Abstract
Objective:
Macrophage Activation Syndrome (MAS) is a life-threatening cytokine storm syndrome that occurs in patients with underlying rheumatologic diseases. Preclinical and clinical data suggest that interferon (IFN)γ is pathogenic in MAS, yet how IFNγ causes disease remains unknown. In this manuscript, we sought to determine whether IFNγ-dependent signals synergize with systemic innate immune responses to drive cytokine storm in a murine model of MAS.
Methods:
IFNγ-deficient mice were treated with five doses of a Toll-like receptor (TLR)9 agonist (CpG1826), IFNγ, or a combination of the two stimuli over the course of ten days. MAS immunopathology was assessed by measuring cytopenias, hepatitis, hepatosplenomegaly, and the induction of inflammatory myelopoiesis. Mixed bone marrow chimeras were created to determine if TLR9- and IFNγR1- dependent signals induce enhanced myelopoiesis in a cell-intrinsic or cell-extrinsic manner.
Results:
IFNγ-deficient mice do not develop features of MAS when treated with repeated doses of a TLR9 agonist or IFNγ individually. In contrast, IFNγ-deficient mice treated with both a TLR9 agonist and IFNγ develop cytopenias, hepatitis, and hepatosplenomegaly reproducing major clinical features of MAS. TLR9- and IFNγ- dependent signals synergize to enhance myeloid progenitor function and induce myelopoiesis in vivo, which occurs through cell-extrinsic mechanisms and correlates with induction of disease.
Conclusion:
These data demonstrate that TLR9-driven signals potentiate the effects of IFNγ to initiate murine MAS, and provide evidence that induction of inflammation-induced myelopoiesis is a common TLR- and IFNγ- dependent pathway that may contribute to the pathogenesis of MAS.
Introduction
Macrophage activation syndrome (MAS) is a life-threatening clinical syndrome resulting from immune dysregulation and uncontrolled inflammation in patients with rheumatologic conditions (1). Morbidity and mortality from MAS remains high despite the advent of targeted immunosuppressive therapies and improvements in intensive care measures to support failing organs (1). The pathogenesis of this rare and devastating condition remains poorly defined, which impedes the development of rational and targeted therapies to treat patients with MAS.
Preclinical and translational studies suggest that IFNγ is pathogenic in MAS (2–4), which prompted a clinical trial to study the safety and efficacy of IFNγ neutralization in patients with systemic juvenile idiopathic arthritis (SJIA) who develop MAS (ClinicalTrials.gov, NCT03311854). Despite the growing interest in IFNγ as a therapeutic target, the mechanisms leading to IFNγ-mediated immunopathology in MAS remain unclear. Furthermore, high-dose IFNγ drives anemia and hemophagocytosis in mice, but is insufficient to recapitulate all manifestations of MAS by itself (5). These data suggest that additional inflammatory signals are required in combination with IFNγ to induce MAS immunopathology.
Pattern recognition receptors (PRRs) are expressed by innate immune cells and sense a diverse range of endogenous and exogenous ‘danger’ signals. Toll-like receptors (TLRs) are the best-characterized PRRs and are implicated in the development of MAS. Preclinical models of disease suggest that chronic or exaggerated responses to systemic TLR activation lead to MAS in mice (2, 6, 7). Patients with SJIA, the rheumatic disease with the greatest predisposition to the development of MAS, have an interleukin (IL)-1 and TLR gene expression signature in their peripheral blood mononuclear cells (8). Furthermore, polymorphisms in IRF5, a signaling molecule downstream of TLR activation, result in higher IRF5 expression and a four-fold higher risk of MAS in patients with SJIA (9, 10). These data suggest that TLRs and their downstream signaling pathways contribute to MAS pathogenesis.
Previous work has established that repeated TLR9 activation in wild-type mice induces clinical manifestations of MAS including cytopenias, hepatosplenomegaly, hepatitis, and hypercytokinemia (2). This model is an IFNγ-dependent model of MAS, as neutralization of IFNγ abrogates disease and IFNγ−/− mice are protected from TLR9-induced immunopathology (2). Inflammatory monocytes are the main producers of interleukin (IL)-12 and are key to disease pathogenesis in this model (11), as IL-12 neutralization prevents TLR9-induced IFNγ production and ameliorates disease (12). Repeated doses of a TLR9 agonist are required to induce disease, which leads to heightened systemic production of IL-12 after each TLR9 stimulus and drives a feed-forward inflammatory response (11). This feed-forward inflammatory response correlates with the induction of inflammatory myelopoiesis, which skews the hematopoietic output of the host to accelerate the production of new inflammatory monocytes (11). Accumulation of TLR9 responsive inflammatory monocytes correlates with heightened cytokine production following repeated TLR9 activation in vivo, which is thought to drive disease (11).
It remains unclear whether repeated TLR9 activation is simply required for the upstream induction of high levels of IL-12-induced pathogenic IFNγ or whether IFNγ-independent TLR9-dependent signals are additionally necessary for the induction of murine MAS. To differentiate between these possibilities, we stimulated IFNγ-deficient mice with repeated doses of a TLR9 agonist (CpG1826), IFNγ, or a combination of the two stimuli. Consistent with our hypothesis, IFNγ-deficient mice develop MAS when treated with both a TLR9 agonist and IFNγ, but not when treated with these inflammatory signals individually. We provide evidence that induction of inflammatory myelopoiesis requires both TLR9- and IFNγ- dependent signals, which may contribute to disease by accelerating the production of new TLR9 responsive monocytes. These data add to our understanding of MAS pathogenesis, and reveal IFNγ-independent signals as potential therapeutic targets for the treatment of MAS.
Materials and Methods
Vertebrate Animals
Mice used in individual experiments were age- and sex-matched. Efforts were made to ensure equal use of male and female mice in all experiments. IFNγ-deficient, IFNγR1-deficient, and congenically-labeled CD45.1 wild-type SJL C57BL/6 mice were purchased from The Jackson Laboratory. TLR9-deficient mice were originally made by Shizuo Akira (Osaka University). Mouse breeding, animal husbandry, and animal experiments occurred within the specific-pathogen-free animal facilities at The Children’s Hospital of Philadelphia. All animals were cared for according to the institutions’ animal facility guidelines and procedures were performed after institutional ethics boards’ review and approval of all experiments.
Induction of murine MAS
The TLR9 agonist and class B CpG oligonucleotide CpG1826 (Sequence: T*C*C*A*T*G*A*C*G*T*T*C*C*T*G*A*C*G*T*T) was synthesized with a phosphothioate backbone at Integrated DNA Technologies (Coralville, IA). Recombinant murine IFNγ was obtained from Peprotech (Rocky Hill, NJ). Eight-week old IFNγ-deficient mice were treated with five intraperitoneal doses of vehicle (phosphate buffered saline, PBS), 50 μg of the TLR9 agonist CpG1826, 10 μg of IFNγ, or a combination of CpG1826 and IFNγ every other day over the course of ten days. Mice were sacrificed twenty-four hours after the last injection. Splenomegaly and hepatomegaly were calculated by dividing the organ weight by the body weight of the mouse and multiplying by 100. Cheek bleeds were performed to obtain whole blood for complete blood count analysis on a Sysmex XT-2000iV Automated Hematology Analyzer. Whole livers were fixed in 10% formaldehyde, embedded in paraffin, and individual H&E slides were made at the Pathology Core at The Children’s Hospital of Philadelphia. All histology pictures were taken using a Leica DM4000B microscope and SPOT Software 5.1. Foci of >10 inflammatory cells were counted per high power field using a 20X objective.
Processing of organs, whole blood, and sera
Bone marrow cells were flushed from the leg bones of mice with cold PBS and single cell suspensions were generated by mechanical disruption through a 70 μm strainer. Whole spleens were digested with DNase I (Roche) and collagenase (Roche) at 37°C for 30 minutes prior to generating single cell suspensions of splenocytes by mechanically disrupting the spleen through a 70 μm strainer. Red blood cell lysis was performed on all harvested cells using ACK lysis buffer (Lonza). Total cells per organ were counted on a Countess TM Automated Cell Counter from ThermoFisher Scientific.
Mixed bone marrow chimeras
CD45.1 SJL C57BL/6 wild-type hosts were lethally irradiated with 950 rads (centigray, cGy) of x-ray irradiation and rescued with injection of 2.5–5 × 106 mixed bone marrow cells using 90% wild-type (CD45.1) cells and 10% knock-out cells (TLR9-deficient or IFNγR1-deficient). Mixed bone marrow chimeric mice were allowed 4–5 weeks of bone marrow reconstitution prior to induction of murine MAS.
Cellular immunophenotyping
Flow cytometry was performed on a Miltenyi MacsQuant and cell sorting was performed using a BD FACS Aria II. FACS data were analyzed using FlowJo software (Ashland, OR). Fluorescently labeled cells were gated on forward and side scatter to limit inclusion of dead cells, debris and doublets. Live cells were identified by excluding cells staining positive for LIVE/DEAD™ Fixable Aqua Dead Cell Stain from ThermoFisher Scientific. Fc Block (anti-CD16/32, clone 2.4G2) was used prior to staining for all flow cytometry experiments, except for the myeloid progenitor panels that includes staining for the Fc receptor CD16/32. Inflammatory monocytes were identified as Ly6G-Ly6ChiCD115+ cells. Myeloid progenitors were identified as CMPs (Lin-c-Kit+CD105- CD16/32midCD115-), GMPs (Lin-c-Kit+CD105-CD16/32highCD115-), MDPs (Lin-cKit+CD105-CD115+Ly6C-), and cMoPs (Lin-c-Kit+CD105-CD115+Ly6C+). The lineage panel included antibodies against B220, CD4, CD5, CD8a, CD11b, CD11c, CD90.2, CD49b, Ly6G, NK1.1, and Ter119.
Myelopoiesis assays
Bone marrow myeloid progenitors were sorted from mice treated with five doses of PBS, CpG1826, IFNγ, or a combination of both CpG1826 and IFNγ, as described above. Myeloid progenitors (500–2000 per well) were cultured in MEM-α media (Gibco) supplemented with 20% heat-inactivated FBS (Atlanta Biologicals) and penicillin–streptomycin–L-glutamine (Cellgro) at 37°C in 6% CO2. GM-CSF, M-CSF, IL-3, and stem cell factor were obtained from Peprotech and used to stimulate myeloid progenitor cell division and differentiation in liquid cultures. All cytokines were used at 5 ng/mL, except GM-CSF was used at 3.3 ng/mL. After seven days in culture, myeloid progenitor cell progeny were harvested and stained for mature myeloid cell surface markers (Ly6G, Ly6C, and CD11b) using the cellular immunophenotyping protocol described above. Total myeloid cell counts were enumerated on a Miltenyi MacsQuant flow cytometer and gated on individual cell populations in FlowJo.
Statistical Analyses
The numbers of experimental replicates are described in each figure. All statistical analyses were performed using Prism v7.0a (GraphPad). The Mann–Whitney U test was used for all single comparisons. A Two-way ANOVA was used to analyze the interaction between two independent variables on a dependent variable. Numbers of significance symbols correspond with the P value, as follows (i.e., 1 symbol P < 0.05; 2 symbols P < 0.01; 3 symbols P < 0.001; 4 symbols P < 0.0001; NS, not significant).
Results
TLR9-dependent signals potentiate IFNγ-driven immunopathology in murine MAS
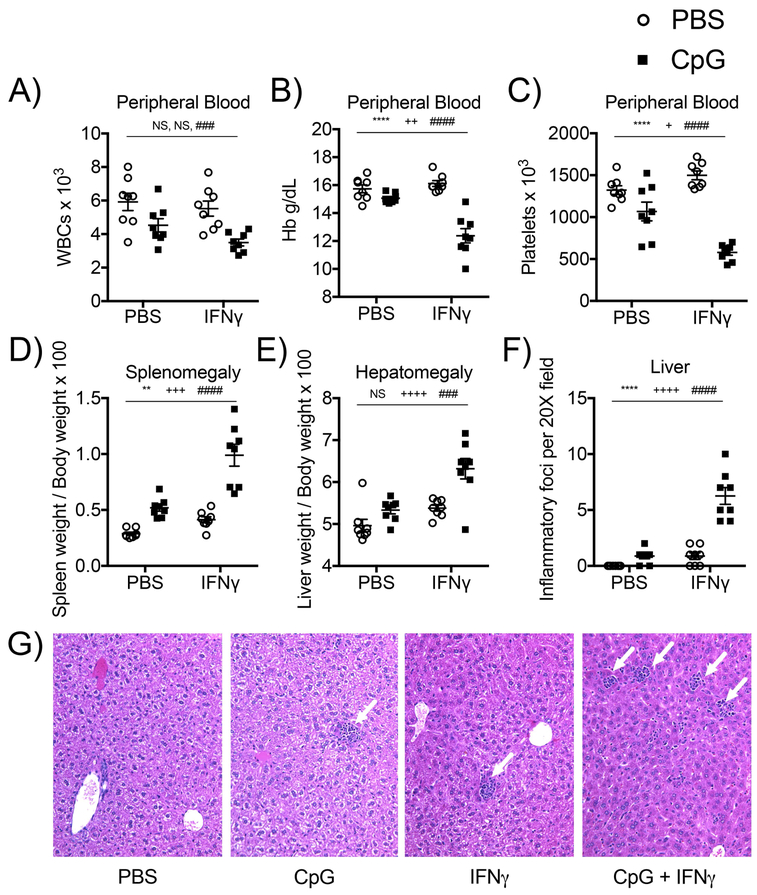
Murine models highlight a role for chronic and exaggerated TLR-driven immune responses as potent inducers of murine MAS (2, 6, 7). In TLR9-mediated MAS, high levels of IFNγ are stimulated downstream of systemic TLR activation, and induction of IFNγ is required to drive MAS immunopathology (2). It remains unclear whether TLR9-driven signals are required solely to induce high levels of IFNγ or whether TLR9-dependent signals in combination with high levels of IFNγ are required to induce murine MAS. To differentiate between these possibilities, IFNγ-deficient mice were treated with repeated doses of a TLR9 agonist alone, IFNγ alone, or a combination of the two stimuli over the course of ten days. The dose of IFNγ used in these experiments recapitulates levels of serum IFNγ (mean ~10 ng/mL, Figure S1) induced downstream of repeated TLR9 activation in wild-type mice described by Behrens et al (2011). IFNγ-deficient mice stimulated with repeated doses of a TLR9 agonist or IFNγ alone did not develop clinical manifestations of MAS (Figure 1). Importantly, the same dose of IFNγ that caused no disease manifestations in IFNγ-deficient mice became pathogenic when delivered in the context of repeated systemic TLR9 activation, as IFNγ-deficient mice treated with repeated doses of both a TLR9 agonist and IFNγ developed robust clinical manifestations of MAS including cytopenias, hepatosplenomegaly, and hepatitis (Figure 1). Levels of ferritin were induced by repeated doses of CpG and were not further increased in the presence of both TLR9- and IFNγ- dependent signals (Figure S1). These data demonstrate that TLR9-dependent signals are required to potentiate IFNγ-dependent immunopathology in murine MAS.
Figure 1. TLR9-dependent signals potentiate IFNγ-driven immunopathology in murine MAS.
IFNγ-deficient mice were treated with five doses of PBS, the TLR9 agonist CpG1826, IFNγ, or the combination of CpG1826 and IFNγ every other day for 10 days. Mice were sacrificed twenty-four hours after the fifth injection. Clinical manifestations of cytokine storm were evaluated by measuring peripheral white blood cell counts (A), anemia (B), thrombocytopenia (C), splenomegaly (D), and hepatomegaly (E). Livers from mice were sectioned and stained with H&E (original magnification, 20X) and hepatic inflammatory foci were enumerated per high power field. (F). One picture per group displays representative liver pathology with white arrows pointing to individual hepatic inflammatory foci. (G). Each graph displays compiled data from three independent experiments (N=8 mice per group). Individual symbols represent one mouse and the horizontal lines represent mean values. Analysis was performed by two-way ANOVA (P values are denoted as follows: * Interaction term; + IFNγ-treated mice vs. mice not treated with IFNγ; # CpG-treated mice vs. mice not treated with CpG; NS = not significant).
Systemic TLR9- and IFNγ- dependent signals synergistically induce inflammatory myelopoiesis
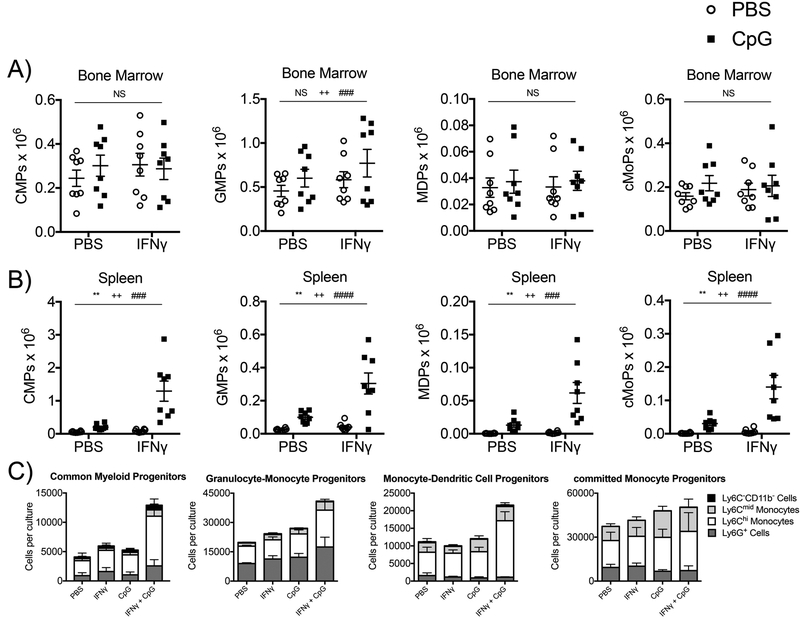
Both TLR- and IFNγ- dependent signals have been implicated in the induction of myelopoiesis during systemic inflammatory responses (13, 14). In TLR9-mediated MAS, induction of enhanced myelopoiesis may be pathogenic, as myeloid progenitors (CMPs – Common Myeloid Progenitors, GMPs – Granulocyte-Monocyte Progenitors, MDPs – Monocyte-Dendritic Cell Progenitors, and cMoPs – committed Monocyte Progenitors) accumulate in the periphery of TLR9-activated mice, which correlates with the accumulation of TLR9 responsive inflammatory monocytes (11). Accumulation of inflammatory monocytes leads to heightened cytokine production and MAS immunopathology downstream of repeated TLR9 activation in vivo (11). Therefore, we sought to determine if combined TLR9- and IFNγ- dependent signals induce inflammatory myelopoiesis to promote immunopathology in murine MAS. Treatment with repeated doses of a TLR9 agonist, IFNγ, or a combination of a TLR9 agonist and IFNγ had minimal effects on numbers of bone marrow myeloid progenitors (Figure 2A). In contrast, treatment of IFNγ-deficient mice with repeated doses of both a TLR9 agonist and IFNγ synergistically induced the accumulation of extramedullary myeloid progenitors in the spleen, whereas treatment with a TLR9 agonist or IFNγ alone failed to do so (Figure 2B). Furthermore, individual populations of sorted myeloid progenitors (CMPs, GMPs, MDPs, and cMoPs) had enhanced output of mature myeloid cells in ex vivo myelopoiesis assays when they were isolated from IFNγ-deficient mice treated with both a TLR9 agonist and IFNγ, but not when they were isolated from IFNγ-deficient mice treated with a TLR9 agonist or IFNγ individually (Figure 2C). These data demonstrate that systemic TLR9- and IFNγ- dependent signals lead to enhanced myeloid progenitor cell production of mature myeloid cells in myelopoiesis assays in vitro, which correlates with the accumulation of myeloid progenitors and MAS immunopathology in vivo. These data support a model involving inflammatory myelopoiesis in the pathogenesis of MAS, whereby both TLR9- and IFNγ-dependent signals combine to accelerate the production of new inflammatory monocytes that accumulate in the periphery and produce heightened systemic immune responses to repeated TLR9 activation in vivo.
Figure 2. Systemic TLR9- and IFNγ- dependent signals synergistically induce inflammatory myelopoiesis.
IFNγ-deficient mice were treated with five doses of PBS, the TLR9 agonist CpG1826, IFNγ, or the combination of CpG1826 and IFNγ every other day for 10 days. Mice were sacrificed twenty-four hours after the fifth injection. Numbers of (A) bone marrow and (B) spleen myeloid progenitors (CMPs, GMPs, MDPs, and cMoPs). (C) Individual populations of bone marrow myeloid progenitors (CMPs, GMPs, MDPs, and cMoPs) were sorted from mice treated with five doses of PBS, the TLR9 agonist CpG1826, IFNγ, or the combination of CpG1826 and IFNγ over the course of ten days. Sorted myeloid progenitors were cultured in vitro for 7 days in media containing M-CSF, GM-CSF, IL-3 and SCF. Mature myeloid cells were enumerated by flow cytometry. Graphs from A and B display compiled data from three independent experiments (N=8 mice per group). Graphs from C display compiled data from two independent experiments (N=4 per group). Individual symbols represent one mouse and the horizontal lines represent mean values. Analysis was performed by two-way ANOVA (P values are denoted as follows: * Interaction term; + IFNγ-treated mice vs. mice not treated with IFNγ; # CpG-treated mice vs. mice not treated with CpG; NS = not significant).
TLR9- and IFNγ- dependent signals indirectly induce inflammatory myelopoiesis in murine MAS
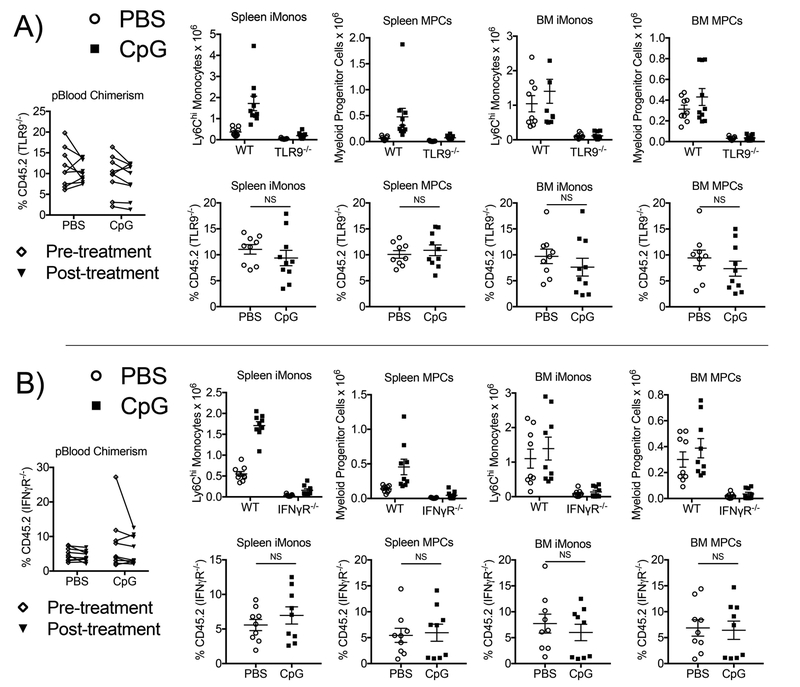
To determine if TLR9- and IFNγ- dependent signals directly or indirectly induce inflammatory myelopoiesis, we generated two cohorts of bone marrow chimeric mice: one cohort with mixed wild-type:TLR9-deficient bone marrow and one cohort with mixed wild-type:IFNγR1-deficient bone marrow. We used a ratio of 90% wild-type and 10% knockout bone marrow for the mixed bone marrow chimeric mice to ensure that chimeric mice generated robust MAS immunopathology (Supplemental Figure 1). TLR9-deficient cells contributed to production of similar percentages of myeloid progenitors and inflammatory monocytes in the spleen and bone marrow of mixed bone marrow chimeric mice whether mice were treated with repeated doses of a TLR9 agonist or vehicle control (Figure 3A). Similar results were observed for wild-type:IFNγR1 mixed bone marrow chimeric mice (Figure 3B) suggesting that activation of both TLR9 and IFNγR1 induce enhanced myelopoiesis through hematopoietic progenitor cell-extrinsic mechanisms. These data imply that both TLR9- and IFNγ- dependent signals stimulate the production of additional inflammatory factors that indirectly induce inflammatory myelopoiesis during murine MAS.
Figure 3. TLR9- and IFNγ- dependent signals indirectly induce inflammatory myelopoiesis in murine MAS.
Congenically-labeled CD45.1 wild-type SJL hosts were lethally irradiated and reconstituted with mixed bone marrow from (A) CD45.1 wild-type mice and CD45.2 TLR9-deficient mice or (B) CD45.1 wild-type mice and CD45.2 IFNγR1-deficient mice at a ratio of 9:1. After 4–5 weeks of bone marrow reconstitution, chimeric mice were treated with five doses of PBS or CpG1826 over the course of ten days. Left panels: Peripheral blood leukocyte chimerism was determined before (white diamonds) and after (black triangles) treatment. Numbers of wild-type (CD45.1) and TLR9- or IFNγR1- deficient (CD45.2) spleen and bone marrow monocytes and total myeloid progenitors were enumerated (right, top). Percentages of wild-type (CD45.1) and TLR9-or IFNγR1- deficient (CD45.2) spleen and bone marrow monocytes and total myeloid progenitors were determined (right, bottom). Each graph displays compiled data from two independent experiments (N=9–10 chimeric mice per group). Individual symbols represent one mouse and the horizontal lines represent mean values. Analysis was performed by Mann-Whitney U tests comparing percentages of TLR9-deficient (A) or IFNγR1-deficient (B) cells between chimeric mice treated with repeated doses of PBS vs. repeated doses of CpG (NS = not significant).
Discussion
A growing body of preclinical and translational studies implicates IFNγ as a central mediator of MAS pathogenesis in patients with SJIA (2–4). However, the mechanisms leading to IFNγ-mediated immunopathology remain unclear, and animal models suggest that IFNγ alone is insufficient to induce all manifestations of MAS (5). In this manuscript, we provide evidence that systemic TLR9-driven signals potentiate IFNγ-mediated immunopathology in a murine model of MAS, as removal of either TLR9- or IFNγ-dependent signals abrogates manifestations of disease in IFNγ-deficient mice. This study adds to our understanding of MAS pathogenesis by delineating a role for both TLR- and IFNγ- dependent processes in the induction of disease, and suggests that these independent signals may contribute to disease pathogenesis by synergistically inducing inflammatory myelopoiesis.
IFNγ is known to potently synergize with TLR-dependent signals to induce cell-intrinsic pro-inflammatory macrophage functions (15). Induction of enhanced myelopoiesis may be a cell-extrinsic correlate that is synergistically induced downstream of IFNγ- and TLR- dependent signals in vivo to drive murine MAS. Intriguingly, increased numbers of extramedullary myeloid progenitors correlates with the induction of disease in TLR9-mediated murine MAS, which both require combined IFNγ- and TLR- dependent signals (11). TLR9- and IFNγ- dependent signals contribute to the induction of inflammatory myelopoiesis through cell-extrinsic mechanisms. These data suggest that additional signals produced downstream of TLR9 and IFNγR activation indirectly induce the accumulation of myeloid progenitors and potentiate their function in vivo. Future efforts directed at identifying the factors and signaling cascades involved in the induction of inflammatory myelopoiesis will be crucial to delineate whether targeting this pathway has therapeutic potential in MAS.
In conclusion, our manuscript supports a role for both TLR- and IFNγ- dependent signals in the pathogenesis of murine MAS, and highlights how the induction of inflammatory myelopoiesis may be a common pathogenic pathway synergistically induced downstream of these inflammatory signals. Future efforts will be required to determine the relevance of these findings to patients with MAS, and to define the cellular and molecular mechanisms that lead to the induction of inflammatory myelopoiesis. Such efforts may reveal novel therapeutic targets to ameliorate the overwhelming inflammatory cascade that leads to the clinical syndrome of MAS.
Supplementary Material
Serum ferritin and IFNγ. IFNγ-deficient mice were treated with five doses of PBS, the TLR9 agonist CpG1826, IFNγ, or the combination of CpG1826 and IFNγ every other day for 10 days. Mice were sacrificed twenty-four hours after the fifth injection and sera was collected by terminal bleed. Serum ferritin (A) and IFNγ (B) levels were measured from the associated sera.
Mixed bone marrow chimeric mice develop robust MAS immunopathology. (A) Wild-type:TLR9-deficient and (B) wild-type:IFNγR1-deficient mixed bone marrow chimeric mice were treated with five doses of PBS or CpG1826 over the course of ten days. Twenty-four hours after the last injection, mice were sacrificed and MAS immunopathology was measured.
Acknowledgments
The authors thank Hamid Bassiri, Martha Jordan, Taku Kambayashi, Gary Koretzky, Paula Oliver, Michael Silverman, and members of their associated laboratories for support and helpful discussions; the flow cytometry core at the University of Pennsylvania for cell sorting; the CHOP Pathology Core Laboratories for processing histology slides; and the CHOP Clinical and Translational Research Center for performing complete blood counts. Funding for this research was provided by the National Heart, Lung, and Blood Institute Grant R01HL112836 and a Howard Hughes Medical Institute early career investigator award to E.M.B., and a Rheumatology Research Foundation Scientist Development Award to L.K.W..
Footnotes
The authors have no conflicts of interest to disclose in association with this manuscript.
Declaration of Interests
The authors declare no competing interests.
References cited
- 1.Weaver LK, Behrens EM. Weathering the storm: Improving therapeutic interventions for cytokine storm syndromes by targeting disease pathogenesis. Curr Treatm Opt Rheumatol. 2017;3(1):33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121(6):2264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bracaglia C, de Graaf K, Pires Marafon D, Guilhot F, Ferlin W, Prencipe G, et al. Elevated circulating levels of interferon-gamma and interferon-gamma-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2016. [DOI] [PubMed] [Google Scholar]
- 4.Prencipe G, Caiello I, Pascarella A, Grom AA, Bracaglia C, Chatel L, et al. Neutralization of IFN-gamma reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J Allergy Clin Immunol. 2017. [DOI] [PubMed] [Google Scholar]
- 5.Zoller EE, Lykens JE, Terrell CE, Aliberti J, Filipovich AH, Henson PM, et al. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med. 2011;208(6):1203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strippoli R, Carvello F, Scianaro R, De Pasquale L, Vivarelli M, Petrini S, et al. Amplification of the response to Toll-like receptor ligands by prolonged exposure to interleukin-6 in mice: implication for the pathogenesis of macrophage activation syndrome. Arthritis Rheum. 2012;64(5):1680–8. [DOI] [PubMed] [Google Scholar]
- 7.Avau A, Mitera T, Put S, Put K, Brisse E, Filtjens J, et al. Systemic juvenile idiopathic arthritis-like syndrome in mice following stimulation of the immune system with Freund’s complete adjuvant: regulation by interferon-gamma. Arthritis Rheumatol. 2014;66(5):1340–51. [DOI] [PubMed] [Google Scholar]
- 8.Fall N, Barnes M, Thornton S, Luyrink L, Olson J, Ilowite NT, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56(11):3793–804. [DOI] [PubMed] [Google Scholar]
- 9.Yanagimachi M, Naruto T, Miyamae T, Hara T, Kikuchi M, Hara R, et al. Association of IRF5 polymorphisms with susceptibility to macrophage activation syndrome in patients with juvenile idiopathic arthritis. J Rheumatol. 2011;38(4):769–74. [DOI] [PubMed] [Google Scholar]
- 10.Graham RR, Kozyrev SV, Baechler EC, Reddy MV, Plenge RM, Bauer JW, et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet. 2006;38(5):550–5. [DOI] [PubMed] [Google Scholar]
- 11.Weaver LK, Chu N, Behrens EM. TLR9-mediated inflammation drives a Ccr2-independent peripheral monocytosis through enhanced extramedullary monocytopoiesis. Proc Natl Acad Sci U S A. 2016;113(39):10944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canna SW, Wrobel J, Chu N, Kreiger PA, Paessler M, Behrens EM. Interferon-gamma mediates anemia but is dispensable for fulminant toll-like receptor 9-induced macrophage activation syndrome and hemophagocytosis in mice. Arthritis Rheum. 2013;65(7):1764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burberry A, Zeng MY, Ding L, Wicks I, Inohara N, Morrison SJ, et al. Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and Toll-like receptor signaling. Cell Host Microbe. 2014;15(6):779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bruin AM, Libregts SF, Valkhof M, Boon L, Touw IP, Nolte MA. IFNgamma induces monopoiesis and inhibits neutrophil development during inflammation. Blood. 2012;119(6):1543–54. [DOI] [PubMed] [Google Scholar]
- 15.Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008;226:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum ferritin and IFNγ. IFNγ-deficient mice were treated with five doses of PBS, the TLR9 agonist CpG1826, IFNγ, or the combination of CpG1826 and IFNγ every other day for 10 days. Mice were sacrificed twenty-four hours after the fifth injection and sera was collected by terminal bleed. Serum ferritin (A) and IFNγ (B) levels were measured from the associated sera.
Mixed bone marrow chimeric mice develop robust MAS immunopathology. (A) Wild-type:TLR9-deficient and (B) wild-type:IFNγR1-deficient mixed bone marrow chimeric mice were treated with five doses of PBS or CpG1826 over the course of ten days. Twenty-four hours after the last injection, mice were sacrificed and MAS immunopathology was measured.