Abstract
Background:
Lung fibrosis is attributed to derangements in extracellular matrix remodeling, a process driven by collagen turnover. We examined the association of two collagen biomarkers, carboxy-terminal telopeptide of collagen type I (ICTP) and amino-terminal propeptide of type III procollagen (PIIINP), with subclinical interstitial lung disease (ILD) in adults.
Methods:
We performed a cross-sectional analysis of 3244 participants age 45–84 years in the Multi-Ethnic Study of Atherosclerosis. Serum ICTP and PIIINP levels were measured at baseline by radioimmunoassay. Subclinical ILD was defined as high attenuation areas (HAA) in the lung fields on baseline cardiac CT scans. Interstitial lung abnormalities (ILA) were measured in 1082 full-lung CT scans at 9.5 years median follow-up. We used generalized linear models to examine the associations of collagen biomarkers with HAA and ILA.
Results:
Median (IQR) for ICTP was 3.2 μg/L (2.6–3.9 μg/L) and for PIIINP was 5.3 μg/L (4.5–6.2 μg/L). In fully adjusted models, each SD increment in ICTP was associated with a 1.3% increment in HAA (95% CI 0.2–2.4%, p = 0.02) and each SD increment in PIIINP was associated with a 0.96% increment in HAA (95% CI 0.06–1.9%, p = 0.04). There was no association between ICTP or PIIINP and ILA. There was no evidence of effect modification by gender, race, smoking status or eGFR.
Conclusions:
Higher levels of collagen biomarkers are associated with greater HAA independent of gender, race and smoking status. This suggests that extracellular matrix remodeling may accompany subclinical ILD prior to the onset of clinically evident disease.
Keywords: Subclinical interstitial lung disease, Collagen biomarkers, Extracellular matrix, Lung fibrosis
1. Introduction
The interstitial lung diseases (ILDs) are a heterogeneous group of conditions characterized by progressive fibrosis and inflammation of the lung parenchyma [1] Clinically, fibrotic ILDs are often diagnosed at an advanced stage, carry a poor prognosis, and have limited treatment options. The investigation of subclinical disease may yield insights into the early pathobiology of ILD, perhaps leading to strategies that prevent incident disease.
The pathogenesis of lung fibrosis has been attributed to recurrent alveolar epithelial injury, inflammation and subsequent dysregulated extracellular matrix remodeling [1–3]. Extracellular matrix remodeling and elements of the collagen cycle can be detected by measuring serum levels of carboxy-terminal telopeptide of collagen type 1 (ICTP) and the amino-terminal propeptide of type III procollagen (PIIINP) [4]. ICTP is a small renally-cleared molecule that represents matrix metalloproteinase (MMP)-dependent degradation of type I collagen. PIIINP levels reflect collagen synthesis and are not dependent on renal clearance [5,6]. Together, ICTP and PIIINP represent aspects of the collagen cycle and serve as collagen biomarkers.
Subclinical ILD represents the asymptomatic phase that may precede the development of clinically significant ILD [7–9]. Our group has developed and validated a novel, automated measure of lung attenuation on computed tomography (CT) called high attenuation areas (HAA), which serves as a quantitative phenotype of subclinical ILD and may reflect early pathological changes in the lung parenchyma [7,10]. Interstitial lung abnormalities (ILA) visualized on chest CT scans are a qualitative phenotype of subclinical ILD that provides complementary information to HAA [11,12]. HAA and ILA are associated with cigarette smoking [7,12], reduced lung function [10,12], and increased all-cause mortality [10,13]. Recently, we have shown that baseline HAA is associated with exertional dyspnea and increased risk of ILD-specific hospitalization and death during a median of 12.2 years follow-up, emphasizing that baseline HAA is a clinically relevant ILD risk factor [14]. We have also shown that elevated levels of MMP-7, interleukin (IL)-6, rheumatoid factor IgM and IgA at baseline are associated with incremental HAA and have stronger associations in ever-smokers, suggesting that HAA may be a marker of lung inflammation and extracellular matrix remodeling [10,15].
In this study, we aimed to examine the association between collagen biomarkers (ICTP and PIIINP) and both HAA and ILA in a large cohort of healthy, community-dwelling adults. We hypothesized that baseline levels of ICTP and PIIINP will be associated with a greater percentage of HAA on baseline CT and also with the presence of ILA on chest CT that were performed approximately 10 years later.
2. Materials and methods
2.1. Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multi-center prospective cohort study investigating subclinical cardiovascular disease (CVD). It consists of 6814 men and women, recruited from six communities in the USA, 45–84 years old at the time of recruitment during Exam 1 (2000–2002), without clinical manifestations of CVD. Participants underwent cardiac CT imaging at Exam 1 and a subset underwent full lung CT imaging at Exam 5 (2010–2012), as previously published [16].
In the current study, we examined participants who had been previously selected for ICTP and PIIINP measurement to test the hypothesis that these measures of collagen turnover would be associated with CVD and arterial elasticity [17–19]. After excluding participants without available banked plasma or baseline blood pressure waveform measurements, the study sampled all 627 participants with adjudicated CVD and a random sample of 56% of all remaining participants (n = 2655). In total, ICTP was measured in 3247 subjects and PIIINP was measured in 3239 subjects.
MESA was approved by Institutional Review Boards at all collaborating centers and all participants provided informed consent.
2.2. Measurement of exposure and covariates
ICTP and PIIINP were measured from frozen plasma stored in ethylenediaminetetraacetic acid from fasting blood samples drawn during Exam 1. Assays were performed between September 2014 and February 2015 (University of Minnesota, under direction of MDG) using commercially available competitive radioimmunoassay kits (UniQ #06099 for ICTP and UniQ #06098 for PIIINP, Orion Diagnostica, Espoo, Finland) [18,20]. Intra-assay coefficients of variation were 9.3–16.5% for PIIINP and 6.3–8.8% for ICTP.
Detailed information regarding covariate data in MESA has been previously published [16].
2.3. HAA on cardiac CT scan
Quantitative measures of CT lung attenuation were measured in the lung fields of cardiac CT scans performed during Exam 1 as previously described [21]. Image attenuation was evaluated by trained readers at the University of Iowa imaging lab, using a modified version of the Pulmonary Analysis Software Suite, who were unaware of the participants’ characteristics or collagen biomarker values. HAA was defined as CT attenuation values of −600 to −250 Hounsfield units, which captures ground glass and reticular opacities [7]. Percent emphysema was defined as the percentage of lung voxels less than −950 Hounsfield units [22].
2.4. ILA on chest CT scan
ILAs were assessed on full lung CT scans performed in years 2010–2012 from a randomly sampled subset of participants who were eligible to return for Exam 5, as previously described [10]. A radiologist visually identified ground glass or reticular opacities, diffuse centrilobular nodularities, non-emphysematous cysts, honeycombing, and/or traction bronchiectasis that affects greater than 5% of any lung zone in a non-dependent manner as previously described [12,23].
2.5. Statistical analysis
Due to the stratified sampling in the original study design, all analyses included inverse probability weighting to account for selection for ICTP and PIIINP measurement. We used multivariable linear regression models to examine associations between both ICTP and PIIINP, and HAA, adjusting for age, gender, race/ethnicity, educational attainment, height, body mass index (BMI), waist circumference, smoking status, cigarette pack-years, estimated glomerular filtration rate (eGFR), high-sensitivity C-reactive protein (hs-CRP), IL-6, d-dimer, study site, percent emphysema, milliamperes (mA) dose, and total volume of imaged lung. These covariates were selected based on our prior work showing associations with HAA and known variables that may confound the measurement of HAA. We treated HAA as a continuous dependent natural log-transformed variable, ILA as a binary dependent variable, and ICTP and PIIINP as continuous independent variables. We used generalized additive models with loess smoothing functions to examine the linearity of the associations of HAA with ICTP and PIIINP with outliers removed. We used logistic regression to examine associations between both ICTP and PIIINP, and ILA, with adjustment for age, gender, race/ethnicity, smoking status, and cigarette pack-years. Missing covariate data was handled using multiple imputations by chained equations [10,24]. Separate regression analyses were performed with values in the top 5th percentile of ICTP and PIIINP removed to account for outliers. Analyses were performed in STATA, v.14.2 (College Station, Texas, USA) and R, v.3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Baseline ICTP levels and baseline HAA were available in 3244 participants. Baseline PIIINP levels were available in 3236 participants. The median (IQR) ICTP level was 3.2 μg/L (2.6–3.9 μg/L, SD 1.33) and the median (IQR) PIIINP level was 5.3 μg/L (4.5–6.2 μg/L, SD 1.54). The median (IQR) HAA was 121.8 cm3 (102.1–147.2 cm3) or 5.6% HAA (4.5–7.2%). Exam 5 ILA readings were available in 1082 who had both ICTP and PIIINP measured at Exam 1. The prevalence of ILA in these participants was 13.7%.
Baseline characteristics are presented in Table 1. Participants had a median (IQR) age of 64 (54–71) years; 49.8% were men; 37.3% were white, 27.7% were African-American, 23.2% were Hispanic and 11.8% were Chinese-American. Fifty percent of participants were former or current smokers and smoked a median (IQR) of 16.5 (6–33) cigarette pack-years. Baseline characteristics were similar across quartiles of ICTP and PIIINP, with and without case-weighting (Table 1 and Tables A1-A3), except for race/ethnicity, weight, BMI, and cigarette pack-years. White race was more prevalent among those with higher ICTP and PIIINP levels, whereas Chinese-American race was more prevalent among those with lower ICTP levels. Hispanic ethnicity was more prevalent with lower PIIINP levels. BMI, weight, and cigarette pack-years tended to be higher among those with higher ICTP or PIIINP levels. HAA percent and ILA stratified across ICTP and PIIINP quartiles are shown in tables A4-A5.
Table 1.
Baseline characteristics stratified by quartiles of ICTP.
| ICTP Quartile |
||||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| ICTP μg/L, range | 1.12–2.59 | 2.6 to 3.16 | 3.17 to 3.89 | 3.9–23.45 |
| Number of subjects | 803 | 819 | 805 | 817 |
| Demographics & anthropometries | ||||
| Age, years | 59 ± 9.3 | 62 ± 9.9 | 64 ± 10.2 | 67 ± 10.5 |
| Male sex | 374 (46.6) | 400 (48.8) | 423 (52.6) | 418 (51.2) |
| Race/ethnicity | ||||
| White | 252 (31.4) | 307 (37.5) | 322 (40.0) | 328 (40.2) |
| African American | 242 (30.1) | 230 (28.1) | 202 (25.1) | 225 (27.5) |
| Chinese American | 121 (15.1) | 94 (11.5) | 100 (12.4) | 69 (8.5) |
| Hispanic | 188 (23.4) | 188 (23.0) | 181 (22.5) | 195 (23.9) |
| Highest Education Level | ||||
| Less than high school | 145 (18.1) | 150 (18.3) | 157 (19.5) | 169 (20.7) |
| High school | 378 (47.2) | 387 (47.3) | 372 (46.3) | 378 (46.3) |
| Bachelor’s or greater | 278 (34.7) | 281 (34.4) | 275 (34.2) | 269 (33.0) |
| Height, cm | 165.7 ± 9.9 | 166.6 ± 10.3 | 166.8 ± 10.2 | 166.8 ± 10.3 |
| Weight, kg | 75.3 ± 15.8 | 77.2 ± 17.0 | 80.1 ± 16.7 | 83.1 ± 18.2 |
| Body mass index, kg/m2 | 27.3 ± 5.0 | 27.7 ± 5.1 | 28.6 ± 5.2 | 29.7 ± 5.9 |
| Body mass index category | ||||
| Underweight | 8 (1.0) | 13 (1.6) | 5 (0.6) | 6 (0.7) |
| Normal | 274 (34.1) | 243 (29.7) | 194 (24.1) | 154 (18.9) |
| Overweight | 327 (40.7) | 337 (41.2) | 323 (40.1) | 311 (38.1) |
| Obese Class I | 138 (17.2) | 157 (19.2) | 188 (23.4) | 215 (26.3) |
| Obese Class II/III | 56 (7.0) | 69 (8.4) | 95 (11.8) | 131 (16.0) |
| Waist circumference, cm | 94.9 ± 13.2 | 96.2 ± 13.3 | 100.1 ± 13.7 | 103.3 ± 15.4 |
| Hip circumference, cm | 103.3 ± 10.3 | 104.3 ± 11.0 | 106.1 ± 11.0 | 108.2 ± 12.3 |
| Smoking Status | ||||
| Never smoker | 365 (45.5) | 350 (42.8) | 379 (47.1) | 352 (43.1) |
| Former smoker | 315 (39.2) | 352 (43.0) | 333 (41.4) | 365 (44.7) |
| Current smoker | 123 (15.3) | 116 (14.2) | 93 (11.6) | 100 (12.2) |
| Cigarette pack-years (among ever-smokers) | 19.5 ± 22.8 | 19.1 ± 21.2 | 21.9 ± 24.5 | 24.4 ± 29.6 |
| Estimated GFR, mL/min/1.73 m2 | 88.2 ± 17.3 | 82.8 ± 16.1 | 80.1 ± 15.6 | 72.3 ± 18.6 |
| Estimated GFR Category | ||||
| Stage 1 | 346 (43.1) | 260 (31.8) | 197 (24.5) | 117 (14.4) |
| Stage 2 | 431 (53.7) | 511 (62.5) | 535 (66.6) | 503 (61.8) |
| Stage 3 | 25 (3.1) | 47 (5.8) | 71 (8.8) | 179 (22) |
| Stage 4 | 0 | 0 | 0 | 14 (1.7) |
| Stage 5 | 0 | 0 | 0 | 1 (0.12) |
| Creatinine, mg/dL | 0.88 ± 0.17 | 0.93 ± 0.19 | 0.96 ± 0.19 | 1.08 ± 0.39 |
| hs-CRP, mg/L | 3.9 ± 5.4 | 3.6 ± 5.7 | 3.3 ± 5.1 | 4.1 ± 6.2 |
| Interleukin-6, pg/mL | 1.4 ± 1.2 | 1.4 ± 1.0 | 1.6 ± 1.2 | 1.9 ± 1.3 |
| D-dimer, μg/L | 0.28 ± 0.36 | 0.32 ± 0.43 | 0.38 ± 1.0 | 0.53 ± 0.99 |
| PIIINP, μg/mL | 4.9 ± 1.2 | 5.2 ± 1.1 | 5.5 ± 1.2 | 6.4 ± 2.0 |
| Computed tomography | ||||
| Total imaged lung volume (gas + tissue), cm3 | 2768 ± 762.5 | 2813 ± 894.8 | 2796 ± 802.0 | 2804 ± 843.0 |
| Emphysema, % | 4.1 ± 4.5 | 4.3 ± 5.0 | 4.6 ± 5.0 | 4.5 ± 4.5 |
| Respiratory diseases | ||||
| Self-reported emphysema | 14 (1.7) | 11 (1.3) | 11 (1.4) | 14 (1.7) |
| Self-reported asthma | 92 (11.5) | 71 (8.7) | 70 (8.7) | 81 (9.9) |
Data presented as mean ± SD and frequency (percentage). All parameters collected at MESA baseline visit in years 2000–2002.
ICTP = collagen type I carboxy-terminal telopeptide, GFR = glomerular filtration rate, Stage 1 = eGFR ≥90mL/min/1.73 m2, Stage 2 = eGFR 60–89 mL/min/1.73 m2, Stage 3 = eGFR 30–59 mL/min/1.73 m2, Stage 4 = eGFR 15–29mL/min/1.73 m2, Stage 5 = eGFR < 15mL/min/1.73 m2, hs-CRP = high-sensitivity C-reactive protein, PIIINP = procollagen type III N-terminal propeptide.
Missing data are as follows: education (n = 5), smoking status (n = 1), asthma (n = 1), smoking pack-years (n = 44), estimated GFR (n = 7), creatinine (n = 7), hs-CRP (n = 16), interleukin-6 (n = 84), d-dimer (n = 12), PIIINP (n = 44).
3.1. ICTP
In an unadjusted model, each SD increment in ICTP was associated with a 2.5% increment in HAA (95% CI 1.5–3.6%, p-value < 0.001, Table 2). In a minimally-adjusted model, accounting for variables related to CT scan characteristics and study site, each SD increment in ICTP was associated with a 1.8% increment in HAA (95% CI 0.8–2.9%, p-value < 0.001, Table 2). In a fully adjusted model, each SD increment in ICTP was associated with a 1.3% increment in HAA (95% CI 0.2–2.4%, p-value = 0.02, Table 2, Fig. 1). The above associations remained statistically significant even after outliers were removed (Table A6).
Table 2.
Association of ICTP and PIIINP with high attenuation areas on CT.
| ICTP | PIIINP | |||||||
|---|---|---|---|---|---|---|---|---|
| % Increment in HAA% per SD Increment in ICTP | 95% CI | P Value | P for Interaction | % Increment in HAA% per SD Increment in PIIINP | 95% CI | P Value | P for Interaction | |
| Unadjusted | 2.5 | 1.5–3.6 | < 0.001 | 2.8 | 1.7–4.0 | < 0.001 | ||
| Minimally Adjusted Modela | 1.8 | 0.8–2.9 | < 0.001 | 2.0 | 0.9–3.1 | < 0.001 | ||
| Full Modelb | 1.3 | 0.2–2.4 | 0.02 | 0.96 | 0.06–1.9 | 0.04 | ||
| Stratified by gender | 0.97 | 0.28 | ||||||
| Male | 1.1 | −0.3–2.5 | 0.14 | 1.4 | 0.06–2.8 | 0.04 | ||
| Female | 1.0 | −0.4–2.5 | 0.17 | 0.3 | −0.9–1.5 | 0.64 | ||
| Stratified by smoking status | 0.88 | 0.43 | ||||||
| Ever-smoker | 1.7 | 0.1–3.4 | 0.04 | 0.6 | −0.8–2.1 | 0.40 | ||
| Never-smoker | 1.2 | −0.3–2.6 | 0.12 | 1.1 | −0.03–2.2 | 0.06 | ||
| Stratified by race/ethnicity | 0.23 | 0.97 | ||||||
| White | 1.2 | −0.5–2.9 | 0.17 | 0.8 | −0.5–2.1 | 0.22 | ||
| African American | 1.9 | 0.1–3.7 | 0.04 | 0.6 | −0.9–2.2 | 0.42 | ||
| Chinese American | −1.6 | −5.3–2.3 | 0.41 | 1.0 | −2.5–4.7 | 0.57 | ||
| Hispanic | 0.9 | −0.9–2.8 | 0.32 | 1.5 | −0.7–3.7 | 0.19 | ||
ICTP = collagen type I carboxy-termmal telopeptide, PIIINP = procollagen type III N-termmal propeptide, HAA = high attenuation areas.
All covariates measured at baseline examinations in 2000–2002. Analyses account for inverse probability weighting for case sampling. SD for ICTP was 1.33. SD for PIIINP was 1.54.
Model adjusted for total imaged lung volume, site and mA dose.
Full model adjusted for age, gender, race/ethnicity, educational attainment, height, body mass index, waist circumference, current smoker status, cigarette pack-years in ever-smokers, estimated glomerular filtration rate, high-sensitivity C-reactive protein, interleukin-6, d-dimer, study site, percent emphysema, mA dose, and total volume of imaged lung.
Fig. 1.
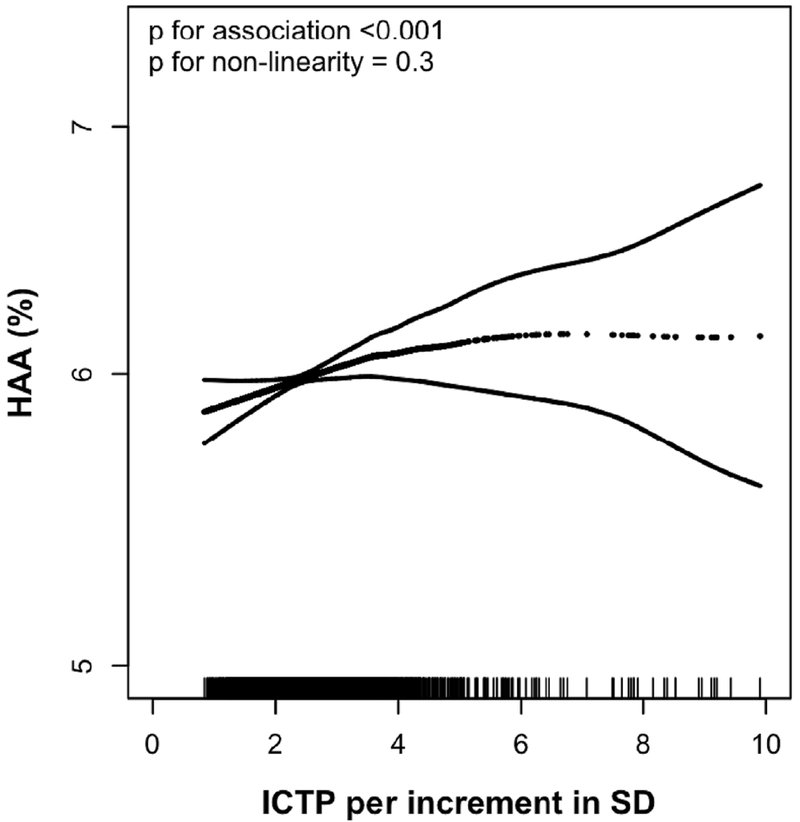
Continuous relationship between collagen type I carboxy-terminal telopeptide (ICTP) and predicted high attenuation area (HAA) after adjustment and accounting for case-sampling with inverse probability weighting. Smoothed regression line with correlating 95% confidence intervals are adjusted for age, gender, race/ethnicity, educational attainment, height, body mass index, waist circumference, current smoker status, cigarette pack-years in ever-smokers, estimated glomerular filtration rate, study site, percent emphysema, mA dose, and total volume of imaged lung. Each vertical mark of the rug plot on the border of the x-axis equates to one participant, p values for association and non-linearity are shown for the association between ICTP and HAA in a fully adjusted multivariable model.
In an unadjusted model, the odds of ILA increased by 17% per SD increment in ICTP (OR 1.17, 95% CI 1.00–1.37, p-value = 0.052, Table 3). In a fully adjusted model, this association was no longer significant (OR 1.05, 95% CI 0.88–1.26, p-value = 0.55, Table 3, Figure A1).
Table 3.
Association of ICTP and PIIINP with interstitial lung abnormalities on CT measured 10 years after biomarker assessment.
| ICTP | PIIINP | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio for ILA per SD Increment in ICTP | 95% CI | P Value | P for Interaction | Odds Ratio for ILA per SD Increment in PIIINP | 95% CI | P Value | P for Interaction | |
| Unadjusted | 1.17 | 1.00–1.37 | 0.05 | 0.87 | 0.72–1.04 | 0.13 | ||
| Adjusteda | 1.05 | 0.88–1.26 | 0.55 | 0.87 | 0.70–1.08 | 0.22 | ||
| Stratified by gender | 0.98 | 0.69 | ||||||
| Male | 1.00 | 0.78–1.29 | 0.98 | 0.82 | 0.58–1.14 | 0.23 | ||
| Female | 1.07 | 0.82–1.39 | 0.64 | 0.90 | 0.67–1.21 | 0.49 | ||
| Stratified by smoking status | 0.20 | 0.13 | ||||||
| Ever-smoker | 1.17 | 0.94–1.45 | 0.17 | 0.98 | 0.76–1.28 | 0.90 | ||
| Never-smoker | 0.91 | 0.68–1.20 | 0.49 | 0.68 | 0.47–0.99 | 0.04 | ||
| Stratified by race/ethnicity | 0.25 | 0.45 | ||||||
| White | 0.80 | 0.57–1.12 | 0.20 | 0.63 | 0.43–0.92 | 0.02 | ||
| African American | 1.23 | 0.90–1.67 | 0.19 | 0.99 | 0.70–1.39 | 0.94 | ||
| Chinese American | 0.90 | 0.56–1.44 | 0.66 | 1.01 | 0.59–1.73 | 0.97 | ||
| Hispanic | 1.30 | 0.85–1.99 | 0.22 | 0.90 | 0.53–1.54 | 0.70 | ||
ICTP = collagen type I carboxy-terminal telopeptide, PIIINP = procollagen type III N-terminal propeptide, ILA = interstitial lung abnormalities.
All covariates measured at baseline examinations in 2000–2002. Analyses account for inverse probability weighting for case sampling. SD for ICTP was 1.33. SD for PIIINP was 1.54.
Adjusted for age, gender, race/ethnicity, current-smoker status and cigarette pack-years in ever-smokers.
There was no evidence of effect modification of the ICTP-HAA association by gender, smoking status, or race/ethnicity (p value for interaction is 0.97 for gender, 0.88 for smoking status, and 0.23 for race/ethnicity, Table 2). Results were similar for the ICTP-ILA association and no effect modification by gender, smoking status or race/ethnicity was identified (p value for interaction is 0.98 for gender, 0.2 for smoking status, and 0.25 for race/ethnicity, Table 3).
3.2. PIIINP
In an unadjusted model, each SD increment in PIIINP was associated with a 2.8% increment in HAA (95% CI 1.7–4%, p-value < 0.001, Table 2). In a minimally-adjusted model, accounting for variables related to CT scan characteristics and study site, each SD increment in PIIINP was associated with a 2% increment in HAA (95% CI 0.9–3.1%, p-value < 0.001, Table 2). In a fully adjusted model, each SD increment in PIIINP was associated with a 0.96% increment in HAA (95% CI 0.06–1.9%, p-value = 0.04, Table 2, Figure A2). Analyses with outliers removed found that associations in unadjusted and minimally adjusted models remained statistically significant, however, in fully adjusted models the relationship between HAA and PIIINP was no longer significant (Table A6).
In both unadjusted and fully adjusted models, the odds of ILA decreased by 13% per SD increment in PIIINP, although this was not statistically significant (unadjusted OR 0.87, 95% CI 0.72–1.04, p-value = 0.13; fully-adjusted OR 0.87, 95% CI 0.7–1.08, p-value = 0.22, Table 3, Figure A3).
There was no evidence of effect modification of the PIIINP-HAA association by gender, smoking status, or race/ethnicity (p value for interaction is 0.28 for gender, 0.43 for smoking status, and 0.97 for race/ethnicity, Table 2). Similarly, no effect modification was identified for the PIIINP-ILA association by gender, smoking status or race/ethnicity (p value for interaction is 0.69 for gender, 0.13 for smoking status, and 0.45 for race/ethnicity, Table 3).
4. Discussion
We have shown that greater serum levels of both ICTP and PIIINP, two collagen biomarkers, were associated with greater HAA, a quantitative, clinically relevant, subclinical ILD phenotype, independent of gender, race/ethnicity, kidney function, and smoking status, in a large cohort of community dwelling individuals. Together with prior data linking serum MMP-7 and IL-6 levels with HAA [10], these data suggest that HAA captures, at least in part, extracellular matrix remodeling of the lung in adults without clinically evident ILD.
While our study is the first to report an association between increased serum collagen biomarkers and subclinical ILD, several prior studies examined associations between increased collagen biomarkers and outcomes in idiopathic pulmonary fibrosis (IPF) [25–29]. The PROFILE study demonstrated in 189 IPF participants that six neoepitopes from MMP-cleaved collagens were significantly higher in subjects with progressive disease compared to those with stable IPF at 6 months, of which two of the neoepitopes measured at baseline were associated with increased long-term mortality. This suggests that circulating serum markers of collagen fragments may serve as a surrogate measurement of extracellular matrix remodeling leading to progressive fibrosis and worsening clinical status in patients with clinically significant IPF [25].
Our findings complement this work by demonstrating associations between collagen biomarkers and a CT-derived subclinical ILD phenotype, HAA, in otherwise healthy community dwelling adults. Our findings suggest that collagen biomarker activity may be occurring very early in the disease pathogenesis, prior to clinically relevant ILD being discovered. Previously we have shown that HAA is associated with MMP-7 in a subset of this cohort, suggesting an association with extracellular matrix remodeling [10]. In the current study we were unable to assess the association with MMP-7, ICTP, PIIINP and HAA, since there was little overlap between those who had MMP-7 measured and those who had ICTP and PIIINP measured [18,30].
There were several limitations to our study. First, the magnitudes of our observed associations are small. Nevertheless, these associations may be biologically relevant, in line with previous work examining subclinical ILD phenotypes [7,10,14,15]. Indeed, it would be surprising if we had detected large, clinically relevant associations, since all study subjects were community dwelling adults sampled without regard to health status. Second, this is a cross-sectional observational study, limiting any causal inferences to be made from our results. Third, our study did not find an association with collagen biomarkers and the presence of ILA during 10 year follow-up. This may reflect the changing roles in underlying pathogenesis, where in HAA there may be early collagen biomarker activity represented by ICTP and PIIINP but once ILA has developed perhaps ICTP and PIIINP are no longer the specific primary markers involved in disease progression and other collagen fragments are more prevalent at that stage. In addition, selection bias may have occurred due to death or loss to follow-up between the baseline visit and ILA assessment. Also, previous work has shown that the within person-correlation for ICTP and PIIINP measured during the baseline exam and again at approximately 10 years later (when ILA was assessed) was close to zero [18]. This demonstrates that baseline measurements of ICTP and PIIINP are unlikely to reflect fibrotic processes 10 years later and may explain why we did not find a significant association. The inverse association between ILA and PIIINP presented in figure A3 is difficult to interpret and given the lack of statistical significance should not be further extrapolated to determine type of CT abnormality being assessed by ILA. We do not believe that the lack of an association between ILA and ICTP and PIIINP should invalidate the association found between HAA and ICTP and PIIINP, which may represent early collagen biomarker activity. These associations are biologically plausible and consistent with our groups’ prior work validating HAA [7,10,14,15]. Whether these same collagen biomarkers play a role and remain active throughout the disease pathogenesis is unknown. Fourth, HAA was measured on cardiac CT scans, which do not provide full images of the lung fields, therefore raising the possibility of incomplete measurements. However, our group has previously shown that HAA on cardiac CT strongly agrees with HAA on full-lung CT scans, since ILD primarily affects the lower lobes of the lung [7].
5. Conclusions
Serum collagen biomarkers are associated with HAA, a clinically-relevant quantitative CT marker of subclinical ILD, independent of gender, race/ethnicity, eGFR or smoking status, in community-dwelling adults. Future studies should investigate whether HAA and collagen biomarkers might help identify adults at-risk for the development of clinically evident ILD.
Supplementary Material
Acknowledgments
Support/funding
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, by grants R01-HL-103676, K24-HL-131937, R01-HL-077612, R01-HL-093081, RC1-HL100543, and R01 HL098382 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR/NCATS. The investigators were also supported by the Pulmonary Fibrosis Foundation, the Rheumatology Research Foundation Scientist Development Award and the Rocco Guinta Research Fund.
Role of the sponsors
The sponsors had a role in the design of the MESA study and the collection of the data. The sponsors had no role in the analysis of the data or the writing of the manuscript.
Other contributions
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations list
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- CT
Computed tomography
- CVDL
Cardiovascular disease
- eGFR
Estimated glomerular filtration rate
- HAA
High attenuation areas
- hs-CRP
High sensitivity C-reactive protein
- ICTP
Carboxy-terminal telopeptide of collagen type I
- IL
Interleukin
- ILA
Interstitial lung abnormalities
- ILD
Interstitial lung disease
- IPF
Idiopathic pulmonary fibrosis
- IQR
Interquartile range
- mA
Milliamperes
- MESA
Multi-ethnic study of atherosclerosis
- MMP
Matrix metalloproteinase
- PIIINP
Amino-terminal propeptide of type III procollagen
- SD
Standard deviation
Footnotes
This work was presented in the form of a poster presentation at the American Thoracic Society International Conference, Washington, DC, May 23rd, 2017.
Conflicts of interest
DJL has received consulting fees from Genentech/Roche, Boehringer-Ingelheim, Gilead, Pharmakea, Veracyte, Patara Pharmaceuticals, Degge Group and the France Foundation related to IPF; Columbia University has received funding for clinical trials in IPF from Boehringer-Ingelheim, Gilead, Bayer, Global Blood Therapeutics and Fibrogen; Columbia University has received funding from the Pulmonary Fibrosis Foundation for DJL’s consulting services; DJL has received fees for serving as a Deputy Editor for the Annals of the American Thoracic Society and as a Statistical Editor for Thorax. SMK reports grants from NIH during the conduct of the study and non-financial support from the ATS. He has received personal fees from the European Respiratory Journal for serving on an editorial board. The University of Pennsylvania has received grants from Actelion, United Therapeutics, Gilead, Lung Biotech and Bayer for CME courses.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.rmed.2018.06.001.
References
- [1].Travis WD, Costabel U, Hansell DM, et al. , An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias, Am. J. Respir. Crit. Care Med 188 (6) (2013) 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fingerlin TE, Murphy E, Zhang W, et al. , Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis, Nat. Genet 45 (6) (2013) 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Margaritopoulos GA, Romagnoli M, Poletti V, Siafakas NM, Wells AU, Antoniou KM, Recent advances in the pathogenesis and clinical evaluation of pulmonary fibrosis, Eur. Respir. Rev. : An Official Journal of the European Respiratory Society. 21 (123) (2012) 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McKleroy W, Lee TH, Atabai K, Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis, Am. J. Physiol. Lung Cell Mol. Physiol 304 (11) (2013) L709–L721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chalikias GK, Tziakas DN, Biomarkers of the extracellular matrix and of collagen fragments, Clinica chimica acta; international journal of clinical chemistry. 443 (2015) 39–47. [DOI] [PubMed] [Google Scholar]
- [6].Fessler JH, Fessler LI, Biosynthesis of procollagen, Annu. Rev. Biochem 47 (1978) 129–162. [DOI] [PubMed] [Google Scholar]
- [7].Lederer DJ, Enright PL, Kawut SM, et al. , Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study, Am. J. Respir. Crit. Care Med 180 (5) (2009) 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rosas IO, Ren P, Avila NA, et al. , Early interstitial lung disease in familial pulmonary fibrosis, Am. J. Respir. Crit. Care Med. 176 (7) (2007) 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tsushima K, Sone S, Yoshikawa S, Yokoyama T, Suzuki T, Kubo K, The radiological patterns of interstitial change at an early phase: over a 4-year follow-up, Respir. Med 104 (11) (2010) 1712–1721. [DOI] [PubMed] [Google Scholar]
- [10].Podolanczuk AJ, Oelsner EC, Barr RG, et al. , High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study, Eur. Respir. J 48 (5) (2016) 1442–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tanizawa K, Handa T, Nagai S, et al. , Clinical impact of high-attenuation and cystic areas on computed tomography in fibrotic idiopathic interstitial pneumonias, BMC Pulmonary Medicine. 15 (2015) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Washko GR, Hunninghake GM, Fernandez IE, et al. , Lung volumes and emphysema in smokers with interstitial lung abnormalities, N. Engl. J. Med 364 (10) (2011) 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Putman RK, Hatabu H, Araki T, et al. , Association between interstitial lung abnormalities and all-cause mortality, JAMA. 315 (7) (2016) 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Podolanczuk AJ, Oelsner EC, Barr RG, et al. , High attenuation areas on chest CT and clinical respiratory outcomes in community-dwelling adults, Am. J. Respir. Crit. Care Med 196 (11) (2017) 1434–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bernstein EJ, Barr RG, Austin JH, et al. , Rheumatoid arthritis-associated autoantibodies and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis, Thorax 71 (12) (2016) 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bild DE, Bluemke DA, Burke GL, et al. , Multi-ethnic study of atherosclerosis: objectives and design, Am. J. Epidemiol. 156 (9) (2002) 871–881. [DOI] [PubMed] [Google Scholar]
- [17].Chirinos JA, Kips JG, Jacobs DR Jr.et al. , Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis), J. Am. Coll. Cardiol 60 (21) (2012) 2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Duprez DA, Gross MD, Sanchez OA, et al. , Collagen turnover markers in relation to future cardiovascular and noncardiovascular disease: the multi-ethnic study of atherosclerosis, Clin. Chem 63 (7) (2017) 1237–1247. [DOI] [PubMed] [Google Scholar]
- [19].Duprez DA, Jacobs DR Jr., Lutsey PL, et al. , Association of small artery elasticity with incident cardiovascular disease in older adults: the multi-ethnic study of atherosclerosis, Am. J. Epidemiol 174 (5) (2011) 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Valentini G, Vettori S, Cuomo G, et al. , Early systemic sclerosis: short-term disease evolution and factors predicting the development of new manifestations of organ involvement, Arthritis Res. Ther 14 (4) (2012) R188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carr JJ, Nelson JC, Wong ND, et al. , Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study, Radiology 234 (1) (2005) 35–43. [DOI] [PubMed] [Google Scholar]
- [22].Barr RG, Bluemke DA, Ahmed FS, et al. , Percent emphysema, airflow obstruction, and impaired left ventricular filling, N. Engl. J. Med 362 (3) (2010) 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Washko GR, Lynch DA, Matsuoka S, et al. , Identification of early interstitial lung disease in smokers from the COPDGene Study, Acad. Radiol 17 (1) (2010) 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bernstein EJ, Peterson ER, Sell JL, et al. , Survival of adults with systemic sclerosis following lung transplantation: a nationwide cohort study, Arthritis & rheumatology. 67 (5) (2015) 1314–1322. [DOI] [PubMed] [Google Scholar]
- [25].Jenkins RG, Simpson JK, Saini G, et al. , Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study, The Lancet. Respiratory medicine. 3 (6) (2015) 462–472. [DOI] [PubMed] [Google Scholar]
- [26].Munch J, Avanesov M, Bannas P, et al. , Serum matrix metalloproteinases as quantitative biomarkers for myocardial fibrosis and sudden cardiac death risk stratification in patients with hypertrophic cardiomyopathy, J. Card. Fail 22 (10) (2016) 845–850. [DOI] [PubMed] [Google Scholar]
- [27].Ricard-Blum S, Chossegros P, Guerret S, Trepo C, Grimaud JA, Chevallier M, The carboxy-terminal cross-linked telopeptide of type I collagen (ICTP) is a potential serum marker of ongoing liver fibrosis, Clinica chimica acta; international journal of clinical chemistry. 248 (2) (1996) 187–195. [DOI] [PubMed] [Google Scholar]
- [28].Safdar Z, Tamez E, Chan W, et al. , Circulating collagen biomarkers as indicators of disease severity in pulmonary arterial hypertension, JACC. Heart failure. 2 (4) (2014) 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stolz D, Leeming DJ, Kristensen JH, et al. , Systemic biomarkers of collagen and elastin turnover are associated with clinically relevant outcomes in COPD, Chest 151 (1) (2017) 47–59. [DOI] [PubMed] [Google Scholar]
- [30].Duprez DA, Gross MD, Kizer JR, Ix JH, Hundley WG, Jacobs DR Jr., Predictive value of collagen biomarkers for heart failure with and without preserved ejection fraction: MESA (Multi-Ethnic study of atherosclerosis), J Am Heart Assoc. 7 (5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


