Abstract
Hodgkin lymphoma (HL) commonly occurs in adolescents and young adults (AYA), defined by the National Cancer Institute as people diagnosed with cancer between the ages of 15 and 39 years. Despite therapeutic advances, the AYA population has derived less incremental benefit compared to both paediatric and adult counterparts. Although the exact aetiology is unclear, contributing factors probably include differences in disease biology, delayed diagnosis, decreased participation in clinical trials and treatment adherence secondary to complex social factors. As such, while HL remains highly curable, there is not a clear consensus regarding the management of patients within this age range, specifically whether paediatric or adult regimens are preferred or how best to incorporate emerging therapeutic advancements. Ongoing clinical trials, as well as continued collaborative efforts are required to address the needs of this population, investigate the potential for unique biological factors and allow for optimization of treatment. Here we review current prognostic and treatment strategies for paediatric and adult patients with HL and highlight complexities around the management of this patient population.
Keywords: Adolescent, young adult, Hodgkin lymphoma, lymphoma, AYA
Hodgkin Lymphoma:
Epidemiology:
Hodgkin lymphoma (HL) is an uncommon lymphoma comprising approximately 0.5% of cancer diagnoses each year in developed countries (Ferlay, et al 2010, Siegel, et al 2018). This translates to an annual incidence of 8,500 individuals per year in the United States and a crude incidence rate of 2.49 per 100,000 lymphoid malignancies in Europe (Sant, et al 2010, Siegel, et al 2018). HL occurs in a bimodal distribution, with peaks occurring in patients between the ages of 15 and 30 years and in those older than 55 years (Ansell 2016). Given the increased incidence in younger patients, HL is one of the most common malignancies to occur in the adolescent and young adult (AYA) population, which is defined by the National Cancer Institute as people diagnosed with cancer between the ages of 15 and 39 years.
Biology:
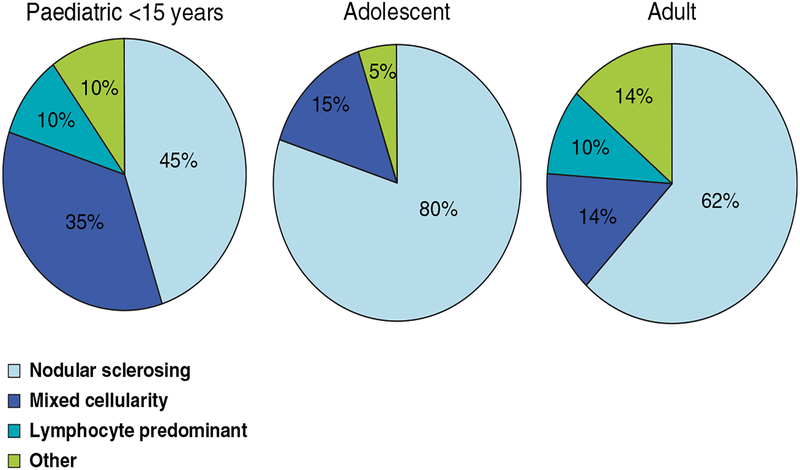
HL is divided into two distinct entities, classical HL (cHL) and the rare subtype, nodular lymphocyte predominant HL (NLPHL). cHL is further classified into four subtypes: nodular sclerosis, mixed cellularity, lymphocyte depleted and lymphocyte rich HL. Nodular sclerosis cHL, the most common subtype, comprises up 80% of cases in the AYA population (Ansell 2016, Hochberg, et al 2009) (Figure 1). Mixed cellularity HL is seen more commonly in paediatric populations, though it also occurs in a small proportion of AYA patients, and is associated with poorer outcomes.
Figure 1:
Distribution of HL subtypes by age. Adapted with permission from: Hochberg, J., Waxman, I.M., Kelly, K.M., Morris, E. & Cairo, M.S. (2009) Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science.
In developed countries, the risk of developing HL has been linked to increased socioeconomic status, specifically in paediatric and AYA patients with nodular sclerosis HL. Conversely, mixed cellularity and lymphocyte depleted HL are more frequent among patients of lower socioeconomic status and are commonly associated with Epstein–Barr virus (EBV) (Hu, et al 1988). The risk of HL is also increased in patients with immunodeficiency, such as in autoimmune disease, solid organ or stem cell transplantation, human immunodeficiency virus (HIV), or use of immunosuppressive medications. Relatives of individuals with HL are at higher risk of developing the disease, though whether this is related to shared genetics or environmental factors in unknown.
cHL is histologically characterized by the presence of Reed-Sternberg cells, characteristic multinucleated cells, surrounded by a microenvironment of inflammatory cells. Immunophenotyping of neoplastic cells is required to distinguish cHL and NLPHL. In cHL, the Reed-Sternberg cells typically express CD15 and CD30 and lack expression of B-cell markers, CD19, CD20 and CD79b, though rarely B-cell antigens can be seen on a subset of cells (von Wasielewski, et al 1997).
Staging and Prognostication:
Paediatric and adult patients with HL are staged using the Ann Arbor Staging System with Cotswold modification (Lister, et al 1989). The Lugano classification further modernized the staging of the disease, with the formal incorporation of fluorodeoxyglucose (FDG) positron emission tomography (PET)–computed tomography (CT) (Cheson, et al 2014). While HL is highly curable, risk-adapted strategies using interim PET scans have become increasingly important to help guide management in paediatric and adult populations, to both de-escalate therapy and avoid late toxicities, and intensify therapy to improve outcomes. Prognostic tools are uniquely defined in paediatric and adult oncology.
In paediatric patients, risk stratification varies by group but stage and presence of bulky disease are key tools to categorize patients into low, intermediate and high-risk groups. Early response to initial therapy as measured by CT or PET has also been a useful measure to guide subsequent treatment strategies (Schwartz, et al 2009, 2017). Clinical prognostic tools developed for adult populations are not associated with outcome in paediatric patients and include features that are not relevant to this population. A recent retrospective analysis from a Children’s Oncology Group (COG) trial of paediatric patients aged less than < 22 years with intermediate-risk cHL identified that stage IV disease, the presence of a large mediastinal mass, albumin <35 g/l and fever were independent predictors of event-free survival (EFS) (Schwartz, et al 2017). These features were used to validate a prognostic score, the Childhood Hodgkin’s International Prognostic Score (CHIPS), in which each predictor was assigned one point. Four-year EFS ranged from 93.1% for a CHIPS score of 0 to 69.2% for a CHIPS score of 3 (Schwartz, et al 2017) (Table IA). Additional studies to prospectively validate this tool and assess its applicability to other treatment cohorts are ongoing. The application of this tool to older AYA patients remains unknown.
Table I:
Prognostic scoring systems for (A) paediatric and (B) adult classical Hodgkin lymphoma [Created from data in Schwartz, et al (2017), Hasenclever and Diehl (1998)]
Table IA. Childhood Hodgkin International Prognostic Score (CHIPS)
| Prognostic Feature | CHIPS score | EFS |
|---|---|---|
| Stage IV disease (1 point) | 0 | 93.1% |
| Large mediastinal mass (1 point) | 1 | 88.5% |
| Albumin <35 g/l (1 point) | 2 | 77.6% |
| Fever (1 point) | 3 | 69.2% |
In adults, prognostic factors for limited-stage disease have been identified by both the German Hodgkin Study Group (GHSG) and the European Organization for Research and Treatment of Cancer (EORTC). Prognostic factors include the presence of a large mediastinal mass, an elevated erythrocyte sedimentation rate, involvement of multiple nodal sites, extranodal disease and age >50 years (Ansell 2016, Tubiana, et al 1989). These prognostic scores have maintained the ability to predict progression-free survival (PFS) and overall survival (OS) in patients with early stage HL between the ages of 16 and 59 years of age when treated with modern regimens (Klimm, et al 2013). For patients with advanced stage HL, the International Prognostic Score (IPS) has identified seven factors, including albumin <40 g/l, haemoglobin <105 g/, male sex, stage IV disease, age ≥45 years, white blood cell (WBC) count >15 ×109/l, and lymphocyte count <0.600 ×109/lor <8% of WBC count, that predict freedom from progression (FFP) (Hasenclever and Diehl 1998) (Table IB). The IPS score was developed using outcome data from over 25 centres, including patients aged between 15 and 65 years (Hasenclever and Diehl 1998).
Table IB.
Adult International Prognostic Score (IPS)
| Prognostic Feature | Score | 5-year FFS | 5-year OS |
|---|---|---|---|
| Albumin < 40 g/l (1 point) | 0 | 84% | 89% |
| Haemoglobin <105 g/l (1 point) | 1 | 77% | 90% |
| Male sex (1 point) | 2 | 67% | 81% |
| Stage IV disease (1 point) | 3 | 60% | 78% |
| Age ≥45 years (1 point) | 4 | 51% | 61% |
| WBC count >15 × 109/l (1 point) | 5 or more | 42% | 59% |
| Lymphocyte count <0.6 × 109/l or <8% of WBC count (1 point) |
WBC: white blood cell.
Recent advances in understanding the biology of cHL have led to the identification of novel prognostic and predictive biomarkers as well as novel treatment approaches. Gene expression profiling, for example, has established gene signatures that are predictive of outcome (Steidl, et al 2010, 2012). For example, a 23-gene expression classifier from the E2496 Intergroup Trial was able to predict survival in adult patients with advanced stage cHL (Scott, et al 2013). In paediatric patents, however, the 23-gene model was not predictive of outcome and a distinct 16-gene predictor is being validated (Mottok et al 2015). More recently, CD274 (also termed PD-L1) and PDCD1LG2 (PD-L2) alterations have also been identified as defining, prognostic features of cHL, allowing for immune evasion from an effective anti-tumour response. Specifically, amplification of chromosome 9p24.1 was found to be associated with advanced stage of disease and shorter PFS in patients raging from 15 to 60 years of age, and provided the preclinical rationale for checkpoint inhibition in both paediatric and adult patients with relapsed/refractory disease (Roemer, et al 2016). Studies of the molecular genetics of HL have identified mutations in JAK-STAT and nuclear factor (NF)-kappa B signalling, although further studies are required to clarify prognostic and therapeutic implications of these abnormalities and whether AYA populations express unique genetic patterns (Tiacci, et al 2018).
Treatment:
The choice of therapy for AYA patients is typically determined by the treatment setting and referral patterns. The majority of patients who are younger than 18 years are seen in paediatric centres, where many patients are treated in the context of clinical trials. For patients treated by adult oncologists, many are treated in the community setting. Considerable variability exists between the treatment of adult and paediatric patients, including the choice of chemotherapeutic agents and the role of radiation. In the current era in both the paediatric and adult settings, however, there has been increasing focus on balancing the risk of relapse with the risk of secondary side effects with de-intensification for low-risk patients. Use of PET-adapted strategies are also being used to guide subsequent treatment, especially the need for radiation, after initial chemotherapy. While this approach has been applied to all patients with HL independent of age, variability among age groups still results in uncertainty regarding the optimal treatment approach in the AYA population.
Adult Therapeutic Approaches:
As for patients with paediatric HL, the management of adult HL aims to balance the competing risk of relapse with late treatment-associated toxicity, particularly related to radiotherapy. This is especially relevant for the AYA population, where the risk of treatment-associated toxicities, including secondary malignancies, cardiac disease and infertility, can cause substantial morbidity and mortality.
The standard approach to early-stage disease evolved to combined modality therapy after the addition of chemotherapy to radiation was associated with improved outcomes. More recently, studies have focused on reducing the dose and field of radiation or omitting radiation all together in addition to limiting the number of cycles of chemotherapy. The German HD10 trial consisted of four treatment arms containing either 2 or 4 cycles of ABVD (adriamycin, bleomycin, vinblastine and dacarbazine) and 20 or 30 Gy of involved-field radiation therapy (IFRT) in patients with early-stage, favourable disease according to the GHSG schema (Engert, et al 2010). At five years, the rate of freedom from treatment failure (FFTF) was 93% (95% confidence interval [CI]; 90.5 to 94.8) with four cycles of ABVD as compared to 91.1% (95% CI; 88.3 to 93.2) with two cycles. There was also no significant difference in FFTF (p=1.00) or OS (p=0.61) between 20 and 30 Gy IFRT. These findings suggested that fewer cycles of chemotherapy and lower doses of radiation provide adequate disease control in early-stage disease. Subsequent trials for patients with early-stage disease have compared combined modality therapy to chemotherapy alone. The National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) HD.6 trial, for instance, demonstrated that at 12 years, the FFP was 87% versus 92% in patients receiving ABVD alone as compared to ABVD plus subnodal radiation (hazard ratio [HR] for disease progression, 1.91, 95% CI; 0.99 to 3.69), with an OS of 94% versus 87% (HR for death from ABVD alone, 0.5; 95% CI; 0.25 to 0.99) (Meyer, et al 2012). Although this study used subtotal nodal radiotherapy, which is no longer standard of care, and the study closed early due to poor accrual, the results did demonstrate favourable outcomes with chemotherapy alone, particularly in patients achieving a complete remission after 2 cycles of ABVD. More recently, using interim PET as a prognostic biomarker, several studies have compared combined modality to chemotherapy alone in patients achieving a negative interim study in early-stage disease. While combined modality therapy has been associated with minor improvements in PFS as compared to chemotherapy alone, OS has not improved, and radiation use has led to increased late toxicity (Radford, et al 2015, Raemaekers, et al 2014). It should also be noted that the prognosis is excellent with either approach, with the RAPID trial demonstrating a 3-year PFS of 94.6% (95% CI; 91.5 to 97.7) vs. 90.8% (95% CI; 86.9 to 94.8), in patients who received and did not receive radiation after chemotherapy (Radford, et al 2015).
As reflected in the National Comprehensive Cancer Network (NCCN) Guidelines (NCCN 2018), which are expert consensus recommendations widely used in the United States, there are multiple acceptable approaches for patients with early stage disease. In practice, therapy is typically individualized based on comorbidities, age and patient preference. For younger patients with favourable, early-stage disease, avoidance of radiation is generally preferred. An interim negative PET scan is also commonly used to select patients likely to have favourable outcomes with chemotherapy alone, though there is little prospective data to support this approach in patients with bulky disease.
For patients with advanced stage disease, chemotherapy is the mainstay of treatment. ABVD is the most widely used regimen in the US (Gallamini, et al 2007, Hoskin, et al 2009). Escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone) is used commonly in Europe and is associated with improved PFS as compared to ABVD, though at the expense of increased toxicity, including risk of infection and infertility (Borchmann, et al 2018, Federico, et al 2009). Given the lack of OS benefit in multiple clinical trials and improving options for salvage therapy in relapsed patients, the regimen has not been widely adopted in North America (Skoetz, et al 2017).
Risk-adapted studies have also been performed in advanced stage HL. The RATHL (response-adapted therapy for advanced Hodgkin lymphoma) trial found that in patients with advanced stage disease, omission of bleomycin in interim PET-negative patients did not decrease efficacy (Johnson, et al 2016) (Table II). The absolute difference in 3-year PFS for ABVD as compared to AVD (doxorubicin, vinblastine and dacarbazine) was 1.6 percentage points (95% CI; −3.2 to 5.3) (Johnson, et al 2016). More recently, brentuximab vedotin (BV), an anti-CD30 antibody-drug conjugate that is highly active in the relapsed/refractory setting, was also tested in advanced stage patients. The ECHELON study compared ABVD to BV plus AVD (BV+AVD) in patients with advanced stage disease (Connors, et al 2018) (Table II). The trial demonstrated modest improvement in modified PFS, with an absolute difference of 4.9 percentage points (HR for an event of progression, death, or modified progression, 0.77, CI; 0.6 to 0.98, p=0.04) with BV+AVD as compared with ABVD without difference in OS with short follow-up. Trials incorporating checkpoint inhibitors in the frontline setting for patients with advanced disease are also in development.
Table II:
Trials demonstrating available treatment options for AYA patients with advanced stage HL
| Trial | Inclusion criteria | Age | Treatment | EFS/PFS/FFS/FFTF | OS | |
|---|---|---|---|---|---|---|
| Paediatric trials |
COG-AHOD0031 (Friedman et al 2014) |
I/IIA with bulk I/IIB IIIA IVA |
≦22 years | ABVE-PC: → rapid early responders (RER): ABVE x 2 +/− IFRT → slow early responders (SER): ABVE x 2 +/− DECA x 2 + IFRT |
4-year EFS: 85% RER: 87.9% (+IFRT) vs. 84.3% (-IFRT) SER: 79.3% (+DECA) vs. 75.2% (-DECA) |
4-year OS: 97.8% RER: 98.5% SER: 95.3% |
|
COG-59704 (Kelly et al 2011) |
II/IIIB with bulk IV |
<21 years | BEACOPP x 4: → rapid early responders: COPP/ABV x 4 (female) → rapid early responders: ABVD x 2 + IFRT (male) → slow early responders: BEACOPP x 4 + IFRT |
5-year EFS: 94% |
5-year OS: 97% |
|
|
CPOH-HD-2002 (Mauz-Korholz et al 2010) |
IIB-IV | <18 years | OEPA x 2:→ COPDAC x 2–4 (male) + IFRT OPPA x 2:→ COPP x 2–4 (female) + IFRT |
3-year EFS: 90.2% (male) 84.7% (female) |
||
| Adult trials |
RATHL (Johnson et al 2016) |
IIA with bulk, IIB-IV | ≥18 years | ABVD x 2: → PET negative: ABVD x 4 vs. AVD x 4 → PET positive: BEACOPP vs. BEACOPP-14 |
3-year PFS: PET negative: ABVD: 85.7% AVD: 84.4 % PET positive: BEACOPP and BEACOPP-14: 67.5% |
3-year OS: PET negative: ABVD: AVD: 97.2% PET positive: BEACOPP and BEACOPP-14: 87.7% |
|
ECHELON (Connors et al 2018) |
III-IV | ≥18 years | ABVD vs. BV+AVD | 2-year modified PFS: ABVD: 77.2% A+AVD: 82.1% |
||
|
HD15 (Engert et al 2012) |
IIB with large mediastinal mass or extranodal disease III-IV |
18–60 years | Randomized to: → Escalated BEACOPP x 8 → Escalated BEACOPP x 6 → BEACOPP x 8 |
5-year FFTF: 8 x B esc: 84.4% 6 x B esc: 89.3% 8 x B: 85.4% |
5-year OS: 8 x B esc: 91.9% 6 x B esc: 95.3% 8 x B: 94.5% |
ABVD: doxorubicin, vinblastine, bleomycin and dacarbazine; ABVE: doxorubicin, bleomycin, vinblastine, etoposide;AVD: doxorubicin, vinblastine and dacarbazine; AYA: adolescent and young adult; BEACOPP: bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone; BV: brentuximab vedotin; COG: Children’s Oncology Group; COPDAC: cyclophosphamide, vincristine, prednisone, dacarbazine; DECA: dexamethasone, etoposide, cisplatin, cytarabine; EFS: event-free survival; FFS: failure-free survival; FFTF: freedom from treatment failure; IFRT: involved filed radiotherapy; OEPA: vincristine, etoposide, prednisone, doxorubicin; OPPA: vincristine, procarbazine, prednisone, doxorubicin; OS: overall survival; PC: prednisone, cyclophosphamide; PET: positron emission tomography; RATHL: Response-adapted therapy for advanced Hodgkin lymphoma; RER: rapid early responders; SER: slow early responders.
Paediatric Therapeutic Approaches:
While ABVD is the most common therapy used in adults, paediatric regimens use alternate combinations of chemotherapy with the goal of decreasing the cumulative doses of anthracyclines, bleomycin and alkylating agents. Patients are typically treated with risk-adapted regimens with chemotherapy with or without radiotherapy. Commonly used chemotherapy regimens include ABVE-PC (doxorubicin, bleomycin, vinblastine, etoposide-prednisone cyclophosphamide) or OEPA (vincristine, etoposide, prednisone, doxorubicin) with COPDAC (cyclophosphamide, vincristine, prednisone and dacarbazine) in intermediate/high risk patients (Donaldson, et al 2007, Dorffel, et al 2013, Nachman, et al 2002, Tebbi, et al 2012). If radiotherapy if used, the doses range from 15 to 25 Gy, which are lower than those used in the adult setting. In early-stage patients, EFS ranges from 89 to 100% with an OS of greater than 95% (Dorffel, et al 2013).
Paediatric patients with intermediate or high-risk disease receive additional chemotherapy cycles, followed by response-based IFRT. The German Society of Paediatric Oncology and Hematology (GPOH) and the European paediatric and adolescent Hodgkin lymphoma network (Euronet-HD) use OEPA-COPDAC (Mauz-Korholz, et al 2010) (Table II). The COG has developed the use of ABVE-PC, in order to enhance efficacy with the use of dose-dense drug delivery while also reducing the risk of late-term toxicity associated with cumulative chemotherapy (Schwartz, et al 2009). The COG has also reported favourable outcomes for patients with high-risk disease treated with followed by less intensive response-based treatment approaches in rapid responders (Kelly, et al 2011) (Table II). Trials that incorporate BV, an antibody drug conjugate that targets CD30, in the upfront setting, as have been performed in adult patients, are currently underway. While the omission of radiation was initially limited to trials in patients with early-stage disease, the COG AHOD0031 trial demonstrated that it is safe to omit radiation in intermediate-risk patients who have a rapid early response (RER) as determined by CT followed by complete response with chemotherapy (Friedman, et al 2014) (Table II). In this study, the EFS was 87.9% (95% CI; 83.7 to 91.1) in patients receiving radiation as compared to 84.3% (95% CI; 79.8% to 87.9%) in patients who received chemotherapy alone (Friedman, et al 2014).
Comparison of paediatric and adult regimens in the AYA population:
No prospective trials have been conducted in AYA HL patients comparing adult versus paediatric regimens with regard to efficacy and toxicity. Data from the British Columbia Cancer Agency Lymphoid Cancer database demonstrated that adolescents between the ages of 16 and 21 years and young adults between the ages of 22 and 45 years have similar outcomes to adults when treated with adult regimens (Foltz, et al 2006). The GHSG similarly demonstrated that adolescents and young adults treated on the HD4 and HD9 trials had similar outcomes as adult patients (Eichenauer, et al 2009). When similar analyses have been performed using paediatric regimens, similar findings were seen. For example, outcomes for adolescent patients between the ages of 15 to 21 years had comparable outcomes to those less than 15 years of age in two Pediatric Oncology Group/COG trials, using dose-dense, response-based chemotherapy in combination with low dose IFRT (Fernandez, et al 2017).
There have been efforts to retrospectively compare outcomes for adolescent patients treated with either a paediatric or adult regimen. For example, outcomes were compared between 114 adolescent patients between the ages of 17 to 21 years treated on Eastern Cooperative Oncology Group Intergroup adult E2496 study and 391 patients of the same age who were treated on the COG AHOD0031 study (Henderson, et al 2018). Patients on the E2496 trial, who had advanced stage disease or bulky localized disease, were randomized to receive either ABVD or Stanford V (doxorubicin, vinblastine, chloromethine, vincristine, bleomycin, etoposide and prednisone), with radiation added for all patients with bulky disease and those with pre-treatment disease >5 cm who were receiving the Stanford V regimen (Gordon, et al 2013). Patients in the COG trial, on the contrary, included patients with bulky or extranodal stage I or stage II-IV disease, who were treated with response-based ABVE-PC with or without IFRT (Friedman, et al 2014). When comparing the two studies, it was shown that adolescents treated on the E2495 trial had 5‐year failure-free survival (FFS) and OS rates of 68% and 89%, respectively, as compared to 81% and 97%, in the COG AYAs. For patients treated on the E2495 trial, FFS was also noted to be inferior in younger patients between the ages of 17 and 21 years as compared to young adults between the ages of 22 and 44 years. It should be noted that there were important differences in trial design and patient characteristics between these trials, with more patients with advanced stage disease in the adult trial. Patients treated in the COG trial were also more likely to receive radiation, though at a lower dose than typically used in adult regimens.
While data suggests a potential advantage of paediatric regimens for younger AYA patients, prospective trials will be required to clarify the optimal treatment approach. Furthermore, longer follow-up of late toxicities, such as secondary malignancies and cardiac disease, will also be important to consider when comparing paediatric and adult regimens and the impact of radiation.
Outcomes and complexities associated with AYA populations:
While the use of adult versus paediatric regimens remains an important issue for AYA patients with HL, there are also a variety of complex social and psychological factors that play a fundamental role in the care of this population. It has been recognized that despite advances in cancer care, survival rates in the AYA population are lower than their paediatric and adult counterparts, especially when compared to outcomes of paediatric trials (Bleyer 2002, Trama, et al 2016). Poorer outcomes in the AYA populations across cancer types have been attributed to a broad range of disparities specific to this age group (Isenalumhe, et al 2016, Keegan, et al 2016, Shaw, et al 2015).
One potential factor contributing to poorer outcomes relates to delays in diagnosis and treatment. This is typically multifactorial in origin, given both decreased suspicion of malignancy and decreased access to healthcare among patients. This is especially true in the United States, where patients between the ages of 18 and 24 years and 25 and 34 years are more likely than any other groups to be uninsured, thus deterring use of healthcare resources (Isenalumhe, et al 2016). Lack of insurance has specifically been identified as a negative prognostic factor for HL, as patients without insurance coverage or of lower socioeconomic status were more likely to have advanced stage disease at the time of diagnosis (Smith, et al 2012). In another study, public health insurance or lack of insurance was associated with worse HL-specific survival (Keegan, et al 2016). In countries with universal access to healthcare, there have also been disparities regarding in access to care for the AYA population, with longer waiting times for AYA as compared to paediatric patients (Fernandez and Barr 2006).
Across countries, AYA patients are also less likely to take part in clinical trials as compared to paediatric and adult patients (Barr, et al 2016, Fern and Whelan 2010, Parsons, et al 2011, Roth et al 2016). While the majority of paediatric patients are treated at comprehensive cancer centres, a large subset of AYA patients are treated in community centres that may have decreased access to trial participation. One study demonstrated that in the United States, only 2% of patients between the ages of 20 and 29 years enrol in trials, as compared to approximately 60% in younger patients (Fern and Whelan 2010). In the United Kingdom, the nadir for clinical trial enrolment was in patients between the ages of 35 and 39 years, with only 7.5% participation (Fern and Whelan 2010). However, even among cancer centres that have clinical trial options, there is reported to be decreased participation among the AYA population (Isenalumhe, et al 2016). How the perceptions and attitudes of the AYA population influence clinical trial enrolment remains less well understood, although may also be a contributing factor.
Psychosocial aspects of care are also important to consider in the AYA population. Social situations can vary considerably, ranging from patients who are dependent on their parents to those who are primary providers for young children. Despite variability, it is common for patients to be unable to proceed with age-appropriate milestones related to education, career development or relationships, which can add significant stress throughout cancer treatment. Many patients also experience financial hardship, with limited sick leave at entry level positions and high amounts of educational debt (Isenalumhe, et al 2016).
Survivorship in the AYA population:
For AYA patients with HL there is also a recognized “cost of cure” associated with both late toxicities of treatment and long-term psychosocial sequelae (Barr, et al 2016). Although there have been efforts to reduce the dose or eliminate the use of radiation, those patients who require radiation are at risk of a variety of late toxicities, most notably secondary malignancies and cardiovascular disease (Ng 2014). Risk of cancer, specifically breast, lung and gastrointestinal, are directly related to the dose of radiation (Inskip, et al 2009, Ng 2014, Travis and Gilbert 2005). It is therefore important to recommend appropriate cancer and cardiovascular screening depending on the treatment and dose of radiation received (Ng 2014). Even in the absence of radiation, patients may be at increased risk of secondary malignancies, such as leukaemia, following treatment with chemotherapy alone. While regimens such as ABVD do not appear to increase the risk of leukaemia, more intensive regimens such as BEACOPP, are thought to be leukaemogenic (Eichenauer, et al 2014).
While other late toxicities may not impact on mortality, they can significantly impair quality of life. For example, concerns regarding fertility are important considerations in the AYA population. While the majority of frontline treatment options for patients with HL are unlikely to impair fertility, the risk can vary according to the age of the patient and dose and intensity of the treatment. Specifically, infertility increases with higher doses of alkylating agents and is higher after salvage regimens and conditioning for autologous stem cell transplantation (Harel, et al 2011). It is important to discuss these risks with patients and provide referral to reproductive endocrinology if desired. Additional support and resources should also be provided to patients who are concerned about this issue or struggling with infertility following treatment.
AYA patients are often in the midst of physical, emotional and social development throughout their treatment. As such, they may be at risk for impaired health-related quality of life (HRQoL) later in life. One study identified that AYA lymphoma survivors experience clinically relevant impairments in HRQoL in several domains, with physical, role, cognitive, emotional, social functioning, fatigue and financial difficulties (Husson, et al 2017).
It is important for providers to be aware of both the physical and emotional impact of HL in the AYA population in order to appropriately guide follow-up, cancer and cardiac screening and referral for social or psychological support.
Advocacy and Future Directions:
In 2006, the National Cancer Institute (NCI) and the Lance Armstrong Foundation conduced a Progress Review Group (PRG) in order to address the needs of AYA oncology patients, examine the state of current research and identify the scientific gaps and future requirements to improve outcomes (Bleyer 2007, Kahn, et al 2017). They provided a comprehensive guide to inform future collaborative efforts to improve the care for this population. Key recommendations included the need to identify distinguishing characteristics of the AYA population, improve education and communication, improve research tools for AYA population, ensure excellence in cancer care delivery and improve advocacy (Bleyer 2007) (Table III). Since these guidelines were published, there has been increased attention to the AYA population among academic centres, research foundations and collaborative groups. In 2015, the first Lymphoma Research Foundation (LRF) AYA Symposium was held to create a research agenda specific to lymphoma AYAs and a framework to enhance their care (Kahn, et al 2017). Similarly, the European Network for Teenagers and Young Adults with Cancer, which was created in the context of the European Network for Cancer Research in Children and Adolescents (ENCCA), is promoting the development of AYA-specific practice guidelines, educational programmes, healthy lifestyles and greater involvement in patient support organizations (Stark, et al 2016). Research efforts that were developed at these meetings are currently ongoing and, with time, questions to many of the unanswered questions regarding AYA care will hopefully be answered.
Table III:
Recommendations by the National Cancer Institute and Lance Armstrong Foundation Progress Review Group [Created from data in Bleyer (2007)]
| Recommendations: | |
|---|---|
| Identify the characteristics that distinguish the unique cancer burden in the AYA oncology patient. | |
| Provide education, training, and communication to improve awareness, prevention, access, and quality cancer care for AYAs. | |
| Create the tools to study the AYA cancer problem. | |
| Ensure excellence in service delivery across the cancer control continuum (i.e., prevention, screening, diagnosis, treatment, survivorship, and end of life). | |
| Strengthen and promote advocacy and support of the AYA cancer patient. | |
AYA: adolescent and young adult.
In order to effectively address the recognized barriers to cancer care in the AYA population, it will be imperative for paediatric and adult cooperative groups to work together when designing clinical trials and studying the underlying disease biology of HL. Academic and community oncologists will also be required to collaborate to encourage clinical trial enrolment and ensure adequate resources, including psychosocial supports, for patients within this age range. Additionally, as novel therapeutic treatments make their way into clinic, it will be imperative to think about the role of these drugs in the AYA population, with special focus on AYA-specific issues, including impact on fertility and late toxicities.
References:
- Ansell SM (2016) Hodgkin lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol, 91, 434–442. [DOI] [PubMed] [Google Scholar]
- Barr RD, Ferrari A, Ries L, Whelan J & Bleyer WA (2016) Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA Pediatr, 170, 495–501. [DOI] [PubMed] [Google Scholar]
- Bleyer A (2007) Adolescent and young adult (AYA) oncology: the first A. Pediatr Hematol Oncol, 24, 325–336. [DOI] [PubMed] [Google Scholar]
- Bleyer WA (2002) Cancer in older adolescents and young adults: epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol, 38, 1–10. [DOI] [PubMed] [Google Scholar]
- Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, Zijlstra JM, Markova J, Meissner J, Feuring-Buske M, Huttmann A, Dierlamm J, Soekler M, Beck HJ, Willenbacher W, Ludwig WD, Pabst T, Topp MS, Hitz F, Bentz M, Keller UB, Kuhnhardt D, Ostermann H, Schmitz N, Hertenstein B, Aulitzky W, Maschmeyer G, Vieler T, Eich H, Baues C, Stein H, Fuchs M, Kuhnert G, Diehl V, Dietlein M & Engert A (2018) PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet, 390, 2790–2802. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol, 32, 3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, Younes A, Alekseev S, Illes A, Picardi M, Lech-Maranda E, Oki Y, Feldman T, Smolewski P, Savage KJ, Bartlett NL, Walewski J, Chen R, Ramchandren R, Zinzani PL, Cunningham D, Rosta A, Josephson NC, Song E, Sachs J, Liu R, Jolin HA, Huebner D, Radford J & Group E-S (2018) Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N Engl J Med, 378, 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson SS, Link MP, Weinstein HJ, Rai SN, Brain S, Billett AL, Hurwitz CA, Krasin M, Kun LE, Marcus KC, Tarbell NJ, Young JA & Hudson MM (2007) Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin’s disease. J Clin Oncol, 25, 332–337. [DOI] [PubMed] [Google Scholar]
- Dorffel W, Ruhl U, Luders H, Claviez A, Albrecht M, Bokkerink J, Holte H, Karlen J, Mann G, Marciniak H, Niggli F, Schmiegelow K, Schwarze EW, Potter R, Wickmann L & Schellong G (2013) Treatment of children and adolescents with Hodgkin lymphoma without radiotherapy for patients in complete remission after chemotherapy: final results of the multinational trial GPOH-HD95. J Clin Oncol, 31, 1562–1568. [DOI] [PubMed] [Google Scholar]
- Eichenauer DA, Bredenfeld H, Haverkamp H, Muller H, Franklin J, Fuchs M, Borchmann P, Muller-Hermelink HK, Eich HT, Muller RP, Diehl V & Engert A (2009) Hodgkin’s lymphoma in adolescents treated with adult protocols: a report from the German Hodgkin study group. J Clin Oncol, 27, 6079–6085. [DOI] [PubMed] [Google Scholar]
- Eichenauer DA, Thielen I, Haverkamp H, Franklin J, Behringer K, Halbsguth T, Klimm B, Diehl V, Sasse S, Rothe A, Fuchs M, Boll B, von Tresckow B, Borchmann P & Engert A (2014) Therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood, 123, 1658–1664. [DOI] [PubMed] [Google Scholar]
- Engert A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, Berger B, Greil R, Willborn KC, Wilhelm M, Debus J, Eble MJ, Sokler M, Ho A, Rank A, Ganser A, Trumper L, Bokemeyer C, Kirchner H, Schubert J, Kral Z, Fuchs M, Muller-Hermelink HK, Muller RP & Diehl V (2010) Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med, 363, 640–652. [DOI] [PubMed] [Google Scholar]
- Federico M, Luminari S, Iannitto E, Polimeno G, Marcheselli L, Montanini A, La Sala A, Merli F, Stelitano C, Pozzi S, Scalone R, Di Renzo N, Musto P, Baldini L, Cervetti G, Angrilli F, Mazza P, Brugiatelli M & Gobbi PG (2009) ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol, 27, 805–811. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Parkin DM & Steliarova-Foucher E (2010) Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer, 46, 765–781. [DOI] [PubMed] [Google Scholar]
- Fern LA & Whelan JS (2010) Recruitment of adolescents and young adults to cancer clinical trials--international comparisons, barriers, and implications. Semin Oncol, 37, e1–8. [DOI] [PubMed] [Google Scholar]
- Fernandez CV & Barr RD (2006) Adolescents and young adults with cancer: An orphaned population. Paediatr Child Health, 11, 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez KS, Schwartz CL, Chen L, Constine LS, Chauvenet A & de Alarcon PA (2017) Outcome of adolescents and young adults compared to children with Hodgkin lymphoma treated with response-based chemotherapy on pediatric protocols: A Children’s Oncology Group report. Pediatr Blood Cancer, 64, e26681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz LM, Song KW & Connors JM (2006) Hodgkin’s lymphoma in adolescents. J Clin Oncol, 24, 2520–2526. [DOI] [PubMed] [Google Scholar]
- Friedman DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, Kessel S, De Alarcon PA, Chen AR, Kobrinsky N, Ehrlich P, Hutchison RE, Constine LS & Schwartz CL (2014) Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol, 32, 3651–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, Patti C, Loft A, Di Raimondo F, D’Amore F, Biggi A, Vitolo U, Stelitano C, Sancetta R, Trentin L, Luminari S, Iannitto E, Viviani S, Pierri I & Levis A (2007) Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol, 25, 3746–3752. [DOI] [PubMed] [Google Scholar]
- Gordon LI, Hong F, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, Wagner H, Stiff PJ, Cheson BD, Gospodarowicz M, Advani R, Kahl BS, Friedberg JW, Blum KA, Habermann TM, Tuscano JM, Hoppe RT & Horning SJ (2013) Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol, 31, 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel S, Ferme C & Poirot C (2011) Management of fertility in patients treated for Hodgkin’s lymphoma. Haematologica, 96, 1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenclever D & Diehl V (1998) A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med, 339, 1506–1514. [DOI] [PubMed] [Google Scholar]
- Henderson TO, Parsons SK, Wroblewski KE, Chen L, Hong F, Smith SM, McNeer JL, Advani RH, Gascoyne RD, Constine LS, Horning S, Bartlett NL, Shah B, Connors JM, Leonard JI, Kahl BS, Kelly KM, Schwartz CL, Li H, Friedberg JW, Friedman DL, Gordon LI & Evens AM (2018) Outcomes in adolescents and young adults with Hodgkin lymphoma treated on US cooperative group protocols: An adult intergroup (E2496) and Children’s Oncology Group (COG AHOD0031) comparative analysis. Cancer, 124, 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg J, Waxman IM, Kelly KM, Morris E & Cairo MS (2009) Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol, 144, 24–40. [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Lowry L, Horwich A, Jack A, Mead B, Hancock BW, Smith P, Qian W, Patrick P, Popova B, Pettitt A, Cunningham D, Pettengell R, Sweetenham J, Linch D & Johnson PW (2009) Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol, 27, 5390–5396. [DOI] [PubMed] [Google Scholar]
- Hu E, Hufford S, Lukes R, Bernstein-Singer M, Sobel G, Gill P, Pinter-Brown L, Rarick M, Rosen P & Brynes R (1988) Third-World Hodgkin’s disease at Los Angeles County-University of Southern California Medical Center. J Clin Oncol, 6, 1285–1292. [DOI] [PubMed] [Google Scholar]
- Husson O, Prins JB, Kaal SE, Oerlemans S, Stevens WB, Zebrack B, van der Graaf WT & van de Poll-Franse LV (2017) Adolescent and young adult (AYA) lymphoma survivors report lower health-related quality of life compared to a normative population: results from the PROFILES registry. Acta Oncol, 56, 288–294. [DOI] [PubMed] [Google Scholar]
- Inskip PD, Robison LL, Stovall M, Smith SA, Hammond S, Mertens AC, Whitton JA, Diller L, Kenney L, Donaldson SS, Meadows AT & Neglia JP (2009) Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol, 27, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenalumhe LL, Fridgen O, Beaupin LK, Quinn GP & Reed DR (2016) Disparities in Adolescents and Young Adults With Cancer. Cancer Control, 23, 424–433. [DOI] [PubMed] [Google Scholar]
- Johnson P, Federico M, Kirkwood A, Fossa A, Berkahn L, Carella A, d’Amore F, Enblad G, Franceschetto A, Fulham M, Luminari S, O’Doherty M, Patrick P, Roberts T, Sidra G, Stevens L, Smith P, Trotman J, Viney Z, Radford J & Barrington S (2016) Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. N Engl J Med, 374, 2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JM, Ozuah NW, Dunleavy K, Henderson TO, Kelly K & LaCasce A (2017) Adolescent and young adult lymphoma: collaborative efforts toward optimizing care and improving outcomes. Blood Adv, 1, 1945–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan TH, DeRouen MC, Parsons HM, Clarke CA, Goldberg D, Flowers CR & Glaser SL (2016) Impact of Treatment and Insurance on Socioeconomic Disparities in Survival after Adolescent and Young Adult Hodgkin Lymphoma: A Population-Based Study. Cancer Epidemiol Biomarkers Prev, 25, 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM, Sposto R, Hutchinson R, Massey V, McCarten K, Perkins S, Lones M, Villaluna D & Weiner M (2011) BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children’s Oncology Group. Blood, 117, 2596–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimm B, Goergen H, Fuchs M, von Tresckow B, Boll B, Meissner J, Glunz A, Diehl V, Eich HT, Engert A & Borchmann P (2013) Impact of risk factors on outcomes in early-stage Hodgkin’s lymphoma: an analysis of international staging definitions. Ann Oncol, 24, 3070–3076. [DOI] [PubMed] [Google Scholar]
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA & Tubiana M (1989) Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol, 7, 1630–1636. [DOI] [PubMed] [Google Scholar]
- Mauz-Korholz C, Hasenclever D, Dorffel W, Ruschke K, Pelz T, Voigt A, Stiefel M, Winkler M, Vilser C, Dieckmann K, Karlen J, Bergstrasser E, Fossa A, Mann G, Hummel M, Klapper W, Stein H, Vordermark D, Kluge R & Korholz D (2010) Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J Clin Oncol, 28, 3680–3686. [DOI] [PubMed] [Google Scholar]
- Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, Horning SJ, Dar AR, Shustik C, Stewart DA, Crump M, Djurfeldt MS, Chen BE & Shepherd LE (2012) ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med, 366, 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottok A, Johnston R, Chan FC (2015) Prediction of Primary Treatment Outcome Using Gene Expression Profiling of Pre-Treatment Biopsies Obtained from Childhood and Adolescent Hodgkin Lymphoma Patients. Blood, 126, 175. [Google Scholar]
- Nachman JB, Sposto R, Herzog P, Gilchrist GS, Wolden SL, Thomson J, Kadin ME, Pattengale P, Davis PC, Hutchinson RJ & White K. (2002) Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol, 20, 3765–3771. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. (2018) Hodgkin Lymphoma (Version 3.2018). https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf. Accessed September 14, 2018. [Google Scholar]
- Ng AK (2014) Current survivorship recommendations for patients with Hodgkin lymphoma: focus on late effects. Blood, 124, 3373–3379. [DOI] [PubMed] [Google Scholar]
- Parsons HM, Harlan LC, Seibel NL, Stevens JL & Keegan TH (2011) Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol, 29, 4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, Wimperis J, Culligan D, Popova B, Smith P, McMillan A, Brownell A, Kruger A, Lister A, Hoskin P, O’Doherty M & Barrington S (2015) Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med, 372, 1598–1607. [DOI] [PubMed] [Google Scholar]
- Raemaekers JM, Andre MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, Brice P, Ferme C, van der Maazen R, Gotti M, Bouabdallah R, Sebban CJ, Lievens Y, Re A, Stamatoullas A, Morschhauser F, Lugtenburg PJ, Abruzzese E, Olivier P, Casasnovas RO, van Imhoff G, Raveloarivahy T, Bellei M, van der Borght T, Bardet S, Versari A, Hutchings M, Meignan M & Fortpied C (2014) Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol, 32, 1188–1194. [DOI] [PubMed] [Google Scholar]
- Roemer MG, Advani RH, Redd RA, Pinkus GS, Natkunam Y, Ligon AH, Connelly CF, Pak CJ, Carey CD, Daadi SE, Chapuy B, de Jong D, Hoppe RT, Neuberg DS, Shipp MA & Rodig SJ (2016) Classical Hodgkin Lymphoma with Reduced beta2M/MHC Class I Expression Is Associated with Inferior Outcome Independent of 9p24.1 Status. Cancer Immunol Res, 4, 910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, O’Mara AM, Seibel NL, Dickens DS, Langevin AM, Pollock BH & Freyer DR (2016) Low Enrollment of Adolescents and Young Adults On to Cancer Trials: Insights From the Community Clinical Oncology Program. J Oncol Pract, 12, e388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, Marcos-Gragera R, Maynadie M, Simonetti A, Lutz JM, Berrino F & Group HW (2010) Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood, 116, 3724–3734. [DOI] [PubMed] [Google Scholar]
- Schwartz CL, Chen L, McCarten K, Wolden S, Constine LS, Hutchison RE, de Alarcon PA, Keller FG, Kelly KM, Trippet TA, Voss SD & Friedman DL (2017) Childhood Hodgkin International Prognostic Score (CHIPS) Predicts event-free survival in Hodgkin Lymphoma: A Report from the Children’s Oncology Group. Pediatr Blood Cancer, 64, e26278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CL, Constine LS, Villaluna D, London WB, Hutchison RE, Sposto R, Lipshultz SE, Turner CS, deAlarcon PA & Chauvenet A (2009) A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood, 114, 2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DW, Chan FC, Hong F, Rogic S, Tan KL, Meissner B, Ben-Neriah S, Boyle M, Kridel R, Telenius A, Woolcock BW, Farinha P, Fisher RI, Rimsza LM, Bartlett NL, Cheson BD, Shepherd LE, Advani RH, Connors JM, Kahl BS, Gordon LI, Horning SJ, Steidl C & Gascoyne RD (2013) Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol, 31, 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PH, Reed DR, Yeager N, Zebrack B, Castellino SM & Bleyer A (2015) Adolescent and Young Adult (AYA) Oncology in the United States: A Specialty in Its Late Adolescence. J Pediatr Hematol Oncol, 37, 161–169. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD & Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin, 68, 7–30. [DOI] [PubMed] [Google Scholar]
- Skoetz N, Will A, Monsef I, Brillant C, Engert A & von Tresckow B (2017) Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev, 5, CD007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EC, Ziogas A & Anton-Culver H (2012) Association between insurance and socioeconomic status and risk of advanced stage Hodgkin lymphoma in adolescents and young adults. Cancer, 118, 6179–6187. [DOI] [PubMed] [Google Scholar]
- Stark D, Bielack S, Brugieres L, Dirksen U, Duarte X, Dunn S, Erdelyi DJ, Grew T, Hjorth L, Jazbec J, Kabickova E, Konsoulova A, Kowalczyk JR, Lassaletta A, Laurence V, Lewis I, Monrabal A, Morgan S, Mountzios G, Olsen PR, Renard M, Saeter G, van der Graaf WT & Ferrari A (2016) Teenagers and young adults with cancer in Europe: from national programmes to a European integrated coordinated project. Eur J Cancer Care (Engl), 25, 419–427. [DOI] [PubMed] [Google Scholar]
- Steidl C, Diepstra A, Lee T, Chan FC, Farinha P, Tan K, Telenius A, Barclay L, Shah SP, Connors JM, van den Berg A & Gascoyne RD (2012) Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood, 120, 3530–3540. [DOI] [PubMed] [Google Scholar]
- Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC & Gascoyne RD (2010) Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med, 362, 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbi CK, Mendenhall NP, London WB, Williams JL, Hutchison RE, Fitzgerald TJ, de Alarcon PA, Schwartz C & Chauvenet A (2012) Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: a report from the Children’s Oncology Group. Pediatr Blood Cancer, 59, 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiacci E, Ladewig E, Schiavoni G, Penson A, Fortini E, Pettirossi V, Wang Y, Rosseto A, Venanzi A, Vlasevska S, Pacini R, Piattoni S, Tabarrini A, Pucciarini A, Bigerna B, Santi A, Gianni AM, Viviani S, Cabras A, Ascani S, Crescenzi B, Mecucci C, Pasqualucci L, Rabadan R & Falini B (2018) Pervasive mutations of JAK-STAT pathway genes in classical Hodgkin lymphoma. Blood, 131, 2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trama A, Botta L, Foschi R, Ferrari A, Stiller C, Desandes E, Maule MM, Merletti F & Gatta G (2016) Survival of European adolescents and young adults diagnosed with cancer in 2000–07: population-based data from EUROCARE-5. Lancet Oncol, 17, 896–906. [DOI] [PubMed] [Google Scholar]
- Travis LB & Gilbert E (2005) Lung cancer after Hodgkin lymphoma: the roles of chemotherapy, radiotherapy and tobacco use. Radiat Res, 163, 695–696. [PubMed] [Google Scholar]
- Tubiana M, Henry-Amar M, Carde P, Burgers JM, Hayat M, Van der Schueren E, Noordijk EM, Tanguy A, Meerwaldt JH & Thomas J (1989) Toward comprehensive management tailored to prognostic factors of patients with clinical stages I and II in Hodgkin’s disease. The EORTC Lymphoma Group controlled clinical trials: 1964–1987. Blood, 73, 47–56. [PubMed] [Google Scholar]
- von Wasielewski R, Mengel M, Fischer R, Hansmann ML, Hubner K, Franklin J, Tesch H, Paulus U, Werner M, Diehl V & Georgii A (1997) Classical Hodgkin’s disease. Clinical impact of the immunophenotype. Am J Pathol, 151, 1123–1130. [PMC free article] [PubMed] [Google Scholar]