Abstract
An integral component of the antiviral response, Type I IFNs require regulation to modulate immune activation. We identify β-arrestin 2 as a key modulator of Type I IFN in primary human macrophages, an essential component of the innate immune response. β-arrestin 2 was selectively activated by CCL2/CCR2 signaling, which induced a decrease in IFN-α, but not IFN-β expression. siRNA knockdown of β-arrestin 2 demonstrated its role in IFNAR1 internalization, as well as STAT1 and IRF3 activation. As a result, cytokine responses were not propagated following HIV infection and TLR3 activation. However, remnants of IFN signaling remained intact, despite β-arrestin 2 activation, as IFN-β, IFN-γ, IFN-λ1, IRF7, TRAIL, and MxA expression were sustained. Similar effects of β-arrestin 2 on IFN signaling occurred in hepatocytes, suggesting that arrestins may broadly modulate IFN responses in multiple cell types. In summary, we identify a novel role of β-arrestin 2 as an integral regulator of Type I IFN through its internalization of IFNAR1 and a subsequent selective loss of downstream IFN signaling.
INTRODUCTION
Virus recognition by the immune system requires a well-coordinated interplay of pathogen recognition, potent, nonspecific innate responses, highly specific adaptive responses, and pathogen clearance. Macrophages are integral to each of these components of the innate immune response. During virus infection, macrophages produce Type I Interferons (IFN) that signal through the IFN receptor (IFNAR) to promote autocrine and paracrine signaling to limit viral replication (1, 2). While critical for protecting the host early during infection, these potent Type I IFN responses are transient and decline upon initiation of adaptive immunity. However, when infection or other chronic immune stimuli continues without regulatory mechanisms, sustained IFN contribute to chronic immune activation, autoimmunity, oncogenesis, and neurologic disease (3).
Modulation of Type I IFN occurs through many mechanisms, including limiting pathogen recognition by pattern recognition receptors, altering IFNAR cellular localization, transcriptional and epigenetic regulation of IFNAR adapter proteins, posttranscriptional modifications by noncoding RNA’s, negative feedback loops, and posttranslational modifications of key transcription factors (4, 5). While there has been much focus on identifying regulatory mechanisms of the Type I IFN pathway, relatively little emphasis has been placed on characterizing mechanisms specific to IFN-α. We previously demonstrated that the non-human primate brain expresses unique IFN-α subtypes compared to peripheral organs (6). Furthermore, we identified a lack of coordination of the IFN-α and IFN-β responses in brain during simian immunodeficiency virus infection (7). In that study, we determined that astrocyte-mediated CCL2 was the key regulatory factor that promoted this altered IFN response, IFN-β expression without IFN-α, in the brain (7, 8).
In our current study, we characterized the mechanisms by which CCL2 alters Type I IFN responses in primary human macrophages by focusing on the cellular scaffolding protein, β-arrestin. β-arrestins, comprised of the β-arrestin 1 and β-arrestin 2 isoforms (also known as arrestin-2 and arrestin-3, respectively), are best characterized for regulating G protein-coupled receptor (GPCR) signaling and recycling (9). β-arrestins also serve in immunomodulatory roles through their GPCR-independent signaling activities, including regulate signaling downstream of receptor protein tyrosine kinases, cytokine receptors, and ion channel receptors (10). While not completely characterized, the two arrestin isoforms may act in a functionally distinct manner to differentially immune responses (11).
We evaluated the contribution of CCR2 signaling and β-arrestin activation to Type I IFN signaling in primary human macrophages. We determined that CCL2 promotes β-arrestin activation that induced an inhibition of IFN-α expression in unstimulated cells, as well as in those stimulated with a TLR3 agonist or infected with HIV. However, IFN-β, IFN-γ, and IFN-λ1 expression was unaffected, indicating a selective and preferential regulation of IFN-α. There was a functional consequence to inhibiting IFN-α, as IFN-induced cytokines IL-10, TNF-α, IL-6, and CXCL10 were significantly decreased. siRNA knockdown identified β-arrestin 2 as the specific isoform required for decreasing IFN-α expression, which occurred through IFNAR1 internalization from the cell surface. This loss of extracellular IFNAR1 rendered the macrophages unable to properly elicit IFN responses, indicated by a loss of STAT1 and IRF3 activation. Interestingly, β-arrestin 2 did not mediate its effects through IRF7, IFN-β, TRAIL, or MxA, as knockdown did not alter expression of these IFN-stimulated genes. In summary, we identified β-arrestin 2 as a critical and highly specific regulator of Type I IFN that suppresses IFN signaling in both uninfected cells prior to viral exposure, and, following infection, which specifically regulates the IFN-α arm of innate immune responses.
MATERIALS AND METHODS
Generation of Primary Human Monocyte-Derived Macrophages (MDM)
Blood was obtained from HIV-seronegative adult donors according to established protocols at the Johns Hopkins University (Protocol NA_00078178, Molecular Determinants of Retroviral Pathogenesis PI: Witwer). No identifying information about the blood donors was collected for this study, including gender, in accordance with our established protocol with the Johns Hopkins University Institutional Review Board. Blood was separated by Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) density gradient centrifugation to obtain PBMC. To obtain MDM, PBMC were cultured adherently in plastic dishes with Macrophage Media (DMEM (Gibco, Grand Island, NY) supplemented with 10% FBS (Gibco), 5% Human Serum (Corning, Corning, NY), 1% HEPES (Gibco), 1% Pen-Strep (Gibco), 1% Glutamine (Gibco), 1% penicillin–streptomycin (Gibco), and 10 ng/mL M-CSF (R&D Systems, Minneapolis, MN)) at 37°C, 5% CO2 for three days, the cells washed, and cultured for an additional three days in fresh Macrophage Media. After a total of six days of culture the cells were determined to be mature MDM and used for downstream experiments.
HIV Infection of MDM
Purified HIV-189.6 was obtained from the NIH AIDS Reagent Program. Viral stocks were developed by purifying the supernatant of infected MDM by 20% sucrose/PBS gradient centrifugation for 2 hours at 36,000 rpm, 4°C. Viral pellets were resuspended in Macrophage Media and stored in aliquots at −80°C. There were no freeze-thaw cycles before use and each aliquot of virus was used only once. Infectious viral load of the viral stocks was determined by HIV p24 ELISA (Perkin Elmer) according to the manufacturer’s protocol.
MDM were inoculated with 20 ng/mL HIV89.6 or remained uninfected as a control. After 24 hours, the media was changed to remove the virus and fresh Macrophage Media added to the cells. MDM then were cultured for an additional five days to facilitate HIV infection. Uninfected control cells were also cultured in fresh media for an additional five days. One hundred microliters of supernatant from HIV-infected MDM cultures was collected every 24 hours from days 2–6 post-infection. Supernatant was also collected from uninfected MDM cultures on day 6 as a control. The supernatants were collected, aliquoted, and stored at −80°C for quantitation of HIV p24 protein as a measure of viral replication. There were no freeze-thaw cycles before use and each supernatant aliquot was used only once.
For chemokine studies, uninfected and HIV-infected MDM were treated with 100 ng/mL CCL2, CCL5, CCL7, CCL8 (Peprotech, Rocky Hill, NJ) or vehicle control for 24 hours, after which time the cells were lysed for RNA extraction. The supernatants of vehicle and CCL2 treated MDM were collected, aliquoted, and stored at −80°C for determination of soluble IFN-α. There were no freeze-thaw cycles before use and each supernatant aliquot was used only once.
Determination of HIV Viral Replication
HIV p24 ELISA was performed to measure the amount of virus using a 1:10–1:50 dilution of MDM supernatant according to the manufacturer’s protocol. Viral loads were log-transformed for statistical analyses.
Cell Lines
The human liver hepatocellular carcinoma Huh-7 cell line was kindly provided by Dr. Charles Rice of Rockefeller University (12). The cell line originated from the liver tumor of a 57-year-old male. Huh-7 cells were grown in Huh media (DMEM (Gibco) supplemented with 10% FBS (Gibco) and 1% Pen-Strep (Gibco)) at 37°C, 5% CO2.
MDM Treatment with TLR Agonists
MDM and Huh-7 cells were treated with 10 μg/mL of the TLR3 agonist polyinosinic:polycytidylic acid (pIC; Sigma-Aldrich, St. Louis, MO) in the presence or absence of 100 ng/mL CCL2 or vehicle control for 0.5, 2, 6, 10, 18, and 24 hours, supernatants collected, and the cells lysed for RNA extraction. Due to donor variability, the time point at which the maximal decrease in IFN-α mRNA occurred for each individual donor was used for analysis.
CCR2 Inhibition
MDM were pretreated with 10 μM of the CCR2 inhibitor 2-carboxyethylgermanium (IV) sesquioxide (Sigma) for 45 minutes. After pretreatment, cells were treated with 100 ng/mL CCL2 for 0.5, 2, 6, 10, 18, and 24 hours, after which time the cells were lysed for RNA extraction. The media was not changed after pretreatment and the inhibitor remained present for the duration of CCL2 treatment. Due to donor variability, the time point at which the maximal decrease in IFN-α mRNA occurred for each individual donor was used for analysis.
G Protein Inhibition
MDM were pretreated with 10 μM of the Gα inhibitor, substance P peptide, or the Gβγ inhibitor, gallein, (Sigma) for 45 minutes. After pretreatment, cells were treated with 100 ng/mL CCL2 for 0.5, 2, 6, 10, 18, and 24 hours, after which time the cells were lysed for RNA extraction. The media was not changed after pretreatment and the inhibitor remained present for the duration of CCL2 treatment. Due to donor variability, the time point at which the maximal decrease in IFN-α mRNA occurred for each individual donor was used for analysis.
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted from MDM using the RNeasy PLUS Mini Kit according to the manufacturer’s protocol with the modification of on column DNase digestion (RNase free DNase kit (Qiagen)) using RQ1 RNase free DNase (Promega, Madison, WI) in the enzyme mix. RNA purity and concentration was determined with a Nanodrop spectrophotometer (Nanodrop technologies, Wilmington, DE). Quantification of IFN-α, IFN-β, TRAIL, MxA, and 18S mRNA was assessed by RT-PCR using the CFX96 Touch Real-Time PCR Detection System (Bio Rad, Hercules, CA) and a cycling protocol of 50°C for 30 min, 95°C for 15 min, and 45 cycles of denaturation (15 sec at 94°C), anneal (15 sec at 55°C), and amplification (30 sec at 60°C). Oligonucleotide primers and probes used for gene amplification are listed in Table 1. It is important to note that we designed pan-IFN-α primers specific to the regions conserved among all 13 subtypes. The 2-ΔΔCt method was used for determination of the fold change in gene expression relative to 18S.
Table 1:
Oligonucleotides primers used for qRT-PCR.
| Name | Sequence |
|---|---|
| Pan-IFNα Primer Forward | 5’-ATGAGATGATCCAGCAGACCT-3’ |
| Pan-IFNα Primer Reverse | 5’-GATCTCATGATTTCTGCTCTGACAACC-3’ |
| Pan-IFNα Probe | 5’-AGAAGAAATACAGCCCTTGTGCC-3’ |
| IFN-β Primer Forward | 5’-GCCTCAAGGACAGGATGAACTT-3’ |
| IFN-β Primer Forward | 5’- GCGTCCTCCTTCTGGAACTG-3’ |
| IFN-β Probe | 5’- CATCCCTGAGGAAATTAAGCAGCCGC-3’ |
| Trail Primer Forward | 5’-GAGAGTATGAACAGCCCCTG-3’ |
| Trail Primer Reverse | 5’-AGGACCTCTTTCTCTCACTAGG-3’ |
| Trail Probe | 5’-TGGCAACTCCGTCAGCTCGTTA-3’ |
| MxA Primer Forward | 5’-AGGAGTTGCCCTTCCCAGA-3’ |
| MxA Primer Reverse | 5’-TCGTTCACAAGTTTCTTCAGTTTC A-3’ |
| MxA Probe | 3’-ACCAGCGGGCATCTGGTCACGA-3’ |
| 18S Primer Forward | 5’-TAGAGGGACAAGTGGAGTTC-3’ |
| 18S Primer Reverse | 5’-CGCTGAGCCAGTCAGTGT-3’ |
| 18S Probe | 5’-AGCAATAACAGGTCTGTGATG-3’ |
Detection of Soluble IFN-α
Amicon Ultra-0.5 mL Centrifugal Filters (Millipore, Billerica, MA) were used to concentrate supernatants 30-fold prior to IFN-α quantification. Soluble IFN-α was detected in the concentrated supernatant by using the VeriKine-HS Human Interferon Alpha ELISA (PBL Assay Science, Piscataway, NJ), according to the manufacturer’s directions. The limit of detection was 40 pg/mL.
Luminex Multiplexed Cytokine Analysis
The Bioplex 200 platform (BioRad, Hercules, CA) was used to determine the concentration of multiple target proteins in MDM supernatants. The Luminex bead based immunoassay was performed following the manufacturer’s protocol and concentrations were determined using 5 parameter log curve fits using Bioplex Manager 6.0 (BioRad) with vendor provided standards and quality controls. The Human Cytokine/Chemokine Panel HCYTOMAG-60K was used to measure TGFα, IFNα2, IL-10, IL-12(p70), IL-15, IL-1β, IL-6, MCP-1, and TNF-α (Millipore, Bilerica NY).
LEGENDPlex Multiplex Bead Based Cytokine Analysis
The Biolegend LEGENDPlex platform (San Diego, CA) was used to determine the concentration of multiple target proteins in MDM supernatants by flow cytometric analyses. The LegendPlex bead based immunoassay was performed following the manufacturer’s protocol and concentrations were determined using 7 parameter log curve fits with vendor provided standards and quality controls. The Human Anti-Virus Response Panel was used to measure IL-1β, IL-6, TNF-α, IP-10, IFN-λ1, IL-8, IL-12p70, IFN-α2, IFN-λ2/3, GM-CSF, IFN-β, IL-10, and IFN-λ.
Flow Cytometry
MDM and Huh7 cells were recovered from tissue culture plates using TrypLE Express (Invitrogen Grand Island, NY) for 10–15 min at 37°C, 5% CO2 as previously described (13). After recovery, the cells were washed once with PBS (Gibco) and separated for extracellular and intracellular staining. For extracellular staining, the cells were washed once with cold FACS buffer (PBS supplemented with 2% Human Serum (Corning, Manassas, VA) and 500,000 cells per tube were stained with fluorochrome-coupled antibodies specific for CCR2, IFNAR1, TLR3, CD14, or corresponding isotype-matched negative control antibodies in the dark, on ice for 30 min. Following staining the cells were washed with FACS buffer, fixed with pre-warmed BD Fix Buffer III for 10 min at 37°C, and filtered using BD FACS tubes with cell strainer caps with 35-μm pores. The samples were stored at 4 °C wrapped in foil up to 1 week prior to flow cytometric analysis.
For intracellular staining, 1 × 106 cells per tube were fixed with pre-warmed BD Fix Buffer III for 10 min at 37°C. The cells were washed twice with PBS and permeabilized by slowly adding pre-chilled BD Perm Buffer and incubating on ice for 30 min. The cells were then washed twice with FACS buffer, resuspended at a density of 5 × 106/mL, and stained with fluorochrome-coupled antibodies specific for TLR7, IFNAR1, pSTAT1, pIRF3, pIRF7, STAT1, IRF3, IRF7, or corresponding isotype-matched negative control antibodies in the dark for 30 min. Following staining, the cells were washed with FACS buffer, filtered, and stored at 4 °C wrapped in foil up to 1 week prior to flow cytometric analysis.
For cells that received both extracellular and intracellular staining, extracellular staining occurred prior to the intracellular staining protocol. All antibodies were titered to determine optimal concentrations for staining. At least 2 × 105 events were acquired with a BD LSRFortessa cytometer and Diva software version 6.1.3. FACS data were analyzed using FlowJo version 10.3 (FlowJo, Ashland, OR). Representative gating strategies are shown in MDM were recovered from tissue culture plates using TrypLE Express, washed with PBS, and resuspended at a density of 1 × 106 cells with 250 μL pre-warmed human serum free Macrophage Media. The cells were loaded with a calcium sensitive dye using the BD Calcium Assay Kit according to the manufacturer’s protocol for 1 hour at 37°C. After cooling to room temperature for 20 min, the cells were acquired using Diva software with the BD LSRFortessa cytometer for one min, 100 ng/mL CCL2 added, the cells vortexed briefly and acquired for an additional three min. The data were analyzed using FlowJo version 10.3 in kinetics mode.
siRNA Knockdown of β-Arrestin 1 and 2
siRNA knockdown was performed using the forward transfection of primary human macrophages protocol as described by Troegeler et al. (14). Briefly, 4 × 106 PBMC were plated in a six-well plate in macrophage media to obtain MDM. Lipid-siRNA complexes were obtained by mixing the respective siRNA at a final concentration of 200 nM with 440 μL of warm, non-supplemented DMEM medium and 45 μL HiPerFect transfectant reagent (Qiagen), per well, for 20 minutes at room temperature. While the lipid-siRNA complexes were forming, the MDM were washed twice with PBS and 1 mL of antibiotic-free macrophage media added to the cells. The lipid-siRNA complexes were added drop wise onto the MDM and the cells incubated at 37°C, 5% CO2 for six hours, after which time an additional two mL of antibiotic-free macrophage media was added to the cells. Transfection conditions included siβ-Arrestin 1, siβ-Arrestin 2, combined siβ-Arrestin 1 and 2, siGLO Red, and a non-targeting siRNA (Thermo Fisher Scientific, Dharmacon. Cell viability and transfection efficiency was determined by flow cytometry at 24 and 48 hours after transfection. Cell viability was assessed utilizing the BD FITC Annexin V Apoptosis Detection Kit, according to manufacturer’s instructions. Transfection efficiency was assessed by determining the frequency of PE positive cells in siGLO Red transfected cells compared to untransfected control cells.
Western Blot
MDM were lysed with RIPA buffer (Cell Signaling, Beverly, MA) containing a protease/phosphatase inhibitor cocktail (Cell Signaling), and 30 μg of protein electrophoresed on a 4–12% polyacrylamide gel (Bio-Rad), and transferred to nitrocellulose membranes. Membranes were probed with antibodies to Phospho-Stat1 (Tyr701), Stat1, β-actin (Cell Signaling Technology), β-arrestin 1, β-arrestin 2, or GAPDH (Thermo Fisher Scientific). Densitometric analysis was performed using Image Lab Software 6.0 (Bio-Rad).
Quantification and Statistical Analysis
Statistical analyses were performed using Prism software version 7.0 (GraphPad Software, Inc., San Diego, CA). For experiments with MDM, n equals the number of individual donors used. For experiments with Huh-7 cells, n equals the number of independent experiments performed, where three technical replicates were used for each experiment. All experiments were performed with at least n ≥ 5 to perform the D’Agostino-Pearson omnibus normality test. For all experiments, the reference group was set to 1 and the relative pooled average fold change in gene/protein expression was determined. All data are represented as median ± SEM. Statistical significance for experiments with MDM was determined by Friedman nonparametric ANOVA with Dunn’s multiple comparisons test, where vehicle treatment served as the reference group. Wilcoxon matched-pairs signed rank test was used to determine statistical significance for paired analyses between two groups. Pearson’s test was used to determine statistical significance for the correlation between HIV p24 protein concentration and the fold change in IFN-α mRNA. Statistical significance for Huh-7 experiments was determined by RM one way ANOVA with Greenhouse-Geisser correction and Dunnett’s multiple comparisons test, where vehicle treatment served as the reference group. Paired t test was used to determine statistical significance for paired analyses between two groups in experiments with Huh-7 cells. *p≦0.05. **p≦0.01. ***p≦0.001.
RESULTS
Interdonor Variability Within the Type I IFN Pathway
Due to the accessibility and ease of genetic homogeneity, much of the seminal work characterizing Type I IFN was performed using rodent models. This resulted in many advances and greatly strengthened the overall understanding of the IFN response. However, it is not clear whether these pathways are regulated in the same way in other species (15, 16). Human innate immune responses are incompletely understood. This is particularly true when considering the mononuclear phagocyte system, which differs substantially between humans and mice (17). There is a paucity of data evaluating the Type I IFN pathway in human macrophages, despite the integral roles these cells play as a critical bridge between the innate and adaptive immune system. In this study, we evaluated the Type I IFN pathway in primary human macrophages using monocyte-derived macrophages (MDM) that were obtained by adherent culture of peripheral blood mononuclear cells (PBMC).
The basal, endogenous levels of IFN-α, IFN-β, TRAIL, and MxA were examined by qRT-PCR in MDM from 25 human donors. We detected all of the 13 IFN-α subtypes with the use of pan-IFN-α primers. The individual donor with the lowest basal expression of each gene was selected as a “reference donor.” This individual had the highest Ct value, which was set to one, and the fold change in gene expression for all remaining 24 individuals was determined relative to this donor. Interdonor variability was highly prevalent in the Type I IFN pathway, which was most noticeable for expression of IFN-α (Figure 1A). The vast majority of individuals (75%) expressed more than 100,000 times more IFN-α mRNA than the “reference donor.” Variability in expression was also found for IFN-β, and to a lesser extent TRAIL and MxA (Figure 1A). In contrast, there was almost no interdonor variability for the “housekeeping gene” 18S. These data suggest that Type I IFN induction during both homeostatic and pathogenic states will vary widely due to interdonor variability in basal gene expression.
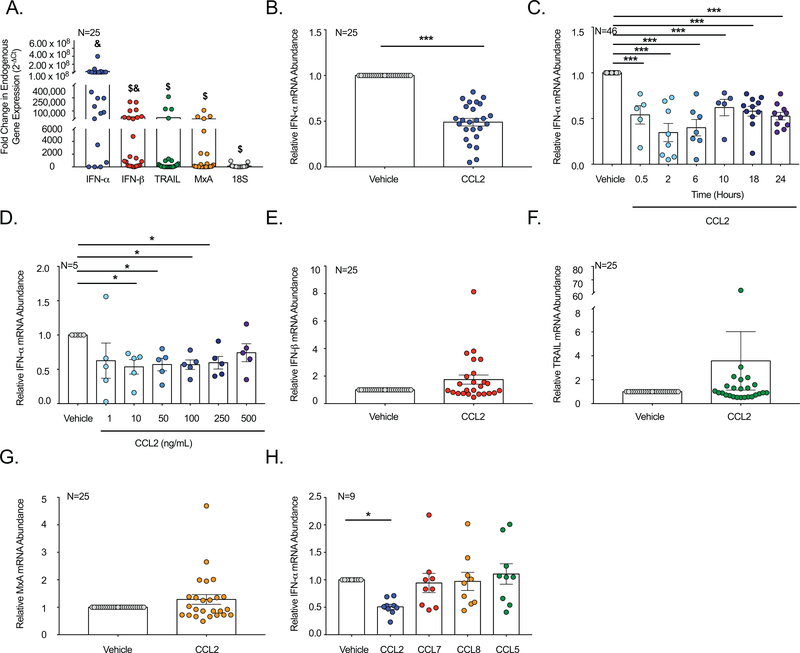
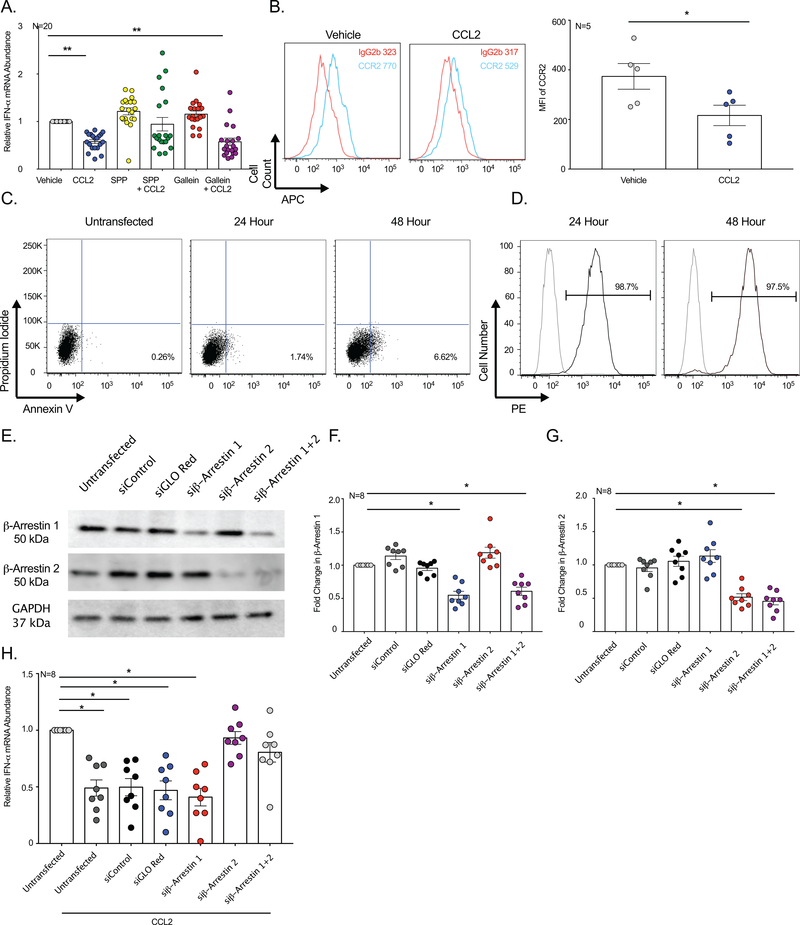
Figure 1: CCL2 Decreases Endogenous IFN-α in Primary Human Macrophages.
(A) Endogenous levels of IFN-α, IFN-β, TRAIL, MxA, and 18S mRNA were quantified in MDM from 25 donors. & indicates statistical significance relative to 18S. $ indicates statistical significance relative to IFN-α. (B, E-G). Uninfected MDM from 25 donors were treated with CCL2 or vehicle and (B) IFN-α, (E) IFN-β, (F) TRAIL, and (G) MxA quantified. (C) MDM from 46 donors were treated with CCL2 for 0.5, 2, 6, 10, 18, and 24 hours and qRT-PCR performed. (D) Uninfected MDM from five donors were treated with 1–500 ng/mL CCL2 or vehicle and qRT-PCR performed. (H) Uninfected MDM from nine donors were treated with CCL2, CCL7, CCL8, CCL5, or vehicle and qRT-PCR performed.
CCL2 Selectively Decreases Endogenous IFN-α
CCR2 was examined based on previous studies demonstrating its role in Type I IFN regulation in nonhuman primates (7, 8). We determined that CCL2 significantly decreased endogenous levels of IFN-α mRNA in MDM by 0.5 ± 0.1 fold, relative to vehicle treated cells (Figure 1B). While CCL2 substantially inhibited IFN-α mRNA for all donors, there was variability in the magnitude of the decrease (Figure 1B) and the duration of time needed for this to occur (Figure 1C). The decrease in IFN-α mRNA was not dependent on the concentration of CCL2 and occurred, on average, between 10–500 ng/mL (Figure 1D). One hundred ng/mL of CCL2 decreased IFN-α mRNA most consistently among donors and was used for all experiments in this study. These findings underscore the importance of studying primary human cells whenever possible to assess not only the immune response, but also to identify variability within human immunologic pathways.
To determine whether CCL2 modulated other components of the Type I IFN pathway, we evaluated IFN-β, MxA, and TRAIL, genes that are coordinately regulated with IFN-α. Unlike that which occurred for IFN-α, CCL2 had no significant effect on IFN-β (Figure 1E), MxA (Figure 1F), or TRAIL (Figure 1G) mRNA. These data suggest that CCL2 promotes a unique, non-canonical Type I IFN response in macrophages wherein IFN-α is selectively inhibited, but not IFN-β or other downstream IFN-stimulated mediators.
To evaluate the specificity of IFN-α inhibition to CCL2, we treated MDM with CCL7 and CCL8, additional ligands of CCR2. While CCL2, CCL7, and CCL8 all bind to CCR2, they do so with differing potencies that result in different levels of β-arrestin activation. CCL2 is the most potent CCR2 ligand and recruits β-arrestin with the fastest kinetics, with the highest potency, and induces strong β-arrestin activation for the longest duration of time, as compared to CCL7 and CCL8 (18). We also examined CCL5, as the active site of its primary receptor, CCR5, has 82% homology with CCR2 (19). Following treatment with each chemokine, qRT-PCR was performed to quantify IFN-α mRNA. CCL2 significantly decreased IFN-α mRNA in MDM (Figure 1H). However, CCL7, CCL8, and CCL5 had no effect on IFN-α whose mRNA levels remained, on average, similar to that of vehicle treated cells (Figure 1H). These data demonstrate specificity of CCL2 in IFN-α inhibition.
CCL2 Inhibits IFN-α in the Context of Viral Infection and TLR3 Activation
We next characterized the contribution of CCL2 to Type I IFN in the context of viral infection. For these studies, we infected MDM with HIV or treated with polyinosinic:polycytidylic acid (pIC), a TLR3 agonist. On average, HIV and pIC increased IFN-α mRNA by 2.3 ± 0.5 and 3.9 ± 0.8 fold, respectively, relative to mock infected MDM (Figure 2A). However, there was substantial interdonor variability in the magnitude to which HIV or pIC induced IFN-α. For the HIV infected cells, the magnitude of the increase in IFN-α mRNA significantly correlated with HIV replication, measured by quantitating the viral p24 protein in the cell supernatant (p=0.01, R2=0.507).
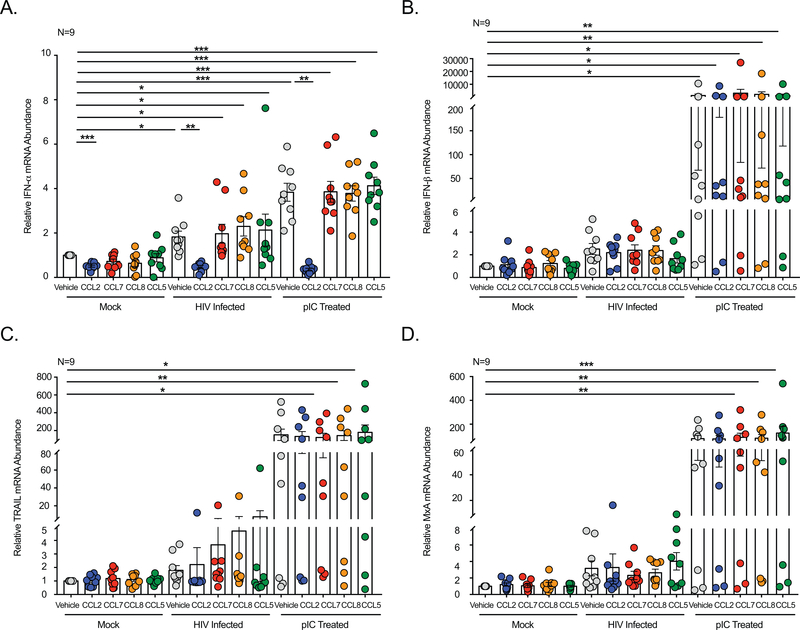
Figure 2: CCL2 Selectively Inhibits HIV- and TLR3-Induced IFN-α.
Following treatment/infection with vehicle, HIV, or pIC, MDM from nine donors were treated with CCL2, CCL7, CCL8, CCL5 or vehicle and qRT-PCR performed to quantify (A) IFN-α, (B) IFN-β, (C) TRAIL, or (D) MxA.
The mock infected, HIV infected, and pIC treated MDM were then treated with CCL2, CCL7, CCL8, and CCL5 and IFN-α mRNA quantified. CCL2 significantly decreased endogenous IFN-α mRNA in mock infected MDM (Figure 2A). Similarly, despite the increase in IFN-α induced by viral infection and exposure to a viral mimetic, CCL2 significantly decreased HIV- and pIC-induced IFN-α to below that present in vehicle-treated, uninfected MDM (0.5 ± 0.1 and 0.3 ± 0.4 fold, respectively) (Figure 2A). In contrast, CCL7, CCL8, and CCL5 had no effect on IFN-α mRNA, strongly suggesting that there is a component of the CCL2-CCR2 signaling cascade unique to CCL2 that is required to inhibit IFN in the context of viral infection.
To more completely evaluate the effects of CCL2 on the Type I IFN pathway, we also examined IFN-β and the IFN stimulated genes TRAIL and MxA. While not significant due to interdonor variability, HIV caused a trend towards an increase in IFN-β (2.6 fold), TRAIL (1.8 fold), and MxA (3.2 fold) mRNA, as compared to mock infected cells (Figure 2B-2D). pIC more strongly induced Type I IFN responses and significantly increased each of these genes (Figure 2B-2D). Unlike that which occurred for IFN-α, CCL2 did not decrease IFN-β, TRAIL, or MxA in mock, HIV infected, or pIC treated MDM (Figure 2B-2D).
The IFN-modulatory effects of CCL2 on IFN-α were also evaluated in the context of IFN-β stimulation, as it is rapidly induced during HIV infection, pIC treatment, as well as more generally in the context of viral infection. While CCL2 significantly decreased IFN-α mRNA, there was no clear trend on the combined effects of CCL2 and IFN-β treatment on IFN-α, where the alpha IFN subtype was decreased, increased, or remained unchanged in 37%, 25%, and 38% remained unchanged of individuals, respectively (Supplemental Figure 2A). There was also no effect of CCL2 on IFN-β-induced IFN-β, TRAIL, or MxA mRNA (Supplemental Figure 2B-D). These findings confirm that CCL2 selectively decreases IFN-α in macrophages when uninfected and also in the context of viral infection. Furthermore, they indicate that CCL2 promotes an altered, non-canonical Type I IFN signaling pathway wherein IFN-α, IFN-β, and downstream IFN inducible genes TRAIL and MXA are differentially regulated.
CCL2 Abrogates IFN-α and IFN-α Inducible Cytokines, Without Altering IFN-β, IFN-γ, and IFN-λ1
In addition to transcriptional regulation, Type I IFNs mediate their antiviral effects at the protein level and through IFN-induced cytokines. We examined soluble IFN-α produced in the MDM supernatant by performing a pan-IFN-α ELISA that has specificity for all 13 subtypes. As anticipated, IFN-α was not detectable for the majority of donors in the vehicle treated, uninfected MDM (Figure 3A-3B). IFN-α is expressed at very low levels in the absence of viral or inflammatory stimuli and cannot be quantified even when utilizing highly sensitive assays (20–23). However, it is a rapidly inducible protein, and HIV infection (Figure 3A) and pIC treatment (Figure 3B) significantly increased IFN-α in the MDM supernatant. Consistent with our mRNA findings, the increased levels of IFN-α induced by HIV and pIC were significantly decreased following CCL2 treatment (Figure 3A-3B). This demonstrates that CCL2 regulated IFN-α at both the mRNA and protein levels.
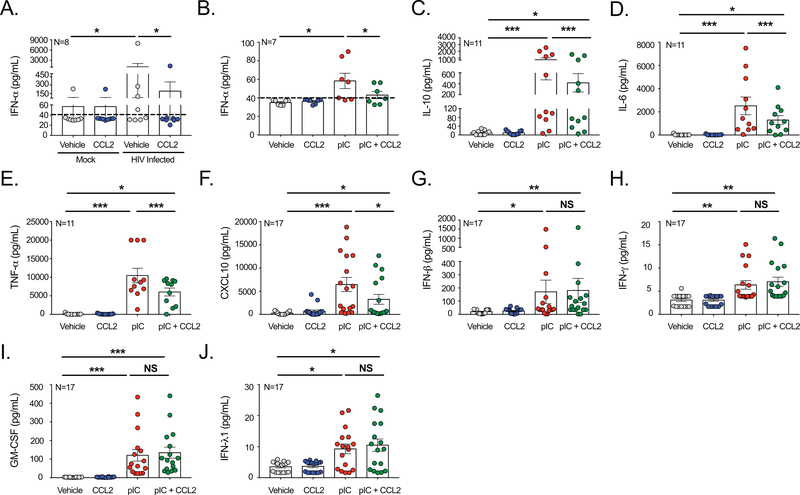
Figure 3: CCL2 Selectively Inhibits IFN-Induced Cytokines.
Supernatant from (A) HIV infected and (B) pIC treated MDM was collected following treatment in the presence or absence of CCL2 and IFN-α protein levels determined. The limit of detection is indicated by a dashed line. (C-E) MDM from 11 donors were treated with pIC in the presence or absence of CCL2, the supernatant collected, and (C) IL-10, (D), IL-6, and (E) TNF-α quantified by Luminex analysis. (F-J) MDM from 17 donors were treated with pIC in the presence or absence of CCL2, the supernatant collected, and (F) CXCL10, (G) IFN-β, (H) IFN-γ, (I) GM-CSF, and (J) IFN-λ1 quantified by LegendPlex Multiplex analysis.
Following their induction, IFNs produce a cascade of signaling events that result in the production of additional cytokines and antiviral factors to facilitate clearance of the pathogen. To determine whether CCL2 affected IFN-induced cytokines, we performed multiplexed analysis of the supernatants of pIC treated MDM. We focused specifically on pIC as it more strongly induced Type I IFN responses in MDM than HIV (Figure 2). While undetectable in vehicle treated cells, pIC significantly induced IL-10 (Figure 3C), IL-6 (Figure 3D), TNF-α (Figure 3E), and CXCL10 (Figure 3F) in the MDM, though the magnitudes of the increase were highly donor dependent. Following CCL2 treatment, there was a consistent and significant decrease in each of the pIC-induced cytokines (Figures 3C-3F). These findings indicate that there is a functional consequence to CCL2-mediated IFN-α inhibition through regulation of cytokine responses during viral infection.
As additional IFN subtypes also contribute to the antiviral response and promote cytokine production, we evaluated whether IFN-β, IFN-γ, and IFN-λ1 protein levels were altered by CCL2. While minimally expressed in vehicle treated cells, pIC significantly induced IFN-β (Figure 3G) and IFN-λ1(Figure 3J) for all donors. The effects of pIC on IFN-γ (Figure 3H) did not reach statistical significance; however, its inducible cytokine GM-CSF (Figure 3I) was significantly increased following TLR3 activation. Unlike that which occurred for IFN-α, CCL2 had no effect on IFN-β, IFN-λ1, IFN-γ (Figure 3G-3J). Interdonor variability in cytokine production is highlighted in Supplemental Figure 3. These data strongly demonstrate the specificity of CCL2 signaling for inhibiting IFN-α while maintaining additional IFN subtypes.
CCR2 is Required for CCL2-Mediated Decrease in IFN-α
Upon determining that CCL2 promoted a decrease in IFN-α at both the mRNA and protein levels, we next sought to identify the mechanisms by which this occurred. We began by focusing on CCR2, the predominant receptor for CCL2. While CCL2 may bind to other receptors, CCL2 uptake in myeloid cells occurs primarily through CCR2 (24, 25). For this reason, we focused specifically on CCR2 to evaluate the mechanisms by which CCL2 decreased IFN-α. We first characterized the expression of CCR2 on MDM. Although present on all donors, CCR2 was highly variable among individuals (Figure 4A). Upon CCL2 binding to CCR2, intracellular calcium is released from intracellular stores to propagate signal transduction. Therefore, calcium flux can be used as an indicator of receptor function. Because of the highly variable nature of CCR2 among individuals, we wanted to ensure appropriate CCR2 function for all donors and utilized calcium influx as our readout. Despite this interdonor variability, CCR2 was consistently functional among all donors evaluated (Figure 4B). Some individuals had prolonged calcium flux that lasted over four minutes (Figure 4B, Donors 1–4), while the calcium influx occurred more acutely in other donors and returned to basal levels within seconds of CCL2 treatment (Figure 4B, Donors 5–8). The duration and pattern of calcium influx was not related to the amount of CCR2 present.
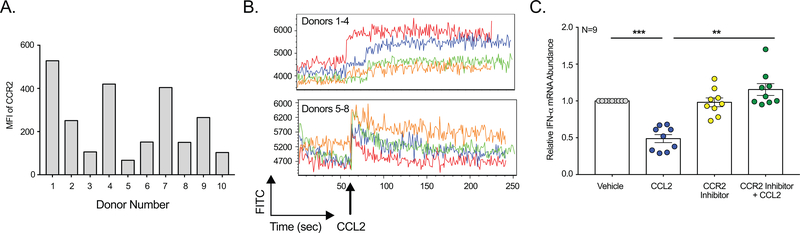
Figure 4: CCR2 is Functional and Required for Decrease in IFN-α.
(A) Flow cytometry was performed on MDM using antibodies specific to CCR2 or an isotype matched negative control. After subtracting the contribution from the isotype matched control, the median fluorescence intensity (MFI) of CCR2 from 10 donors was determined. (B) MDM from eight donors were loaded with a calcium sensitive dye and flow cytometric determination of calcium influx was determined. Arrow indicates CCL2 addition. (C) MDM from nine donors were treated with CCL2 following pretreatment with the CCR2 inhibitor propagermanium or vehicle and qRT-PCR performed to quantitate IFN-α.
While CCR2 was expressed and functional on the MDM, its contribution to IFN-α regulation required investigation of a direct functional role. To evaluate this, MDM were pretreated with a small molecule inhibitor of CCR2, 2-carboxyethylgermanium (IV) sesquioxide, also known as propagermanium. Following pretreatment, MDM were treated with CCL2 or vehicle, and IFN-α mRNA evaluated by qRT-PCR. The CCR2 inhibitor abrogated completely the inhibitory effect of CCL2 and restored IFN-α mRNA to basal levels (Figure 4C). These data demonstrate that a CCR2-dependent mechanism is required to mediate the decrease in IFN-α by CCL2.
β-arrestin 2 is Required for CCL2-Mediated Decrease in IFN-α
We next examined components of the CCR2 signaling pathway that contributed to regulation of the Type I IFN pathway. Following CCL2 binding, Gα, Gβ, and Gγ proteins are recruited to CCR2 to initiate the signal transduction cascade. We pretreated the MDM with a small molecule inhibitor of all Gα subtypes, substance P peptide (SPP), and gallein, an inhibitor of Gβγ, to evaluate the contribution of these G proteins to IFN regulation. Following pretreatment, MDM were treated with CCL2 or vehicle, and IFN-α mRNA evaluated by qRT-PCR. Consistent with our previous experiments, CCL2 significantly decreased IFN-α mRNA (Figure 5A). On average, Gα blockade reversed the effect of CCL2 and restored IFN-α to basal levels. However, there was a subset of individuals for whom this did not occur (Figure 5A). Similar to CCL2 treatment alone, Gβγ inhibition prior to CCL2 treatment also promoted a significant decrease in IFN-α (Figure 5A). Of note, the inhibitors alone did not significantly affect IFN-α. These data suggest that Gβγ signaling does not contribute to an inhibition of Type I IFN in macrophages, while Gα may be involved for a subset of individuals.
Figure 5: β-Arrestin 2 is Required for CCL2-Mediated Decrease in IFN-α.
(A) MDM from 20 donors were treated with CCL2 following pretreatment with the Gα inhibitor SPP, Gβγ inhibitor gallein, or vehicle and qRT-PCR performed to quantitate IFN-α. (B) CCR2 cell surface expression was analyzed by flow cytometry after MDM from five donors were treated with CCL2 or vehicle. Data from one representative histogram (left) and CCR2 MFI (right) from five independent donors are shown. (C-H) MDM from five independent donors were transfected with siRNA’s with specificity for β-arrestin 1, β-arrestin 2, both β-arrestins, non-targeting siRNA control, control fluorescent siGLO Red, or remained untransfected. (C) MDM viability was assessed by flow cytometric analysis 24 and 48 hours after transfection by propidium iodide and annexin V. The frequency of apoptotic cells is denoted in the bottom right quadrant. (D) PE fluorescent signal was assessed in untransfected MDM (grey line) and cells transfected with siGLO Red (black) 24 and 48 hours after transfection. The frequency of PE positive cells is demonstrated in representative histograms. (E-G) Western blot was performed following transfection with the indicated siRNA’s to assess β-arrestin 1, β-arrestin 2, and GAPDH. (E) One representative experiment is shown. Densitometric analysis was performed and following normalization to GAPDH, the pooled average fold change in (F) β-arrestin 1 or (G) β-arrestin 2 was determined. (H) Twenty-four hours post-transfection, MDM were treated with vehicle or CCL2 and qRT-PCR performed to quantify IFN-α.
The contributions of G proteins, specifically Gα, on Type I IFN signaling were not consistent among all donors and we next examined β-arrestin, a subsequent component of CCR2 signaling that has been implicated previously in modulating inflammatory responses. β-arrestin rapidly promotes CCR2 internalization following CCL2 binding to prevent receptor desensitization (18). To ensure that this occurred in our MDM, we assessed CCR2 on the MDM cell surface. Within 15 minutes, CCL2 significantly decreased cell surface CCR2, as compared to vehicle (Figure 5B). However, CCR2 was not completely internalized and approximately 44% of the receptor was extracellular (Figure 5B). Within 45 minutes, CCR2 was restored to levels equivalent to that of vehicle treated cells (data not shown), indicating that receptor recycling occurred. Already well known to occur, these data confirm that CCL2 promotes β-arrestin activation, as indicated by CCR2 internalization.
After ensuring CCL2 promoted β-arrestin activation in macrophages, we next examined its contribution to IFN regulation. There are two functionally distinct β-arrestin isoforms that are recruited to GPCRs in a context dependent fashion (26). We performed siRNA knockdown to identify the β-arrestin isoform(s) that regulate Type I IFN responses. Primary human MDM were transfected with a pool of four siRNA’s to mediate knockdown of β-arrestin 1, β-arrestin 2, or dual knockdown of both β-arrestins. siRNA transfection was not toxic to the MDM (Figure 5C) and resulted in a transfection efficiency of 98% that lasted up to 48 hours, as determined by co-transfection of a fluorescent siRNA, siRNA GLO Red (Figure 5D. The siRNA’s were specific to each β-arrestin, and knockdown of one β-arrestin did not affect expression of the other (Figure 5E-5G). Single and dual knockdown of β-arrestin 1 (Figure 5F) and β-arrestin 2 (Figure 5G) resulted in approximately a 50% reduction of each arrestin.
To determine which β-arrestin isoform induced dysregulated Type I IFN, MDM were treated with CCL2 24 hours after siRNA knockdown and IFN-α mRNA evaluated. CCL2 promoted a significant decrease in IFN-α mRNA for untransfected MDM, and also in cells transfected with a non-targeting control siRNA or fluorescent siGLO Red reporter, as compared to untransfected cells treated with vehicle (Figure 5H). Similarly, MDM transfected with siβ-arrestin 1 had a significant decrease in IFN-α mRNA upon CCL2 treatment, relative to vehicle treated cells. In contrast, MDM treated with siβ-arrestin 2 and dual siβ-arrestin 1 and 2 were refractory to CCL2 inhibition of IFN-α (Figure 5H), identifying β-arrestin 2 as the specific arrestin isoform recruited to CCR2 that mediates IFN-α inhibition.
β-Arrestin 2 Internalizes IFNAR1 and Inhibits IFN Signaling
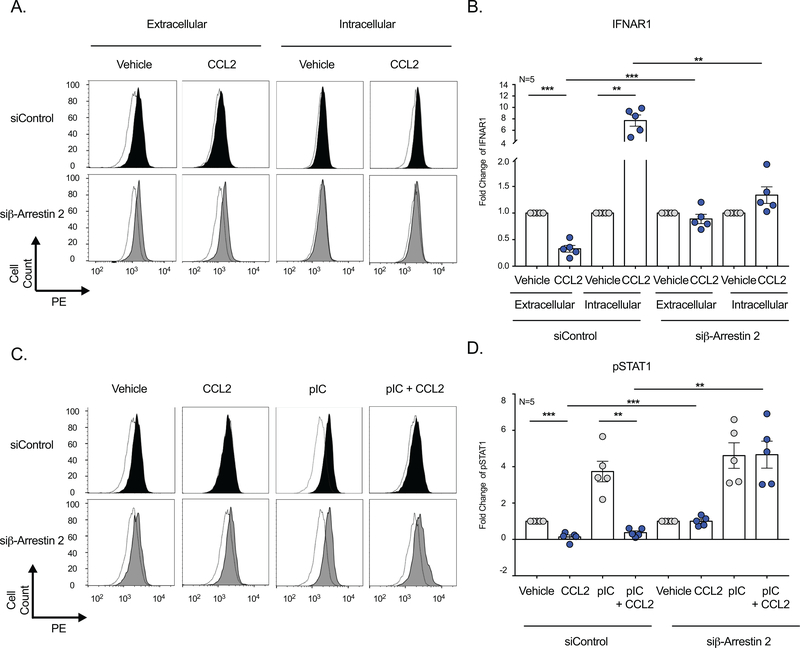
While we determined that CCL2-CCR2 mediated β-arrestin 2 activation promoted non-canonical IFN signaling, the mechanisms by which this occurred were not completely understood. We examined IFNAR1, the receptor for IFN-α/β, whose function and expression regulates Type I IFN. The effect of β-arrestin 2 on IFNAR1 localization was determined following transfection with a control siRNA (siControl) or a pool of four siRNA’s with specificity for β-arrestin 2 by flow cytometry.
We first evaluated IFNAR1 on the cell surface, where IFN-α/β bind to induce downstream signaling. For these studies, we used an antibody with specificity for the extracellular domain of the receptor. IFNAR1 was expressed on the surface of vehicle treated MDM (Figure 6A), which was significantly decreased following CCL2 treatment in siControl (0.3 ± 0.06 fold) (Figure 6A-6B) and siβ-arrestin 1 MDM (data not shown). However, this did not occur in siβ-arrestin 2 MDM, as CCL2 did not alter extracellular IFNAR1 in these cells. To more completely characterize the effects of β-arrestin 2 on IFNAR1, we permeabilized the MDM to examine intracellular levels of the receptor. Importantly, we utilized the same antibody with specificity for the extracellular domain of IFNAR1, whose intracellular presence would be indicative of receptor internalization.
Figure 6: β-Arrestin 2 Promotes IFNAR1 Internalization and Inhibits STAT1 Activation.
MDM from five independent donors were transfected with a non-targeting siRNA control or siRNA’s with specificity for β-arrestin 2. (A-B) MDM were treated with vehicle or CCL2 and flow cytometry performed to examine extracellular and intracellular IFNAR1. (A) Representative histograms are shown for IFNAR1 (filled) and the isotype control antibody (open) for MDM transfected with siControl or siβ-arrestin 2. (B) IFNAR1 MFI was determined, vehicle treated cells were set to 1, and the pooled average fold change in IFNAR1 was determined. (C-D) MDM were treated with pIC in the presence or absence of CCL2 and flow cytometry performed to examine pSTAT1. (C) Representative histograms are shown for pSTAT1 (filled) and the isotype control antibody (open) for MDM transfected with siControl or siβ-arrestin 2. (D) pSTAT1 MFI was determined, vehicle treated cells were set to 1, and the pooled average fold change in pSTAT1 was determined.
The extracellular domain of IFNAR1 was not detected intracellularly in vehicle treated cells, suggesting that the receptor was primarily present on the cell surface during basal conditions (Figure 6A). There was a significant increase of the IFNAR1 extracellular domain within the MDM upon CCL2 treatment in siControl cells (7.7 ± 1.0 fold), suggesting that IFNAR1 internalization occurred (Figure 6A-6B). Similarly, siβ-arrestin 1 MDM also had a significant increase in intracellular IFNAR1 upon CCL2 treatment (data not shown), which did not occur in MDM transfected with siβ-arrestin 2 (Figure 6A-6B). These findings demonstrate that β-arrestin 2 specifically mediated, and was required for, IFNAR1 internalization.
To determine the consequence of the loss of extracellular IFNAR1, we evaluated Tyr701 STAT1 phosphorylation (pSTAT1) in MDM, as it is downstream of the receptor and integral to IFN signal transduction. CCL2 significantly decreased pSTAT1 in vehicle treated MDM following siControl and siβ-arrestin 1 (not shown) transfection, but had no effect in cells transfected with siβ-arrestin 2 (Figure 6C-6D). Similarly, CCL2 significantly decreased pIC-induced pSTAT1 in siControl and siβ-arrestin 1 cells, but not MDM that received siβ-arrestin 2. In fact, β-arrestin 2 knockdown restored pSTAT1 in CCL2 treated cells to levels similar to that present during vehicle treatment (Figure 6C-6D). The effects of CCL2 on pIC-induced STAT1 phosphorylation was confirmed by Western Blot (Supplemental Figure 4A). Of note, the basal levels of STAT1 varied widely among MDM donors (Supplemental Figure 4B). These data demonstrate, for the first time, that β-arrestin 2 mediates IFNAR1 internalization, which inhibits downstream STAT1 activation and promotes non-canonical Type I IFN responses in macrophages.
β-Arrestin 2 Inhibition of Type I IFN Signaling Is Not Restricted to Macrophages
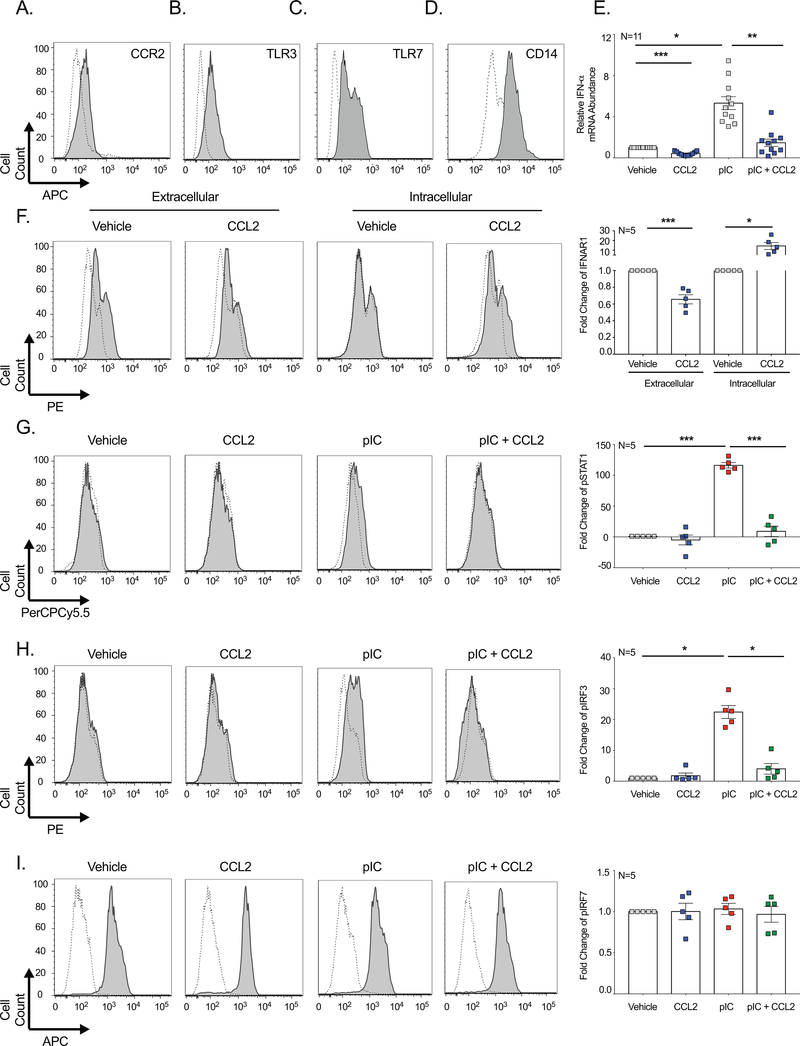
Due to the ubiquitous nature of β-arrestin, we were interested in determining whether its inhibitory effects on IFN signaling were applicable to other cells types. We chose to evaluate hepatocytes because they are the primary targets of Hepatitis C and other hepatic viruses. We used the well characterized Huh-7 hepatocyte cell line that is known to respond to TLR agonists, including pIC (27, 28).
CCR2 was present on Huh-7 cells at levels similar to that of macrophages (Figure 7A). Additionally, TLR3 (Figure 7B), TLR7 (Figure 7C), and CD14 (Figure 7D) were highly expressed in Huh-7 cells, indicating that they are capable of recognizing and responding to viral and bacterial pathogens. As Huh-7 cells expressed both CCR2 and TLR3, they represented an ideal model to evaluate the impact of CCL2-induced β-arrestin activation on IFN-α. Similar to our findings in MDM, CCL2 significantly decreased endogenous IFN-α mRNA in vehicle treated Huh-7 cells (Figure 7E). Furthermore, CCL2 significantly decreased pIC-induced IFN-α and restored it to levels similar to that present in unstimulated cells (Figure 7E). In agreement with our MDM studies, there was no effect of CCL2 on IFN-β, TRAIL, or MxA mRNA in Huh-7 cells (data not shown). Additionally, CCL7, CCL8, and CCL5 had no effect on IFN-α mRNA (data not shown), suggesting that CCL2/CCR2-mediated β-arrestin activation was required to inhibit Type I IFN.
Figure 7: β-Arrestin 2-Induced IFN Regulation is Not Restricted to Macrophages.
Huh-7 cells were stained with fluorochrome coupled antibodies to evaluate (A) CCR2, (B) TLR3, (C) TLR7, and (D) CD14. Histograms representative of five experiments are shown. (E) In 11 experiments, Huh-7 cells were treated with pIC in the presence or absence of CCL2 and qRT-PCR performed to quantitate IFN-α. (F) In five experiments, Huh-7 cells were treated with CCL2 or vehicle and flow cytometry performed to determine extracellular and intracellular IFNAR1. (G-I) In five experiments, Huh-7 cells were treated with CCL2, pIC, combined CCL2 and pIC, or vehicle control. Intracellular flow cytometry was performed to determine (G), pSTAT1, (H) pIRF3, or (I) pIRF7. Representative histograms (left) show signal for the protein of interest (filled histogram) and the isotype control antibody (dashed, open). The MFI was determined, vehicle treated cells were set to 1, and the pooled average fold change for each protein of interest was determined (right).
To determine whether the same non-canonical Type I IFN pathway occurred in hepatocytes, we evaluated the extracellular and intracellular presence of IFNAR1. IFNAR1 was highly expressed on the cell surface in vehicle treated Huh-7 cells, which was significantly decreased with CCL2 (Figure 7F). In contrast, intracellular IFNAR1 was undetectable during basal conditions, and was significantly increased by CCL2 (Figure 7F). Upon determining that IFNAR1 internalization occurred, we also evaluated the effects of CCL2 on STAT1 phosphorylation. Huh-7 cells had almost no detectable pSTAT1 during basal conditions, but were highly responsive to pIC, which significantly induced pSTAT1 by 116.0 ± 4.5 fold, relative to vehicle treated cells (Figure 7G). As expected, CCL2 significantly decreased pSTAT1 in pIC treated cells (9.0 ± 8.5 fold). These data indicate that the CCL2-induced, β-arrestin-mediated internalization of IFNAR1, and subsequent loss of downstream STAT1 signaling, is not restricted to macrophages.
Huh-7 cells readily divide and because we were not limited by cell number, we evaluated the effects of CCL2 on additional components of the Type I IFN pathway. Similar to that which occurred for STAT1, CCL2 significantly decreased pIC-induced IRF3 Ser396 phosphorylation (Figure 7H). Interestingly, the entire Type I IFN pathway was not altered, as CCL2 had no effect on IRF7 S477/S479 phosphorylation in vehicle or pIC-treated treated cells (Figure 7I). This suggests specificity to the β-arrestin 2-induced mechanism by which CCL2 decreased IFN-α, by selectively inhibiting IFNAR1, STAT1, and IRF3, but not IRF7.
DISCUSSION
In this study, we identified a novel pathway by which CCL2-CCR2 signaling induced β-arrestin 2 activation, which promoted IFNAR1 internalization. This is, to our knowledge, the first evidence that β-arrestin 2 mediates IFNAR1 internalization. We confirmed our findings with the use of CCR2/CCR5 ligands that do not promote β-arrestin activation and by siRNA knockdown. Due to the loss of receptor from the cell surface, macrophages were unable to propagate canonical IFN signaling, and STAT1 and IRF3 activation were inhibited. As a result, IFN-α mRNA, protein, and downstream IFN stimulated cytokines were not produced. There was selectivity to β-arrestin 2-mediated regulation of the Type I IFN pathway, as IFN-β, TRAIL, MxA, and IRF7 were not altered, demonstrating that an arm of IFN signaling remained functionally intact. Interestingly, these effects on Type I IFN occurred in unstimulated cells, and also upon HIV infection and TLR3 activation, demonstrating that β-arrestin 2 abrogated IFN responses even in the absence of viral stimuli. Together, our findings identify β-arrestin 2 as a highly specific regulator of Type I IFN during both physiologic and pathogenic states.
We performed the majority of these studies in primary human MDM, and identified significant interdonor variability within the Type I IFN and CCR2 systems. Donor responses varied and contributed to differing endogenous levels of Type I IFN mRNA, concentrations of CCL2 to which MDM were responsive, cell surface CCR2 expression, basal levels of STAT1 and pSTAT1, intracellular calcium concentrations, and even the duration of time required to elicit a decrease in IFN-α. These findings demonstrate that primary human macrophages reflect donor-specific genetic differences in the Type I IFN pathway, and also provide insight into the multiple levels of regulation that exist to elicit such diverse responses. Interdonor differences reflect important distinctions in humans that would not have been appreciated if another model had been chosen to perform the studies. A more complete understanding of the genetic, epigenetic, environmental, and other mechanisms that underlie the variability among humans is critical to develop better animal models of disease and more effective therapeutics.
We determined that β-arrestin 2 hindered Type I IFN responses in both macrophages and Huh-7 cells, suggesting that this CCL2-CCR2-induced, β-arrestin 2-mediated pathway represents a conserved regulatory mechanism of the innate immune system that is not restricted to one cell type. While best characterized on myeloid cells, CCR2 is also present on many cells of the immune system, including T cells, neutrophils, and dendritic cells. CCR2 is also highly abundant in non-immune cells, such as neurons. This suggests that any CCR2-expressing cell has the potential to respond to CCL2 by selectively inhibiting IFN-α. However, this altered Type I IFN pathway may not be exclusively induced by the CCL2-CCR2 axis. β-arrestins are recruited to all GPCRs, the largest and most diverse group of cell surface receptors (29). Furthermore, β-arrestins are expressed in every cell type. This suggests that in the appropriate context, β-arrestin has the potential to hinder Type I IFN responses in multiple cell types following activation by a number of receptors. GPCRs represent the most pharmacologically relevant drug targets (30), and β-arrestin biased drugs are of major interest. Therefore, β-arrestin-biased therapeutics may have unexpected implications for cancer and infectious, chronic inflammatory, autoimmune, and neurologic disorders through their alteration of Type I IFN.
Our work provides important insights into the functional differences between the two β-arrestin isoforms. β-arrestin 1 and β-arrestin 2 have 78% sequence homology and were initially thought to be functionally redundant (26). However, the arrestin isoforms have functionally distinct roles that have implications for many immunologic disease processes (31). While β-arrestin 1 and β-arrestin 2 primarily localize to the cytoplasm, they may also reside within distinct cellular compartments. β-arrestin 1 accumulates within the nucleus because it lacks the nuclear export signal present within β-arrestin 2 (32, 33). This difference in subcellular localization provides β-arrestin 1 the unique ability to negatively regulate transcriptional activity. In this capacity, β-arrestin 1 was shown to directly inhibit nuclear STAT1 phosphorylation (34). However, the role of β-arrestin 1 in STAT1 activation is not fully understood as others found opposing results (35). Our current work is in agreement with the latter, as we found that β-arrestin 1 knockdown did not restore IFN-α mRNA, suggesting that it did not contribute to STAT1 dephosphorylation. This reflects a need for a more complete understanding of the underlying functional differences between the β-arrestin isoforms, particularly with respect to innate immune responses. Our work addresses this gap, in part, by identifying an isoform specific role of β-arrestin 2 in IFNAR1 internalization, STAT1 and IRF3 phosphorylation, and inhibition of IFN-α and IFN-induced cytokines.
We identified β-arrestin 2 as a critical modulator of IFNAR1, a non-GPCR it had not been previously identified as associating with. This finding expands substantially our understanding of immunoregulatory roles of β-arrestins. While best known for associating with GPCRs, there is a limited understanding of β-arrestin-mediated internalization of other receptors, including PPARγ and TGF-β receptor (36, 37). Our findings expand this knowledge to include β-arrestin 2 as a regulator of IFNAR1 internalization. IFNAR1 is a highly dynamic receptor that readily undergoes endocytosis and degradation upon ligand binding, which drastically inhibits downstream signaling (38, 39). While the roles of IFN-α/β in promoting IFNAR1 recycling are understood (40–42), substantially less is known about IFNAR1 internalization following stimulation by ligands that do not directly interact with the receptor. There is no evidence that CCL2 directly interacts with IFNAR1, and our studies demonstrated that IFNAR1 endocytosis occurred as a result of CCL2/CCR2-mediated β-arrestin 2 activation. Interestingly, IFNAR1 signaling is required for CCL2 production and leukocyte migration (43). Additionally, CCL2 is directly induced by IFN-α and is not produced in the presence of IFN neutralizing antibodies (44, 45). This suggests a complex interplay between IFNAR1, Type I IFN, and the CCL2-CCR2 axis, wherein they reciprocally regulate each other’s expression and downstream functions. Our findings provide key insights into novel regulatory mechanisms of IFNAR1 by β-arrestin 2 and add to our understanding of IFNAR1/CCL2 reciprocal regulation.
Our studies focused specifically on IFN-α, which has not been characterized as comprehensively as IFN-β and IFN-γ. Despite having a similar potency to these IFNs and its role as an important component of host antiviral defenses, IFN-α has been largely understudied because of its many isoforms that make quantification and mechanistic studies difficult. Because of donor specific variability in predominant IFN-α subtypes, we utilized pan-IFN-α primers and antibodies to ensure we could detect changes in IFN-α mRNA and protein for all donors. Upon doing so, we determined that IFN-α, but not IFN-β, was specifically decreased following β-arrestin 2 activation. This loss of coordinated Type I IFN signaling indicates a tightly regulated means of modulating Type I IFN responses, which may represent a protective host mechanism to maintain some components of IFN signaling while abolishing others.
We determined that this lack of canonical Type I IFN signaling occurred as a result of differential IRF3 and IRF7 activation. While IRF3 and IRF7 are integral components of the Type I IFN response, they are nonredundant proteins and have unique functions. Upon recognition of appropriate stimuli, IRF3 becomes activated and promotes transcription of IFN-β and early IFN-stimulated genes (46). Soon after this initial response, IRF7 becomes the primary IFN transcriptional regulator as IRF3 is readily degraded. IRF7 then plays a substantial role in IFN signal amplification by promoting autocrine signaling through IFNAR1 that allows for the rapid and continued production of both IFN-α and IFN-β (47–49). IRF7 has been coined the “master regulator” of IFN because it also maintains production of low levels of Type I IFN even in the absence of functional IRF3 (50, 51). We found that CCL2 prevented IRF3 activation upon stimulation by the TLR3 agonist pIC. In contrast, CCL2 had no effect on IRF7 activation. These findings suggest that CCL2-induced β-arrestin 2 selectively hinders IRF3 activation, but allows for sustained IRF7 activation to maintain a limited repertoire of IFN responses, including the production of IFN-β, TRAIL, and MxA. β-arrestin 2 may maintain diminished IFN responses to allow cells to respond to pathogenic states in a limited capacity without having a sustained inflammatory response mediated by IFN-α and IFN-induced cytokines IL-6, TNF-α, and CXCL10.
It is important to acknowledge that the consequence of decreasing IFN-α has not been addressed fully in our studies, but is likely to be dependent on the anatomic site, disease state, and infection versus chronic inflammatory condition. Inhibiting IFN-α may have dire consequences, as it is critical for inducing downstream signaling that elicits robust antiviral innate immune responses. However, there is a duality to this paradigm, wherein IFN-α elicits strong inflammatory responses that are damaging if allowed to persist chronically. In such instances, inhibition of IFN-α is beneficial (3, 52, 53). This is highlighted by Type I interferonopathies, a spectrum of human genetic disorders that arise due to mutations in the IFN pathway that result in prolonged IFN-α production (54, 55). The most common of these disorders is Aicardi–Goutières syndrome, which predominantly affects the brain (56), suggesting that the central nervous system is particularly sensitive to sustained IFN-α responses (57). Thus, we propose that the CCL2/CCR2 induced, β-arrestin 2-dependent mechanisms identified in this study present a novel immunoregulatory feedback mechanism to fine-tune the balance of beneficial and deleterious Type I IFN responses that may occur in multiple cell types and have broad implications for states of both health and disease.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge all of the members of the Retrovirus Laboratory for their valuable insights and helpful discussions. We thank Mr. Christopher Thoburn of the Flow Cytometry and Immune Monitoring Core for his help with Luminex multiplexed cytokine analysis and Dr. Feilim Mac Gabhann for his assistance with statistical analyses.
Footnotes
This work was supported by the National Institutes of Health research grants K99/R00 DA044838 (DWW), R01 AI127142 (JEC), R01 NS076357 (JEC), R01 NS077869 (JEC), and the Johns Hopkins University Provost’s Postdoctoral Fellowship (DWW). LCA was supported by the Hopkins Postbaccalaureate Research Education Program (NIH R25 GM109441), EJ was supported by the Johns Hopkins Internship in Brain Sciences Program (NIH R25 MH100711), and DWW received pilot funds from the Translational Research in NeuroAIDS and Mental Health Center (NIH R25 MH080661). This research has been facilitated by the infrastructure and resources provided by the Johns Hopkins University Center for AIDS Research, a NIH funded program (P30 AI094189). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.De Weerd NA, and Nguyen T 2012. The interferons and their receptors—distribution and regulation. Immunology and cell biology 90: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pestka S, Krause CD, and Walter MR 2004. Interferons, interferon-like cytokines, and their receptors. Immunological Reviews 202: 8–32. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G 2010. Type I interferon: friend or foe? Journal of Experimental Medicine 207: 2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arimoto K-I, Miyauchi S, Stoner SA, Fan J-B, and Zhang D-E 2018. Negative regulation of type I IFN signaling. Journal of Leukocyte Biology 103: 1099–1116. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, He C, Wang L, and Ge B 2017. Post-translational regulation of antiviral innate signaling. Eur. J. Immunol. 47: 1414–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaritsky LA, Dery A, Leong WY, Gama L, and Clements JE 2013. Tissue-specific interferon alpha subtype response to SIV infection in brain, spleen, and lung. Journal of Interferon & Cytokine Research 33: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alammar L, Gama L, and Clements JE 2011. Simian immunodeficiency virus infection in the brain and lung leads to differential type I IFN signaling during acute infection. The Journal of Immunology 186: 4008–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaritsky LA, Gama L, and Clements JE 2012. Canonical type I IFN signaling in simian immunodeficiency virus-infected macrophages is disrupted by astrocyte-secreted CCL2. The Journal of Immunology 188: 3876–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Violin JD, and Lefkowitz RJ 2007. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends in Pharmacological Sciences 28: 416–422. [DOI] [PubMed] [Google Scholar]
- 10.Sharma D, and Parameswaran N 2015. Multifaceted role of |[beta]|-arrestins in inflammation and disease. Genes and Immunity 16: 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang D, Xie T, Liang J, and Noble PW 2013. β-arrestins in the Immune System. Progress in molecular biology and translational science 118: 359–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blight KJ, McKeating JA, and Rice CM 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. Journal of Virology 76: 13001–13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams DW, Tesfa L, and Berman JW 2015. Novel flow cytometric analysis of the blood–brain barrier. Cytometry Part A 87: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troegeler A, Lastrucci C, Duval C, Tanne A, Cougoule C, Maridonneau-Parini I, Neyrolles O, and Lugo-Villarino G 2014. An efficient siRNA-mediated gene silencing in primary human monocytes, dendritic cells and macrophages. Immunology and Cell Biology 92: 699–708. [DOI] [PubMed] [Google Scholar]
- 15.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences 110: 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mestas J, and Hughes CCW 2004. Of mice and not men: differences between mouse and human immunology. The Journal of Immunology 172: 2731–2738. [DOI] [PubMed] [Google Scholar]
- 17.Schneemann M, and Schoeden G 2007. Macrophage biology and immunology: man is not a mouse. Journal of Leukocyte Biology 81: 579–580. [DOI] [PubMed] [Google Scholar]
- 18.Berchiche YA, Gravel S, Pelletier M-E, St-Onge G, and Heveker N 2011. Different effects of the different natural CC chemokine receptor 2b ligands on beta-arrestin recruitment, Gαi signaling, and receptor internalization. Molecular Pharmacology 79: 488–498. [DOI] [PubMed] [Google Scholar]
- 19.Kothandan G, Gadhe CG, and Cho SJ 2012. Structural insights from binding poses of CCR2 and CCR5 with clinically important antagonists: a combined in silico study. PloS one 7: e32864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton JA, Whitty GA, Kola I, and Hertzog PJ 1996. Endogenous IFN-alpha beta suppresses colony-stimulating factor (CSF)-1-stimulated macrophage DNA synthesis and mediates inhibitory effects of lipopolysaccharide and TNF-alpha. The Journal of Immunology 156: 2553–2557. [PubMed] [Google Scholar]
- 21.Zhou P, Tachedjian M, Wynne JW, Boyd V, Cui J, Smith I, Cowled C, Ng JHJ, Mok L, Michalski WP, Mendenhall IH, Tachedjian G, Wang L, and Baker ML 2016. Contraction of the type I IFN locus and unusual constitutive expression of IFN-α in bats. In vol. 114 2696–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogel SN, and Fertsch D 1984. Endogenous interferon production by endotoxin-responsive macrophages provides an autostimulatory differentiation signal. Infection and Immunity 45: 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gough DJ, Messina NL, Clarke C, Johnstone RW, and Levy D 2012. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 36: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpe S, Cameroni E, Moepps B, Thelen S, Apuzzo T, and Thelen M 2012. CCR2 Acts as Scavenger for CCL2 during Monocyte Chemotaxis. PloS one 7: e37208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, and Berman JW 2013. Mechanisms of HIV Entry into the CNS: Increased Sensitivity of HIV Infected CD14+CD16+ Monocytes to CCL2 and Key Roles of CCR2, JAM-A, and ALCAM in Diapedesis. PloS one 8: e69270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava A, Gupta B, Gupta C, and Shukla AK 2015. Emerging functional divergence of β-arrestin isoforms in GPCR function. Trends in Endocrinology & Metabolism 26: 628–642. [DOI] [PubMed] [Google Scholar]
- 27.Li K, Chen Z, Kato N, Gale M, and Lemon SM 2005. Distinct poly (IC) and virus-activated signaling pathways leading to interferon-β production in hepatocytes. Journal of Biological Chemistry 280: 16739–16747. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Guo M, Wang X, Li J, Wang Y, Ye L, Dai M, Zhou L, Persidsky Y, and Ho W 2013. TLR3 activation efficiency by high or low molecular mass poly I:C. Innate Immunity 19: 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajagopal S, and Shenoy SK 2018. GPCR desensitization: Acute and prolonged phases. Cellular Signalling 41: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, and Gloriam DE 2017. Trends in GPCR drug discovery: new agents, targets and indications. Nature Reviews Drug Discovery 16: 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan H 2013. β-Arrestins 1 and 2 are critical regulators of inflammation. Innate Immunity 20: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith JS, and Rajagopal S 2016. The β-arrestins: multifunctional regulators of G protein-coupled receptors. Journal of Biological Chemistry 291: 8969–8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeppner CZ, Cheng N, and Richard DY 2012. Identification of a nuclear localization sequence in β-arrestin-1 and its functional implications. Journal of Biological Chemistry 287: 8932–8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mo W, Zhang L, Yang G, Zhai J, Hu Z, Chen Y, Chen X, Hui L, Huang R, and Hu G 2008. Nuclear β-Arrestin1 Functions as a Scaffold for the Dephosphorylation of STAT1 and Moderates the Antiviral Activity of IFN-γ. Molecular Cell 31: 695–707. [DOI] [PubMed] [Google Scholar]
- 35.Pelzel C, Begitt A, Wenta N, and Vinkemeier U 2013. Evidence against a Role for β-Arrestin1 in STAT1 Dephosphorylation and the Inhibition of Interferon-γ Signaling. Molecular Cell 50: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang L, Hu W, Xin S, Zhao J, and Pei G 2011. β-Arrestin-1 Protein Represses Adipogenesis and Inflammatory Responses through Its Interaction with Peroxisome Proliferator-activated Receptor-γ (PPARγ). Journal of Biological Chemistry 286: 28403–28413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Kirkbride KC, How T, Nelson CD, Mo J, Frederick JP, Wang X-F, Lefkowitz RJ, and Blobe GC 2003. ß-Arrestin 2 mediates endocytosis of type III TGF-ß receptor and down-regulation of its signaling. Science 301: 1394–1397. [DOI] [PubMed] [Google Scholar]
- 38.Marijanovic Z, Ragimbeau J, Kumar K, Fuchs SY, and Pellegrini S 2006. TYK2 activity promotes ligand-induced IFNAR1 proteolysis. Biochemical Journal 397: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marijanovic Z, Ragimbeau J, Van Der Heyden J, Uze G, and Pellegrini S 2007. Comparable potency of IFNα2 and IFNβ on immediate JAK/STAT activation but differential down-regulation of IFNAR2. Biochemical Journal 407: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng H, Qian J, Varghese B, Baker DP, and Fuchs S 2011. Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2. Molecular and Cellular Biology 31: 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carbone CJ, Zheng H, and Fuchs S 2014. Endocytosis of the IFNAR1 chain of Type 1 interferon receptor is regulated by diverse E2 ubiquitin conjugation enzymes. Uspehi Molekulârnoj Onkologii 1: 61–73. [Google Scholar]
- 42.Kumar K, Varghese B, Banerjee A, Baker DP, Constantinescu SN, Pellegrini S, and Fuchs S 2008. Basal ubiquitin-independent internalization of interferon α receptor is prevented by Tyk2-mediated masking of a linear endocytic motif. Journal of Biological Chemistry 283: 18566–18572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann MH, Torres-Domínguez LE, Price PJR, Brandmüller C, Kirschning CJ, and Sutter G 2016. CCL2 expression is mediated by type I IFN receptor and recruits NK and T cells to the lung during MVA infection. Journal of Leukocyte Biology 99: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 44.Veckman V, Miettinen M, Matikainen S, Lande R, Giacomini E, Coccia E, and Julkunen I 2003. Lactobacilli and streptococci induce inflammatory chemokine production in human macrophages that stimulates Th1 cell chemotaxis. Journal of Leukocyte Biology 74: 395–402. [DOI] [PubMed] [Google Scholar]
- 45.Tisoncik JR, Korth MJ, Simmons C, Farrar J, Martin TR, and Katze MG 2012. Into the eye of the cytokine storm. Microbiology and Molecular Biology Reviews 76: 16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juang YT, Lowther W, Kellum M, Au W-C, Lin R, Hiscott J, and Pitha PM 1998. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proceedings of the National Academies of Sciences 95: 9837–9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marie I, Durbin JE, and Levy DE 1998. Differential viral induction of distinct interferon‐α genes by positive feedback through interferon regulatory factor‐7. The EMBO Journal 17: 6660–6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato M, Hata N, Asagiri M, Nakaya T, and FEBS TT 1999. Positive feedback regulation of type I IFN genes by the IFN‐inducible transcription factor IRF‐7. Journal of Leukocyte Biology 441: 106–110. [DOI] [PubMed] [Google Scholar]
- 49.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, and Taniguchi T 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Trends in Endocrinology & Metabolism 13: 539–548. [DOI] [PubMed] [Google Scholar]
- 50.Savitsky D, Tamura T, Yanai H, and Taniguchi T 2010. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol. Immunother. 59: 489–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, and Taniguchi T 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature Genetics 434: 772–777. [DOI] [PubMed] [Google Scholar]
- 52.Gresser I, Maroger LM, Rivière Y, Guillon J-C, Tovey M, Woodrow D, Sloper J, and Moss J 1980. Interferon-Induced Disease in Mice and Rats. Annals of the New York Academy of Sciences 350: 12–20. [DOI] [PubMed] [Google Scholar]
- 53.Dejager L, Vandevyver S, Ballegeer M, Van Wonterghem E, An L-L, Riggs J, Kolbeck R, and Libert C 2014. Pharmacological inhibition of type I interferon signaling protects mice against lethal sepsis. Journal of Infectious Disease 209: 960–970. [DOI] [PubMed] [Google Scholar]
- 54.Crow YJ 2015. Type I interferonopathies: Mendelian type I interferon up-regulation. Current opinion in immunology 32: 7–12. [DOI] [PubMed] [Google Scholar]
- 55.Rice GI, del Toro Duany Y, Jenkinson EM, Forte G, Anderson BH, Ariaudo G, Bader-Meunier B, Baildam EM, Battini R, Beresford MW, Casarano M, Chouchane M, Cimaz R, Collins AE, Cordeiro NJV, Dale RC, Davidson JE, De Waele L, Desguerre I, Faivre L, Fazzi E, Isidor B, Lagae L, Latchman AR, Lebon P, Li C, Livingston JH, Lourenco CM, Mancardi MM, Masurel-Paulet A, McInnes IB, Menezes MP, Mignot CM, OSullivan J, Orcesi S, Picco PP, Riva E, Robinson RA, Rodriguez D, Salvatici E, Scott C, Szybowska M, Tolmi JL, Vanderver A, Vanhuille C, Vieira JP, Webb K, Whitney RN, Williams SG, Wolfe LA, Zuberti SM, and Hur S 2014. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nature Genetics 46: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Livingston JH, and Crow YJ 2016. Neurologic Phenotypes Associated with Mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1: Aicardi–Goutières Syndrome and Beyond. Neuropediatrics 47: 355–360. [DOI] [PubMed] [Google Scholar]
- 57.Blank T, and Prinz M 2017. Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 195: 1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.