Supplemental Digital Content is available in the text.
Keywords: atherosclerosis, cardiovascular diseases, cholesterol, lipoproteins, stroke
Abstract
Objective—
To assess the role of HDL (high-density lipoprotein)-mediated cholesterol mass efflux capacity (CMEC) in incident cardiovascular disease and carotid plaque progression.
Approach and Results—
We measured CMEC in 2 cohorts aged 45 to 84 years at baseline derived from the MESA (Multi-Ethnic Study of Atherosclerosis). Cohort 1 comprised 465 cases with incident cardiovascular disease events during 10 years of follow-up and 465 age- and sex-matched controls; cohort 2 comprised 407 cases with progression of carotid plaque measured by ultrasonography at 2 exams >10 years and 407 similarly matched controls. Covariates and outcome events were ascertained according to the MESA protocol. CMEC level was modestly correlated with HDL cholesterol (R=0.13; P<0.001) but was not associated with age, sex, race/ethnicity, body mass index, diabetes mellitus, alcohol use, smoking status, or statin use. Higher CMEC level was significantly associated with lower odds of cardiovascular disease (odds ratio, 0.82 per SD of CMEC [95% CI, 0.69–0.98; P=0.031] in the fully adjusted model) in cohort 1 but higher odds of carotid plaque progression (odds ratio, 1.24 per SD of CMEC [95% CI, 1.04–1.48; P=0.018] in the fully adjusted model) in cohort 2 but without dose-response effect. In subgroup analysis within cohort 1, higher CMEC was associated with lower risk of incident coronary heart disease events (odds ratio, 0.72 per SD of CMEC (95% CI, 0.5–0.91; P=0.007) while no association was found with stroke events.
Conclusions—
These findings support a role for HDL-mediated cholesterol efflux in an atheroprotective mechanism for coronary heart disease but not stroke.
Highlights.
We measured HDL (high-density lipoprotein)-mediated cholesterol mass efflux capacity in samples from the MESA (Multi-Ethnic Study of Atherosclerosis). Higher cholesterol mass efflux capacity level was significantly associated with lower odds of incident cardiovascular disease in fully adjusted multivariate models.
Higher cholesterol mass efflux capacity level was associated with lower risk of incident coronary heart disease events, but no association was found with risk of stroke.
Higher cholesterol mass efflux capacity level was associated with higher risk of carotid plaque progression, but no dose-response relationship was found for this association, suggesting that the positive finding is of minimal or unclear significance.
These findings support a role for HDL-mediated cholesterol efflux in an atheroprotective mechanism for coronary heart disease but not stroke.
At a therapeutic level, these findings suggest that therapies that increase cholesterol efflux, such as infusions of cholesterol poor reconstituted HDL that are in phase 3 clinical studies, or upregulation of ABCA1/G1 (ATP binding cassette transporter subfamily ABCA member 1/G member 1), for example, by LXR (liver X receptor) activator treatment have potential to decrease coronary heart disease risk but possibly not stroke.
In population studies plasma HDL (high-density lipoprotein) cholesterol levels show an inverse relationship to cardiovascular disease (CVD) risk, independent of other risk factors.1 Studies in cell culture and animal models suggest that HDL has the ability the reduce atherosclerosis by promoting the removal of cholesterol from macrophage foam cells, reducing macrophage-related inflammatory processes in atherosclerotic plaques.2,3 The relevance of HDL-mediated macrophage cholesterol efflux to human atherosclerosis was suggested by the discovery that the cholesterol efflux capacity (CEC) of HDL was inversely related to coronary atheroma burden in subjects undergoing coronary angiography.4 Moreover, in a longitudinal design from the Dallas Heart Study5 and a nested case-control design from the Epic-Norfolk study,6 the CEC of HDL was inversely related to incident coronary heart disease (CHD). Importantly, these relationships were seen even after adjustment for HDL-cholesterol level, suggesting that CEC is measuring a key function of HDL relevant to CHD. However, the inverse relationship of CEC to CHD has not always been observed. In one study increased HDL-mediated cholesterol efflux was associated with an increased incidence of CHD.7 In the JUPITER study (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) of rosuvastatin, on treatment CEC measurements only showed a weak inverse correlation with CHD, while baseline samples did not show any relationship.8 The reasons for these discrepancies are unknown but could be related to sample size, study design, or methods for measuring CEC. Therefore, to further assess the relationship between CEC and incident CHD, we performed a nested case-control study in the MESA (Multi-Ethnic Study of Atherosclerosis) cohort, measured CEC in baseline samples, and then related these measurements to incident CVD.
See accompanying editorial on page 2
Moreover, only one study in 203 healthy White subjects has reported an inverse relationship between CEC and carotid-intima thickness,4 whereas another study found no relationship after multivariate adjustment between CEC and lipid-rich necrotic cores as determined by carotid magnetic resonance imaging.9 Therefore, in a second subsample from the MESA cohort, we investigated the relationship of CEC to progression of carotid artery plaque. To date, all studies have used a radioactive or fluorescent cholesterol tracer to measure efflux of cholesterol from cultured macrophages to HDL over the course of several hours. Because there is a bidirectional exchange of cholesterol between cells and HDL, the efflux of labeled cholesterol from cells may be partly or wholly counter-balanced by the uptake of nonlabeled cholesterol from HDL. Thus, the efflux of labeled cholesterol is not an accurate measure of the net movement (efflux minus influx) of cholesterol between cells and HDL. We used a cholesterol mass efflux assay in which the change in cholesterol mass in media is directly measured10 to circumvent this problem. We determined the cholesterol mass efflux capacity (CMEC) of the HDL fraction in serum samples from subjects in the MESA cohort. Our studies provide confirmation of the inverse relationship between CEC and CHD. Unexpectedly they also revealed a positive relationship between CMEC and carotid plaque progression and no relationship to nonhemorrhagic stroke.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Participants and Baseline Measures
The MESA is a population-based study of 6814 men and women aged 45 to 85 years, without known clinical CVD at time of entry, recruited from 6 US communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan, NY; and St Paul, MN). Sampling and recruitment procedures have been reported.11 Questionnaires were used to assess age, sex, race/ethnicity, educational and income levels, occupational information, smoking status, and medication use for diabetes mellitus, lipid lowering, and hypertension. Classification of race/ethnicity was based on self-identification using questions based on the United States 2000 census questionnaire. Physical activity,12 alcohol intake, and diet13 were measured using questionnaires. Height and weight were measured, and body mass index was computed as kg/m2. Blood pressure was measured 3 times at 1 minute intervals after a standardized protocol.14 Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-reported high blood pressure and on treatment with medication for hypertension.15 Diabetes mellitus was defined as being on treatment with insulin or oral medication for diabetes mellitus or fasting glucose ≥126 mg/dL.16 The baseline exam was conducted between August 1, 2000 and July 30, 2002. Follow-up at 10 years (MESA exam 5) was 76% (n=4655) of those alive. Centrally trained and certified study staff performed all participant measurements. Institutional Review Board approval was obtained at all MESA sites. Consent was obtained from all participants.
Laboratory Measurements
Fasting blood specimens were analyzed for serum glucose, total cholesterol, HDL cholesterol, and triglyceride levels. LDL (low-density lipoprotein) cholesterol was calculated in plasma specimens having a triglyceride value <400 mg/dL using the Friedewald formula.17
Plasma HDL Preparation
ApoB-containing particles were precipitated from serum by adding 100 µL of serum to 40 µL of 20% polyethylene glycol (Sigma P-2139 in 200 mmol/L glycine, pH10) solution. This mixture was incubated at room temperature for 15 minutes then was centrifuged at 4000 rpm for 20 minutes. The supernatant, containing HDL fractions, was removed and used for experiments as previously described.10
Cholesterol Mass Efflux Measurements
Cholesterol efflux measurements were performed at Columbia University in a completely blinded fashion and data transmitted to the University of Washington for unblinding. THP-1 monocytes (ATCC TIB-202, Manassas, VA) were cultured in RPMI (Roswell Park Memorial Institute) 1640 medium supplemented with 10% fetal bovine serum at 37°C in 5% Co2. Cells were treated with 100 nmol/L Phorbol myristate acetate for 24 hours to facilitate differentiation into macrophages. Then, adherent macrophages were incubated with 50 µg/mL acetyl-LDL and 3 µmol/L LXR (liver X receptor) agonist (TO901317) for 24 hours before cholesterol efflux studies.
CMEC Analysis
CMEC was analyzed in DMEM containing 0.2% BSA in the presence of polyethylene glycol-HDL matched by volume (ratio 7:1). After 6 hours incubation with HDL, the mass of total cholesterol was determined from the collected media by colorimetric assay. The HDL-mediated CMEC was calculated by subtraction of cholesterol mass of the medium cultured with or without cells. This allows the determination of the net cholesterol efflux driven by HDL particles reflecting the ability of HDL to remove cellular cholesterol.10 The assay was run in triplicate; the intra-assay coefficient of variation was 4.6%.
Cardiovascular Events
Participants were followed for incident CVD events for a median of 10.2 years from their baseline examinations. In addition to 5 follow-up MESA examinations, a telephone interviewer contacted each participant every 9 to 12 months to inquire about interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. To verify self-reported diagnoses, copies were requested of all death certificates and medical records for all hospitalizations and outpatient cardiovascular diagnoses. Next of kin interviews for out of hospital cardiovascular deaths were obtained.18 Medical records were obtained for ≈99% of reported hospitalized cardiovascular events and information on 97% of reported outpatient cardiovascular diagnostic encounters. Follow-up telephone interviews were completed in 90% of living participants. Trained personnel abstracted medical records suggesting possible cardiovascular events. Two physicians independently reviewed all abstracted medical records for end point classification and assignment of incidence dates, using prespecified criteria.
Carotid Ultrasonography
B-mode ultrasound images of the right and left common carotid, bifurcation, and internal carotid artery segments were recorded on Super-VHS videotape with a Logiq 700 ultrasound system using the M12L transducer (General Electric Medical Systems, CCA frequency 13 MHz) at MESA exams 1 and 5 as described previously.19 Carotid plaque score (range 0–12) was defined as the number of carotid plaques in the internal, bifurcation, and common segments of both carotid arteries.19,20 Carotid plaque was defined as a discrete, focal wall thickening ≥1.5 cm or focal thickening at least 50% greater than the surrounding intima-media thickening.21 Progression was defined as an increase in the carotid plaque score from exam 1 to 5, which almost always was because of the appearance of a new plaque.19 For carotid plaque presence and score, intrareader reproducibility was κ=0.83 (95% CI, 0.70–0.96) and inter-reader reproducibility was κ=0.89 (95% CI, 0.72–1.00).19
Statistical Analysis
Cases, defined as participants who had an incident CVD event between exams 1 and 5, were matched by age (5-year intervals) and sex to controls, defined as those who did not have a CVD event (cohort 1; Figure). Controls were required to have at least as much event-free follow-up time as cases. Similarly, cases with carotid plaque progression were matched by the same factors to controls, defined as participants who did not have any increase in carotid plaque score between MESA exams 1 and 5 (cohort 2). All CVD cases were used. Of the total 1923 cases of carotid plaque progression, a random sample was selected based on age- and sex-matching to controls. Bivariate displays of values for the predictor of interest and covariates were used to compare case versus control groups. Conditional logistic regression models (conditional on the matching) were used to examine the relationship between cholesterol efflux and case-control status. A series of staged models were used to control for potential confounders. Model 1 adjusted for age, sex, race/ethnicity, body mass index, and study site. Model 2 additionally adjusted for diabetes mellitus, current smoking, total and HDL cholesterol, statin and antihypertensive medication use, and systolic blood pressure. Model 3 additionally adjusted for pack-years of smoking, alcohol use, intentional exercise, and Mediterranean diet score.22
Figure.
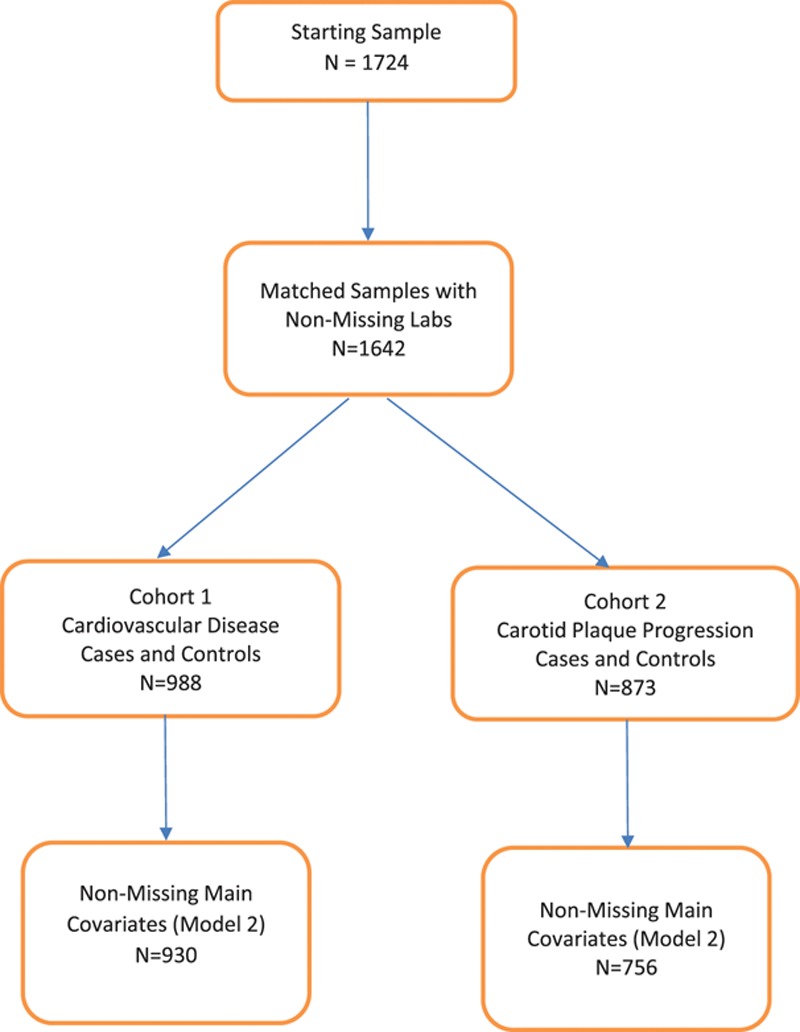
Flowchart.
Results
After exclusion of matched cases and controls with missing values for laboratory data or other key covariates, 930 subjects (465 cases and matched 465 controls) were available for analysis for incident CVD and 814 subjects (407 cases and 407 matched controls) for progression of carotid artery plaque (Figure). Cases and controls did not differ with respect to age and sex because they were matched on these criteria (Table 1). As expected, cases had higher prevalence or levels of body mass index, diabetes mellitus, smoking, hypertension, systolic and diastolic blood pressure, and lower level of intentional exercise and HDL cholesterol (48.5 versus 50.6 mg/dL for the CVD cases and controls, respectively; 49.9 versus 51.8 mg/dL for the carotid plaque progression cases versus controls). Mean CMEC was lower in the CVD cases versus controls (2.9 versus 3.1 mg/dL) but higher in the carotid artery plaque progression cases versus controls (3.1 versus 2.8 mg/dL; Table 1).
Table 1.
Participant Characteristics for Incident CVD and Carotid Plaque Progression by Case and Control Status
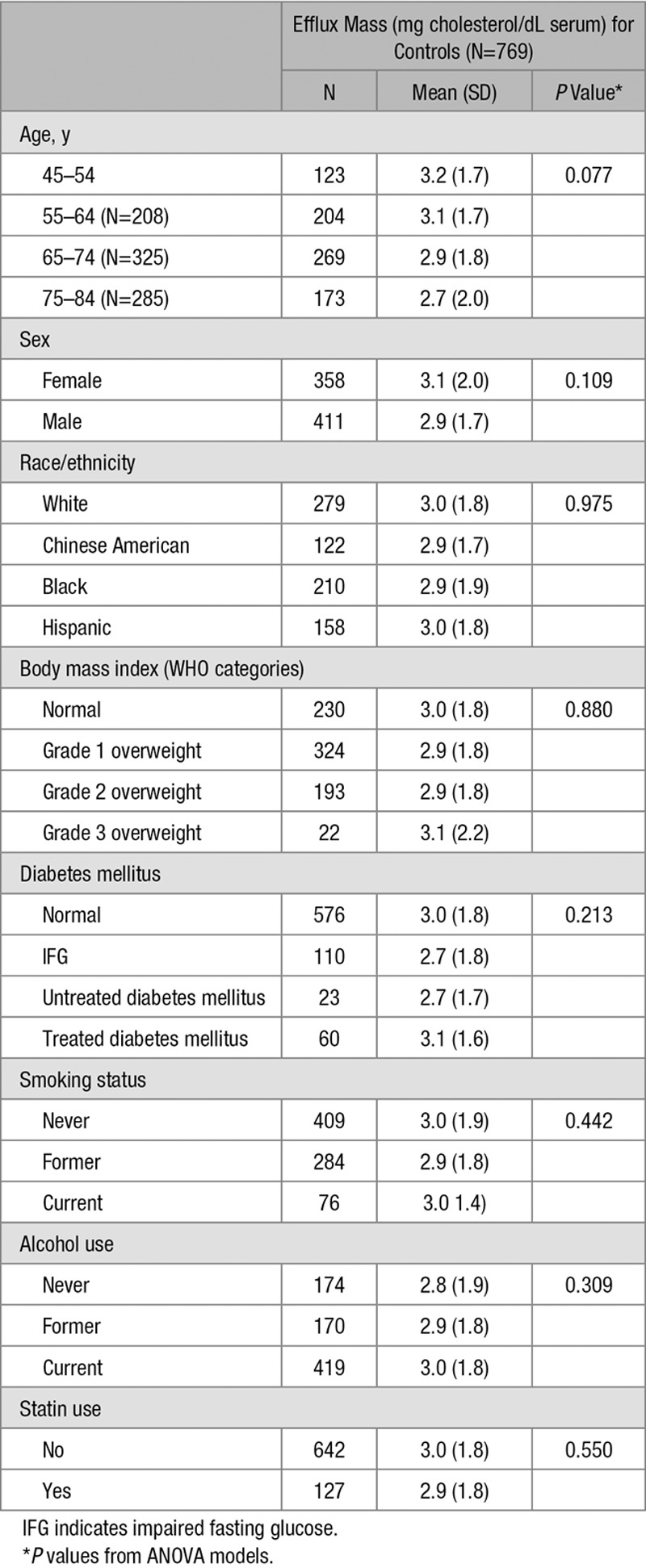
Table 2 shows mean CMEC levels in the control group classified by demographic and clinical characteristic. There was no statistically significant difference by age category, sex, race/ethnicity, body mass index category, diabetes mellitus category, alcohol use, smoking status, or statin use. CMEC was modestly correlated with HDL cholesterol (R=0.13; P<0.001), total cholesterol (R=0.08; P=0.036), and age (R=0.02; P=.009) but not with triglyceride level or homeostatic model assessment–insulin resistance (Tables I and II in the online-only Data Supplement).
Table 2.
Average Efflux Mass Among Controls, by Baseline Characteristics
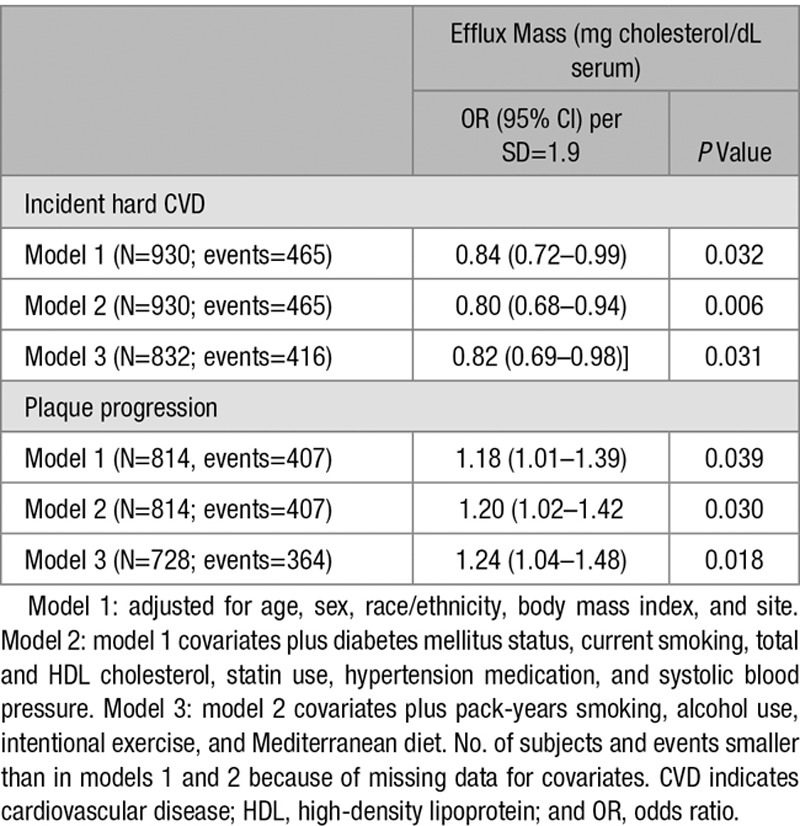
In conditional logistic regression models examining the association of CMEC with incident CVD, higher level of CMEC was associated with lower odds of incident CVD, with little difference in magnitude of the effect size or P value with adjustment for covariates (Table 3, top), with odds ratio of 0.82 per SD of CMEC (95% CI, 0.69–0.98; P=0.031) in the fully adjusted model. In contrast, a higher level of CMEC was associated with greater odds of carotid artery plaque progression (Table 3, bottom), with an odds ratio of 1.24 per SD of CMEC (95% CI, 1.04–1.48; P=0.018) in the fully adjusted model.
Table 3.
Conditional Logistic Regression Models for Incident Hard CVD and Plaque Progression (OR and 95% CI per SD of Efflux Mass)
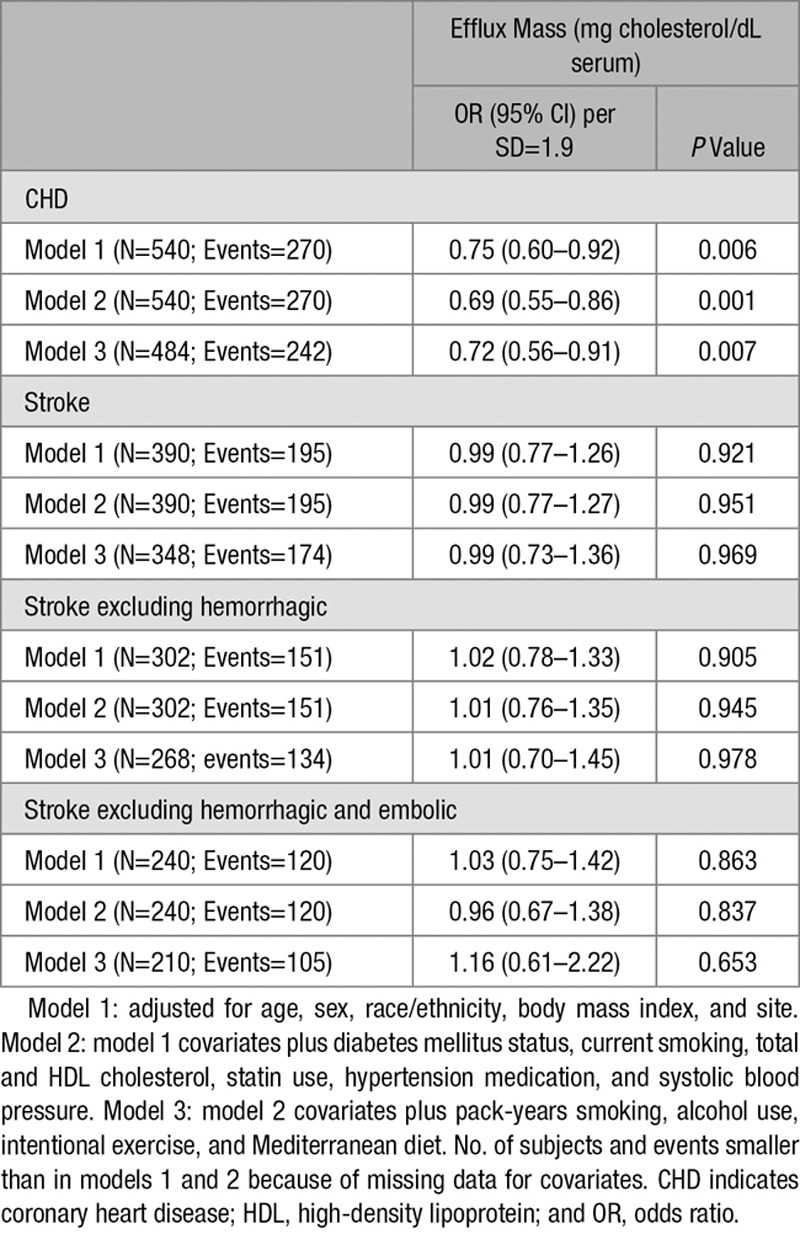
We further examined the association between higher level of CMEC and lower risk of incident CVD events by separately analyzing CHD events and stroke events. As shown in Table 4, higher CMEC level was associated with lower odds of CHD events (odds ratio, 0.72 per SD of CMEC (95% CI, 0.56–0.91; P=0.007) in the fully adjusted model while no association was found with stroke events or with stroke events after exclusion of hemorrhagic strokes (odds ratio, 1.01 per SD of CMEC (95% CI, 0.70–1.45; P=0.978). These findings in cohort 1, in a separate sample of cases and controls, were consistent with the lack of protection of higher CMEC level against carotid plaque progression in cohort 2. For both incident CVD and plaque progression, we tested for potential interactions between CMEC and sex, race/ethnicity, diabetes mellitus, hypertension, chronic kidney disease, smoking, and statin use; none was significant at the P<0.05 level. Additionally, statin use was not associated with CMEC level in our controls (Table 2). For these reasons, we did not pursue additional sensitivity analyses stratified by statin use. Models with results for all covariates are shown for carotid plaque progression, incident CVD, incident CHD, and incident stroke in Tables III through VI in the online-only Data Supplement, respectively. There was no evidence of a dose-response relationship in the association between CMEC and plaque progression (Table VII in the online-only Data Supplement).
Table 4.
Conditional Logistic Regression for Incident Stroke and CHD Events Separately (OR and 95% CI per SD of Efflux Mass)
Discussion
Using a novel assay based on the ability of HDL to stimulate the efflux of cholesterol from cholesterol-loaded macrophages, we have shown a strong, independent relationship between CMEC and incident CVD, and specifically CHD, in the MESA cohort. Our study involving 465 cases and 465 controls in a population-based prospective study with a 10-year follow-up, provides powerful support for the protective role of HDL-mediated cholesterol efflux in CVD, consistent with a majority of previous studies. This relationship was entirely driven by an inverse relationship between efflux and CHD, whereas there was no relationship with stroke or with nonhemorrhagic stroke. Although we found a positive relationship between efflux and carotid plaque progression overall, we did not find a dose-response relationship, possibly suggesting that the positive association is minimal and of unclear significance.
Our study supports the relationship between CEC and CHD, although finding a distinct relationship to carotid plaque progression and stroke. The findings on carotid plaque progression and stroke were found in different sub-cohorts, suggesting these were not chance observations. Mutharasan et al9 found no association after multivariate adjustment between cholesterol efflux and presence by carotid magnetic resonance imaging of plaque with lipid-rich necrotic core, in 402 individuals in the Chicago Aging Study, while in the Dallas Heart Study CEC was inversely associated with a composite CVD end point that included thrombotic stroke and an inverse relationship with thrombotic stroke considered as a subgroup (n=37).5 The reasons for the different results are uncertain but could include sample size, imaging modality, study design, and different efflux assays. The differences between the inverse relationship of CMEC to CHD and the variable relationship to carotid plaque progression and nonhemorrhagic/nonembolic stroke points to differences in these disease processes. Myocardial infarction reflects rupture or erosion of coronary plaques, leading to thrombotic occlusion whereas nonhemorrhagic stroke represents several processes, a minority of which represent carotid plaque ulceration and thrombus formation. In addition, in the largest epidemiological studies plasma HDL-cholesterol levels were inversely related to CHD but have no clear relationship to stroke.1 Interestingly, in the REVEAL study (Randomized Evaluation of the Effects of Anacetrapib Through Lipid-Modification), the CETP (cholesteryl ester transfer protein) inhibitor anacetrapib, which dramatically raised HDL and moderately reduced non-HDL cholesterol, benefited CHD but had no impact on nonhemorrhagic stroke.23 In contrast, in FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk trial), a PCSK9 (proprotein convertase subtilisin/kexin type 9) neutralizing antibody markedly lowered LDL and slightly raised HDL, with benefit for both CHD and stroke.24 Statin-mediated LDL lowering is generally associated with reduced nonhemorrhagic stroke.25 A limitation of our study is that there were too few stroke events (N=28) in cohort 2 to assess the association with carotid plaque. However, in a previous publication from the full MESA cohort, carotid plaque but not intima-media thickening was associated with incident stroke or transient ischemic attack.26
The evidence that HDL mediates an atheroprotective effect, at least in part by promotion of cholesterol efflux, has been strongly supported by animal studies. Infusion of HDL27 or increased expression of the main HDL protein, apoA-1,28,29 consistently reduces atherosclerosis. Cholesterol efflux from macrophages to HDL is mediated primarily by the ATP binding cassette transporters ABCA1 (ATP binding cassette transporter subfamily ABCA member 1) and ABCG1 (ATP binding cassette transporter subfamily G member 1). Knocking out these transporters in macrophages or endothelial cells results in an increase in atherosclerosis, driven in part by increased inflammatory processes and, for endothelial cells, decreased eNOS (endothelial nitric oxide synthase) activity.30,31 Differences in flow characteristics between coronary and carotid circulations may modify the response to HDL-mediated CMEC. Our studies were conducted by measuring efflux of cholesterol to apoB-depleted serum and thus not a direct measurement of HDL-mediated efflux. However, previous studies have shown that cholesterol efflux to apoB-depleted serum is largely driven by HDL.4 Our CMEC assay used human THP-1 macrophages, in which ABCA1 and ABCG1 are strongly upregulated by cholesterol loading and treatment with an LXR activator. ABCA1 and ABCG1 mediate unidirectional net cholesterol efflux from cells to apoA-1 or HDL, and the THP-1 cell assay is probably largely measuring ABCA1/ABCG1-driven cholesterol efflux.32 Most previous studies have used cAMP-treated murine J774 macrophages in which ABCA1 is upregulated but also with contributions of ABCG1, SR-B1 (scavenger receptor class B type 1), and aqueous diffusion to total cholesterol efflux.4 The latter 2 processes are bidirectional and thus can result in an increase in radioactive cholesterol in HDL without any change in net cholesterol efflux.32,33 Thus, the contributions of specific and nonspecific components of the isotopic efflux assay may vary with different samples. Although this can be compensated by large sample size and statistical adjustment for HDL-cholesterol levels, it may reduce the sensitivity and specificity of the CEC measurement.
The so-called HDL hypothesis based largely on the epidemiological relationships—that increasing HDL-cholesterol levels therapeutically will produce a consistent and proportionate reduction in CHD risk—has been called into question. First, clinical trials with agents that raise HDL-cholesterol level, notably niacin34 and some CETP inhibitors,35,36 have not shown consistent benefit. Notably, however, these agents while raising HDL cholesterol effectively (especially CETP inhibitors) only modestly increased CEC.10,37 Most recently, the REVEAL trial with the CETP inhibitor anacetrapib, the largest trial and the first to go to completion, did show a highly significant reduction in CHD.23 Although the magnitude of the CHD benefit appeared to correlate with the reduction in non-HDL cholesterol, there are likely wide confidence intervals to the CHD assessments, and a role of increased HDL levels and increased CEC cannot be excluded.38 Second, Mendelian Randomization studies have shown that while single nucleotide polymorphisms affecting LDL cholesterol and triglyceride levels have the expected relationship to CHD, HDL-cholesterol associated single nucleotide polymorphisms did not, perhaps suggesting that HDL cholesterol is not in the causal pathway of atherosclerosis.39,40
Our studies strongly support that HDL is directly involved in an atheroprotective mechanism and is not simply a biomarker for other processes more directly related to atherosclerosis. However, because cholesterol efflux measurements generally show weak correlations with HDL-cholesterol measurements they may not be measuring the same factor that was originally discovered in epidemiological studies of HDL-cholesterol levels. Apart from a weak trend of decreasing CMEC with aging and a modest (R=0.13) correlation with HDL-C, we did not detect any relationship to covariates. Prior studies also have not found the strong relationship of CEC to specific HDL composition or size measurements at least as determined by nuclear magnetic resonance.41 In addition, a recent study suggests that CEC does not have strong genetic determination.41 In small studies, HDL particle number measured by ion mobility shift assays does have a relationship both to ABCA1-mediated cholesterol efflux and CHD.42,43 It will be important in the future studies to define the physical or compositional factors in HDL that are responsible for differences in its ability to promote cholesterol efflux from macrophages.
On a therapeutic level, our study suggests that therapies that increase cholesterol efflux, such as infusions of cholesterol poor reconstituted HDL that are in phase 3 clinical studies, or upregulation of ABCA1/G1, for example, by LXR activator treatment have potential to decrease CHD risk. However, our findings suggest that these treatments may benefit CHD but not stroke.
Acknowledgments
We thank the other investigators, the staff, and the participants of the MESA study (Multi-Ethnic Study of Atherosclerosis) for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
Contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, by grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources, and by grants R1HL127637 (Dr Shea), P01 HL092969, P01 HL128203, and P30 DK017047 (J. Heinecke), and R1HL107653 (A.R. Tall). This publication was developed under STAR research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the US Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication.
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.118.311366.
Nonstandard Abbreviations and Acronyms
ABCA1: ATP binding cassette transporter subfamily ABCA member 1
ABCG1: ATP binding cassette transporter subfamily G member 1
CEC: cholesterol efflux capacity
CETP: cholesteryl ester transfer protein
CHD: coronary heart disease
CMEC: cholesterol mass efflux capacity
CVD: cardiovascular disease
eNOS: endothelial nitric oxide synthase
HDL: high-density lipoprotein
HOMA-IR: homeostatic model assessment–insulin resistance
LDL: low-density lipoprotein
LXR: liver X receptor
MESA: Multi-Ethnic Study of Atherosclerosis
SR-B1: scavenger receptor class B type 1
References
- 1.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rader DJ, Tall AR. The not-so-simple HDL story: is it time to revise the HDL cholesterol hypothesis? Nat Med. 2012;18:1344–1346. doi: 10.1038/nm.2937. doi: 10.1038/nm.2937. [DOI] [PubMed] [Google Scholar]
- 4.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khera AV, Demler OV, Adelman SJ, Collins HL, Glynn RJ, Ridker PM, Rader DJ, Mora S. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation. 2017;135:2494–2504. doi: 10.1161/CIRCULATIONAHA.116.025678. doi: 10.1161/CIRCULATIONAHA.116.025678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutharasan RK, Thaxton CS, Berry J, Daviglus ML, Yuan C, Sun J, Ayers C, Lloyd-Jones DM, Wilkins JT. HDL efflux capacity, HDL particle size, and high-risk carotid atherosclerosis in a cohort of asymptomatic older adults: the Chicago Healthy Aging Study. J Lipid Res. 2017;58:600–606. doi: 10.1194/jlr.P069039. doi: 10.1194/jlr.P069039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yvan-Charvet L, Kling J, Pagler T, Li H, Hubbard B, Fisher T, Sparrow CP, Taggart AK, Tall AR. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol. 2010;30:1430–1438. doi: 10.1161/ATVBAHA.110.207142. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–454. doi: 10.1093/aje/kwn350. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR., Jr. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2006;83:1369–1379. doi: 10.1093/ajcn/83.6.1369. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 15.Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI). Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 16.Expert Committee on the Diagnosis and Classifications of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 19.Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, Astor BC, Sheppard L, Kronmal RA, Stein JH. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:3257–3262. doi: 10.1161/STROKEAHA.114.005669. doi: 10.1161/STROKEAHA.114.005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollander M, Bots ML, Del Sol AI, Koudstaal PJ, Witteman JC, Grobbee DE, Hofman A, Breteler MM. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: the Rotterdam study. Circulation. 2002;105:2872–2877. doi: 10.1161/01.cir.0000018650.58984.75. [DOI] [PubMed] [Google Scholar]
- 21.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Abiemo EE, Alonso A, Nettleton JA, Steffen LM, Bertoni AG, Jain A, Lutsey PL. Relationships of the Mediterranean dietary pattern with insulin resistance and diabetes incidence in the Multi-Ethnic Study of Atherosclerosis (MESA). Br J Nutr. 2013;109:1490–1497. doi: 10.1017/S0007114512003339. doi: 10.1017/S0007114512003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217–1227. doi: 10.1056/NEJMoa1706444. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 24.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 25.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e002262. doi: 10.1161/CIRCIMAGING.114.002262. doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 29.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci USA. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerterp M, Murphy AJ, Wang M, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westerterp M, Tsuchiya K, Tattersall IW, Fotakis P, Bochem AE, Molusky MM, Ntonga V, Abramowicz S, Parks JS, Welch CL, Kitajewski J, Accili D, Tall AR. Deficiency of ATP-binding cassette transporters A1 and G1 in endothelial cells accelerates atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2016;36:1328–1337. doi: 10.1161/ATVBAHA.115.306670. doi: 10.1161/ATVBAHA.115.306670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 34.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 35.Barter PJ, Caulfield M, Eriksson M, et al. ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 36.Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. ACCELERATE Investigators. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933–1942. doi: 10.1056/NEJMoa1609581. doi: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 37.Nicholls SJ, Ruotolo G, Brewer HB, Kane JP, Wang MD, Krueger KA, Adelman SJ, Nissen SE, Rader DJ. Cholesterol efflux capacity and pre-beta-1 HDL concentrations are increased in dyslipidemic patients treated with evacetrapib. J Am Coll Cardiol. 2015;66:2201–2210. doi: 10.1016/j.jacc.2015.09.013. doi: 10.1016/j.jacc.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Tall AR, Rader DJ. Trials and Tribulations of CETP Inhibitors. Circ Res. 2018;122:106–112. doi: 10.1161/CIRCRESAHA.117.311978. doi: 10.1161/CIRCRESAHA.117.311978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. doi: 10.1038/ng.2795. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koekemoer AL, Codd V, Masca NG, Nelson CP, Musameh MD, Kaess BM, Hengstenberg C, Rader DJ, Samani NJ. Large-scale analysis of determinants, stability, and heritability of high-density lipoprotein cholesterol efflux capacity. Arterioscler Thromb Vasc Biol. 2017;10:1956–1962. doi: 10.1161/ATVBAHA.117.309201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monette JS, Hutchins PM, Ronsein GE, Wimberger J, Irwin AD, Tang C, Sara JD, Shao B, Vaisar T, Lerman A, Heinecke JW. Patients with coronary endothelial dysfunction have impaired cholesterol efflux capacity and reduced HDL particle concentration. Circ Res. 2016;119:83–90. doi: 10.1161/CIRCRESAHA.116.308357. doi: 10.1161/CIRCRESAHA.116.308357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, Oda MN, Zhao XQ, Heinecke JW. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114:1733–1742. doi: 10.1161/CIRCRESAHA.114.303454. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]


