Abstract
Dysregulated glucose and redox metabolism are near universal features of cancers. They therefore represent potential selectively toxic metabolic targets. This review outlines the preclinical and clinical data for targeting glucose and hydroperoxide metabolism in cancer, with a focus on drug strategies that have the most available evidence. In particular, inhibition of glycolysis using 2-deoxyglucose, and inhibition of redox metabolism using the glutathione pathway inhibitor buthionine sulfoximine and the thioredoxin pathway inhibitor auranofin, have shown promise in preclinical studies to increase sensitivity to chemotherapy and radiation by increasing intracellular oxidative stress. Combined inhibition of glycolysis, glutathione, and thioredoxin pathways sensitizes highly glycolytic, radioresistant cancer models in vitro and in vivo. Although the preclinical data support this approach, clinical data are limited to exploratory trials using a single drug in combination with either chemotherapy or radiation. Open research questions include optimizing drug strategies for targeting glycolysis and redox metabolism, determining the appropriate timing for administering this therapy with concurrent chemotherapy and radiation, and identifying biomarkers to determine the cancers that would benefit most from this approach. Given the quality of preclinical evidence, dual targeting of glycolysis and redox metabolism in combination with chemotherapy and radiation should be further evaluated in clinical trials.
Introduction:
Increased glucose utilization is a nearly universal feature of cancers and was described by Warburg nearly a century ago.1,2 This has been proposed as an adaptation to hypoxia as malignant lesions outgrow their blood supply, and as a phenotype that is selected for in environments with scarce oxygen and glucose.3-5 It has also formed the basis for the widespread clinical use of [F-18]fluorodeoxy glucose positron emission tomography ([F-18]FDG-PET) in oncology as a tool for staging, prognosis, and monitoring treatment response.6,7 The mechanism of increased [F-18]FDG signal seen in cancers relative to normal tissues is related to increased transport by GLUT transporters and phosphorylation by hexokinases compared to most normal tissues. [F-18]FDG becomes trapped after its phosphorylation by hexokinases and does not proceed any further through glycolysis.8-10 [F-18]FDG-PET derived imaging metrics have been shown to be prognostic in lymphomas, head and neck, lung, and gynecologic cancers and numerous other cancer sites, and decreased [F-18]FDG signal during or following treatment is a favorable prognostic sign.11-17 These extensive clinical data suggest that increased glucose utilization is related to the aggressiveness of a cancer and how well it is able to tolerate standard therapies such as chemotherapy and radiation.
Altered redox metabolism and particularly changes in glutathione and thioredoxin metabolism, have likewise been noted in many cancer types, and upregulation of these pathways has been linked to cancer progression.18 Cancer cells produce more reactive oxygen species (ROS) than non-cancerous cells, and upregulate antioxidant systems, such as the glutathione and thioredoxin pathways, to manage this excess of ROS, particularly hydroperoxide species.18-21 Glutathione in particular has been shown to play an important role in cancer initiation, and the combination of inhibiting the glutathione and thioredoxin pathways has been shown to be toxic to a number of different cancer cells.18,22,23 Increased glycolysis and increased activity of the pentose phosphate pathway may also help cancer cells manage oxidative stress by providing reducing equivalents, for example nicotinamide adenine dinucleotide phosphate (NADPH), that can be used by ROS scavenging pathways, connecting the increased glucose consumption and upregulation redox metabolic pathways seen in many cancers.24
Despite the evidence of the importance of alterations in both glycolysis and redox metabolism to cancer initiation and progression, no current standard-of-care therapies specifically target these metabolic pathways, and clinical investigations have been limited. There are accumulating preclinical data that targeting hydroperoxide metabolism at both the glutathione and thioredoxin pathways increases ROS and is toxic to cancer cells, and that targeting glycolysis in addition to these pathways further increases redox stress and toxicity.18,22,24 Therapies that increase oxidative stress may be particularly valuable as a means to sensitize cancers to radiation. Damage from radiation therapy is classically thought to be mediated by ROS created by ionizing radiation, and upregulation of ROS scavenging pathways may therefore help cancers mitigate damage from radiation.25 Inhibiting both glycolysis and hydroperoxide metabolism has therefore also been proposed as a potential radiosensitization strategy.23,26 Here we aim to review the preclinical and clinical data on therapies targeting glycolysis and the glutathione and thioredoxin pathways, either alone or in combination, with particular focus on how these therapies might improve radiation response.
Targeting Glycolysis:
There are a number of potential therapeutic targets within the early steps of glucose uptake and metabolism (Figure 1). Furthest upstream, the GLUT family of transporters can be targeted for anticancer effect27, and some cancers express unique GLUT isoforms.28 WZB117 is an inhibitor of GLUT1 that has been shown to inhibit cancer growth and cause cell-cycle arrest, senescence, and death in vitro and in vivo through a mechanism that appears to be dependent on ATP, and it has also been shown to decrease the tumor-initiating capacity of cancer stem cells (CSCs) derived from a number of different cancers.29,30 The protease inhibitor ritonavir, approved for the treatment of HIV, has an off-target inhibitory effect on the GLUT4 transporter, and has been proposed as a therapy for multiple myeloma, which is dependent upon expression of the GLUT4 transporter.31
Figure 1:
Potential targets of glucose metabolism for anticancer therapy. Blocking glucose metabolism serves to inhibit both a major energy source for cancer cells, as well as a major source of reducing equivalents (e.g. NADPH). The major glucose metabolic pathways, and potential drug strategies to target them are shown. Drugs are shown in bold. Abbreviations: HK, hexokinase; 2-DG, 2-deoxyglucose; LND, lonidamide; G6PD, glucose-6-phosphate dehydrogenase; 6-AN, 6-aminonicotinamide; DHEA, dehydroepiandrosterone; PPP, pentose phosphate pathway; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; 3-BrPA, 3-bromopyruvate; LDHA, lactate dehydrogenase A; TCA cycle, tricarboxyclic acid cycle.
Hexokinase, which phosphorylates glucose after it has entered the cell, is another potential upstream target in glucose metabolism. A number of drugs have been evaluated in the preclinical and clinical setting as hexokinase inhibitors, including 2-deoxyglucose (2-DG), and lonidamine. 32,33 Lonidamide has been evaluated in clinical trials, but widespread clinical utility of lonidamide has been limited by significant hepatic and pancreatic toxicities.34 As with [F-18]FDG, 2-DG is taken up by GLUT transporters and phosphorylated by hexokinase, at which point it becomes trapped within cells and is not metabolized any further. Excess accumulation of 2-DG leads to cell cycle inhibition and ultimately cell death in a variety of preclinical cancer models.27,33,35 There is also evidence that increased oxidative stress in cancer cells relative to normal cells mediates selective toxicity of 2-DG for cancers.36
Much of the preclinical and clinical work studying 2-DG has been in glioblastoma multiforme (GBM). 2-DG selectively inhibits proliferation of GBM cell lines relative to normal human astrocytes.35 Use of 2-DG has also been shown to inhibit cancer growth and carcinogenesis in non-central nervous system cancers (e.g. mammary cancers).37 However, 2-DG alone may not act as an effective anticancer therapeutic. For example, although glucose deprivation has been shown to induce apoptosis through a ROS dependent mechanism in GBM derived cell lines but not normal human astrocytes, treatment with 2-DG actually prevents apoptosis through depletion of ATP.38 Controversy remains over the precise mechanism of 2-DG toxicity when this drug is administered a monotherapy.39,40 Hypoxia and activation of the hypoxia-inducible-factor-1 (HIF1) transcription factor have been linked to 2-DG monotherapy resistance41, and some studies have demonstrated activation of pro-survival pathways in cancer cells after 2-DG treatment, leading some investigators to propose 2-DG combination therapies rather than 2-DG monotherapy as the most appropriate strategy.42,43
Numerous combination therapies, utilizing 2-DG in addition to another metabolic agent, a chemotherapeutic drug, or radiation therapy, have, however, proven to be effective strategies. For example, the combination of 2-DG and the oxidative phosphorylation inhibitor oligomycin has been shown to synergistically suppress growth and mobility in GBM cell lines, and the combination of 2-DG and inhibition of NADPH oxidase 4 likewise inhibits proliferation and angiogenesis in GBM cells.35,44 In addition, 2-DG has been shown to enhance the efficacy of systemic and chemotherapeutic agents, such as cisplatin, topoisomerase, and trastuzumab, in vivo and in vitro27,45-47, and the combination of 2-DG and docetaxel has been evaluated in a phase I trial in patients with advanced solid tumors with tolerable and reversible side effects including hyperglycemia and QTc prolongation. Most germane to this review, 2-DG has been shown to increase the efficacy of radiation therapy in a number GBM and other cancer cell lines, with evidence supporting that this is due to disruptions in thiol metabolism.49-52
The enhanced response to radiation seen in GBM cell lines has formed the basis for a number of clinical trials combining 2-DG with radiation therapy. The published trials have for the most part been small phase I/II trials evaluating the safety and tolerability of administering 2-DG prior to radiation therapy using escalating doses of 2-DG, and have used large fractions of RT (5 Gy) delivered once a week 20-30 minutes after 2-DG administration. These trials have reported that such a regimen is tolerated, with acute side effects of restlessness, nausea, and vomiting, and without any apparent significant long-term toxicities.53-55 There are currently no open randomized controlled clinical trials evaluating the efficacy of 2-DG as an anti-cancer agent alone or in combination with radiation or chemotherapy. Limiting factors include unacceptable side effects at high doses used to limit glucose metabolism in cancer cells, and limited efficacy for glycolysis inhibition when lower doses of 2-DG monotherapy are used.56
Finally, although separate from glycolysis, the pentose phosphate pathway represents another potential target for anticancer therapy (Figure 1). The pentose phosphate pathway is one of the primary sources of cellular NADPH, which provides electrons for ROS scavengers like glutathione and thioredoxin. For example, 6-aminonicotinamide (6-AN) is an inhibitor of glucose-6-phosphate dehydrogenase (G6PD) that has shown anticancer activity in vitro, either alone or in combination with radiation, but that also has significant side effects.33 Other strategies for inhibiting G6PD have also been investigated, for example using dehydroepiandrosterone (DHEA) in combination with 2-DG.24 Whether the pentose phosphate pathway can effectively be targeted for anticancer therapy in humans remains an open question.
Targeting Hydroperoxide Metabolism:
Cancer cells are thought to generate excess ROS as a result of unrestrained growth, and genetic and metabolic alterations that uncouple glycolysis, the pentose phosphate pathway and the tricarboxylic acid (TCA) cycle. In order to manage this excess oxidative stress, cancer cells upregulate ROS scavenging pathways, and as such are selectively sensitive to therapies that inhibit these pathways.18,57-59 The glutathione and thioredoxin pathways, which scavenge hydroperoxide radicals, are two pathways that have been shown to be upregulated in many cancers. Targeting these pathways can be toxic in itself, or sensitize cancers to other therapies that further increase oxidative stress such as radiation therapy and some chemotherapies (Figure 2).
Figure 2:
Potential targets of hydroperoxide metabolism for anticancer therapy. The glutathione and thioredoxin pathways are the two major thiol reactive oxygen species scavenging pathways. Many cancers rely on these pathways to manage increased oxidative stress from excess growth and dysregulated metabolism, and targeting them could increase sensitivity to therapies that further increase oxidative stress, like radiation therapy.
Targeting Glutathione Metabolism:
As outlined above, glutathione has been identified as an important factor in carcinogenesis, and as one of the most abundant antioxidant molecules in cells it plays a key role in maintaining redox balance, particularly in cancer cells.18,58 One of the most widely used strategies to target glutathione metabolism is the drug buthionine sulfoximine (BSO), which inhibits γ-glutamylcysteine synthase, the enzyme responsible for the first step in the synthesis of glutathione.60 A number of preclinical studies have demonstrated that BSO can be used to sensitize cancer cells to a number of therapies including arsenic61, cisplatin62, mephalan63, and radiation.64 Some of these studies have demonstrated that BSO also sensitizes cancer cells to therapies that are not conventionally thought of as inducing ROS on their own (e.g. cisplatin), and that glutathione depletion may have an effect on mediating cell death pathways like apoptosis.62
BSO was first studied in clinical trials in combination with mephalan over 20 years ago. These phase I trials in small cohorts of patients with advanced refractory cancers studied different doses and dosing schedules (e.g. BSO every 12 hours versus as a continuous infusion), the biologic activity of BSO, namely reduced glutathione levels in the peripheral blood and within tumors, and toxicities associated with its use. These trials found that BSO given in combination with melphalan is fairly well tolerated, with low grade nausea and vomiting the most common acute toxicity, and a more frequent incidence of severe (grade 4) myelosuppression. They have also reported depletion of glutathione in the peripheral blood of <10% of pretreatment values, and depletion to ≤20% of pretreatment values within tumors.65-67 More recently, the combination of BSO and melphalan has been tested in a pilot study of children with recurrent neuroblastoma, again assessing for tolerability and toxicity, biochemical efficacy, and clinical efficacy.68 This trial showed the combination of these drugs decreased blood mononuclear cell glutathione levels and, an 18% response rate was observed. Grade 3-4 leukopenia and thrombocytopenia in these patients were common, and two grade 5 toxicities were reported (acute renal tubular necrosis and diffuse cerebral edema) after the initiation of BSO.
Targeting Thioredoxin Metabolism:
The other major thiol-based ROS scavenging pathway in mammalian cells is the thioredoxin system. Thioredoxin donates electrons to peroxiredoxins, which then remove hydroperoxide radicals. Thioredoxin is then converted back to its reduced form by thioredoxin reductase, using electrons donated by NADPH. Along with the glutathione system, this is one of the key regulators of intracellular ROS.69
There are a number of drug strategies that have been proposed to inhibit the thioredoxin pathway. These include auranofin, a gold complex initially developed for treating rheumatoid arthritis that acts as a thioredoxin reductase inhibitor and sulfasalazine, an inhibitor of the XC- cystine transporter also used in rheumatoid arthritis and other inflammatory conditions (Figure 2).18,23 Auranofin has been shown to inhibit both mitochondrial and cytosolic thioredoxin reductase, resulting in an increase in ROS levels.70 As a result of the increased oxidative stress, auranofin causes endoplasmic reticulum stress and mitochondrial dysfunction, which can be reversed using the thiol antioxidant n-acetylcysteine (NAC), suggesting that this effect is mediated by ROS71,72
Auranofin has shown activity against a number of cancer types in preclinical studies. This includes diverse disease sites such as chronic lymphocytic and chronic myeloid leukemias, multiple myeloma, osteosarcoma, and gastric cancer.71-75 In each of these cases, the toxicity due to auranofin appears to be mediated by increased ROS, which trigger programmed cell death pathways. Auranofin alone can also act as a radiosensitizer in both normoxic and hypoxic cancer cells, and appears to do so through increased ROS generation, which can be reversed by NAC, as well by inducing mitochondrial dysfunction.76
Combined Inhibition of Glucose and Hydroperoxide Metabolism
As outlined above, inhibition of glutathione alone does not result in significant toxicity to cancer cells. The glutathione and thioredoxin pathways are the two major thiol ROS scavenging systems in mammalian cells, and selective targeting of one pathway often results in increased activity of the other.69 For example, thioredoxin pathway proteins are overexpressed in cancer cells depleted of glutathione.18 Therefore, inhibition of both pathways may be needed to induce ROS dependent toxicity, and a number of studies have found that many cancers are sensitive to the simultaneous inhibition of both the glutathione and thioredoxin pathways.18,22,77 Combining glutathione and thioredoxin pathway inhibition has also been shown to sensitize cancer cells, including breast cancer stem cells, to radiation therapy, and to inhibit cancer cell migration and invasion.23 The toxicity of combined glutathione and thioredoxin pathway inhibition and the resulting sensitization to radiation therapy are mediated by ROS, and reversible using exogenous ROS scavengers like NAC.
Altered glucose metabolism, particularly through the generation of NADPH in the pentose phosphate pathway, provides another means for cancer cells to manage excess oxidative stress. The pyruvate produced by glycolysis may also help mediate ROS scavenging.77,78. Furthermore, treatment with 2-DG has been shown to increase levels of superoxide and hydrogen peroxide in cancers.36 Therefore, the combination of inhibition of glucose metabolism and inhibition of the glutathione and thioredoxin pathways may increase oxidative stress in cancer cells even further (Figure 3). As all of these pathways are upregulated in many cancer types this approach therefore offers a potential means of selectively targeting one of the more reproducible observations with respect to dysregulated metabolism seen in many cancers. The combination of glycolysis inhibition using 2-DG, pentose phosphate pathway inhibition using DHEA, and thioredoxin pathway inhibition using auranofin is significantly more toxic than when any one of these pathways is inhibited alone as documented in human breast and prostate cancer cells, and importantly this combination treatment is significantly more toxic to breast cancer cells than normal mammary cells.24 As such, the dual targeting of glycolysis in combination with redox metabolism holds great promise for cancer therapy.
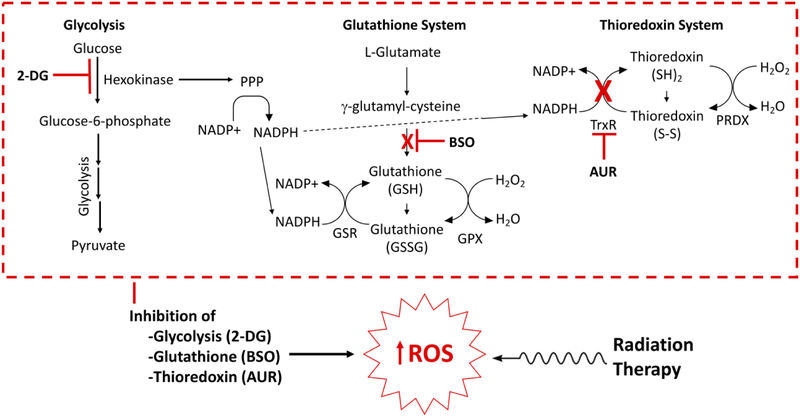
Figure 3:
Simultaneous inhibition of the glycolysis and the glutathione and thioredoxin systems reduces cancer cells’ defenses against reactive oxygen species, and can be used as a strategy to sensitize cancer cells to therapies that increase oxidative stress, like radiation therapy. An example inhibitory combination drug strategy using 2-DG, BSO, and AUR is shown. Abbreviations: 2-DG, 2-deoxyglucose; BSO, buthionine sulfoximine; AUR, auranofin; PPP, pentose phosphate pathway; GSR, glutathione reductase; GPX, glutathione peroxidase; TrxR, thioredoxin reductase; PRDX, peroxiredoxin.
Our group has recently evaluated the combination of glycolysis, glutathione and thioredoxin inhibition as a means to radiosensitize cervical cancer cell lines.26 Increased [F-18]FDG uptake is an established prognostic factor in human cervical cancers, and persistent tumor [F-18]FDG uptake following definitive chemo-radiation therapy portends a poor outcome.15,17 These clinical observations suggest that glucose metabolism is a marker for aggressive, radiation-resistant cervical cancers. Cervical cancer may therefore be well suited to a treatment approach using inhibition of glycolysis in combination with inhibition of hydroperoxide metabolism to increase oxidative stress and enhance sensitivity to standard of care radiation therapy. We have demonstrated that combining 2-DG, buthionine sulfoximine, and auranofin results in significant toxicity to a number of cervical cancer cell lines that is mediated by an increase in ROS. Furthermore, treatment with this combination of drugs results in decreased tricarboxylic acid cycle activity, as well as AMPK activation in cell lines sensitive to this drug combination. The mechanism of cell death from this combination treatment appears to be dependent on expression of specific pathways, i.e. MYC. In MYC transformed cells, this therapy causes a caspase and PARP dependent autophagic cell death, whereas non-MYC transformed cells die by an AMPK dependent non-autophagic death. These data also demonstrated that this drug combination was an effective radiosensitization strategy in vivo.26 This high quality preclinical evidence supports further investigation of this drug combination as an adjunct to radiotherapy in the context of clinical trials for cervical cancer.
Future Directions:
An important future direction in this field is selection of the best drug strategy to target aerobic glycolysis in tumors. A number of glycolytic enzymes downstream of hexokinase have been explored as potential cancer therapeutic targets, and are at various stages of preclinical versus clinical development. Small molecule inhibitors of 6-phosphofructo-2-kinase are being developed and have been shown to influence tumor growth in animal models.79 As the first enzymatic step within glycolysis associated with NADH production, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) represents a unique therapeutic target.80,81 Inhibition of GAPDH leads to accumulation of glucotrioses, which, during the course of their metabolism generate methylgyoxal, which is toxic to the cell. Enzymes responsible for the metabolism of methyglyoxal are dependent upon reduced glutathione (GSH). Therefore, in the presence of limited GSH, targeting GAPDH represents an attractive opportunity to not only limit ATP production via glycolysis, but also to enhance intracellular levels of oxidative stress by simultaneously limiting NADH production and generating a toxic metabolite that consumes intracellular GSH.
A number of GAPDH inhibitors have been tested in the preclinical setting. For example, 3-bromopyruvate has been well studied in this role and has entered early phase clinical trials.82-84 Recently, Gunda et al demonstrated that pretreatment with 3-bromopyruvate abrogated radiation resistance in MUC1 overexpressing pancreatic cancer models. Pyruvate kinase (PK) catalyzes the near final step of conversion of phyophoenolpyruvate (PEP) to pyruvate. Early studies showed that knockdown of PKM2 in tumor cells and replacement with the PKM1 isoform reversed the Warburg effect85, and preclinical studies of PKM2 inhibition have demonstrated anti-cancer effect.86 Although it is now clear that expression of PKM2 is not cancer-specific, additional study of PKM2 regulation and biology may lead to effective and selective anti-cancer strategies. It is important to note that PKM2 is an inefficient enzyme, and backflow of glycolytic intermediates can be diverted to the PPP to generate NADPH that can be used to metabolize ROS.58 PKM2 itself is inhibited directly by ROS, and detailed understanding of this relationship will be important to design rationale drug or radiation combinations with PKM2 inhibitors.87 Further downstream, inhibitors of lactate dehydrogenase A (LDHA), such as the pyruvate analog oxamate, have been shown to increase efficacy of systemic agents such as paclitaxel and traztuzumab.27,88 Further preclinical studies, particularly in vivo, supporting the use of these drug strategies in the context of radiotherapy should be performed.
A second important future direction is optimization of drug strategies to increase intracellular oxidative stress in cancer cells. In this review, we have focused on dual targeting of glutathione and thioredoxin pathways with BSO and auranofin, the most well studied drugs in each category. Other drug strategies exist to deplete cells of GSH reserves, including inhibitors of cysteine/glutamate transporter XCT (sulfasalazine), inhibitors of glutathione peroxidase (GPX), and glutathione disulphide mimetics (NOV-002).89,90 It is important to note that glutamine can be converted to glutamate, which is required for GSH synthesis. As such, developing drug strategies that inhibit glutamine metabolism will, by definition, decrease intracellular glutathione levels. A number of drug strategies are being developed to target glutamine metabolism, and detailed study of the effects of these drugs on intracellular levels of oxidative stress will need to be performed. Although it is well accepted that radiation treatment results in increased ROS, the precise source, species and timing of ROS increases after RT exposure are not well known and may be unique in certain cancer types. Understanding the precise mechanism and timing of ROS fluctuations after RT exposure will be important for the successful timing of administration of ROS inducing agents. Preclinical studies that incorporate variations in dose and schedule for ROS inducing agents in the context of single fraction and multi-fraction radiation schedules should be performed.
Determining the most appropriate biomarker to identify cancers that are uniquely susceptible to inhibition of glycolysis and redox metabolism is also critical. Historically, increased uptake of [F18]FDG-PET by tumors has been used to identify tumors that engage in increased rates of aerobic glycolysis; however, it is important to note that this imaging modality is limited to glucose uptake, and does not directly visualize glucose metabolism. Significant improvement in imaging technology, specifically the development of magnetic resonance imaging with hyperpolarized substrates, will allow for direct visualization of enzymatic steps within glycolysis and other metabolic pathways in tumors (i.e., the pyruvate to lactate transition.) Ongoing research efforts in our own group are evaluating novel PET imaging strategies that can be used to noninvasively monitor levels of tumor oxidative stress.91
Conclusions:
Dysregulated metabolism is a hallmark of cancer cells. Specifically, cancers demonstrate changes in both glucose metabolism and redox metabolism to help them meet high energy and growth requirements in environments with limited resources, and manage excess ROS that result from excess growth and aberrant metabolic pathways. Both glycolysis and thiol-mediated hydroperoxide metabolism represent targetable pathways that are upregulated in many cancer types, and this work has reviewed the preclinical and clinical literature on the efficacy of such an approach. The available clinical data are restricted to inhibitors of a single pathway (e.g. glycolysis or the glutathione pathway) given in combination with radiation or chemotherapy, and have been limited to early phase trials in small cohorts. The preclinical data reviewed here demonstrate that the combination of glucose and hydroperoxide metabolism is an effective treatment strategy across a number of cancer types, particularly when also combined with radiation or chemotherapy, and far more effective than inhibiting any one single pathway on its own. Ongoing research to identify the most appropriate biomarker for susceptibility to inhibition of glycolysis and redox metabolism, including development of novel imaging strategies, should be performed.
Acknowledgments
Grant Support: This work was supported in part by NIH R01CA181745 to JK Schwarz.; and by ASTRO Resident Research Seed Grant 531448 and the RSNA Resident Research Grant to JM Floberg.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Warburg O: Über den Stoffwechsel der Carcinomzelle. Die Naturwissenschaften 12(50):1131–1137, 1924 [Google Scholar]
- 2.Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell 144(5):646–74, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Gatenby RA, Gillies RJ: Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4(11):891–9, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Graeber TG, Osmanian C, Jacks T: Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379(6560):88–91, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Gatenby RA, Vincent TL: An evolutionary model of carcinogenesis. Cancer Res 63(19):6212–20, 2003 [PubMed] [Google Scholar]
- 6.Hawkins RA, Phelps ME: PET in clinical oncology. Cancer Metastasis Rev 7(2):119–42, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Gambhir SS: Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2(9):683–93, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Bos R, vand Der Hoeven JJ, van Der Wall E: Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 20(2):379–87, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Rivenzon-Segal D, Boldin-Adamsky S, Seger D: Glycolysis and glucose transporter 1 as markers of response to hormonal therapy in breast cancer. Int J Cancer 107(2):177–82, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Mathupala SP, Rempel A, Pedersen PL: Aberrant glycolytic metabolism of cancer cells: a remarkable coordination of genetic, transcriptional, post-translational, and mutational events that lead to a critical role for type II hexokinase. J Bioenerg Biomembr 29(4):339–43, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Berghmans T, Dusart M, Paesmans M: Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 3(1):6–12, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Haioun C, Itti E, Rahmouni A: [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood 106(4):1376–81, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Halfpenny W, Hain SF, Biassoni L: FDG-PET. A possible prognostic factor in head and neck cancer. Br J Cancer 86(4):512–6, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brun E, Kiellen E, Tennvall J: FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck 24(2):127–35, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kidd EA, Siegel BA, Dehdashti F: The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer 110(8):1738–44, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Westerterp M, van Westreenen HL, Reitsma JB: Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy--systematic review. Radiology 236(3):841–51, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Schwarz JK, Siegel BA, Dehdashti F: Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA 298(19):2289–95, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Harris IS, Treloar AE, Inoue S: Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27(2):211–22, 2015. [DOI] [PubMed] [Google Scholar]
- 19.DeNicola GM, Karreth FA, Humpton TJ: Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475(7354):106–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diehn M, Cho RW, Lobo NA: Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458(7239):780–3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schafer ZT, Grassian AR, Long L: Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461(7260):109–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobhakumari A, Love-Homan L, Fletcher EV: Susceptibility of human head and neck cancer cells to combined inhibition of glutathione and thioredoxin metabolism. PLoS One 7(10):e48175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodman SN, Spence JM, Ronnfeldt TJ: Enhancement of Radiation Response in Breast Cancer Stem Cells by Inhibition of Thioredoxin- and Glutathione-Dependent Metabolism. Radiat Res 186(4):385–395, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Fath MA, Scarbrough PM: Combined inhibition of glycolysis, the pentose cycle, and thioredoxin metabolism selectively increases cytotoxicity and oxidative stress in human breast and prostate cancer. Redox Biol 4:127–35, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzam EI, Jay-Gerin JP, Pain D: Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 327(1-2):48–60, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rashmi R, Huang X, Floberg JM: Radioresistant Cervical Cancers Are Sensitive to Inhibition of Glycolysis and Redox Metabolism. Cancer Res 78(6):1392–1403, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Butler EB, Tan M: Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis 4:e532, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adekola K, Rosen ST, Shanmugam M: Glucose transporters in cancer metabolism. Curr Opin Oncol 24(6):650–4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibuya K, Okada M, Suzuki S: Targeting the facilitative glucose transporter GLUT1 inhibits the self-renewal and tumor-initiating capacity of cancer stem cells. Oncotarget 6(2):651–61, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Cao Y, Zhang W: A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther 11(8):1672–82, 2012 [DOI] [PubMed] [Google Scholar]
- 31.McBrayer SK, Cheng JC, Singhal S: Multiple myeloma exhibits novel dependence on GLUT4, GLUT8, and GLUT11: implications for glucose transporter-directed therapy. Blood 119(20):4686–97, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Mjiyad N, Caro-Maldonado A, Ramirez-Peinado S: Sugar-free approaches to cancer cell killing. Oncogene 30(3):253–64, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Pelicano H, Martin DS, Xu RH: Glycolysis inhibition for anticancer treatment. Oncogene 25(34):4633–46, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Price GS, Page RL, Riviere JE: Pharmacokinetics and toxicity of oral and intravenous lonidamine in dogs. Cancer Chemother Pharmacol 38(2):129–35, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Kennedy CR, Tilkens SB, Guan H: Differential sensitivities of glioblastoma cell lines towards metabolic and signaling pathway inhibitions. Cancer Lett 336(2):299–306, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Aykin-Burns N, Ahmad IM, Zhu Y: Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J 418(1):29–37, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Z, Jiang W, McGinley JN: 2-Deoxyglucose as an energy restriction mimetic agent: effects on mammary carcinogenesis and on mammary tumor cell growth in vitro. Cancer Res 65(15):7023–30, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Jelluma N, Yang X, Stokoe D: Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol Cancer Res 4(5):319–30, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Kurtoglu M, Gao N, Shang J: Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther 6(11):3049–58, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Zhong D, Liu X, Schafer-Hales K: 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther 7(4):809–17, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Maher JC, Wangpaichitr M, Savaraj N: Hypoxia-inducible factor-1 confers resistance to the glycolytic inhibitor 2-deoxy-D-glucose. Mol Cancer Ther 6(2):732–41, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Zhong D, Xiong L, Liu T: The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J Biol Chem 284(35):23225–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maschek G, Savaraj N, Priebe W: 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res 64(1):31–4, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Gupta P, Jagavelu K, Mishra DP: Inhibition of NADPH Oxidase-4 Potentiates 2-Deoxy-D-Glucose-Induced Suppression of Glycolysis, Migration, and Invasion in Glioblastoma Cells: Role of the Akt/HIF1alpha/HK-2 Signaling Axis. Antioxid Redox Signal 23(8):665–81, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Dwarakanath B, Jain V: Targeting glucose metabolism with 2-deoxy-D-glucose for improving cancer therapy. Future Oncol 5(5):581–5, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Simons AL, Fath MA, Mattson DM: Enhanced response of human head and neck cancer xenograft tumors to cisplatin combined with 2-deoxy-D-glucose correlates with increased 18F-FDG uptake as determined by PET imaging. Int J Radiat Oncol Biol Phys 69(4):1222–30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simons AL, Ahmad IM, Mattson DM: 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res 67(7):3364–70, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raez LE, Papadopoulos K, Ricart AD: A phase I dose-escalation trial of 2-deoxy-Dglucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 71(2):523–30, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Varshney R, Dwarakanath BS, Jain V: Radiosensitization by 6-aminonicotinamide and 2-deoxy-D-glucose in human cancer cells. International Journal of Radiation Biology 81(5):397–408, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Kalia VK, Prabhakara S, Narayanan V: Modulation of cellular radiation responses by 2-deoxy-D-glucose and other glycolytic inhibitors: implications for cancer therapy. J Cancer Res Ther 5 Suppl 1:S57–60, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Dwarkanath BS, Jain VK: Energy linked modifications of the radiation response in a human cerebral glioma cell line. Int J Radiat Oncol Biol Phys 17(5):1033–40, 1989 [DOI] [PubMed] [Google Scholar]
- 52.Lin X, Zhang F, Bradbury CM: 2-Deoxy-D-glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism. Cancer Res 63(12):3413–7, 2003 [PubMed] [Google Scholar]
- 53.Mohanti BK, Rath GK, Anantha N: Improving cancer radiotherapy with 2-deoxy-Dglucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys 35(1):103–11, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Singh D, Banerji AK, Dwarakanath BS: Optimizing cancer radiotherapy with 2-deoxy-dglucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol 181(8):507–14, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Dwarakanath BS, Singh D, Banerji AK: Clinical studies for improving radiotherapy with 2-deoxy-D-glucose: present status and future prospects. J Cancer Res Ther 5 Suppl 1:S21–6, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Vander Heiden MG: Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10(9):671–84, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Panieri E, Santoro MM: ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 7(6):e2253, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorrini C, Harris IS, Mak TW: Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12(12):931–47, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Trachootham D, Alexandre J, Huang P: Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8(7):579–91, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Griffith OW: Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem 257(22):13704–12, 1982 [PubMed] [Google Scholar]
- 61.Davison K, Cote S, Mader S: Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia 17(5):931–40, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Rudin CM, Yang Z, Schumaker LM: Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer Res 63(2):312–8, 2003 [PubMed] [Google Scholar]
- 63.Skapek SX, Colvin OM, Griffith OW: Enhanced melphalan cytotoxicity following buthionine sulfoximine-mediated glutathione depletion in a human medulloblastoma xenograft in athymic mice. Cancer Res 48(10):2764–7, 1988 [PubMed] [Google Scholar]
- 64.Leung SW, Mitchell JB, al-Nabulsi I: Effect of L-buthionine sulfoximine on the radiation response of human renal carcinoma cell lines. Cancer 71(7):2276–85, 1993 [DOI] [PubMed] [Google Scholar]
- 65.Bailey HH, Mulcahu RT, Tutsch KD: Phase I clinical trial of intravenous L-buthionine sulfoximine and melphalan: an attempt at modulation of glutathione. J Clin Oncol 12(1):194–205, 1994 [DOI] [PubMed] [Google Scholar]
- 66.O'Dwyer PJ, Hamilton TC, LaCreta FP: Phase I trial of buthionine sulfoximine in combination with melphalan in patients with cancer. J Clin Oncol 14(1):249–56, 1996 [DOI] [PubMed] [Google Scholar]
- 67.Bailey HH, Ripple G, Tutsch KD: Phase I study of continuous-infusion L-S,R-buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst 89(23):1789–96, 1997 [DOI] [PubMed] [Google Scholar]
- 68.Anderson CP, Matthay KK, Perentesis JP: Pilot study of intravenous melphalan combined with continuous infusion L-S,R-buthionine sulfoximine for children with recurrent neuroblastoma. Pediatr Blood Cancer 62(10):1739–46, 2015 [DOI] [PubMed] [Google Scholar]
- 69.Lu J, Holmgren A: The thioredoxin antioxidant system. Free Radic Biol Med 66:75–8, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Gandin V, Fernandes AP, Rigobello MP: Cancer cell death induced by phosphine gold(I) compounds targeting thioredoxin reductase. Biochem Pharmacol 79(2):90–101, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Fiskus W, Saba N, Shen M: Auranofin induces lethal oxidative and endoplasmic reticulum stress and exerts potent preclinical activity against chronic lymphocytic leukemia. Cancer Res 74(9):2520–32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou P, Chen M, Ji J: Auranofin induces apoptosis by ROS-mediated ER stress and mitochondrial dysfunction and displayed synergistic lethality with piperlongumine in gastric cancer. Oncotarget 6(34):36505–21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Topkas E, Cai N, Cumming A: Auranofin is a potent suppressor of osteosarcoma metastasis. Oncotarget 7(1):831–44, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raninga PV, Di Trapani G, Vuckovic S: Inhibition of thioredoxin 1 leads to apoptosis in drug-resistant multiple myeloma. Oncotarget 6(17):15410–24, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen X, Shi X, Zhao C: Anti-rheumatic agent auranofin induced apoptosis in chronic myeloid leukemia cells resistant to imatinib through both Bcr/Abl-dependent and - independent mechanisms. Oncotarget 5(19):9118–32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, Bouzakoura S, de Mey S: Auranofin radiosensitizes tumor cells through targeting thioredoxin reductase and resulting overproduction of reactive oxygen species. Oncotarget 8(22):35728–35742, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fath MA, Ahmad IM, Smith CJ: Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clin Cancer Res 17(19):6206–17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray LR, Tompkins SC, Taylor EB: Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci 71(14):2577–604, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clem B, Telang S, Clem A: Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 7(1):110–20, 2008 [DOI] [PubMed] [Google Scholar]
- 80.Ganapathy-Kanniappan S, Geschwind JF: Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 12:152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ganapathy-Kanniappan S, Kunjithapatham R, Geschwind JF: Glyceraldehyde-3-phosphate dehydrogenase: a promising target for molecular therapy in hepatocellular carcinoma. Oncotarget 3(9):940–53, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ko YH, Smith BL, Wang Y: Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun 324(1): p.269–75, 2004 [DOI] [PubMed] [Google Scholar]
- 83.Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res 29(12):4909–18, 2009 [PMC free article] [PubMed] [Google Scholar]
- 84.Pereira da Silva AP, El-Bacha T, Kyaw N: Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate. Biochem J 417(3):717–26, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Christofk HR, Vander Heiden MG, Harris MH: The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452(7184):230–3, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Goldberg MS, Sharp PA: Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J Exp Med 209(2):217–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anastasiou D, Poulogiannis G, Asara JM: Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334(6060):1278–83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao Y, Liu H, Liu Z: Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res 71(13):4585–97, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorrini C, Harris IS, Mak TW: Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12(12):931–47, 2013 [DOI] [PubMed] [Google Scholar]
- 90.Hangauer MJ, Viswanathan VS, Ryan MJ: Drug-tolerant persistent cancer cells are vulnerable to GPX4 inhibition. Nature 551(7679):247–250, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chu W, Chepetan A, Zhou D: Development of a PET radiotracer for non-invasive imaging of the reactive oxygen species, superoxide, in vivo. Org Biomol Chem 12(25):4421–31, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]