Abstract
Objectives:
The peroneal artery is a well-established target for bypass in patients with critical limb ischemia (CLI). The objective of this study was to evaluate the outcomes of peroneal artery revascularization in terms of wound healing and limb salvage in patients with CLI.
Methods:
Patients presenting between 2006 and 2013 with critical limb ischemia (Rutherford IV- VI) and isolated peroneal runoff were included in the study. They were divided into patients who underwent bypass to the peroneal artery and those who underwent endovascular peroneal artery intervention. Demographics, comorbidities, and follow up data were recorded. Wounds were classified by Wound, Ischemia, foot Infection (WIfI) score. The primary outcome was wound healing; secondary outcomes included mortality, major amputation, and patency.
Results:
Two hundred limbs with peroneal bypass and 138 limbs with endovascular peroneal intervention were included with mean follow-up of 24.0 ± 26.3 and 14.5 ± 19.1 months, respectively (P = .0001). The two groups were comparable in comorbidities with the exception of the endovascular group having more patients with cardiac and renal disease and diabetes mellitus, but fewer patients with smoking history. Based on WIfI criteria, ischemia scores were worse in bypass patients, but wound and foot infection scores were worse in endovascular patients. Peri-operatively, bypass patients had higher rates of myocardial infarction (4.5% vs. 0%, P = .012) and incisional complications (13.0% vs. 4.4%, P = .008). At 12 months, the bypass group compared to the endovascular group had better primary patency (47.9% vs. 23.4%, P = .002) and primary assisted patency (63.6% vs. 42.2%, P = .003) and a trend toward better secondary patency (74.2% vs. 63.5%, P = .11). There were no differences in the rate of wound healing (52.6% vs. 37.7% at one year, P = .09) or freedom from major amputation (81.5% vs. 74.7% at one year, P = .37). In a multivariate analysis, neuropathy was associated with improved wound healing, while WIfI wound score, cancer, chronic renal insufficiency, and smoking were associated with decreased wound healing. Treatment modality was not a significant predictor (P = .15).
Conclusions:
Endovascular peroneal artery intervention results in poorer primary and primary assisted patency rates than surgical bypass to the peroneal artery, but provides similar wound healing and limb salvage rates with a lower rate of complications. In appropriately selected patients, endovascular intervention to treat the peroneal artery is a low-risk intervention that may be sufficient to heal ischemic foot wounds.
Introduction:
Critical limb ischemia (CLI) leads to high rates of major amputation and mortality rates as high as 40% at two years1,2. Patients with tibial artery disease have a higher risk of amputation compared to those with isolated femoropopliteal disease3. Tibial disease is commonly associated with chronic debilitating comorbidities, including diabetes mellitus (DM), coronary artery disease (CAD), and chronic kidney disease (CKD) or end-stage renal disease (ESRD), which place these patients at high risk of peri-operative morbidity and mortality4,5. Historically, distal bypass was the main treatment to avoid limb loss from CLI, but endovascular interventions for infrapopliteal disease have been shown to be similar to distal bypass in limb salvage and amputation-free survival with fewer peri-operative complications6. Although distal bypasses to peroneal artery have good results7,8, there is less evidence that endovascular interventions on an isolated peroneal runoff are effective. We have previously found at our institution that, among all patients undergoing endovascular tibial intervention, those with isolated peroneal runoff had poorer rates of wound healing9, potentially questioning the efficacy of peroneal endovascular intervention compared to peroneal bypass.
The objectives of this study were (1) to evaluate the outcomes after revascularization of an isolated peroneal artery runoff, and (2) to compare peroneal bypass to endovascular peroneal intervention in patients undergoing lower extremity revascularization for critical limb ischemia. We specifically sought to determine whether the two treatment modalities were associated with similar rates of wound healing, limb salvage, and patency rates.
Methods:
This study was approved by the Investigational Review Board at the University of Pittsburgh; due to the retrospective nature of the study, the need for informed consent was waived. We performed a chart review of all consecutive patients presenting to the University of Pittsburgh Medical Center (UPMC) between 2006 and 2013 with CLI (Rutherford stage IV to VI) and tibial occlusive disease who underwent revascularization of an isolated peroneal artery by either surgical bypass or endovascular intervention. Bypasses to the tibioperoneal trunk were included if the peroneal artery was the only runoff. Patients were excluded if the anterior or posterior tibial artery was successfully recanalized by endovascular intervention in the same setting. Thus, our cohort consisted of all patients in whom the peroneal artery was the sole runoff after revascularization. Patients treated for more proximal disease (either by endovascular intervention or bypass) in the same procedure were included. Patients were excluded if the procedure was not conducted at our institution, or an operative report was not available.
Patient demographics, past medical history (diabetes mellitus, chronic kidney disease (CKD), hypertension, hyperlipidemia, coronary artery disease (CAD), coronary artery bypass graft (CABG), congestive heart failure (CHF), history of myocardial infarction, connective tissue disorder, chronic obstructive pulmonary disease (COPD), cancer, cerebrovascular disease (CVA), lower extremity neuropathy and history of smoking), and prior medications were recorded. Wounds were classified based on Wound, Ischemia, foot Infection (WifI) criteria, which have been validated for use in infrapopliteal lesions10,11. Anatomic lesions were characterized by conventional angiography in all patients, and the runoff score, which estimates the runoff resistance and helps to predict infrainguinal revascularization outcomes, was used to quantify the pedal disease burden12. Though runoff scores are designed for determination of bypass outflow resistance, a runoff score was also calculated for the endovascular group as if a peroneal bypass was performed to provide an objective comparison of distal runoff. The runoff score for a peroneal artery bypass is calculated by scoring the degree of occlusion in the pedal arch and the collaterals from the peroneal artery to the anterior and posterior tibial arteries on a 0–3 scale (with 3 indicating complete occlusion) and combining these scores into a 1–10 scale (by double-weighting the pedal arch score, single-weighting the collateral score, and adding one point)13. Details of the intervention, complications of the initial treatment, subsequent interventions, amputations, primary patency, primary assisted patency and secondary patency, mortality and wound healing were all reviewed.
The primary endpoint was successful wound healing, defined as the first time point of complete wound healing as documented either in outpatient or subsequent hospitalization records. The vascular surgeon coordinated wound care in all patients. Wounds were evaluated in the office on a biweekly basis to optimize the wound care strategy. Dressing changes and other aspects of wound care may have been provided by the patient or family members, visiting nurses, or staff at a skilled nursing facility as appropriate for each patient’s individual needs. The secondary endpoints were primary, primary assisted, and secondary patency rates, major amputation (above the ankle level), and death. The subset of patients without tissue loss were excluded for study of wound healing, but included in all other analyses.
Excel (Microsoft, Redmond, WA, USA) was used to compile data. Stata/SE 13.0 (StataCorp, College Station, TX) was used to conduct statistical analyses, under the guidance of a statistician. Categorical data were compared using the Chi-squared test and continuous data were compared with Student’s T-test. Thirty-day outcomes were analyzed by logistic regression. Kaplan-Meier and cumulative incidence analyses were used to calculate time-dependent patency rates, amputation free-survival, wound healing and survival and the log-rank test was used for comparisons between groups. All predictors with P < .20 in the univariate analysis were included in a backward stepwise elimination to create a multivariate Cox regression model. A P-value of .05 was considered significant for all statistical tests, and standard error was <10% in all depicted Kaplan-Meier curves.
Results:
From 450 limbs that had endovascular intervention or bypasses to the peroneal artery or the tibioperoneal trunk, 112 were excluded because the peroneal artery was not the only runoff. Of the remaining 338 limbs, 200 underwent bypass and 138 underwent endovascular intervention. There were 44 bypass patients (22.0%) and 13 endovascular patients (9.4%) with rest pain but no wounds. Mean follow-up was 24.0 ± 26.3 and 14.5 ± 19.1 months, respectively (P = .0001). Baseline characteristics were mostly similar. Patients who underwent endovascular intervention were more likely to have diabetes mellitus (P = .03), CKD (P < .001), ESRD (P = .001), and history of myocardial infarction (P = .005), but less likely to have a smoking history (P < .001). They were more likely to be on dual antiplatelet therapy (P = .049) but less likely to be on statin therapy (P = .01). Pre-operative ABI was lower in the bypass group. When wounds were scored by the WIfI criteria, the wound and foot infection scores were worse in the endovascular group, while the ischemia scores were worse in the bypass group. Pedal runoff scores were nearly identical between the two groups (Table 1).
Table 1:
Baseline characteristics, including WIfI scoring at baseline. ABI: ankle-brachial index. WIfI: Wound, Ischemia, foot Infection.
| Bypass (N = 200) |
Endovascular (N = 138) |
P-value | |
|---|---|---|---|
| Age, mean ± SD | 74.1 ± 12.3 | 76.1 ± 11.4 | .12 |
| Male, n (%) | 117 (58.5) | 79 (57.3) | .82 |
| Diabetes, n (%) | 122 (61.0) | 100 (72.5) | .03 |
| Chronic renal insufficiency, n (%) | 37 (18.5) | 59 (42.8) | < .001 |
| End stage renal disease, n (%) | 12 (6.0) | 24 (17.4) | .001 |
| Hypertension, n (%) | 174 (87.0) | 125 (90.6) | .31 |
| Hyperlipidemia, n (%) | 105 (52.5) | 78 (56.5) | .47 |
| Coronary artery disease, n (%) | 121 (60.5) | 95 (68.8) | .12 |
| History of myocardial infarction, n (%) | 29 (14.5) | 37 (26.8) | .005 |
| Chronic obstructive pulmonary disease, n (%) | 43 (21.5) | 22 (15.9) | .20 |
| Cancer, n (%) | 35 (17.5) | 30 (21.7) | .33 |
| Lower extremity neuropathy, n (%) | 52 (26.0) | 39 (28.3) | .65 |
| Smoking: | |||
| Never, n (%) | 68 (34.0) | 77 (55.8) | < .001 |
| Former, n (%) | 84 (42.0) | 41 (29.7) | |
| Current, n (%) | 48 (24.0) | 20 (14.5) | |
| Antiplatelet: | |||
| None, n (%) | 51 (25.5) | 33 (23.9) | |
| Aspirin, n (%) | 93 (46.5) | 48 (34.8) | .049 |
| Clopidogrel, n (%) | 21 (10.5) | 17 (12.3) | |
| Dual, n (%) | 35 (17.5) | 40 (29.0) | |
| Anticoagulation, n (%) | 50 (25.0) | 29 (21.0) | .40 |
| Statin, n (%) | 124 (62.0) | 66 (47.8) | .01 |
| Prior ipsilateral intervention: | |||
| Endovascular, n (%) | 43 (21.5) | 23 (16.7) | .27 |
| Open, n (%) | 55 (27.5) | 31 (22.5) | .30 |
| Pre-operative ABI: | |||
| ≥ 0.6, n (%) | 13 (8.8) | 42 (40.4) | |
| 0.30 – 0.59, n (%) | 67 (44.7) | 30 (28.9) | < .001 |
| < 0.3, n (%) | 49 (32.7) | 15 (14.4) | |
| Non-compressible, n (%) | 21 (14.0) | 17 (16.4) | |
| WIfI Wound Score: | |||
| None, n (%) | 44 (22.0) | 13 (9.4) | |
| Minor, n (%) | 56 (28.0) | 42 (30.4) | .01 |
| Major, n (%) | 88 (44.0) | 67 (48.6) | |
| Extensive, n (%) | 12 (6.0) | 16 (11.6) | |
| WIfI Ischemia Score: | |||
| None, n (%) | 3 (1.5) | 14 (10.1) | |
| Mild, n (%) | 9 (4.5) | 21 (15.2) | < .001 |
| Moderate, n (%) | 31 (15.5) | 21 (15.2) | |
| Severe, n (%) | 104 (52.0) | 43 (31.2) | |
| Unknown, n (%) | 53 (26.5) | 39 (28.3) | |
| WIfl Foot Infection Score: | |||
| None, n (%) | 149 (74.5) | 83 (60.1) | |
| Mild, n (%) | 36 (18.0) | 30 (21.7) | .004 |
| Moderate, n (%) | 15 (7.5) | 25 (18.1) | |
| Severe, n (%) | 0 (0.0) | 0 (0.0) | |
| Pedal runoff score, mean ± SD | 7.42 ± 1.71 | 7.47 ± 1.79 | .81 |
Among patients who underwent bypass, the inflow was suprageniculate in 92.0% and infrageniculate in 8.0%. The target artery was the peroneal artery in 92.5% and the tibioperoneal trunk in 7.5%. Great saphenous vein was used in 70.5%, with the remainder requiring alternative autologous vein (23.5%) or prosthetic conduit (6.0%). Thirty-eight patients (19.0%) underwent a concomitant procedure to improve the inflow, either by endovascular or open intervention. Among patients who underwent endovascular intervention, the peroneal artery was the only treated tibial artery in 75.4%; in the remainder of patients, an attempted recanalization of the anterior and/or posterior tibial arteries was unsuccessful. In the peroneal artery, balloon angioplasty was performed in all cases, along with atherectomy in 5.8% and adjunctive stenting in 6.5%. An iatrogenic dissection of the target artery after balloon angioplasty was found in 14 cases (10.1%) and was treated by observation in 8 cases, stenting in 3 cases and prolonged inflation in 3 cases. Ninety-four patients (68.1%) underwent concomitant femoropopliteal or iliac intervention. One patient underwent a common femoral artery to below-knee popliteal artery bypass and peroneal artery balloon angioplasty in the same setting (a femoro-peroneal bypass was not performed due to conduit limitations). The remainder received endovascular intervention.
Post-operative outcomes are summarized in Table 2. Hospital stay was longer after bypass surgery than endovascular intervention (7.26 days vs 4.80 days, P < .001). At 30 days, patients who underwent a bypass had higher rates of myocardial infarction (4.5% vs 0.0%, P = .01) and surgical site complications (13.0% vs 4.4%, p= .008) and re-operations for surgical site complications (7.0% vs 0.0%, P = .001). There was no difference between groups in 30-day major adverse limb event (MALE) or major adverse cardiovascular event (MACE) rates. The overall number of re-interventions was similar in both groups. Post-operative ABI was higher in the bypass group, and patients were more likely to have improvement in their ABI after bypass compared to endovascular intervention (40.5% vs 28.3%, P < .001). Overall 30-day mortality and major amputation were 4.4% and 5.0% respectively with no difference between groups (P = .64 and P = .21, respectively).
Table 2:
Post-operative outcomes. UTI: Urinary Tract Infection, DVT: Deep Venous Thrombosis, TIA: Transient Ischemic Attack.
| Bypass (N = 200) |
Endovascular (N = 138) |
P-value | |
|---|---|---|---|
| Hospital stay (days), mean ± SD | 7.26 ± 4.90 | 4.80 ± 5.40 | < .001 |
| Complications (30-day): | |||
| Cardiopulmonary arrest, n (%) | 9 (4.5) | 7 (5.1) | .81 |
| Death, n (%) | 8 (4.0) | 7 (5.1) | .64 |
| UTI/Urinary retention, n (%) | 16 (8.0) | 11 (8.0) | .99 |
| DVT, n (%) | 3 (1.5) | 1 (0.7) | .65 |
| Minor amputation, n (%) | 29 (14.5) | 20 (14.5) | .999 |
| Major amputation, n (%) | 13 (6.5) | 4 (2.9) | .21 |
| Myocardial Infarction, n (%) | 9 (4.5) | 0 (0.0) | .01 |
| Renal Failure, n (%) | 11 (5.0) | 12 (8.7) | .25 |
| Pneumonia, n (%) | 2 (1.0) | 3 (2.2) | .40 |
| Stroke/TIA, n (%) | 4 (2.0) | 0 (0.0) | .15 |
| Wound complication, n (%) | 26 (13.0) | 6 (4.4) | .008 |
| Infection, n (%) | 16/26 (61.5) | 2/6 (33.3) | |
| Hematoma, n (%) | 8/26 (30.8) | 4/6 (66.7) | |
| Other, n (%) | 5/26 (19.2) | 0 (0.0) | |
| Reoperation for wound, n (%) | 14 (7.0) | 0 (0.0) | .001 |
| Graft hemorrhage, n (%) | 6 (3.0) | - | - |
| Graft thrombosis, n (%) | 18 (9.0) | - | - |
| 30-Day Safety | |||
| MALE, n (%) | 23 (11.5) | 15 (10.9) | .86 |
| MACE, n (%) | 12 (6.0) | 7 (5.1) | .72 |
| Post-operative ABI: | |||
| ≥ 0.6, n (%) | 81 (68.1) | 39 (39.8) | |
| 0.30−0.59, n (%) | 9 (7.6) | 9 (9.2) | < .001 |
| < 0.3, n (%) | 4 (3.4) | 6 (6.1) | |
| Non-compressible, n (%) | 25 (21.0) | 44 (44.9) | |
At 12 months, primary patency was better in the bypass group (47.9% vs. 23.4%, P = .002), as was primary assisted patency (63.6% vs. 42.2%, P = .004; Figures 1A and 1B). There was a trend toward better secondary patency in the bypass group (74.2% vs. 63.5%, P = .12; Figure 1C). During the follow-up period, the mean number of re-interventions was similar between groups (0.62 vs. 0.72, P = .43). Fifteen patients (10.9%) who had originally undergone endovascular peroneal intervention underwent a subsequent peroneal bypass, and 11 patients (5.5%) who had originally undergone peroneal bypass underwent a subsequent endovascular peroneal intervention.
Figure 1:
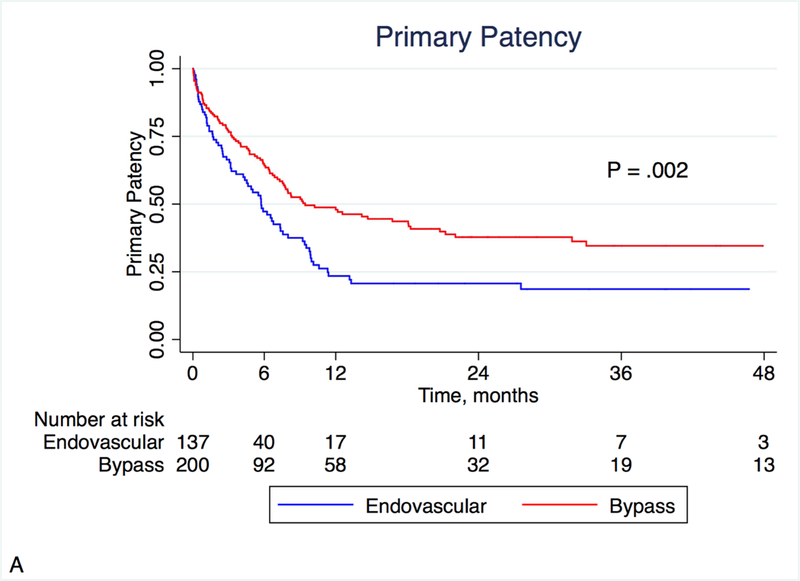
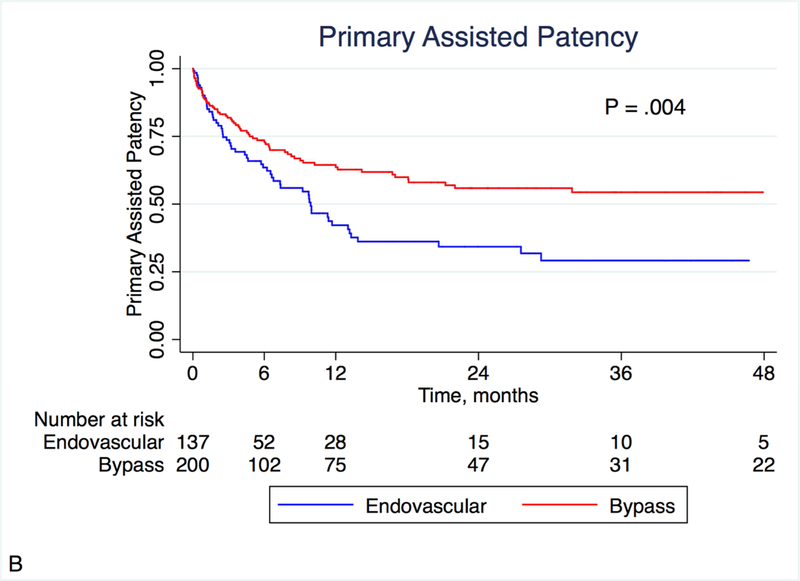
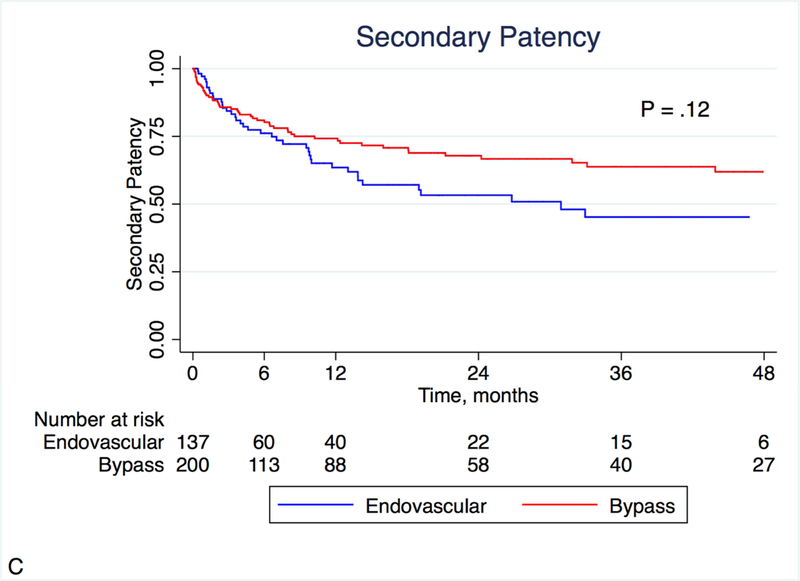
Kaplan-Meier estimates of patency, stratified by treatment modality. Primary (A) and primary assisted patency (B) were significantly different, with a similar non-significant trend noted in secondary patency (C).
There was no difference between the bypass and endovascular groups with respect to either wound healing (in the 281 patients with wounds; P = .09) or major amputation (P = .37) on Kaplan-Meier analysis (Figures 2 and 3; patients were censored for analysis of wound healing if they underwent a major amputation). At one year, life-table estimated wound healing rates were 52.6% in the bypass group and 37.7% in the endovascular group (P = .09), suggesting potentially faster healing in the bypass group. In a multivariate analysis, neuropathy was associated with improved wound healing (HR 1.82, 95% CI 1.02–3.24, P = .042) while extensive wound (HR 0.25, 95% CI 0.09–0.75, P = .013), cancer (HR 0.45, 95% CI 0.22–0.89, P = .023), CKD (HR 0.45, 95% CI 0.25–0.83, P = .010) and smoking (HR 0.37, 95% CI 0.14–0.93, P = .036) were associated with decreased wound healing. Treatment modality was not a significant predictor of wound healing in the multivariate model (P = 0.15; Table 3). Overall survival was better for patients who underwent a peroneal bypass compared to the endovascular group (P = .0001; Figure 4). At 4 years, survival was 27.5% in the bypass group and 14.5% in the endovascular group.
Figure 2:
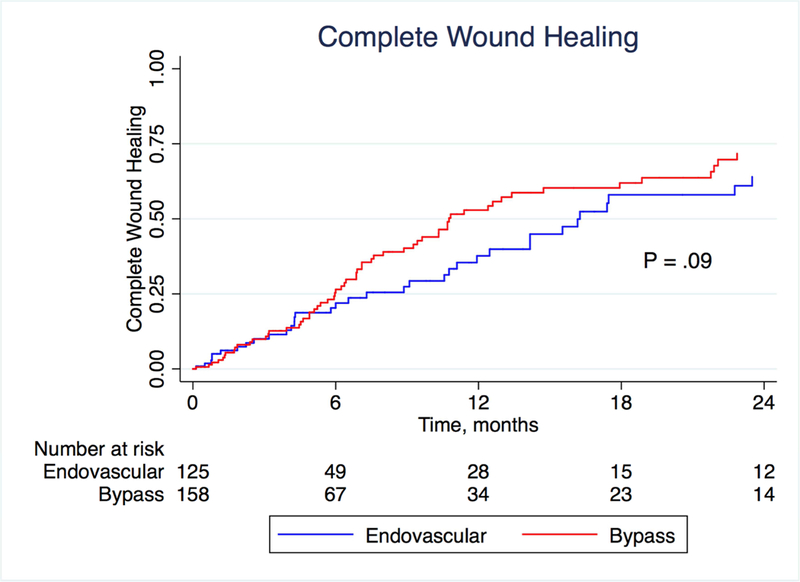
Kaplan-Meier estimates of complete wound healing, stratified by treatment modality.
Figure 3:
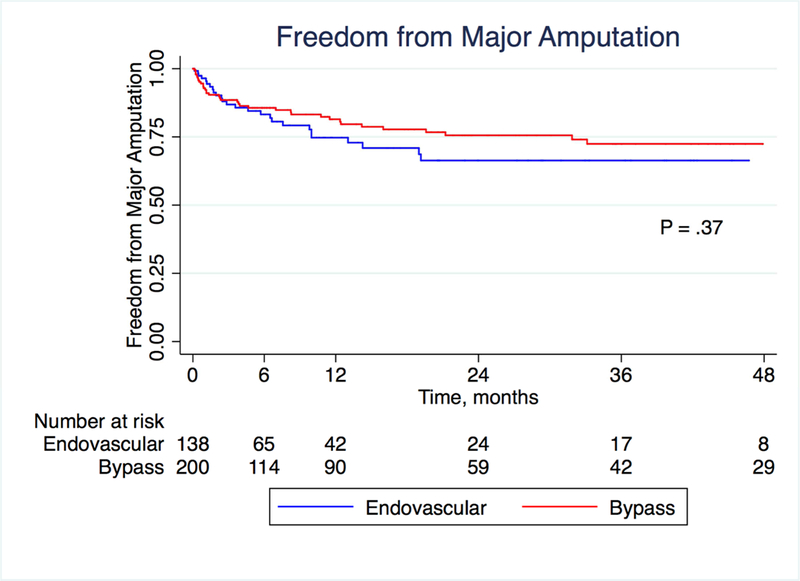
Kaplan-Meier estimates of freedom from major amputation, stratified by treatment modality.
Table 3:
Multivariate predictors of wound healing. Note that in this context, HR > 1 is favorable and indicates higher rate of successful healing.
| Predictor | Hazard Ratio | P-Value |
|---|---|---|
| Neuropathy | 1.82 | .04 |
| Bypass vs. Endovascular | 1.49 | .15 |
| WIfl wound score | ||
| Minor | 1 | - |
| Major | 0.79 | .39 |
| Extensive | 0.25 | .01 |
| Smoking | ||
| Never | 1 | - |
| Former | 1.04 | .88 |
| Current | 0.37 | .04 |
| Cancer | 0.45 | .02 |
| Chronic renal insufficiency | 0.45 | .01 |
Figure 4:
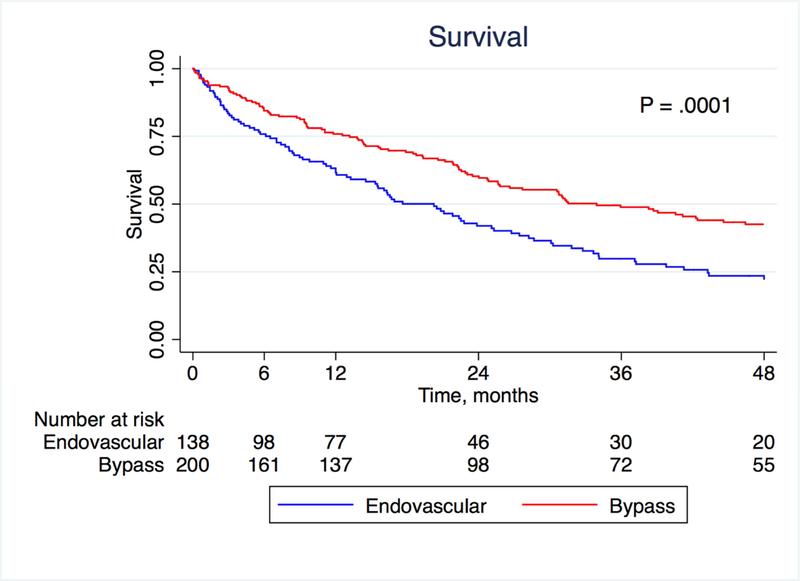
Kaplan-Meier estimates of survival, stratified by treatment modality.
Discussion:
In this study, we sought to describe the patency and limb-related outcomes in patients undergoing revascularization of an isolated peroneal runoff, and to determine whether patients undergoing endovascular intervention had similar outcomes to those undergoing surgical bypass. We performed a retrospective chart review of such patients at our institution to compare these two groups of patients.
In the infrapopliteal arteries, surgical bypass continues to be the standard against which any novel therapy is compared. Patients with CLI often have a number of medical comorbidities that place them at higher risk of peri-operative complications and long-term mortality3,14. Endovascular tibial intervention provides an attractive alternative in part because it provides improved peri-operative outcomes. Indeed, in our study we found that patients undergoing endovascular peroneal intervention had shorter lengths of stay and fewer cardiac and surgical site complications without any increase in adverse limb outcomes, compared to those undergoing peroneal bypass. In patients who are felt to be poor surgical candidates, our results showed that 30-day outcomes were favorable after endovascular intervention.
In follow-up of our cohort, we found that bypass was associated with improved patency compared to endovascular intervention. This difference was most notable in primary patency rates, which were approximately twice as high in the bypass group. The difference was less impressive and no longer statistically significant when comparing secondary patency rates, which suggests that re-interventions are more critical to the success of an endovascular treatment strategy. Our patency findings imply that re-intervention rates may be higher in the endovascular patients; however, we noted no difference in the mean number of re-interventions between the two groups. We suspect this is the case for two reasons. First, follow-up was shorter in the endovascular group and a longer follow-up period may have shown more re-interventions in patients who received endovascular intervention. Second, we have differing strategies with regard to re-interventions in these two groups of patients. In patients with a bypass, reinterventions are performed for any >50% stenosis on surveillance duplex ultrasound studies to prevent graft thrombosis regardless of whether clinical endpoints (such as wound healing or resolution of rest pain) are achieved. In patients with an endovascular intervention, a re-stenosis or occlusion is generally not treated unless clinical symptoms recur or fail to improve.
Despite the differences in patency rates between the two treatment modalities, we observed no difference in long-term limb-related outcomes in our cohort. The rates of wound healing and major amputation were similar between groups. Our Kaplan-Meier estimated wound healing rates were somewhat low in both groups; in addition to severe arterial insufficiency, the patient population studied had high rates of diabetes and renal dysfunction, both known to impair wound healing. Furthermore, poor nutritional status is likely common in this population but was difficult to assess retrospectively. We did note a higher long-term mortality rate in the patients who underwent endovascular intervention, without any associated increase in 30-day mortality. We suspect this is a result of selection bias, in that patients who were felt to be good candidates for a distal bypass operation were likely at decreased risk of all-cause mortality. This is supported by the higher rates of medical comorbidities in the endovascular group.
The peroneal artery is unique in that it is often relatively spared of atherosclerotic disease compared to the anterior and posterior tibial arteries, but terminates at the ankle and perfuses the foot indirectly via collateral branches; in light of traditional mantra that in-line flow must be achieved to heal wounds, the anatomy of the peroneal artery calls to question whether it would be an appropriate bypass target in patients with CLI. Darling, et al previously studied bypasses to the peroneal artery as compared to those to a dorsalis pedis artery, finding a 76% secondary patency and 93% limb salvage rate at 5 years; the authors concluded the peroneal artery was a good alternative to a pedal target in the presence of conduit limitations or foot infection in close proximity to the pedal target8. No studies to date have specifically described the patency rates of peroneal artery endovascular interventions in a cohort of patients with an isolated peroneal artery runoff. Two studies, however, have compared the outcomes after infrainguinal intervention in patients with peroneal artery-only runoff to those with other runoff15,16. A large fraction of patients in both studies underwent isolated femoropopliteal interventions, and thus their patency rates cannot be compared to ours; however, both groups of investigators concluded that isolated peroneal runoff by itself does not negatively affect outcomes after revascularization. Similar to these prior studies, we found overall acceptable patency and limb salvage rates with any form of peroneal artery revascularization, further supporting the use of the peroneal artery as a bypass or endovascular intervention target despite its lack of direct perfusion to the foot.
There are a number of limitations to this retrospective study. Most notably, selection bias plays a role in the treatment modality that patients are offered. Patients who underwent endovascular intervention had higher rates of many medical comorbidities and are inherently different than patients who were offered a surgical bypass. Specifically, the endovascular group had higher rates of diabetes and renal impairment than the bypass group; both of these risk factors are well- known to be associated with calcified lesions in the infrapopliteal segment3. We did account for this to the extent possible with the use of multivariate regression modeling techniques, but cannot account for other unknown or unmeasured confounders that distinguish the two groups. High rates of disease recurrence after balloon angioplasty in calcified lesions may explain the inferior patency of endovascular intervention in this arena. Conduit limitations may have affected the treatment decision, as most surgeons would favor endovascular intervention more strongly in patients without adequate vein. Bias between surgeons with regard to indications is also possible, and may affect outcomes of the two therapies, but the collaborative nature of our practice likely limits this effect. In addition, we were unable to study more granular characteristics of wounds, such as their size, location on the foot, or intensity or quality of wound care, which may impact their healing. Likewise, more granular arterial lesion characteristics such as length or degree of calcification were not available for review. Furthermore, high rates of both mortality and loss to follow-up decreased the number of patients in whom accurate wound healing documentation could be acquired.
Prospective, randomized studies in this area, such as the Best Endovascular vs. Best Surgical Therapy in Patients With Critical Limb Ischemia (BEST-CLI) trial17, would benefit from comparison groups with more similar baseline risk factors and improved documentation and characterization of wound location and severity. However, the immense heterogeneity in clinical and anatomic disease patterns poses a significant challenge to completion of these studies and interpretation of their results, and thus surgeons must often rely on retrospective work to address such clinical questions. Based on our observational findings, endovascular peroneal interventions seem to allow for fewer perioperative complications and similar wound healing and limb salvage rates compared to bypass surgery, at the cost of less durable patency rates.
Conclusions:
Endovascular peroneal artery intervention results in lower primary and primary assisted patency rates compared to surgical bypass to the peroneal artery, but may offer similar rates of wound healing and limb salvage in short-term follow-up. In appropriately selected patients, endovascular intervention to treat the peroneal artery is a low-risk intervention that may be sufficient to heal ischemic foot wounds.
Acknowledgments
This work is partially funded by a grant from the National Institutes of Health (grant number 5T32HL098036–08).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg 2015;62:1642–1651. [DOI] [PubMed] [Google Scholar]
- 2.Farber A, Eberhardt RT. The Current State of Critical Limb Ischemia. JAMA Surg 2016;151:1070. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Shi Y, Wang Y, Li X. Patterns of Disease Distribution of Lower Extremity Peripheral Arterial Disease. Angiology 2015;66:211–8. [DOI] [PubMed] [Google Scholar]
- 4.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-society consensus for the management of peripheral arterial disease. Int Angiol 2007;26:81–157. [PubMed] [Google Scholar]
- 5.Alzamora MT, Forés R, Baena-DÍez JM, Pera G, Toran P, Sorribes M, et al. The peripheral arterial disease study (PERART/ARTPER): prevalence and risk factors in the general population. BMC Public Health 2010;10:38.20529387 [Google Scholar]
- 6.Söderström MI, Arvela EM, Korhonen M, Halmesmäki KH, Albäck AN, Biancari F, et al. Infrapopliteal percutaneous transluminal angioplasty versus bypass surgery as first-line strategies in critical leg ischemia: a propensity score analysis. Ann Surg 2010;252:765–73. [DOI] [PubMed] [Google Scholar]
- 7.Yasa H, Cakir C, Tetik O, Akyüz M, Gökalp O, Karahan N, et al. Bypass grafting for infrapopliteal occlusive disease with poor distal flow on angiography. Anadolu Kardiyol Derg 2008;8:444–8. [PubMed] [Google Scholar]
- 8.Darling RC, Chang BB, Shah DM, Leather RP. Choice of peroneal or dorsalis pedis artery bypass for limb salvage. Semin Vasc Surg 1997;10:17–22. [PubMed] [Google Scholar]
- 9.Fernandez N, McEnaney R, Marone LK, Rhee RY, Leers S, Makaroun M, et al. Predictors of failure and success of tibial interventions for critical limb ischemia. J Vasc Surg 2010;52:834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills JL, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfl). J Vasc Surg 2014;59:220–34. [DOI] [PubMed] [Google Scholar]
- 11.Darling JD, McCallum JC, Soden PA, Meng Y, Wyers MC, Hamdan AD, et al. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system following infrapopliteal endovascular interventions for critical limb ischemia. J Vasc Surg 2016;64:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biancari F, Albäck A, Ihlberg L, Kantonen I, Luther M, Lepäntalo M. Angiographic runoff score as a predictor of outcome following femorocrural bypass surgery. Eur J Vasc Endovasc Surg 1999;17:480–5. [DOI] [PubMed] [Google Scholar]
- 13.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J Vasc Surg 1997;26:517–38. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Chen J, Mohler ER, Xie D, Shlipak MG, Townsend RR, et al. Risk Factors for Peripheral Arterial Disease among Patients with Chronic Kidney Disease. Am J Cardiol 2012;110:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abularrage CJ, Conrad MF, Haurani MJ, Crawford RS, Hackney LA, Lee H, et al. Long-term outcomes of patients undergoing endovascular infrainguinal interventions with singlevessel peroneal artery runoff. J Vasc Surg 2011;53:1007–13. [DOI] [PubMed] [Google Scholar]
- 16.Dosluoglu HH, Cherr GS, Lall P, Harris LM, Dryjski ML. Peroneal artery-only runoff following endovascular revascularizations is effective for limb salvage in patients with tissue loss. J Vasc Surg 2008;48:137–43. [DOI] [PubMed] [Google Scholar]
- 17.Menard MT, Farber A, Assmann SF, Choudhry NK, Conte MS, Creager MA, et al. Design and Rationale of the Best Endovascular Versus Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST-CLI) Trial. J Am Heart Assoc 2016;5:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


