Abstract
Objective:
Synovitis is a feature of knee osteoarthritis (OA) and meniscal tear and has been associated with articular cartilage damage. Our study examined the associations between baseline and changes in effusion-synovitis and changes in cartilage damage in a cohort with OA and meniscal tear.
Methods:
We analyzed data from the Meniscal Tear in Osteoarthritis Research (MeTeOR) trial of surgery vs. physical therapy for treatment of meniscal tear. We performed semiquantitative grading of effusion-synovitis and cartilage damage on magnetic resonance imaging (MRI), and dichotomized effusion-synovitis as none/small (‘minimal’) and medium/large (‘extensive’). We assessed the association between baseline and changes in effusion-synovitis on changes in cartilage damage size and depth over 18 months, using Poisson regression models. Analyses were adjusted for demographics, treatment, and baseline cartilage damage.
Results:
We analyzed 221 participants. Over 18 months, effusion-synovitis was persistently minimal in 45.3% and persistently extensive in 21.3%. The remaining 33.5% had minimal synovitis on one occasion and extensive on the other. In adjusted analyses, extensive effusion-synovitis at baseline was associated with a relative risk (RR) of 1.7 (95% CI 1.1, 2.6) for progression of cartilage damage depth. Compared to those with persistently minimal effusion-synovitis, persistently extensive effusion-synovitis had a statistically significant increased risk of progression of cartilage damage depth (RR 2.0 95% CI 1.1, 3.4).
Conclusions:
The presence of extensive effusion-synovitis is associated with subsequent progression of cartilage damage over 18 months. Persistence of extensive effusion-synovitis over time was associated with the greatest risk of concurrent cartilage damage progression.
Introduction
Osteoarthritis (OA) of the knee is a prevalent and disabling disorder traditionally ascribed to degeneration of articular cartilage. OA is increasingly recognized as a disorder of the entire joint, with a prominent role for inflammation, manifesting as synovitis. The presence of synovitis has been associated with pain, incidence and progression of OA, and meniscal tears.(1-8) An estimated 91% of patients with symptomatic knee OA will have meniscal tear on MRI.(9) As synovitis is a prominent feature of both OA and meniscal tear, the role of synovitis in patients with concurrent OA and meniscal tear warrants investigation.
Synovitis is posited to stem from intra-articular damage, including debris from cartilage destruction or meniscal tear.(5, 6) Even in patients without evidence of articular cartilage damage on magnetic resonance imaging (MRI) the presence of meniscal tear confers twice the odds of joint effusion when compared to patients without meniscal tear.(5) Synovitis further contributes to a catabolic intra-articular milieu and presence of inflammatory cytokines, leading to cartilage damage.(3, 10, 11) Thus intra-articular damage appears to provoke synovitis, which in turn can incite further damage. Previous research has demonstrated an association between synovitis and cartilage damage; however, these studies included patients both with and without OA and did not examine longitudinal changes (including persistence or intermittence) of synovitis on cartilage damage.(3, 12, 13) Further research is needed to determine whether persistent vs. intermittent synovitis over time is associated with progression of cartilage destruction, as this relationship may offer insights for managing patients with synovitis, meniscal tear, and OA.
MRI provides a non-invasive method to investigate longitudinally intra- articular structures of the knee including synovitis. In large studies, non-contrast enhanced MRI is often used in lieu of contrast enhanced studies to reduce cost and avoid adverse reactions; however, non-contrast enhanced images cannot distinguish effusion from synovitis.(14) Thus, effusion-synovitis, hyperintense fluid equivalent signal in the joint cavity comprised of effusion and synovial thickening, has been used in non-contrast enhanced studies as a proxy for synovitis.(14)
The aim of this study is to examine the association between changes in effusion-synovitis and cartilage damage over time in a population with both OA and meniscal tear. We hypothesize that the persistence of extensive effusion- synovitis over time will be associated with greater progression of cartilage damage.
Methods
Study Sample
We used data from participants enrolled in the Meniscal Tear in Osteoarthritis Research (MeTeOR) trial, a randomized clinical trial of arthroscopic partial meniscectomy (APM) versus physical therapy (PT) for the treatment of symptomatic meniscal tear in patients with knee OA.(15) Details of the trial design have been published previously.(16) Three hundred and fifty-one participants were recruited from 7 academic referral centers between June 2008 and August 2011. Men and women ≥ 45 years old with evidence of meniscal tear extending to the meniscal surface on knee MRI were enrolled. To be eligible, subjects had to have osteoarthritis changes on imaging studies including presence of an osteophyte or joint space narrowing on plain radiograph, or of fullthickness cartilage defect on at least one tibial or femoral surface on MRI. We used the MRI OA Knee Score (MOAKS) criteria to assess for cartilage damage on MRI, utilizing water-sensitive sequences (such as intermediate-weighted with and without fat-suppression) which are known to be more sensitive than gradient echo sequences for cartilage focal defects (14, 17). All participants had ongoing knee symptoms at study enrollment that had been present for at least 4 weeks, including pain and at least one of the following symptoms: clicking, catching, popping, giving way, pain with pivot or torque, pain that was episodic, and pain that was acute and localized to one joint line. Exclusion criteria included a chronically locked knee, inflammatory arthritis (i.e., rheumatoid, acute crystalline, spondyloarthritis), or prior surgery on the index knee. Patients with clinically symptomatic chondrocalcinosis were excluded, as this could provide an alternative source of pain and swelling. Those with radiographic Kellgren Lawrence (KL) grade 4 OA (greater than 50% loss of tibiofemoral joint space) were excluded; those with KL grade 0-3 disease were eligible. Patients with bilateral symptomatic meniscal tears were excluded, therefore, the participants each contributed 1 index knee to the study based on reported symptoms. Participants were randomized to APM with PT or to PT alone. Two-hundred and twenty-seven participants had paired MRIs at both baseline and 18-month follow- up available for central reading. Six participants lacked MRI scoring for effusion- synovitis or cartilage damage and were excluded; thus, our cohort consisted of 221 participants (Figure 1).
Figure 1:
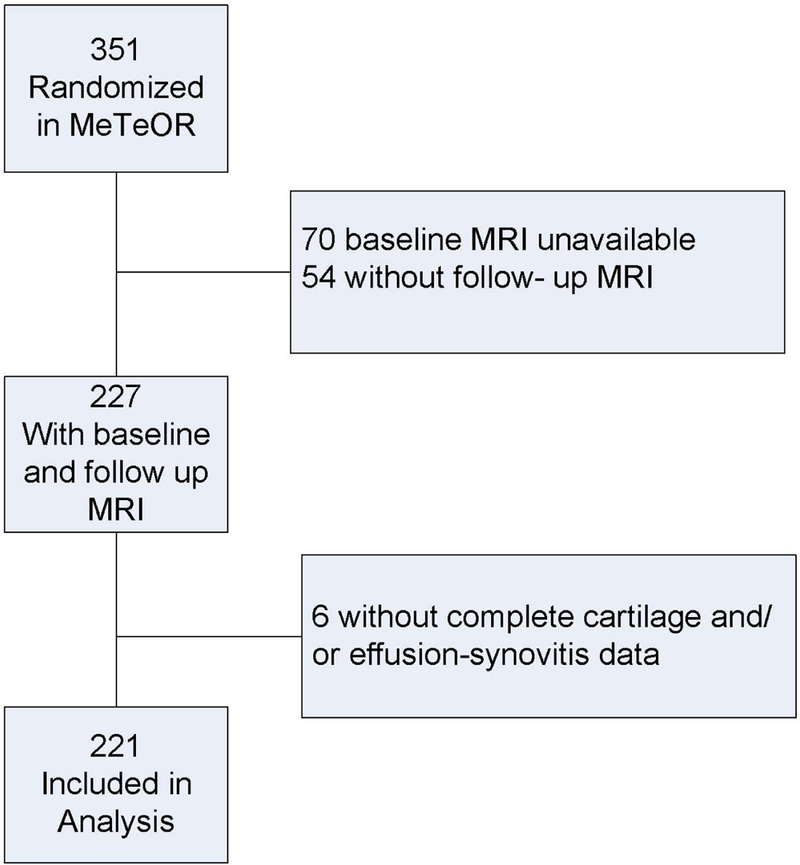
Flow diagram illustrating selection of subjects for this analysis
Our study was approved by the Partners HealthCare Human Research Committee. The MeTeOR clinical trial is registered at clinicaltrials.gov (NCT00597012).
Data Elements
Data on age, sex, and body mass index (BMI, kg/m2) were collected at baseline. The Knee injury and Osteoarthritis Outcome Scale (KOOS) Pain and Function scales were collected at baseline and 18-month follow-up to assess overall patient-reported knee pain in the past week. KOOS Pain and Function were transformed to a 0-100 scale with 0 being least amount of pain/best function and 100 greatest amount of pain/worst function.(18) KL radiographic grade was used as an indication of baseline OA severity. The KL grade was categorized as 0) normal; 1) questionable osteophyte; 2) definite osteophyte; 3) definite narrowing of joint space not exhibiting a bone-on-bone appearance with or without osteophyte.(19)
Imaging Features
Effusion-synovitis
Both baseline and 18 month follow-up MRIs were re-read by a single experienced musculoskeletal radiologist and scored according to the MOAKS criteria.(14) In a reliability study of 10 subjects read by another highly experienced reader, the inter-rater reliability intra-class correlation coefficient was 0.98 for MOAKS total OA score.
Effusion-synovitis was assessed by hyperintensity in the joint cavity fat- suppressed T2- or fat-suppressed proton density-weighted MRI.(14) We chose to use effusion-synovitis as our measure of synovitis rather than Hoffa-synovitis (hyperintensity in the Hoffa fat pad), since effusion-synovitis may be more sensitive than Hoffa-synovitis in evaluating synovial thickness on non-contrast MRI.(20) The inter-reader weighted kappa for MOAKs based effusion-synovitis is 0.72.(14) At both baseline and 18 months, effusion-synovitis was categorized as 0) none (physiologic amount); 1) small (fluid continuous in the retropatellar space); 2) medium (slight convexity of the suprapatellar space); or 3) large (evidence of capsular distention) (Figure 2). For all analyses, we dichotomized effusion-synovitis into 1) none to small effusion-synovitis (termed ‘minimal’); and 2) medium to large effusion-synovitis (termed ‘extensive’). Change in effusion- synovitis from baseline to 18-month follow-up was categorized into the following three groups; 1) persistently minimal (graded as none/small at baseline and at 18 months); 2) intermittent (none/small at one time point and medium/large at the other); 3) persistently extensive (medium/large at both baseline and at 18 months).
Figure 2.
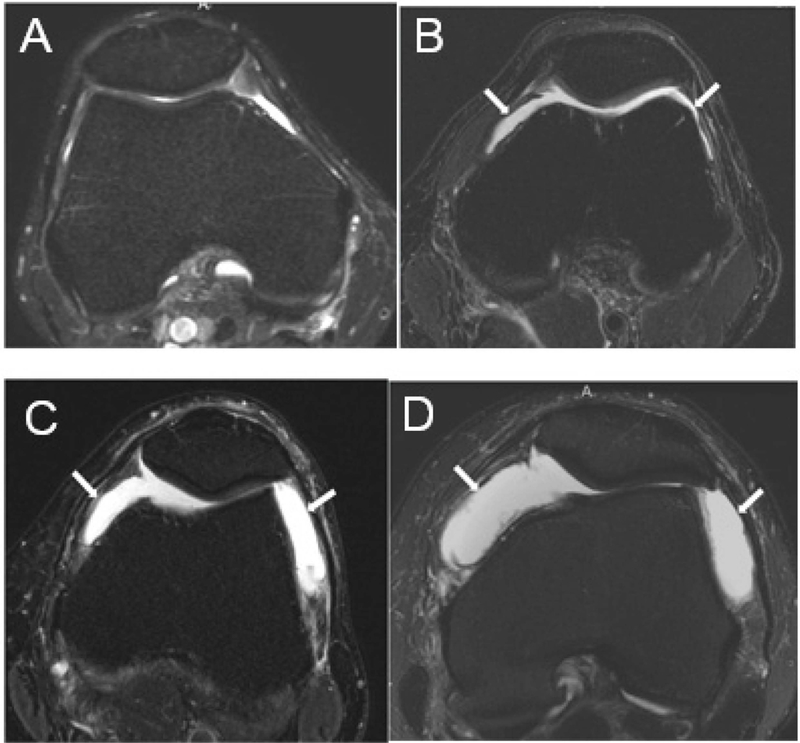
MRI Grading of Effusion-synovitis (A-D) Axial fat-suppressed proton-density weighted MR images through the suprapatellar pouch, showing different grades of effusion-synovitis. (A) Grade 0, with no joint effusion-synovitis. (B) Grade 1 effusion-synovitis is depicted with mild distension of the joint cavity by fluid-equivalent signal within the suprapatellar pouch (arrows). (C) Grade 2 effusion-synovitis with moderate distension of the joint cavity (arrows). (D) Grade 3 effusion-synovitis with marked capsular distention.
Cartilage Damage
We used MOAKS to assess articular cartilage damage (size and depth). MOAKS divides the articular region of the knee into 14 subregions to grade cartilage. Cartilage damage was quantified as size of any cartilage loss (% of surface area in subregion) and depth of cartilage damage (% of loss that is full thickness in subregion). For each subregion, both cartilage damage size and depth were categorized as 0) none; 1) <10%; 2) 10-75%; and 3) >75%. At baseline we used the maximum cartilage damage size and depth across the 14 regions for analysis. Due to small numbers populating some categories and based on the baseline distributions of these variables, the MOAKs baseline cartilage damage size was dichotomized to ≤75% and >75% while baseline cartilage damage depth was dichotomized to <10% and ≥10%. The outcome of interest, progression of cartilage damage, was analyzed in two ways. First, we assessed number of additional subregions affected, defined as the number of subregions with MOAKS grade 0 damage at baseline and grade ≥1 at 18 month follow up. Second, we analyzed the number of subregions with worsening, defined as the number of subregions with an increase of at least one MOAKS grade at 18 month follow up. Both cartilage damage size and depth were evaluated in this manner, as has been done previously.(4, 21) Number of additional subregions affected and number of subregions with worsening damage were dichotomized as: those with no additional subregion affected vs. any additional subregions affected; and, those without any subregions with worsening vs. with any worsening subregion (Figure 3).
Figure 3:
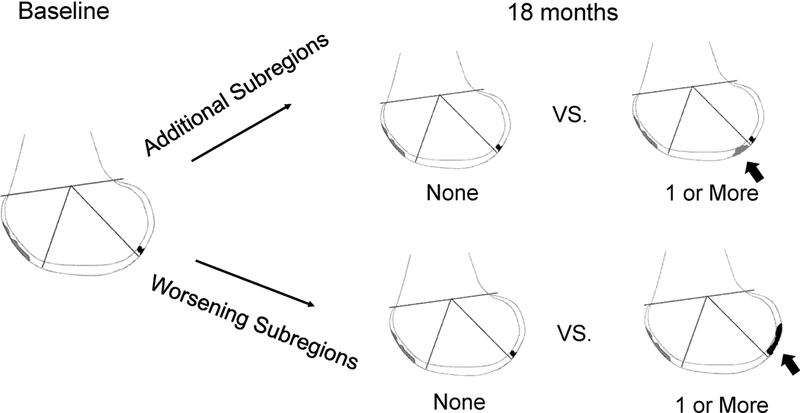
Diagram of outcome, change in cartilage damage over time Adapted from Hunter et al, Osteoarthritis and Cartilage 2011.(14) Reprinted with permission
Statistical Analysis
Baseline characteristics were analyzed using means and percentages. We used a modified Poisson regression with robust error variance to estimate the relative risk of baseline effusion-synovitis and binary change in cartilage damage and the relationship of change in effusion-synovitis and binary change in cartilage damage over 18 months.(22) We chose this approach given the high prevalence of the outcome; when the rare event rate assumption is violated, odds ratios generated from logistic regression generally overestimate risk ratios. Because binomial regression may lead to problems with convergence, Poisson regression with modification to ensure accurate variance estimations has been used to calculate relative risk from binary data.(22, 23) All analyses were adjusted for age, sex, BMI, and baseline cartilage damage (depth or size). Given that the outcome, worsening cartilage damage, occurred frequently in this sample, we present our results as relative risks instead of odds ratios. APM has been associated with increased cartilage damage in this cohort, thus all analyses were also adjusted for treatment received (APM vs. no APM).(24) Over the 18- month follow-up period, 5 patients assigned to APM did not have surgery and were classified as ‘no APM.’ A total of 44 patients crossed over from the PT to the APM arm. Forty-three crossed over between baseline and 14 months and were considered ‘APM’ in this analysis. One patient crossed over from PT to APM at 30 months and was thus classified as ‘no APM’ in this analysis.
In a sensitivity analysis, we re-examined the association between both baseline and change in effusion-synovitis and change in cartilage damage over 18 months after stratifying by three treatment groups; those randomized to APM and receiving APM, those randomized to PT and receiving PT, those who crossed over (randomized to PT and received APM and those randomized to APM and not receiving APM).
In a second sensitivity analysis, we dichotomized effusion-synovitis to none vs. any (small, medium, or large). Change in effusion-synovitis over 18 months was defined as 1) never developed (graded as none at baseline and at 18 months); 2) intermittent (none at one time point and any at the other time point); 3) persistent (any at both baseline and at 18 months). We then reexamined the association between both baseline and change in effusion- synovitis and change in cartilage damage over 18 months after adjusting for age, sex, BMI, and baseline cartilage damage (depth or size).
In a third sensitivity analysis, we re-defined our change in effusion- synovitis categories, as participants with ‘resolution’ of effusion-synovitis over 18 months may differ from those who have persistent, or develop effusion-synovitis over 18 months. Change in effusion-synovitis was defined as 1) persistently minimal (none/small at both time points); 2) resolving (medium/large at baseline and none/small at 18 months); 3) extensive at 18 months (medium/large at baseline and at 18 months and those with none/small at baseline and medium/large at 18 months). We then re-examined the association between change in effusion-synovitis and change in cartilage damage over 18 months after adjusting for age, sex, BMI, and baseline cartilage damage (depth or size).
Results
Baseline Effusion-synovitis and Change in Cartilage Damage
The sample consisted of 221 participants. The mean age was 59 years, 58% were female, with mean BMI 30. In comparison to male participants, females had similar age and BMI, though had a higher average baseline KOOS Pain score of 50 points compared to 40 points for males. At baseline, 48% had extensive effusion-synovitis and 52% had minimal. Thirty-nine percent of participants had cartilage damage size >75%, and 40% had cartilage damage depth ≥10% (Table 1). There were no statistically significant associations between baseline effusion-synovitis and cartilage damage size: in the adjusted model, extensive effusion-synovitis was associated with a 1.4-fold increased risk of having additional subregions with damage (95% CI: 1.0, 2.0) and a 1.2-fold increased risk of cartilage worsening (95% CI: 0.8, 1.6) compared to those with minimal effusion-synovitis. There was a statistically significant association between baseline effusion-synovitis and cartilage damage depth: those with extensive effusion-synovitis had a 1.7 (95% CI 1.1, 2.6) times increased risk of additional subregions with damage and a 1.5 times increased risk (95% CI 1.0, 2.2) for subregions with worsening damage in comparison to those with minimal effusion-synovitis (Table 2).
Table 1:
Baseline Characteristics of cohort
| Overall n=221 |
Change in Effusion-Synovitis over 18 months |
|||
|---|---|---|---|---|
| Persistently minimal n=100 |
Intermittent n=74 |
Persistently extensive n=47 |
||
| Age | 59 (+/−7) | 59 (+/−7) | 58 (+/−8) | 58 (+/−8) |
| BMI kg/m2 | 30 (+/−6) | 28 (+/−5) | 30 (+/−6) | 31 (+/−7) |
| KOOS Pain | 46 (+/−16) | 44 (+/−16) | 44 (+/−15) | 54 (+/−15) |
| KOOS Function | 37 (+/−18) | 34 (+/−17) | 35 (+/−17) | 45 (+/−20) |
| Female, n (%) | 129 (58%) | 59 (59%) | 33 (45%) | 37 (79%) |
| Received APM, n (%) | 143 (65%) | 64 (64%) | 46 (62%) | 33 (70%) |
| KL grade 0, n (%) | 50 (23%) | 26 (26%) | 17 (23%) | 7 (15%) |
| KL grade 1, n (%) | 51 (23%) | 24 (24%) | 23 (31%) | 4 (9%) |
| KL grade 2, n (%) | 58 (26%) | 27 (27%) | 14 (19%) | 17 (36%) |
| KL grade 3, n (%) | 62 (28%) | 23 (23%) | 20 (27%) | 19 (40%) |
| Cartilage damage size ≤75%, n (%) | 135 (61%) | 65 (65%) | 46 (62%) | 24 (51%) |
| Cartilage damage size >75%, n (%) | 86 (39%) | 35 (35%) | 28 (38%) | 23 (49%) |
| Cartilage damage depth <10%, n (%) | 132 (60%) | 73 (73%) | 39 (53%) | 20 (43%) |
| Cartilage damage depth ≥ 10%, n (%) | 89 (40%) | 27 (27%) | 35 (47%) | 27 (57%) |
Mean (SD) unless otherwise noted. BMI; body mass index. KOOS; knee injury and osteoarthritis outcome score. APM; arthroscopic partial meniscectomy
Table 2:
Relative Risk of Baseline and Change in Effusion-Synovitis on Change in Cartilage Damage
| Cartilage Damage Size | Cartilage Damage Depth | |||
|---|---|---|---|---|
| Additional Subregions Affected |
Subregions with Worsening |
Additional Subregions Affected |
Subregions with Worsening |
|
| Baseline Effusion-Synovitis | ||||
| Minimal | ref | ref | ref | ref |
| Extensive | 1.4 (1.0, 2.0) | 1.2 (0.8, 1.6) | 1.7 (1.1, 2.6)* | 1.5 (1.0, 2.2)* |
| Change in Effusion-Synovitis | ||||
| Persistently minimal | ref | ref | ref | ref |
| Intermittent | 1.6 (1.0, 2.4)* | 1.3 (0.9, 1.8) | 1.6 (0.9, 2.6) | 1.5 (1.0, 2.3) |
| Persistently extensive | 1.6 (1.0, 2.6) | 1.2 (0.8, 1.9) | 2.0 (1.1, 3.4)* | 1.7 (1.0, 2.7)* |
p<0.05
Change in Effusion-synovitis and Change in Cartilage Damage
Over 18 months, effusion-synovitis was persistently minimal (none/small at baseline and 18 months) in 45% of participants, intermittent (none/small at one time point and medium/large at the other) in 33%, and persistently extensive (medium/large at baseline and 18 months) in 21%. Of the participants with intermittent effusion-synovitis, 78% had effusion-synovitis at baseline only.
Participants with persistently minimal effusion-synovitis were 59% female and had a mean BMI of 28. Those with intermittent effusion-synovitis were 45% female and had a mean BMI of 30. Participants with persistently extensive effusion-synovitis were 79% female and had a mean BMI of 31. The percent of participants receiving APM among the effusion-synovitis categories ranged from 62% to 70%, but these differences were not statistically significant (Table 1). Reduction in reported pain scores from baseline to 18 months was similar across the effusion-synovitis categories, with those having persistently minimal effusion- synovitis sustaining a reduction in KOOS Pain of 24 points, compared to 26 points in those with intermittent effusion-synovitis and 27 points in those with persistently extensive effusion-synovitis. KOOS Function improved by 21 points in persistently minimal, 23 points in intermittent, and 25 points in those with persistent effusion-synovitis. Over 18 months, 74% had worsening cartilage damage size, and 53% percent had additional subregions affected by cartilage damage size. Fifty-seven percent had worsening cartilage damage thickness, and 46% had additional subregions affected by cartilage damage thickness. In participants with KL grade 0-1 at baseline, 73% had worsening cartilage damage size, and 51% had additional regions affected by cartilage damage size compared to 75% and 55% in those with KL grade 2-3, respectively. Of those with KL grade 0-1, 50% had worsening cartilage damage thickness, and 42% had additional regions affected by cartilage damage thickness, in comparison to 64% and 49% in those with KL grade 2-3 at baseline.
In adjusted models, intermittent and persistent effusion-synovitis over 18 months was associated with concurrent increases cartilage damage for both size and depth (Table 2 and Figure 4). Those with intermittent (RR 1.6, 95% CI 1.0, 2.4) and persistently extensive effusion-synovitis (RR 1.6, 95% CI 1.0, 2.6) had an increased risk of additional subregions affected by cartilage damage size compared to subjects with persistently minimal effusion-synovitis, but there were no significant associations between either synovitis category and the number of subregions with worsening size. Those with intermittent (RR 1.6, 95% CI 0.9, 2.6) and persistently extensive (RR 2.0, 95% CI 1.1, 3.4) effusion-synovitis had an increased risk of having additional subregions affected by cartilage damage depth. Finally, intermittent synovitis was associated with a 1.5 times increased risk (95% CI: 1.0, 2.3) of having additional subregions with worsening in cartilage damage depth, and those with persistently extensive synovitis had a 1.7 times increased risk (95% CI: 1.0, 2.7), compared to those with persistently minimal effusion-synovitis.
Figure 4:
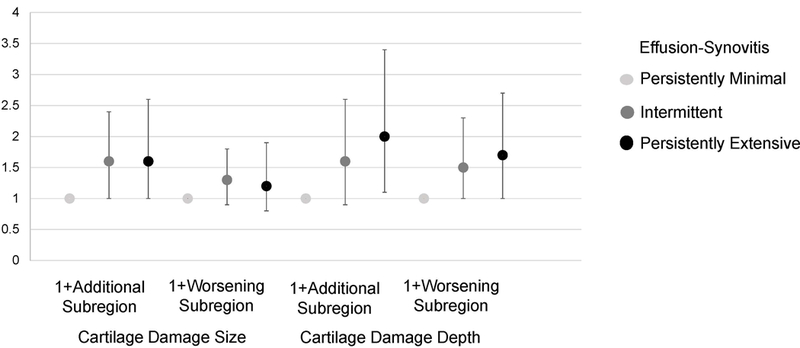
Relative Risk for association of change in effusion-synovitis on change in cartilage damage over 18 months. Adjusted for age, sex, BMI, and baseline cartilage damage and treatment
In the sensitivity analysis adjusting for the 3-level treatment group, the associations between baseline and change in effusion-synovitis on change in cartilage damage were similar to those observed in the main analysis in each treatment stratum (Appendix 1).
In the second sensitivity analysis in which effusion-synovitis was dichotomized at any vs. none, 20% of participants had no effusion-synovitis, and 80% had any effusion-synovitis at baseline. Over 18 months, 11% never developed effusion-synovitis, 19% had intermittent effusion synovitis, and 71% had persistent effusion-synovitis (Appendix 2). In the third sensitivity analysis assessing resolution of effusion-synovitis over 18 months, effusion-synovitis was persistently minimal in 45%, resolved in 26%, and extensive at 18 months in 29% (Appendix 3). In sensitivity analyses 2 and 3, the association of baseline and change in effusion-synovitis on change in cartilage damage were similar to the main analysis.
Discussion
Our study demonstrates a positive association between the presence and persistence of extensive effusion-synovitis and progression of cartilage damage depth over 18 months. Extensive effusion-synovitis at baseline conferred approximately 50-70% increased risk of worsening cartilage damage depth. Over 18 months, intermittent and persistently extensive effusion-synovitis were associated with greater cartilage damage in analyses that adjusted for baseline cartilage damage. Participants with persistently extensive effusion-synovitis demonstrated the greatest risk of subsequent cartilage damage, with a 70-100% increased risk for cartilage damage depth.
In our analyses, effusion-synovitis was associated with a non-statistically significant 20-30% increased risk of worsening cartilage damage size. The notable effect of effusion-synovitis on worsening cartilage damage depth but not on worsening cartilage damage size may be due to a threshold effect, as many participants had subregions with extensive cartilage damage size at baseline.
Several prior studies of patients both with and without OA have investigated the relationship between synovitis and cartilage damage.(3, 12, 13, 25) A study using the MOST cohort (Multicenter Osteoarthritis Study) of patients with or at risk for OA documented that baseline MRI-defined effusion or synovitis was associated with a 3-fold increased odds of more rapid cartilage loss.(3) A subsequent study using MOST and contrast-enhanced MRI demonstrated that patients with synovitis had twice the odds of cartilage damage in a crosssectional analysis.(25)
Wang et al. used MRI to assess the association of synovitis grade and cartilage damage both cross-sectionally and longitudinally in a cohort of older adults.(12) In these longitudinal analyses over 2.7 years, baseline effusion- synovitis was associated with a small risk of worsening cartilage defects (relative risk 1.1) after adjustment for baseline bone marrow lesions (BML) and cartilage defects.(12) These analyses support the findings in our study. Furthermore, the majority of participants that we characterized as ‘intermittent’ had effusion- synovitis at baseline only (78%). Thus, it appears that ‘resolving’ effusion- synovitis still confers a greater risk of future cartilage damage than no synovitis at all. Wang et al. also evaluated a quantitative measure of cartilage volume on MRI and demonstrated a significant negative association between baseline effusion- synovitis and cartilage volume longitudinally.(12) Interestingly, no significant association was documented between baseline effusion synovitis and change in measured cartilage volume after adjustment for baseline BML and cartilage volume.(12)
In the above study by Wang et al., around 60% of the study population had any cartilage defect on MRI. In contrast, our cohort represents an important and prevalent subset of patients with greater intra-articular pathology, as all had imaging evidence of cartilage damage and concurrent meniscal tear. We contribute to the prior knowledge base by demonstrating that the presence of effusion-synovitis over time (persistent or intermittent) appears to be associated with worsening cartilage damage.
A second study, also by Wang et al., used a quantitative measure of MRI- defined effusion-synovitis using the same cohort in which around 60% had cartilage defect on MRI. Change in effusion-synovitis over 2.7 years was calculated, with 29% increasing in size, 50% remaining stable, and 22% decreasing in size. Over 2.7 years, baseline effusion-synovitis area was associated with increased cartilage defects and decreasing cartilage volume.(13) The effect of change in effusion-synovitis on change in cartilage parameters was not assessed. It is of interest that in our study only 7% of participants developed extensive effusion-synovitis over the 18-month period, while 26% had a decrease in grade from extensive to minimal. We cannot rule out the possibility that treatment (APM or PT) affected the presence of effusion-synovitis over time.
Our study has several limitations. We used semi-quantitative grades for effusion-synovitis and cartilage and therefore have less granularity than a strictly quantitative score. However, our semi-quantitative results appear to be consistent with prior studies using quantitative measures.(13) We also recategorized the baseline effusion-synovitis into none vs. any and observed similar findings. MOAKs was developed to score knee OA on MRI, thus the clinical relevance or correlation of progressive cartilage damage on MRI is unknown. As our cohort had baseline OA and meniscal tear, we are unable to ascertain whether intra-articular damage (cartilage and meniscal damage) incited synovitis, though we demonstrate that these pathologies are highly associated. We demonstrate that changes in effusion-synovitis potentially contribute to cartilage damage over time.
In summary, our study demonstrates that, in patients with OA and meniscal tear, changes in synovitis, whether persistently extensive or intermittent, are associated with cartilage damage over time. As synovitis is a potentially modifiable intra-articular feature, further research is warranted to assess whether treatment of synovitis mitigates cartilage degradation.
Acknowledgments
Source of funding: P60 AR047782, R01 AR05557, T32 AR055885, K24 AR 057827
Dr. Guermazi is a shareholder of BICL and a consultant to Pfizer, GE, AstraZeneca, Sanofi, TissueGene, OrthoTrophix, and MerckSerono. Dr. Brophy is a speaker for Arthrex. Dr. Levy is a consultant for Arthrex, and Smith and Nephew, he receives research support from Arthrex, Stryker and Bioment, he receives royalties from Arthrex. He is the deputy editor or sports medicine for CORR. Dr. Losina is the deputy editor of JBJS and a consultant for Samumed.
Dr. MacFarlane has consulted for Flexion Therapeutics. Dr. Mandl is an associate editor at the Annals of Internal Medicine and receives royalties from Up-to-Date. Dr. Marx is the Deputy editor of Sport Medicine for JBJS and the Associate Editor of Evidence Based Orthopedics. He receives royalties for books from Springer and Demos Health Dr. Matava receives grants from Arthrex and Breg and is a consultant to Arthrex and Schwartz Biomedical as well as president of the Southern Orthopedic Association. Dr. Safran- Norton is a shareholder of Merck and Johnson and Johnson. Dr. Stuart is a consultant for Arthrex and receives research support from Stryker and USA Hockey foundation. Dr. J. Wright is an employee of Johnson & Johnson. Dr. R. Wright receives book royalties from Wolters Kluwer Lippincott & Williams.
Appendix 1:
Relative Risk of Baseline and Change in Effusion-Synovitis on Change in Cartilage Damage
| Cartilage Damage Size | Cartilage Damage Depth | ||||
|---|---|---|---|---|---|
| N (%) | Additional Subregions Affected |
Subregions with Worsening |
Additional Subregions Affected |
Subregions with Worsening |
|
| Arthroscopic Partial Meniscectomy (n=100) | |||||
| Baseline Effusion-Synovitis | |||||
| None/Small | 51 (51%) | ref | ref | ref | ref |
| Medium/Large | 49 (49%) | 1.2 (0.7, 2.1) | 1.0 (0.6, 1.6) | 1.5 (0.8, 2.7) | 1.4 (0.8, 2.4) |
| Change in Effusion-Synovitis | |||||
| Persistently minimal | 40 (40%) | ref | ref | ref | ref |
| Intermittent | 35 (35%) | 1.6 (0.8, 2.9) | 1.1 (0.7, 1.8) | 1.2 (0.6, 2.6) | 1.5 (0.8, 2.8) |
| Persistently extensive | 25 (25%) | 1.7 (0.8, 3.3) | 1.1 (0.6, 2.0) | 1.9 (0.9, 4.0) | 1.7 (0.9 3.5) |
| Physical Therapy (n=72) | |||||
| Baseline Effusion-Synovitis | |||||
| None/Small | 38 (53%) | ref | ref | ref | ref |
| Medium/Large | 34 (47%) | 2.1 (0.9, 5.0) | 1.6 (0.8, 3.2) | 2.6 (1.1, 6.2)* | 1.9 (0.9, 4.2) |
| Change in Effusion-Synovitis | |||||
| Persistently minimal | 35 (49%) | ref | ref | ref | ref |
| Intermittent | 26 (36%) | 1.9 (0.8, 4.6) | 1.9 (0.9, 3.9) | 2.6 (1.0, 6.7)* | 1.7 (0.7, 4.1) |
| Persistently extensive | 11 (15%) | 1.3 (0.4, 4.4) | 1.2 (0.4, 3.4) | 2.2 (0.6, 7.5) | 2.0 (0.7, 5.9) |
| Cross-over (n=49) | |||||
| Baseline Effusion-Synovitis | |||||
| None/Small | 27 (55%) | ref | ref | ref | ref |
| Medium/Large | 22 (45%) | 1.2 (0.5, 2.7) | 1.0 (0.5, 2.0) | 1.6 (0.6, 3.8) | 1.3 (0.6, 2.8) |
| Change in Effusion-Synovitis | |||||
| Persistently minimal | 25 (51%) | ref | ref | ref | ref |
| Intermittent | 13 (27%) | 1.2 (0.5, 3.2) | 0.9 (0.4, 2.0) | 1.2 (0.4, 3.7) | 1.3 (0.5, 3.2) |
| Persistently extensive | 11 (22%) | 1.4 (0.5, 4.2) | 1.2 (0.5, 2.7) | 1.9 (0.6, 5.5) | 1.2 (0.5, 3.1) |
p<0.05
Appendix 2:
Relative Risk of Baseline and Change in Effusion-Synovitis on Change in Cartilage Damage
| Cartilage Damage Size | Cartilage Damage Depth | |||
|---|---|---|---|---|
| Additional Subregions Affected |
Subregions with Worsening |
Additional Subregions Affected |
Subregions with Worsening |
|
| Baseline Effusion-Synovitis | ||||
| None | ref | ref | ref | ref |
| Any | 1.2 (0.7, 1.9) | 0.9 (0.6, 1.4) | 1.3 (0.8, 2.3) | 1.3 (0.8, 2.1) |
| Change in Effusion-Synovitis | ||||
| Never Developed | ref | ref | ref | ref |
| Intermittent | 2.4 (0.9, 5.8) | 1.0 (0.5 1.8) | 1.6 (0.6, 4.0) | 1.0 (0.5, 2.3) |
| Persistent | 2.2 (1.0, 5.1) | 1.0 (0.6, 1.7) | 1.9 (0.8, 4.5) | 1.4 (0.7, 2.7) |
Any Effusion-synovitis; comprised of small, medium and large effusion-synovitis
Appendix 3:
Relative Risk of Baseline and Change in Effusion-Synovitis on Change in Cartilage Damage
| Cartilage Damage Size | Cartilage Damage Depth | |||
|---|---|---|---|---|
| Additional Subregions Affected |
Subregions with Worsening |
Additional Subregions Affected |
Subregions with Worsening | |
| Change in Effusion-Synovitis | ||||
| Persistently minimal | ref | ref | ref | ref |
| Resolve | 1.5 (0.9, 2.4) | 1.2 (0.8, 1.8) | 1.6 (1.0, 2.8) | 1.5 (1.0, 2.4) |
| Extensive at 18 months | 1.6 (1.0, 2.5)* | 1.3 (0.9, 1.9) | 1.8 (1.1, 2.9)* | 1.6 (1.0, 2.5)* |
p<0.05
Reference
- 1.Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L, et al. Association of Joint Inflammation With Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis & rheumatology (Hoboken, NJ). 2016;68:654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Niu J, Neogi T, Goggins J, Nevitt MC, Roemer F, et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis and cartilage. 2016;24:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative Imaging Biomarkers of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2016;68:2422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roemer FW, Guermazi A, Hunter DJ, Niu J, Zhang Y, Englund M, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roemer FW, Felson DT, Yang T, Niu J, Crema MD, Englund M, et al. The association between meniscal damage of the posterior horns and localized posterior synovitis detected on T1-weighted contrast-enhanced MRI--the MOST study. Seminars in arthritis and rheumatism. 2013;42:573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis and rheumatism. 2011;63:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace G, Cro S, Dore C, King L, Kluzek S, Price A, et al. Associations Between Clinical Evidence of Inflammation and Synovitis in Symptomatic Knee Osteoarthritis: A Cross-Sectional Substudy. Arthritis Care Res. 2017;69:1340–8. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. The Journal of bone and joint surgery American volume. 2003;85-A:4–9. [DOI] [PubMed] [Google Scholar]
- 10.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature reviews Rheumatology. 2010;6:625–35. [DOI] [PubMed] [Google Scholar]
- 11.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Blizzard L, Halliday A, Han W, Jin X, Cicuttini F, et al. Association between MRI-detected knee joint regional effusion-synovitis and structural changes in older adults: a cohort study. Ann Rheum Dis. 2016;75:519–25. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Blizzard L, Jin X, Chen Z, Zhu Z, Han W, et al. Quantitative Assessment of Knee Effusion-Synovitis in Older Adults: Association With Knee Structural Abnormalities. Arthritis Rheumatol. 2016;68:837–44. [DOI] [PubMed] [Google Scholar]
- 14.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz JN, Brophy RH, Chaisson CE, de Chaves L, Cole BJ, Dahm DL, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. The New England journal of medicine. 2013;368:1675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz JN, Chaisson CE, Cole B, Guermazi A, Hunter DJ, Jones M, et al. The MeTeOR trial (Meniscal Tear in Osteoarthritis Research): rationale and design features. Contemporary clinical trials. 2012;33:1189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roemer FW, Kwoh CK, Hannon MJ, Crema MD, Moore CE, Jakicic JM, et al. Semiquantitative assessment of focal cartilage damage at 3T MRI: a comparative study of dual echo at steady state (DESS) and intermediate- weighted (IW) fat suppressed fast spin echo sequences. Eur J Radiol. 2011;80:15. [DOI] [PubMed] [Google Scholar]
- 18.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. [DOI] [PubMed] [Google Scholar]
- 19.Kellgren J LJ. The Epidemiology of Chronic Rheumatism, Atlas of Standard Radiograph. Oxford: Blackwell Scientific; 1963. [Google Scholar]
- 20.Crema MD, Roemer FW, Li L, Alexander RC, Chessell IP, Dudley AD, et al. Comparison between semiquantitative and quantitative methods for the assessment of knee synovitis in osteoarthritis using non-enhanced and gadolinium-enhanced MRI. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2017;25:267–271. [DOI] [PubMed] [Google Scholar]
- 21.Roemer FW, Guermazi A, Collins JE, Losina E, Nevitt MC, Lynch JA, et al. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort - Methodologic aspects and definition of change. BMC musculoskeletal disorders. 2016;17:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou G A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 23.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American journal of epidemiology. 2003;157:940–3. [DOI] [PubMed] [Google Scholar]
- 24.Collins JE, Losina E, Guermazi A, Katz JN. The risk of osteoarthritis progression after arthroscopic partial meniscectomy (APM); data from an rct of APM vs. physical therapy. Osteoarthritis and Cartilage.25:S59. [Google Scholar]
- 25.Guermazi A, Hayashi D, Roemer FW, Zhu Y, Niu J, Crema MD, et al. Synovitis in knee osteoarthritis assessed by contrast-enhanced magnetic resonance imaging (MRI) is associated with radiographic tibiofemoral osteoarthritis and MRI-detected widespread cartilage damage: the MOST study. J Rheumatol. 2014;41:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


