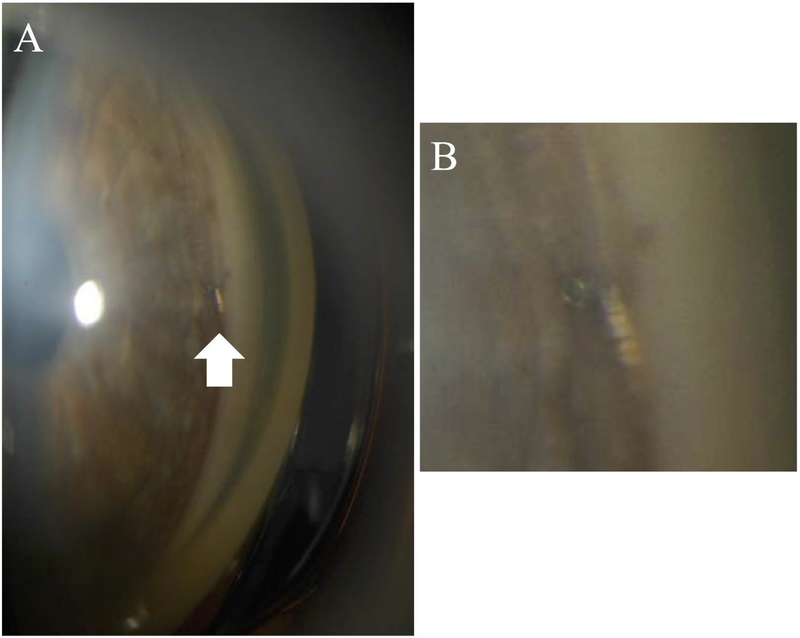
Ab interno access to the SCS can also be achieved using micro-stents, which serve as minimally-invasive glaucoma surgery (MIGS) devices. Examples include the CyPass® Micro-Stent (Alcon, Fort Worth, Texas, US) and iStent® (Glaukos), which are surgically implanted to provide a conduit from the anterior chamber to the SCS to drain the aqueous humor without forming a filtering bleb (figure 4).12
Figure 4.
Gonioscopic photo of the anterior chamber angle of a patient with glaucoma following iStent® (Glaukos) insertion (A, arrow), and magnified image of the implant snorkel in the angle.
Pharmacokinetics of the SCS:
Targeted and effective delivery of drugs to the posterior segment of the eye has been challenging. Topical administration of drops achieves low bioavailability (1–7%) in the anterior segment and almost negligible penetration posteriorly.3,13,14 Systemic treatment is not a viable option in most of the retinal conditions as an intact blood-retina barrier limits penetration and bioavailability of the drugs in the retina.15 Periocular delivery such as subconjunctival or sub-tenon injection penetrate the conjunctivae and ocular surface, but the sclera remains poorly permeable to larger molecules.1 To date, intravitreal injections have been the most popular route for intraocular delivery to the retina due to the ease of administration in clinic under topical anesthesia and high bioavailability of the drug in the retina. The vitreous humor acts a natural reservoir and slowly releases the drug molecules, but also reduces the bioavailability to some target tissues and diffusion to other areas of the eye.16,17 For example, intravitreal injection of steroids for retinal edema can result in cataract formation and increased intraocular pressure due to secondary effects on anterior segment tissues.1 Moreover, the internal limiting membrane limits diffusion of certain molecules from the vitreous to the choroid and the retinal pigment epithelium (RPE).18
The SCS provides a novel route for drug delivery to the posterior segment because it bypasses the internal limiting membrane and may theoretically provide higher bioavailability within the choroid, RPE, and ciliary body while minimizing drug levels in the anterior segment, hence reducing the risk of cataract and glaucoma.9,19 Animal studies have shown that the SCS can accommodate larger amounts of fluid (up to 1ml) compared to intravitreal delivery (10–50um).20 After injection into the SCS, molecules spread in a circumferential pattern. However, this flow in limited and compartmentalized by the scleral spur anteriorly, optic nerve posteriorly and by short posterior ciliary arteries21,22 so anterior segment structures are largely spared.3 In addition, targeted treatment of certain pathologies can be achieved with lower dosages of drug due to the compartmentalized nature of the SCS3,5 where the injected molecules can achieve higher drug levels in the retina, RPE, and choroid. For example, SCS injection of bevacizumab (molecular weight: 149kDa) resulted in 8-fold higher drug levels in the choroid and significantly lower levels in the anterior chamber as compared to intravitreal injection.22 This was also true for smaller molecules such as sodium fluorescein (40kDa), where SCS injection achieved 25–200 times higher concentration in the choroid and retina compared to intravitreal delivery.3
Drug clearance after both SCS and intravitreal injections occur through choroidal flow, and depends in part on the molecular weight of the molecules.23,24 Studies have shown higher levels of fluorescein in the choroid/retina at 2 hours after intravitreal injection compared to SCS injection.3,25 Faster clearance is also seen after SCS injection of bevacizumab, which achieves higher chorioretinal levels at 12 hours but becomes undetectable after 7 days (compared to 1 month for intravitreal injection).25 SCS injection of triamcinolone, on the other hand, resulted in slower clearance of the drug (120 days) compared to intravitreal injection (41 days for non-vitrectomized and 6 days for vitrectomized eye).26 Data suggest that very small molecules (20nm) and very large molecules (2MDa) clear very slowly from the SCS and stay in the SCS for weeks to months.22,27 Moreover, poorly soluble drugs and particles such as triamcinolone acetonide may remain in the SCS for months.19 Further research is needed in this area for the development of molecules and drugs that can take advantage of these properties of the SCS and have longer-lasting, therapeutic levels at the target tissue.5
Pharmacotherapies for the SCS:
Retinal vein occlusion and macular edema:
Studies have evaluated the efficacy of SCS injection in patients with macular edema resulting from diabetes mellitus or retinal vascular occlusion. A small study evaluated the feasibility and efficacy of SCS cannulation and submacular injection of single dose of bevacizumab and triamcinolone in treating macular edema and submacular hard exudates. The authors concluded that this treatment is safe and is associated with significant decrease in macular thickness, resolution of hard exudates, and improvement in vision.28
The use of microneedles for suprachoroidal delivery of triamcinolone acetonide has been evaluated more extensively with the completion of two phase 1/2 open-label studies evaluating the safety and efficacy of this delivery system in both diabetic macular edema and retinal vein occlusion associated with macular edema. The HULK trial evaluated SCS injection of a 4mg triamcinolone acetonide suspension (Zuprata®, Clearside Biomedical) as monotherapy or in conjunction with intravitreal aflibercept (Eylea®, Regeneron Pharmaceuticals) in 20 patients with diabetic macular edema, and demonstrated a decrease in macular thickness and improvement in visual acuity, mostly in treatment naïve eyes.29 The TANZANITE study compared intravitreal aflibercept with and without suprachoroidal triamcinolone acetonide in eyes with macular edema secondary to retinal vein occlusion.19 After 2 months, the mean visual acuity improvement was significantly higher in the combination arm, and at 3 months, the number of retreatments with as-needed intravitreal aflibercept was reduced by 61%. Although suprachoroidal triamcinolone was associated with an increase in intraocular pressure (IOP) in 4 patients (17.4%) compared to none in the aflibercept monotherapy group, all patients were controlled by IOP lowering drops only.19 Interestingly, EDI-OCT of patients who received the suprachoroidal triamcinolone injection also demonstrated a 30% expansion in the SCS thickness up to 3 months after the treatment.30,31 While it is unclear if this may be a pharmacologic or mechanical effect of the SCS injection, the prospect of potentially using OCT imaging to monitor suprachoroidal drug delivery warrants further investigation.
Age-related macular degeneration:
To date, intravitreal injections remain the mainstay delivery method of anti-vascular endothelial growth factor (anti-VEGF) therapies in the eye. SCS injection of current anti-VEGF agents is limited by a short half-life, likely due to the molecular weight of these drugs. Animal studies comparing intravitreal and SCS injection of bevacizumab showed a more sustainable concentrations of drug in the target tissue if injected intravitreally.25 To develop a more sustained anti-VEGF formulation for use in the SCS, one group developed a light-activated, in-situ-forming gel network cross-linked with bevacizumab which can achieve sustained release of the drug from the SCS for up to 4 months in rat models.32
Although there are no existing therapies for non-exudative AMD and geographic atrophy, cell-based therapies are currently under investigation. While most of these strategies rely on subretinal injections that require pars plana vitrectomy, a suprachoroidal approach has gained traction as an alternative to avoid complete penetration of the globe.5 In a recent study, Limoli et al. used growth factor-secreting autologous cell grafts in the suprachoroidal space of patients with non-exudative AMD and showed a significant improvement in visual acuity and microperimetry responses.33,34 A phase 2 multi-center clinical trial (PRELUDE) is also underway to evaluate the safety and efficacy of subretinal stem cell injections accessed by a suprachoroidal surgical approach in patients with AMD-related geographic atrophy (CNTO 2476).35
Non-infectious uveitis:
Preclinical studies in animals have shown that SCS injection of both low and high doses of triamcinolone acetonide (0.2mg and 2mg, respectively) reduce inflammation in a porcine model of acute posterior uveitis. When used intravitreally, however, only the higher dose was effective.36 Dexamethasone-containing suprachoroidal implants has also demonstrated efficacy in a rat model of uveitis.37 Advantages of the SCS approach includes higher concentrations and sustained steroid delivery in the posterior pole, while also reducing drug exposure in the anterior segment. Suprachoroidal delivery of 4 mg triamcinolone acetonide suspension using microneedles has been shown to be safe and effective in eyes with macular edema secondary to non-infectious uveitis in a phase 1/2 open-label clinical study.38 The results of the subsequent phase 3 clinical trial evaluating this treatment (PEACHTREE) were recently announced with positive results. Patients randomized to the treatment group received two SCS injections of triamcinolone 12 weeks apart and the control group received sham procedures. After 24 weeks, 47% of patients in the injection group and 16% in the control group gained ≥ 15 ETDRS letters compared to baseline (P<0.001). Moreover, patients in the treatment group demonstrated a significant decrease in the central subfield thickness compared to the control group, although there was also IOP increase in 11.5% of patients in the treatment group compared to none in the sham group.39
Hereditary retinal diseases:
Gene therapy is opening new horizons for the treatment of degenerative retinal diseases like Leber’s congenital amaurosis and retinitis pigmentosa. Typically, viral vectors carrying the wildtype form of the defective gene are injected into the subretinal space during pars plana vitrectomy.5 In contrast, the SCS provides a less invasive route for delivery of vectors, and has shown some promise in early animal models.40,41
Glaucoma:
Targeted delivery of anti-glaucoma medications to the supraciliary space has been evaluated in 2 animal studies. SCS injection of IOP-lowering drugs in these studies was associated with a long-lasting decrease in IOP. This effect was in a dose-sparing fashion, i.e. the supraciliary dose needed to achieve response was 100-fold less than the dose needed for topical delivery.42,43
Surgery using the SCS:
Retinal prosthesis implantation:
Various locations have been explored for the placement of retinal prostheses including intraocular (epiretinal and subretinal), intrascleral, and extrascleral.44 The first retinal prosthesis that is commercially-available to patients (Argus® II, Second Sight) involves an epiretinal implantation technique that involves pars plana vitrectomy and is challenging to perform, with inherent risks such as retinal detachment and endophthalmitis.45 Implantation of the device in or on the sclera has a theoretically lower risk profile, but may be less effective given the greater distance from the retina and photoreceptors. Because in all these methods the field of vision is determined by the size and surface area of the implanted array, the use of larger electrodes or multiple electrodes arrays could further increase surgical risk when implanting epiretinal or subretinal prostheses.44
To address these challenges, the SCS has been evaluated as a potential location for retinal prosthesis implantation. The first human trial to test suprachoroidal prosthesis implantation was performed by the Bionic Vision Australia (BVA) Research Consortium in 3 patients with end-stage retinitis pigmentosa and visual acuity of bare light perceptions or less in both eyes. No intraoperative complications were noted, and patients were followed for 12 months. All 3 patients, however, developed postoperative subretinal and suprachoroidal hemorrhage, which resolved spontaneously and without complication in 2 patients. The third patient developed fibrosis at the edge of the implant after resolution of the bleeding. Device efficacy was evaluated by phosphine perception and was elicited in all patients, with visual acuity noted to be around 20/8000.46 Following this study, BVA developed a new 44-channel suprachoroidal prosthesis which takes advantage of the increased number and size of electrodes and has been successfully tested in a feline model.47 Other animal studies have also shown that it is feasible to implant wide-field electrode arrays48 as well as multiple electrodes in the suprachoroidal space to increase the field of vision.44
Suprachoroidal buckling surgery:
Scleral buckling is typically achieved using a silicone band sutured onto the sclera to alter the globe contour and reduce retinal traction for treating eyes with rhegmatogenous retinal detachments or myopic maculopathies such as foveoschisis or macular hole detachments. Recently, the concept of injecting a viscoelastic material into the SCS has been explored as an alternative method, especially when the buckling effect does not need to be permanent.49,50 In this technique, a suprachoroidal cannula is introduced into the SCS through a sclerotomy and advanced to reach the target area, which may be a peripheral retinal break in rhegmatogenous retinal detachments or underneath the fovea in foveoschisis or macular hole detachments. Viscoelastic (hyaluronic acid) is then injected to indent the choroid and relieve retinal traction. Various viscoelastic options with different durabilities ranging from weeks to a year are available, depending on the duration of indentation indicated.5,49 While this procedure reduces the potential complications associated with silicone bands such as ocular motility disorders, diplopia, buckle exposure and infection, the learning curve is steep and the risk for choroidal hemorrhage is substantial for inexperienced surgeons.50 These challenges have limited the widespread adoption of this technique.
Glaucoma stenting surgery:
Glaucoma filtration surgery has been the mainstay of surgical therapy for intractable glaucoma, but is associated with risks such as bleb leaks and failures, bleb-related endophthalmitis, overhanging blebs or dellen formation, as well as ptosis or eyelid retraction. A number of new MIGS devices have been developed to lower the IOP such as supraciliary microstents (CyPass®, Alcon) that can be performed at the time of cataract surgery. The pivotal clinical trial evaluating 505 patients randomized to phacoemulsification only or combination surgery with supraciliary microstenting (COMPASS) demonstrated sustained reduction in IOP and glaucoma medication use at 2 years, and has led to the device’s FDA approval for mild-to-moderate primary open angle glaucoma.51 Other supraciliary implants include ab interno (iStent®, Glaukos) and ab externo (Gold Micro Shunt, SOLX Corp) devices that have shown promising results in clinical studies.52 As with most new technologies, however, the learning curve of MIGS procedures and reimbursement concerns for these newer devices has been a barrier for widespread adoption of these therapies.
Summary:
With the advent of new technologies to visualize and access the SCS, clinicians and researchers are identifying novel medical and surgical applications that can take advantage of this potential space. The SCS provides not only a novel route for targeted delivery of pharmacological agents, but can also be exploited for surgical treatment of glaucoma and posterior segment conditions. For drug delivery, understanding the pharmacokinetics of drug distribution and clearance in the SCS and their relationship with molecular weight will help tailor the indications and dosing needed to optimize the use of this approach. For surgical approaches, strategies to reduce the learning curve and avoid high-risk complications such as choroidal hemorrhage will be needed to facilitate their incorporation into clinical practice. Future studies are warranted to further explore the potential of the SCS.
REFERENCES:
- 1.Moisseiev E, Loewenstein A, Yiu G. The suprachoroidal space: from potential space to a space with potential. Clin Ophthalmol. 2016;25(10):173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buggage R, Grossniklaus H. Choroid and suprachoroid In: Tasman W, Jaeger A, eds. Duane’s Foundations of Clinical Ophthalmology. Vol 1: Northwestern University: J. B. Lippincott; 1991. [Google Scholar]
- 3.Chiang B, Jung J, Prausnitz M. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev. 2018;Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yiu G, Pecen P, Sarin N, et al. Characterization of the choroid-scleral junction and suprachoroidal layer in healthy individuals on enhanced-depth imaging optical coherence tomography. JAMA Ophthalmol. 2014;132:174–181. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Eliott D. Accessing the Suprachoroidal Space for Therapeutic Delivery. Int Ophthalmol Clin. 2017;57(4):179–192. [DOI] [PubMed] [Google Scholar]
- 6.Spaide R, Koizumi H, Pozzoni M. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. [DOI] [PubMed] [Google Scholar]
- 7.Michalewska Z, Michalewski J, Nawrocki J. Going deeper and going wider. Retinal Physician. 2013;3:42–48. [Google Scholar]
- 8.Yiu G, Vuong V, Oltjen S, et al. Effect of Uveal Melanocytes on Choroidal Morphology in Rhesus Macaques and Humans on Enhanced-Depth Imaging Optical Coherence Tomography. Invest Ophthalmol Vis Sc. 2016;57(13):5764–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Chang Y, Kim J, Lee T, Lew Y. Imaging Suprachoroidal Layer in Exudative Age-Related Macular Degeneration. Curr Eye Res. 2015;41(5):715–720. [DOI] [PubMed] [Google Scholar]
- 10.Yiu G, Chiu S, Petrou P, et al. Relationship of central choroidal thickness with age-related macular degeneration status. J Ophthalmol. 2015;159(4):617–626. [DOI] [PubMed] [Google Scholar]
- 11.Wong S, Vuong V, Cunefare D, Farsiu S, Moshiri A, Yiu G. Macular Fluid Reduces Reproducibility of Choroidal Thickness Measurements on Enhanced Depth Optical Coherence Tomography. Am J Ophthalmol. 2017;184:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey A, Sarkisian S, Vold S. Ab interno approach to the suprachoroidal space. J Cataract Refract Surg. 2014;40(8):1291–1294. [DOI] [PubMed] [Google Scholar]
- 13.Ghate D, Edelhauser H. Barriers to glaucoma drug delivery. J Glaucoma. 2008;17:147–156. [DOI] [PubMed] [Google Scholar]
- 14.Ethier C, Johnson M, Ruberti J. Ocular biomechanics and biotransport. Annu Rev Biomed Eng. 2004;6:249–273. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Chiang B, Wu X, Prausnitz M. Ocular delivery of macromolecules. J Control Release. 2014;190:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakri S, Snyder M, Reid J, Pulido J, Ezzat M, Singh R. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114:2179–2182. [DOI] [PubMed] [Google Scholar]
- 17.Nomoto H, Shiraga F, Kuno N, et al. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2009;50:4807–4813. [DOI] [PubMed] [Google Scholar]
- 18.Del Amo E, Rimpelä A, Heikkinen E, et al. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017;57:134–185. [DOI] [PubMed] [Google Scholar]
- 19.Campochiaro P, Wykoff C, Brown D, et al. Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study. Ophthalmology. 2017;125(5):622–627. [DOI] [PubMed] [Google Scholar]
- 20.Seiler G, Salmon J, Mantuo R, Feingold S, Dayton P, Gilger B. Effect and distribution of contrast medium after injection into the anterior suprachoroidal space in ex vivo eyes. Invest Ophthalmol Vis Sci. 2011;52(8):5730–5736. [DOI] [PubMed] [Google Scholar]
- 21.Krohn J, Bertelsen T. Corrosion casts of the suprachoroidal space and uveoscleral drainage routes in the human eye Acta Ophthalmol Scand. 1997;75:32–35. [DOI] [PubMed] [Google Scholar]
- 22.Patel S, Berezovsky D, McCarey B, Zarnitsyn V, Edelhauser H, Prausnitz M. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sc. 2012;53:4433–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moseley H, Foulds W. The movement of xenon-133 from the vitreous to the choroid. Exp Eye Res. 1982;34:169–179. [DOI] [PubMed] [Google Scholar]
- 24.Moseley H, Foulds W, Allan D, Kyle P. Routes of clearance of radioactive water from the rabbit vitreous. Br J Ophthalmol. 1984;68:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen T, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron J. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci. 2011;52:4749–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen T, Ferg X, Wabner K, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2006;142(5):777–787. [DOI] [PubMed] [Google Scholar]
- 27.Chiang B, Venugopal N, Grossniklaus H, Jung J, Edelhauser H, Prausnitz M. Thickness and closure kinetics of the suprachoroidal space following microneedle injection of liquid formulations. Invest Ophthalmol Vis Sci. 2017;58:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzo S, Ebert F, Bartolo E, et al. Suprachoroidal drug infusion for the treatment of severe subfoveal hard exudates. Retina. 32:776–784. [DOI] [PubMed] [Google Scholar]
- 29.Wykoff C Suprachoroidal triamcinolone acetonide with and without intravitreal aflibercept for diabetic macular edema: Phase 1/2 HULK study AAO Subspecialty Days; 2017; New Orleans, LA. [Google Scholar]
- 30.Willoughby A, Vuong V, Cunefare D, et al. Choroidal Changes After Suprachoroidal Injection of Triamcinolone Acetonide in Eyes With Macular Edema Secondary to Retinal Vein Occlusion. Am J Ophthalmol. 2018;186:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willoughby A, Vuong V, Cunefare D, et al. Reply. Am J Ophthalmol. 2018;189:178. [DOI] [PubMed] [Google Scholar]
- 32.Tyagi P, Barros M, Stansbury J, Kompella U. Light-activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Mol Pharm. 2013;10(8):2858–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limoli P, Limoli C, Vingolo E, Scalinci S, Nebbioso M. Cell surgery and growth factors in dry agerelated macular degeneration: visual prognosis and morphological study. Oncotarget. 2014;7:46913–46923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Limoli P, Vingolo E, Limoli C, Scalinci S, Nebbioso M. Regenerative Therapy by Suprachoroidal Cell Autograft in Dry Age-related Macular Degeneration: Preliminary In Vivo Report. J Vis Exp. 2018;132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janssen Research & Development L. A Study to Evaluate the Safety and Clinical Response of Subretinal Administration of CNTO 2476 in Participants With Geographic Atrophy (PRELUDE) 2016; https://clinicaltrials.gov/ct2/show/NCT02659098.
- 36.Gilger B, Abarca E, Salmon J, Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophthalmol Vis Sci. 2013;54:2483–2492. [DOI] [PubMed] [Google Scholar]
- 37.Barbosa Saliba J, Vieira L, Fernandes-Cunha G, et al. Anti-inflammatory effect of dexamethasone controlled released from anterior suprachoroidal polyurethane implants on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2016;57:1671–1679. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein D, Noronha G, Kissner J, Srivastava J, Nguyen Q. Suprachoroidal Corticosteroid Administration: A Novel Route for Local Treatment of Noninfectious Uveitis. Transl Vis Sci Technol. 2016;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.http://ir.clearsidebio.com/news-releases/news-release-details/clearside-biomedical-announces-positive-topline-results-pivota. 3/5/2018.
- 40.Peden M, Min J, Meyers C, et al. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PLoS One. 2011;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Touchard E, Berdugo M, Bigey P, et al. uprachoroidal electrotransfer: a nonviral gene delivery method to transfect the choroid and the retina without detaching the retina. Mol Ther. 2012;20:1559–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang B, Kim Y, Doty A, Grossniklaus H, Schwendeman S, Prausnitz M. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J Control Release. 2016;228:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y, Edelhauser H, Prausnitz M. Targeted delivery of antiglaucoma drugs to the supraciliary space using microneedles. Invest Ophthalmol Vis Sc. 2014;55:7387–7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdallah W, Li W, Weiland J, Humayun M, Ameri H. Implantation of multiple suprachoroidal electrode arrays in rabbits. J Curr Ophthalmol. 2017;9(30):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghodasra D, Chen A, Arevalo J, et al. Worldwide Argus II implantation: recommendations to optimize patient outcomes. BMC Ophthalmol. 2016;16(52). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayton L, Blamey P, Guymer R, et al. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS One. 2014;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abbott C, Nayagam D, Luu C, et al. Safety Studies for a 44-Channel Suprachoroidal Retinal Prosthesis: A Chronic Passive Study. Invest Ophthalmol Vis Sc. 2018;59(3):1410–1424. [DOI] [PubMed] [Google Scholar]
- 48.Villalobos J, Nayagam D, Allen P. A wide-field suprachoroidal retinal prosthesis is stable and well tolerated following chronic implantation. Invest Ophthalmol Vis Sc. 2013;54(5):3751–3762. [DOI] [PubMed] [Google Scholar]
- 49.El Rayes E, Elborgy E. Suprachoroidal Buckling: Technique and Indications. J Ophthalmic Vis Res. 2013;8(4):393–399. [PMC free article] [PubMed] [Google Scholar]
- 50.El Rayes E, Mikhail M, El Cheweiky H, Elsawah K, Maia A. Suprachoroidal buckling for the management of rhegmatogenous retinal detachments secondary to peripheral retinal breaks. Retina. 2017;37:622–629. [DOI] [PubMed] [Google Scholar]
- 51.Vold S, Ahmed I, Craven E, et al. Two-Year COMPASS Trial Results: Supraciliary Microstenting with Phacoemulsification in Patients with Open-Angle Glaucoma and Cataracts. Ophthalmology. 2016;123(10):2103–2112. [DOI] [PubMed] [Google Scholar]
- 52.Figus M, Lazzeri S, Fogagnolo P, Iester M, Martinelli P, Nardi M. Supraciliary shunt in refractory glaucoma. Br J Ophthalmol. 2011;95(11):1537–1541. [DOI] [PubMed] [Google Scholar]