Abstract
Symptomatic normal tissue injury is a common side effect following definitive therapeutic radiation and chemotherapy treatment for a variety of malignancies. These cancer therapy related toxicities may occur acutely during treatment resulting in reduced or missed therapy agent administration or after the completion of therapy resulting in significant chronic morbidities that significantly diminish patient quality of life. Radiation and chemotherapy induce the formation of reactive oxygen species (ROS) both in normal tissues and tumor cells. One type of ROS common to both chemotherapy and radiation therapy is the formation of superoxide (O2•−). Fortunately, due to metabolic differences between cancer and normal cell metabolism, as well as improved targeting techniques, ROS generation following radiation and chemotherapy is generally greater in cancer cells compared to normal tissues. However, the levels of ROS generated in normal tissues are capable of inducing significant toxicity. Thus, several groups are focusing on metabolism-based approaches to mitigate normal tissue effects occurring both during and following cancer therapy. This review will summarize the most current pre-clinical and clinical data available demonstrating the efficacy of small molecule, superoxide dismutase mimetics in minimizing radiation and chemotherapy-induced normal tissue injury, resulting in enhanced patient outcomes.
Introduction
Reactive oxygen species (ROS) generated under physiological conditions serve an essential role as signaling molecules in cell processes ranging from cell proliferation to apoptosis. However, when generated under non-physiologic, stress-related conditions, such as during therapeutic radiation and chemotherapy administration, ROS becomes toxic both to tumor cells and surrounding normal tissue1–3. Radiation and chemotherapy associated normal tissue toxicities may occur acutely during treatment as well as persisting months to years after radiation and chemotherapy are completed. The toxicities from cancer therapies cause changes in mitochondrial metabolism that lead to formation of several ROS.
Superoxide (O2•−) is a critical ROS formed during cancer therapy. At physiologic concentrations, O2•− is essential to normal signaling processes but with increased cellular concentrations induces toxicity. Physiological levels of O2•− are generated during metabolic respiration, and by the activity of cytochrome p450 enzyme isoforms, alpha-ketoglutarate dehydrogenase, glycerol-3-phosphate dehydrogenase, and NADPH-dependent oxidases4. When cells are stressed, more O2•− is generated, as is observed during and following treatment with anti-cancer therapies, including chemotherapy5 and radiation therapy6,7. Superoxide dismutase enzymes (SODs) convert O2•− to hydrogen peroxide (H2O2). The H2O2 is subsequently converted to H2O and O2 by catalase or other peroxidases.
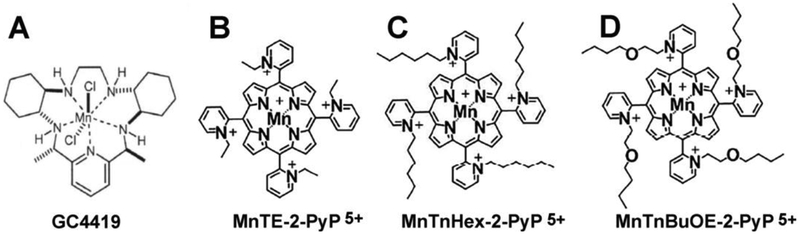
During radiation and chemotherapy, the cooperation between SOD enzymes and peroxidases are not in balance resulting in oxidative damage. In the past 30 years, several groups have developed and utilized small molecule superoxide dismutase mimetics that are administered prior to and/or during radiation and chemotherapy to minimize increased steady-state levels of O2•− in order to mitigate normal tissue injury. The following is a brief review of two such compounds: Galera Compound 4419 (GC4419) and Mn porphyrin-based SOD mimics (Figure 1).
Figure 1. The chemical structures of the SOD mimics discussed in this chapter.
A. GC4419 (M40403) B. MnTE-2-PyP (MnE, BMX-010) C. MnTnHex-2-PyP (MnHex) D. MnTnBuOE-2-PyP (MnBuOE, BMX-001).
GC4419 (formerly known as M40419)
GC4419 is a member of the pentaaza-macrocyclic Mn(II) class of SOD mimetics that catalyzes the dismutation of O2•− into H2O2 at a rate constant of 2 × 107 M−1 s−1, which is comparable to the native SOD enzyme (2 × 109 M−1 s−1)8, 9. GC4419 selectively removes superoxide without reacting with other reactive oxygen species, including nitric oxide, hydrogen peroxide, and peroxynitrite. The protective effects of GC4419 or an active enantiomer (GC4403 formerly known as M40403) against radiation-induced toxicity in cancer but not normal cells have been demonstrated in vitro as well as in animal models.10–12 The selective protection GC4419 for normal and not cancer cells is thought to arise because of fundamental differences in cancer cell mitochondrial oxidative metabolism leading to greater steady-state levels of O2•−, which in turn leads to selective increases in steady-state levels of H2O2 in cancer cells treated with GC4419 and not normal cells.13 Furthermore, GC4419 has been shown to reduce chemoradiation-induced mucositis in clinical trials.14
Mitigating Oral Mucositis
Oral mucositis (OM) is a common side effect of chemoradiation (chemoRT) for head and neck squamous cell carcinoma that is painful, disruptive and difficult to manage15. Approximately 70% of patients receiving concurrent cisplatin and radiation for oral cavity and oropharynx cancer will experience severe oral mucositis (SOM)16, 17. The median time to development of SOM is the 4th week of a 6–7 week treatment course and the median duration is 3–4 weeks. Patients who suffer this painful complication commonly require narcotic analgesics and other medications for pain. OM adversely affects nutrition, hydration, speech, swallowing, quality of life, bacteremia risk, and feeding tube placement and use rates, leading to increased hospitalization, emergency room use, and healthcare costs18–21. Importantly, SOM is also associated with chemoRT treatment breaks, which is known to impact tumor control rates in head and neck cancer22, 23.
Evidence-based management of OM is limited to palliation and pain control with topical agents and systemic analgesics24–32. The only FDA-approved drug to reduce OM, palifermin, is a keratinocyte growth factor that is indicated for mitigating OM only in patients with hematologic malignancies receiving myelotoxic therapy requiring hematologic stem cell support.16, 17, 33. Therefore, there is an urgent need to mitigate OM and several drugs are currently in Phase II/III testing.34
A Phase 1b/2a trial tested GC4419 in combination with intensity-modulated radiation (IMRT) and concurrent cisplatin (either 100 mg/m2 every three weeks or 30–40 mg/m2 weekly) in patients with American Joint Committee on Cancer version 7 Stage IIIIVb oral cavity and oropharynx squamous cell carcinoma.14 The IMRT plan had to include at least 2 oral mucosal sites (right or left buccal mucosa, right or left ventral/lateral oral tongue, floor of mouth, or soft palate) within the 50 Gy cumulative isodose line. GC4419 was delivered as a 60-min IV infusion, ending within 60 minutes prior to each radiation fraction. OM was assessed by trained investigator-evaluators using the World Health Organization (WHO) criteria, in which: Grade 0 = No mucositis; Grade 1 = Pain and erythema; Grade 2 = Ulceration, able to eat solid food; Grade 3 = Ulceration, able to eat only liquids; Grade 4 = Ulceration, inability to eat requiring tube or parenteral feeding. By the WHO criteria, severe OM (SOM) is defined as either Grade 3 or Grade 4 OM. OM was assessed twice weekly with at least a 48-hour interval between assessments during IMRT and weekly thereafter for up to 8 weeks or until the WHO score was < 2. The primary endpoint was to determine safety of dose and schedule escalation concurrent with cisplatin/RT. Secondary endpoints included efficacy of the drug in delaying onset of SOM, decreasing severity and duration of SOM.
The study followed a serial cohort dose-escalation design with 3–6 patients per cohort, testing 15, 30, 50, 75, and then 112 mg of GC4419 per dose delivered before each of the first 14 IMRT fractions. Based on observed safety results in these first 5 cohorts, the protocol was amended to allow testing “duration extension” cohorts of 112 mg of GC4419 prior to each RT fraction in weeks 4, 5, and 6. Subsequent review of safety and OM results through these cohorts led the sponsor to add three cohorts to extend dosing further at reduced doses: 90 mg for 6 or 7 weeks, or 30 mg for 7 weeks.
Nine US centers enrolled 46 patients, the majority of whom were male with stage IVa oropharyngeal cancer treated with definitive high-dose cisplatin with 70 Gy IMRT. 41/46 patients (89%) received all planned GC4419 infusions. Observed safety was acceptable at all dose and duration schedules studied and delivery of planned chemoradiotherapy was not compromised in the presence of GC4419. Grade 3 nausea was the drug-related dose-limiting (DLT) toxicity at 112 mg, but because no single cohort had >1 patient with DLT, the maximum tolerated dose was not considered exceeded. Grade 3 nausea was the reason for dose reduction to 90 mg for subsequent duration extension cohorts. Higher doses of GC4419 also resulted in increased incidence of a transient, infusion-related Grade 1 facial paresthesia that spontaneously resolved shortly following the infusion.
The incidence, duration, and time to onset of SOM appeared improved with GC4419 compared to historical controls. For patients who received 30 mg or 90 mg of GC4419 over the full 6–7 weeks of CRT, the cumulative incidence of SOM was 29% (4/14) through 6 weeks of RT (60 Gy), and 50% (7/14) at any time, with a median time to onset of >50 days, and a median duration of 2.5 days. Tumor control at 1 year was not compromised.
These promising results led to the design of a randomized, double-blind, placebo-controlled Phase 2b trial testing placebo vs 30 mg or 90 mg of GC4419 prior to every RT fraction in the same patient population.35 A total of 44 US and Canadian institutions enrolled 223 oral cavity and oropharynx cancer patients who were randomized 1:1:1 and stratified prospectively by HPV status and cisplatin regimen (weekly vs q3 week). The primary endpoint of duration of SOM was evaluated by intention to treat analysis, with patients who did not experience SOM assigned a duration of zero days. Patients in the placebo arm had a median 19-day duration of SOM, compared with 1.5 days in patients who received the 90-mg dose of GC4419, a 92% relative reduction (p=0.024, unpublished data). Patients in the 90-mg arm demonstrated improvements in incidence of SOM (43% vs 65%, p=0.009), the incidence of grade 4 mucositis (16% vs 30%, p=0.045), and time to onset (median 61 vs 39 days). There were intermediate improvements seen in the 30-mg arm. Safety was comparable across all three arms and GC4419 side effects (i.e., hypotension and oral/facial paresthesia) were mild and transient, usually resolving within 1 hour of infusion completion. The Food and Drug Administration recently granted Breakthrough Therapy and Fast Track designations to GC4419 (Galera Therapeutics, Inc. Press Release, February 28, 2018) for the reduction of the severity and incidence of radiation and chemotherapy-induced oral mucositis. A Phase 3 randomized trial of 90 mg of GC4419 vs placebo is scheduled to begin before the end of 2018.
GC4419 and Aging
Dysregulated expression of genes that encode for mitochondrial electron transport proteins accumulate with aging, resulting in increased leakage of electrons in the mitochondrial electron transport chain and enhanced steady-state levels of O2•− 36–38. This age associated increase in O2•− makes elderly normal tissues more susceptible to toxicity incurred by radiation and chemotherapy10. Mapuskar et al. demonstrated that treatment of fibroblasts from elderly patients with GC4419 prior to exposure of therapeutic radiation doses or platinum based chemotherapy prevented much of the enhanced toxicity associated with aging10. Furthermore, treatment with GC4419 was able to partially restore mitochondrial activity and function without interfering with therapeutic radiation or chemotherapy-induced cancer cell killing10.
Another critical facet necessary for wound healing induced from therapeutic radiation and chemotherapy is the migration of healthy fibroblasts to the injury site. Fibroblasts collected from elderly individuals (≥ 70 years old) have reduced migrating ability compared to fibroblasts from young individuals (≤ 2 years old, Figure 2). Moreover, treatment of the fibroblasts collected from elderly patients with GC4419 restored the migration ability to that of fibroblasts collected from young patients (Figure 2). Thus, SOD mimics may prove to be even more beneficial in protecting from toxicities resulting from cancer treatment in elderly populations.
Figure 2: GC4419 restores the migration ability of fibroblasts collected from elderly individuals to that of the ability of fibroblasts from young individuals.
Exponentially growing young (5 month old foreskin) and old (78 year old male) human dermal fibroblasts were seeded in fibroblast basal media with growth factors. Cells were pre-treated for 24 hours with GC4419 (0.25 μM). Invading fibroblasts were stainedusing Thiazolyl Blue Tetrazolium Bromide (MTT) solution. GC4419 was able to restore the migration ability of fibroblasts from the 78 year old male to that of a 5 month old male.
Mn porphyrin-based SOD mimics (MnPs)
One of the earliest made MnPs was Mn (III) ortho substituted N-pyridyl compound, Mn (III) meso-tetrakis (N-ethylpyridinium-2-yl) porphyrin, MnTE-2-PyP5+ (AEOL10113, BMX-010, MnE, Figure 1)39. The ortho positioned cationic pyridyl nitrogens are close to the Mn center and impose a strong electron-withdrawing effect that results in thermodynamic properties close to those of native SOD enzymes. The penta-positive charge of MnE promotes favorable electrostatics by attracting the negatively charged superoxide, mimicking the SOD enzyme’s kinetics40. Although MnE was shown to be an effective superoxide scavenger in many models, its excessive hydrophilicity inhibited penetration across the blood brain barrier41. Therefore, new drugs were designed and among these were Mn(III) meso-tetrakis (N-n-hexylpyridinium-2-yl) porphyrin, MnTnHex-PyP5+ (MnHex) and Mn(III) meso-tetrakis (N-n-butoxyethylpyridinium-2-yl)porphyrin, MnTnBuOE-2-PyP5+ (BMX-001, MnBuOE, Figure 1). MnHex and MnBuOE are ~ 4,000-fold more lipophilic than MnE42–44 and both MnHex and MnBuOE were able to cross the blood brain barrier and protect the brain against oxidative damage45.
MnPs are reactive not only with superoxide but with numerous small and large reactive oxygen, nitrogen and sulfur species46. MnPs have catalase and glutathione peroxide (GPx)-like activities, peroxynitrite-reducing activity and the ability to oxidize ascorbate42. MnPs act equally as anti-oxidants (reducing O2− to H2O2) and pro-oxidants (oxidizing O2− to O2). If H2O2 is maintained under nM levels, as is the case in normal cells, the catalysis of O2− dismutation results in antioxidant therapeutic effects. Reactions with other molecules besides superoxide can result in MnPs acting as either anti-oxidants or pro-oxidants.
Mn Porphyrins as Radioprotectors
MnPs have been used in a variety of models of radiation by many labs, which will be discussed in detail in the following paragraphs. MnPs protect from gamma, x-ray, and proton beam irradiation. The SOD mimics can protect from one localized large dose of radiation or smaller fractionated doses of radiation. In most cases, MnP is given before radiation exposure and continued after radiation exposure. However, a few studies indicate that beginning treatment with MnP after radiation exposure may also prevent radiation-induced injury. These preclinical studies are discussed in further detail below.
The very first radiation studies with MnP demonstrated that MnE is a potent radioprotector by inhibiting TGF-β signaling and collagen deposition, resulting in the protection of lung tissues from radiation damage47. These data have been confirmed in several subsequent studies showing that MnE inhibits radiation-induced damage in rodent lung tissues when MnE is given prior to radiation48–50. Radiation protection has also been observed when administration of MnPs began 14 days after radiation49. In addition, MnTnHex-2-PyP was effective in protecting irradiated lungs from non-human primates when given 2 hours after RT51.
The GI tract is another organ that is highly sensitive to radiation. Radiation-induced proctitis is a common concern when irradiating tumors in the pelvic region. Archambeau et al. irradiated a small segment of the rectum with 20–30 Gy protons52. MnE was given via i.p. injection one hour before radiation or one hour after radiation and then given weekly for the rest of the experiment. In these models, protection was only observed when MnE was given before radiation. In an acute injury model, by day 10 post-radiation the control group suffered from significant crypt loss and MnE protected from crypt cell death. In a chronic model, MnE prevented rectal dilation and proctitis. At nine months post-radiation, 80% of the irradiated alone mice developed proctitis, while only 20% of mice developed proctitis in the group that received MnE prior to radiation52. Thus, MnE treatment greatly protected the rectal tissues from acute and chronic radiation-induced damage.
Pelvic irradiation is used frequently to treat prostate cancer and unwanted side effects can arise due to radiation exposure, such as erectile dysfunction and bladder incontinence. Rats were irradiated with 5 fractionated doses of 7.5 Gy to the pelvis and MnE was given 24 hours before radiation and then dosed after radiation for 12 weeks. Treatment of rats with MnE protected animals from erectile dysfunction after radiation53. During pelvic irradiation, the addition of MnE was also found to protect from skin and bladder fibrosis54.
MnBuOE protected salivary glands from a single high dose of radiation to the head and neck area when injected twice daily for the duration of study55. These mice were irradiated with single doses up to 15 Gy and treatment with MnBuOE resulted in less inflammation in the irradiated area and protected from saliva loss due to radiation exposure55. The dose-modifying factor for the protection against xerostomia/salivation was 0.77. The study was repeated using ~ one tenth the dose of MnBuOE and the lower dose of MnBuOE still protected against oral mucositis, xerostomia, and salivary gland fibrosis.
Since MnBuOE crosses the blood brain barrier, this drug was also tested for its ability to protect brain tissues from radiation damage45, 56. Protection of neurogenesis was seen when MnBuOE was given sc daily for a week prior to cranial irradiation (5 Gy) and a week following radiation. MnBuOE supported production and long-term survival of newborn neurons in the hippocampal dentate45. In other study, MnBuOE was given before and after whole brain radiation (8–10 Gy) and MnBuOE protected the loss of white matter, specifically loss in axons and myelin in the corpus callosum57. The addition of MnBuOE also maintained rotarod running performance in irradiated mice58. Thus, lipophilic SOD mimics protect both tissue integrity and neuronal function following brain irradiation.
Mechanisms of MnP as Radioprotectors
MnPs regulate immune response.
The first in depth mechanism described for MnE was its ability to inhibit NF-κB signaling in macrophages. Although, these studies did not include radiation, they illustrate that these SOD mimics inhibit inflammation, which plays a role in radiation injury. Piganelli’s work suggests that MnE oxidizes the p50 subunit of NF-κB59. The oxidized p50 no longer binds to DNA, making NF-κB inactive and unable to transcribe pro-inflammatory genes in macrophages59. These findings have also been validated in a variety of ischemia/reperfusion models demonstrating that MnP can prevent injury due to ischemia/reperfusion, in part, by inhibiting NF-κB signaling60, 61.
MnPs inhibit TGF-β signaling.
During lung radiation, MnE treatment reduced serum TGF-β levels in mice47. In subsequent studies conducted in irradiated, hypoxic macrophages, it was demonstrated that MnE treatment reduced levels of superoxide, TGF-β and VEGF levels in these cells62. MnHex also reduced TGF-β, HIF-1α and VEGF signaling in irradiated lungs50. In addition, MnHex inhibited macrophage recruitment and reduced DNA oxidative damage in the irradiated lungs. Therefore, there is solid evidence that MnP treatment results in the reduction of TGF-β signaling and inflammation in the irradiated lung.
The fibroblast is the cell most responsive to TGF-β. In the presence of TGF-β, a fibroblast will differentiate into a myofibroblast, which is the cell type responsible for laying down aberrant extracellular matrix. Using an in vitro fibroblast model, MnE or MnBuOE treatment prevented the activation of regular fibroblasts into myofibroblasts in response to radiation63, 64. In vivo, skin from irradiated mice produce elevated numbers of fibroblasts expressing α-smooth muscle actin (α-SMA), which is a marker for activated myofibroblasts. In contrast, in irradiated, MnE treated mice, no elevation of α-SMA was observed in the skin of these animals54. Thus, indicating that MnP treatment inhibits the activation of fibroblasts and, thus, fibrosis.
A more in depth investigation of the TGF-β signaling pathway revealed that MnE treatment resulted in a small reduction in active TGF-β1 levels, a large reduction of TGF-βRII protein levels (the receptor for active TGFβ−1 levels) and a reduction in SMAD/phosphorylated SMAD protein levels (transcription factors that transcribe fibrotic genes)63. It was also demonstrated that plasminogen activator inhibitor −1 (PAI-1), a target of the TGF-β/SMAD pathway, was also reduced with MnE treatment63. Further studies have also demonstrated that collagen genes, also controlled by the TGF-β/SMAD, are similarly reduced with MnE treatment54. Thus, MnE treatment results in the reduction of the entire TGF-β/SMAD signaling pathway in fibroblasts in response to radiation.
MnPs enhance cytoprotective pathways.
In addition to inhibiting inflammation and TGF-β signaling, MnPs have also been shown to activate the nuclear factor erythroid 2-related factor 2 (NRF2) transcription factor65. NRF2 activation can be regulated by oxidation of redox-sensitive Keap1; however, it is unclear if this is the only mechanism(s) by which MnP are regulating NRF2 at this time. NRF2 upregulates a variety of antioxidant and cytoprotective genes. In a recent report, MnBuOE treatment resulted in the activation of Nrf2 transcription factor, which resulted in the increased transcription of NADPH quinone dehydrogenase 1 (NQO1) mRNA and an increase in MnSOD, catalase and other antioxidant genes in hematopoietic stem cells65.
In another recent report, NQO1 mRNA and protein levels were shown to be highly upregulated with MnE treatment in fibroblasts and these levels were enhanced when MnE treatment was combined with radiation54. Thus, MnP treatment may protect normal cells from radiation damage by upregulating NRF2 signaling and enhancing the expression of antioxidant and cytoprotective proteins. MnP treatment seems to boost the cell’s natural cytoprotective machinery to inhibit damage due to radiation exposure.
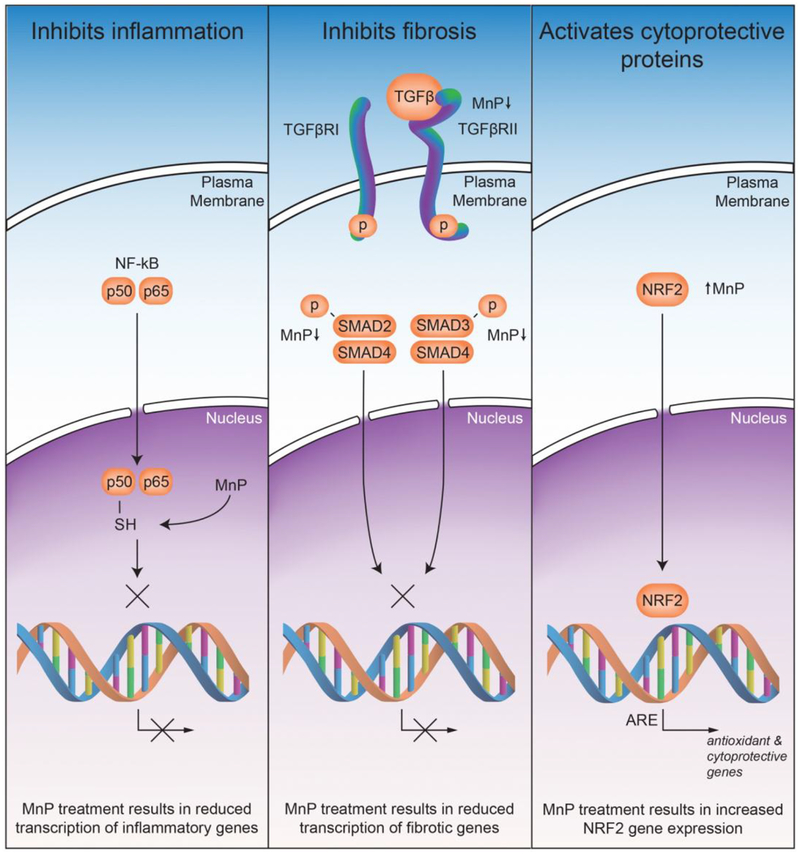
In summary, in normal tissue injury, MnPs suppress oxidative stress by reducing levels of reactive species. Such effects may be due, in part, to the direct removal of reactive species. Yet, more likely, MnPs inhibit those pathways that give rise to reactive species, which promote detrimental effects to the cells (Figure 3). One way in which MnP regulate signaling is through the oxidation of key cysteines of redox sensitive proteins42. The three major pathways identified, NF-κB, TGF-β and NRF2, are all regulated through oxidation of cysteines. There is also potential cross talk between the pathways that could result in the amplification of the changes induced by MnP treatment. However, more work is needed to fully understand how MnP are reducing radiation damage in normal cells.
Figure 3. The identified mechanisms by which MnPs protect normal tissues from radiation damage.
MnPs inhibit inflammation by reducing the ability of NF-κB to bind to DNA though oxidation of the p50 subunit of NF-κB. TGF-β signaling is reduced by MnP treatment through the reduction of active TGF-β, reduction in expression of TGFβRII, and a reduction in the phosphorylation of SMAD2 and SMAD3. These changes result in a reduction of SMAD complexes binding to DNA and transcribing pro-fibrotic genes. MnP treatment enhances Nrf2 binding to antioxidant response element (ARE) to enhance antioxidant and cytoprotective gene expression.
Mn Porphyrins as Anticancer Drugs
MnE or MnBuOE, alone inhibited the ability of human prostate cancer cells to form colonies, invade and migrate in vitro66, 67. MnE also inhibited mouse breast cancer growth at high doses68 and localized high concentrations of MnE on the skin dramatically inhibited skin cancer growth69. Thus, doses of MnP have to be very high to inhibit tumor growth alone. However, if applied along with additional, exogenous sources of H2O2 such as radiation, chemotherapy and ascorbate, the anti-cancer effects are greatly enhanced. Tumor growth inhibition with MnPs combined with radiation were observed in several studies on glioma, head and neck, prostate and breast cancer rodent studies42, 55, 57, 66, 70. MnBuOE promoted the anticancer effect of temozolomide in brain tumors57, 58 and both MnBuOE and MnE sensitize breast tumors to radiation and ascorbate71, 72.
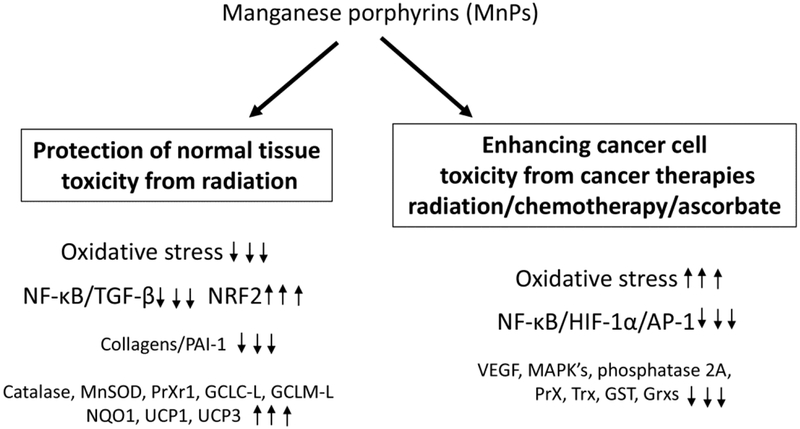
The mechanisms involved in cancer inhibition are less straightforward as compared to the mechanisms involved in protection of normal tissue injury. Two things are essential when considering a mechanism on the molecular level: (i) we need to view cancer as an aggravated oxidative stress injury with inflammation; and (ii) the MnP has no way to distinguish normal from tumor tissue other than differentiating between their redox environments. Cancer tissues have higher H2O2 and MnP levels as compared to normal tissues42, 72. When the normal tissues become highly oxidatively stressed and, thus, contain high H2O2 levels, the MnP would increase injury in the normal tissues52. Conversely, as a single agent, with no additional H2O2 added exogenously, MnP inflicts little damage to the cancer cell42, 68.
MnE affects the activity of several transcription factors that promote tumor progression (Figure 4). Radiation causes tumor hypoxia, which promotes HIF-1α, to enhance VEGF expression and revascularize the tumor73. MnE inhibited HIF-1α activity in hypoxic areas of the tumor after irradiation74. AP-1 controls genes involved in proliferation of skin cancer cells and MnE inhibited skin tumor growth, in part, by inactivating AP-169. NF-κB activity was inhibited in lymphoma cells treated with MnP through S-glutathionylation of cysteines on the NF-κB p65 subunit, which promoted apoptosis75, 76. Subsequently, redox proteomics performed on breast cancer cells treated with MnP/ascorbate, confirmed protein S-glutathionylation of the NF-κB pathway, p38MAPK, p38α (MAPK14), TAB3 and HSP60, phosphatase 2A pathway and endogenous antioxidant defenses, including peroxiredoxins and thioredoxins42. These data are supported by a recent breast cancer study where MnHex suppressed the phosphorylation of several MAPK targets: AKT, p38, ERK and JNK70. These changes in signaling increased oxidative stress and caused the cancer cells to undergo apoptosis42.
Figure 4. MnP affect signaling differently in normal vs. cancer cells undergoing cancer therapy.
In normal tissues, there is an overall reduction in oxidative stress when treated with MnPs and the NF-κB and TGF-β signaling pathways are reduced, while Nrf2 pathways are enhanced. In cancer cells, MnP treatment in combination with cancer therapy results in more oxidative stress, which results in reduction of NF-κB and HIF-1α and their downstream signaling pathways. These changes in signaling have been shown to enhance apoptosis of cancer cells and reduce overall cancer growth.
Clinical trials of Mn porphyrins as radioprotectors
MnBuOE was selected for clinical trials as a radioprotector in cancer patients due to high biodistribution in all tissues, including brain, favorable safety/toxicity properties, and a wealth of preclinical data. In the first Phase I clinical trial, MnBuOE was tested as a radioprotector of normal brain tissues in glioma patients (NCT02655601). MnBuOE has passed the phase I trial, using four escalating doses, with no adverse events. This drug is currently entering Phase II trials. MnBuOE is also in Phase I clinical trials as a radioprotector of normal tissues for head and neck cancer and anal cancer patients.
Conclusions
We are fortunate to witness representatives of two different classes of metal complexes with SOD activity reaching clinical trials at the same time – GC4419 (enantiomer of GC4403) and MnTnBuOE-2-PyP (MnBuOE or BMX-001). Moreover, these compounds are being tested in the same clinical application, radioprotection of normal tissue with head and neck cancer patients. The reason for such coincidence is the: (i) lack of drugs for the protection of normal tissue from radiation; (ii) awareness of the key role of redox signaling pathways in cell biology and (iii) wealth of preclinical data indicating the impact of such compounds on redox signaling pathways in both radioprotection and cancer studies. Both compounds were originally developed as potent SOD mimics. Over time, data has emerged to show that these compounds, directly or indirectly, target several redox-sensitive pathways, which results in the restoration of the cellular physiological redox environment.
Acknowledgments:
This work was supported by an Oberley Award from the Holden Comprehensive Cancer Center (BGA, KAM), CCSG P30-CA086862 (BGA, DRS), R01 CA182804 (DRS), Gateway for Cancer Research award G-17–1500T32-GM007337 (BGA), Department of Radiation Oncology University of Iowa (CMA, KAM), NIH R01CA178888 (ROD), NIH SP20 GM103480 COBRE (ROD), Fred and Pamela Buffet Cancer Center Support Grant P30CA036727 (ROD). North Carolina Biotechnology BIG Award (#2016-BIG-6518) (IBH), BioMimetix JVLLC (USA) (IBH), DCI NIH Core grant, 5-P30-CA14236–29 (IBH). IBH also acknowledges Ivan Spasojevic and his PK/PD Core Laboratory at the Pharmaceutical Research Shared Resource of Duke Cancer Institute (DCI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
IBH and ROD are consultants with BioMimetix JVLLC and hold equities in BioMimetix JVLLC. IBH and Duke University have patent rights and have licensed technologies to BioMimetix JVLLC. KAM, BGA, CMA, and DRS have sponsored research agreements with Galera Therapeutics, Inc.
References
- 1.Burdon RH, et al. , Cell proliferation and oxidative stress. Free Radic Res Commun,7(3–6): p. 149–59 1989. [DOI] [PubMed] [Google Scholar]
- 2.Droge W, Free radicals in the physiological control of cell function. Physiol Rev,82(1): p. 47–95 2002. [DOI] [PubMed] [Google Scholar]
- 3.Sarsour EH, et al. , Redox control of the cell cycle in health and disease. Antioxid Redox Signal,11(12): p. 2985–3011 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handy DE and Loscalzo J, Redox regulation of mitochondrial function. Antioxid Redox Signal,16(11): p. 1323–67 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gille L and Nohl H, Analyses of the molecular mechanism of adriamycin-induced cardiotoxicity. Free Radic Biol Med,23(5): p. 775–82 1997. [DOI] [PubMed] [Google Scholar]
- 6.Azzam EI, et al. , Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett,327(1–2): p. 48–60 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall EJ G AJ, Radiobiology for the radiologist. Sixth Edition ed. 2006, Philadellphia, PA: Lippincott Williams and Wilkins. [Google Scholar]
- 8.Aston K, et al. , Computer-aided design (cad) of mn(ii) complexes: Superoxide dismutase mimetics with catalytic activity exceeding the native enzyme. Inorg Chem,40(8): p. 1779–89 2001. [DOI] [PubMed] [Google Scholar]
- 9.Forman HJ and Fridovich I, Superoxide dismutase: A comparison of rate constants. Arch Biochem Biophys,158(1): p. 396–400 1973. [DOI] [PubMed] [Google Scholar]
- 10.Mapuskar KA, et al. , Mitochondrial superoxide increases age-associated susceptibility of human dermal fibroblasts to radiation and chemotherapy. Cancer Res,77(18): p. 5054–5067 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson JS, Chu Y, Glass J, et al. : The manganese superoxide dismutase mimetic, M40403, protects adult mice from lethal total body irradiation. Free Radic Res 44:529–40, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Murphy CK, Fey EG, Watkins BA, et al. : Efficacy of superoxide dismutase mimetic M40403 in attenuating radiation-induced oral mucositis in hamsters. Clin Cancer Res 14:4292–4297, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Buettner GR, Ng CF, Wang M, Rodgers VG, Schafer FQ. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med. 2006; 41(8):1338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson CM, et al. , Phase 1b/2a trial of the superoxide dismutase mimetic GC4419 to reduce chemoradiotherapy-induced oral mucositis in patients with oral cavity or oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys,100(2): p. 427–435 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonis ST, Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol,45(12): p. 1015–20 2009. [DOI] [PubMed] [Google Scholar]
- 16.Henke M, et al. , Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: A randomized, placebo-controlled trial. J Clin Oncol,29(20): p. 2815–20 2011. [DOI] [PubMed] [Google Scholar]
- 17.Le QT, et al. , Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: A randomized, placebo-controlled study. J Clin Oncol,29(20): p. 2808–14 2011. [DOI] [PubMed] [Google Scholar]
- 18.Elting LS, et al. , Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys,68(4): p. 1110–20 2007. [DOI] [PubMed] [Google Scholar]
- 19.Elting LS, et al. , Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: Demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer,113(10): p. 2704–13 2008. [DOI] [PubMed] [Google Scholar]
- 20.Nonzee NJ, et al. , Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis : Results from a northwestern university costs of cancer program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a veterans administration hospital, or a comprehensive cancer care center. Cancer,113(6): p. 1446–52 2008. [DOI] [PubMed] [Google Scholar]
- 21.Traynor AM, et al. , Comprehensive imrt plus weekly cisplatin for advanced head and neck cancer: The university of wisconsin experience. Head Neck,32(5): p. 599–606 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo G, et al. , Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist,13(8): p. 886–98 2008. [DOI] [PubMed] [Google Scholar]
- 23.Vera-Llonch M, et al. , Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer,106(2): p. 329–36 2006. [DOI] [PubMed] [Google Scholar]
- 24.Allison RR, et al. , Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (mugard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck. Cancer,120(9): p. 1433–40 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber C, et al. , Comparing pain control and ability to eat and drink with standard therapy vs gelclair: A preliminary, double centre, randomised controlled trial on patients with radiotherapy-induced oral mucositis. Support Care Cancer,15(4): p. 427–40 2007. [DOI] [PubMed] [Google Scholar]
- 26.Lalla RV, et al. , Mascc/isoo clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer,120(10): p. 1453–61 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambrecht M, et al. , The effect of a supersaturated calcium phosphate mouth rinse on the development of oral mucositis in head and neck cancer patients treated with (chemo)radiation: A single-center, randomized, prospective study of a calcium phosphate mouth rinse + standard of care versus standard of care. Support Care Cancer,21(10): p. 2663–70 2013. [DOI] [PubMed] [Google Scholar]
- 28.Leenstra JL, et al. , Doxepin rinse versus placebo in the treatment of acute oral mucositis pain in patients receiving head and neck radiotherapy with or without chemotherapy: A phase iii, randomized, double-blind trial (ncctg-n09c6 [alliance]). J Clin Oncol,32(15): p. 1571–7 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao NG, et al. , Phase ii multicenter trial of caphosol for the reduction of mucositis in patients receiving radiation therapy for head and neck cancer. Oral Oncol,50(8): p. 765–9 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarvizadeh M, et al. , Morphine mouthwash for the management of oral mucositis in patients with head and neck cancer. Adv Biomed Res,4: p. 44 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonis ST, et al. , Could the biological robustness of low level laser therapy (photobiomodulation) impact its use in the management of mucositis in head and neck cancer patients. Oral Oncol,54: p. 7–14 2016. [DOI] [PubMed] [Google Scholar]
- 32.Wong KH, et al. , A randomised controlled trial of caphosol mouthwash in management of radiation-induced mucositis in head and neck cancer. Radiother Oncol,122(2): p. 207–211 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spielberger R, et al. , Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med,351(25): p. 2590–8 2004. [DOI] [PubMed] [Google Scholar]
- 34.Sonis ST and Villa A, Phase ii investigational oral drugs for the treatment of radio/chemotherapy induced oral mucositis. Expert Opin Investig Drugs,27(2): p. 147–154 2018. [DOI] [PubMed] [Google Scholar]
- 35.Anderson CM, Lee CM, Saunders D, Curtis A, Dunlap N, Nangia CS, Lee AS, Holmlund J, Brill JM, Sonis ST, Buatti JM; Results of a randomized, placebo (PBO) controlled, double-blind P2b trial of GC4419 (avisopasem manganese) to reduce duration, incidence and severity and delay onset of severe radiation-related oral mucositis (SOM) in patients (pts) with locally advanced squamous cell cancer of the oral cavity (OC) or oropharynx (OP). J Clin Oncol 36, 2018 (suppl; abstr 6006). [Google Scholar]
- 36.Hamilton ML, et al. , Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A,98(18): p. 10469–74 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spitz DR, et al. , Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev,23(3–4): p. 311–22 2004. [DOI] [PubMed] [Google Scholar]
- 38.Suliman HB and Piantadosi CA, Mitochondrial quality control as a therapeutic target. Pharmacol Rev,68(1): p. 20–48 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batinic-Haberle I, et al. , The ortho effect makes manganese(iii) meso-tetrakis(nmethylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J Biol Chem,273(38): p. 24521–8 1998. [DOI] [PubMed] [Google Scholar]
- 40.Batinic-Haberle I, et al. , New class of potent catalysts of o2.-dismutation. Mn(iii) ortho-methoxyethylpyridyl- and di-ortho-methoxyethylimidazolylporphyrins. Dalton Trans,(11): p. 1696–702 2004. [DOI] [PubMed] [Google Scholar]
- 41.Spasojevic I, et al. , Pharmacokinetics of the potent redox-modulating manganese porphyrin, mnte-2-pyp(5+), in plasma and major organs of b6c3f1 mice. Free Radic Biol Med,45(7): p. 943–9 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batinic-Haberle I, et al. , Mn porphyrin-based redox-active drugs - differential effects as cancer therapeutics and protectors of normal tissue against oxidative injury. Antioxid Redox Signal, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tovmasyan A, et al. , Design, mechanism of action, bioavailability and therapeutic effects of mn porphyrin-based redox modulators. Med Princ Pract,22(2): p. 103–30 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weitner T, et al. , Comprehensive pharmacokinetic studies and oral bioavailability of two mn porphyrin-based sod mimics, mnte-2-pyp5+ and mntnhex-2-pyp5+. Free Radic Biol Med,58: p. 73–80 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leu D, et al. , Cns bioavailability and radiation protection of normal hippocampal neurogenesis by a lipophilic mn porphyrin-based superoxide dismutase mimic, mntnbuoe-2-pyp(5). Redox Biol,12: p. 864–871 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batinic-Haberle I, et al. , An educational overview of the chemistry, biochemistry and therapeutic aspects of mn porphyrins - from superoxide dismutation to ho-driven pathways. Redox Biol,5: p. 43–65 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vujaskovic Z, et al. , A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (sod) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med,33(6): p. 857–63 2002. [DOI] [PubMed] [Google Scholar]
- 48.Gauter-Fleckenstein B, et al. , Comparison of two mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic Biol Med,44(6): p. 982–9 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gauter-Fleckenstein B, et al. , Early and late administration of mnte-2-pyp5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med,48(8): p. 1034–43 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gauter-Fleckenstein B, et al. , Robust rat pulmonary radioprotection by a lipophilic mn n-alkylpyridylporphyrin, mntnhex-2-pyp(5+). Redox Biol,2: p. 400–10 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cline JM, et al. , Post-irradiation treatment with a superoxide dismutase mimic, mntnhex-2-pyp(5+), mitigates radiation injury in the lungs of non-human primates after whole-thorax exposure to ionizing radiation. Antioxidants (Basel),7(3) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Archambeau JO, et al. , Superoxide dismutase mimic, mnte-2-pyp(5+) ameliorates acute and chronic proctitis following focal proton irradiation of the rat rectum. Redox Biol,1(1): p. 599–607 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oberley-Deegan RE, et al. , The antioxidant, mnte-2-pyp, prevents side-effects incurred by prostate cancer irradiation. PLoS One,7(9): p. e44178 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shrishrimal S, et al. , The sod mimic, mnte-2-pyp, protects from chronic fibrosis and inflammation in irradiated normal pelvic tissues. Antioxidants (Basel),6(4) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashcraft KA, et al. , Novel manganese-porphyrin superoxide dismutase-mimetic widens the therapeutic margin in a preclinical head and neck cancer model. Int J Radiat Oncol Biol Phys,93(4): p. 892–900 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le O, et al. , Ink4a/arf expression impairs neurogenesis in the brain of irradiated mice. Stem Cell Reports,10(6): p. 1721–1733 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weitzel DH, et al. , Radioprotection of the brain white matter by mn(iii) nbutoxyethylpyridylporphyrin-based superoxide dismutase mimic mntnbuoe-2-pyp5+. Mol Cancer Ther,14(1): p. 70–9 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weitzel DH, et al. , Neurobehavioral radiation mitigation to standard brain cancer therapy regimens by mn(iii) n-butoxyethylpyridylporphyrin-based redox modifier. Environ Mol Mutagen,57(5): p. 372–81 2016. [DOI] [PubMed] [Google Scholar]
- 59.Tse HM, et al. , Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: Implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med,36(2): p. 233–47 2004. [DOI] [PubMed] [Google Scholar]
- 60.Celic T, et al. , Mn porphyrin-based sod mimic, mntnhex-2-pyp(5+), and non-sod mimic, mntbap(3-), suppressed rat spinal cord ischemia/reperfusion injury via nf-kappab pathways. Free Radic Res,48(12): p. 1426–42 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheng H, et al. , Neuroprotective efficacy from a lipophilic redox-modulating mn(iii) n-hexylpyridylporphyrin, mntnhex-2-pyp: Rodent models of ischemic stroke and subarachnoid hemorrhage. J Pharmacol Exp Ther,338(3): p. 906–16 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson IL, et al. , Superoxide dismutase mimetic reduces hypoxia-induced o2*-, tgf-beta, and vegf production by macrophages. Free Radic Res,41(1): p. 8–14 2007. [DOI] [PubMed] [Google Scholar]
- 63.Chatterjee A, et al. , Mnte-2-pyp treatment, or nox4 inhibition, protects against radiation-induced damage in mouse primary prostate fibroblasts by inhibiting the tgf-beta 1 signaling pathway. Radiat Res,187(3): p. 367–381 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosmacek EA, et al. , Mntnbuoe-2-pyp protects normal colorectal fibroblasts from radiation damage and simultaneously enhances radio/chemotherapeutic killing of colorectal cancer cells. Oncotarget,7(23): p. 34532–45 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Y, et al. , A novel redox regulator, mntnbuoe-2-pyp(5+), enhances normal hematopoietic stem/progenitor cell function. Redox Biol,12: p. 129–138 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chatterjee A, et al. , The addition of manganese porphyrins during radiation inhibits prostate cancer growth and simultaneously protects normal prostate tissue from radiation damage. Antioxidants (Basel),7(1) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tong Q, et al. , Mnte-2-pyp reduces prostate cancer growth and metastasis by suppressing p300 activity and p300/hif-1/creb binding to the promoter region of the pai-1 gene. Free Radic Biol Med,94: p. 185–194 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rabbani ZN, et al. , Antiangiogenic action of redox-modulating mn(iii) mesotetrakis(n-ethylpyridinium-2-yl)porphyrin, mnte-2-pyp(5+), via suppression of oxidative stress in a mouse model of breast tumor. Free Radic Biol Med,47(7): p. 992–1004 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Y, et al. , A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res,65(4): p. 1401–5 2005. [DOI] [PubMed] [Google Scholar]
- 70.Shin SW, et al. , Mechanism of the antitumor and radiosensitizing effects of a manganese porphyrin, mnhex-2-pyp. Antioxid Redox Signal,27(14): p. 1067–1082 2017. [DOI] [PubMed] [Google Scholar]
- 71.Evans MK, et al. , Mn porphyrin in combination with ascorbate acts as a prooxidant and mediates caspase-independent cancer cell death. Free Radic Biol Med,68: p. 302–14 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tovmasyan A, et al. , Anticancer therapeutic potential of mn porphyrin/ascorbate system. Free Radic Biol Med,89: p. 1231–47 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moeller BJ, et al. , Radiation activates hif-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell,5(5): p. 429–41 2004. [DOI] [PubMed] [Google Scholar]
- 74.Moeller BJ, et al. , A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Radiat Oncol Biol Phys,63(2): p. 545–52 2005. [DOI] [PubMed] [Google Scholar]
- 75.Jaramillo MC, et al. , Manganese (iii) meso-tetrakis n-ethylpyridinium-2-yl porphyrin acts as a pro-oxidant to inhibit electron transport chain proteins, modulate bioenergetics, and enhance the response to chemotherapy in lymphoma cells. Free Radic Biol Med,83: p. 89–100 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jaramillo MC, et al. , Manganese porphyrin, mnte-2-pyp5+, acts as a pro-oxidant to potentiate glucocorticoid-induced apoptosis in lymphoma cells. Free Radic Biol Med,52(8): p. 1272–84 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]