Abstract
Objective
To identify early predictors of long-term overweight and obesity in pediatric liver transplant recipients.
Methods
Single-center, retrospective review of children who underwent liver transplant before age 6 years. Body Mass Index (BMI), weight, and height percentiles at transplant and post-transplant were calculated. BMI, weight gain trajectories, and failure-to-thrive (FTT) were examined as predictors of overweight/obesity at 3 and 5 years post-transplant.
Results
Children (n=70) were median 0.9 years at transplant. Median BMI percentile increased from 37 (IQR 12–73) at transplant to 83 (IQR 64–97) at 12 months, with median weight percentile 47 (IQR 26 – 67) and height percentile 9 (IQR 2–32). Overweight/obesity prevalence peaked at 3 years post-transplant (44%). Children who were overweight/obese at 3 years post-transplant were more likely to be overweight/obese at transplant, and at 6 and 12 months post-transplant (OR: 9.4, p=0.02, OR: 6.7 p= 0.013, OR: 6.4 p=0.007, respectively). The prevalence of overweight/obesity decreased to 26% at 5 years. Rapid weight gain post-transplant did not predict overweight/obesity at 3 or 5 years. Over 1/3 of children who were FTT at transplant were overweight/obese at 3 or 5 years, but FTT at transplant did not increase later obesity risk.
Conclusions
Most children gain weight rapidly after liver transplant. Nearly half of transplant recipients are overweight/obese at 3 years, but the prevalence decreases by 5 years. Those who become overweight/obese tend to do so within one year post-transplant, making this an important time to identify high-risk children and provide counseling.
Keywords: Obesity, Liver Transplantation, Post-transplant, Failure-to-thrive
INTRODUCTION
Long-term survival after pediatric liver transplant is excellent, exceeding 85% at 5 and 80% at 10 years. 1,2 Attention to the long-term outcomes of these patients—and short-term measures that are associated with long-term outcomes—is key. One important focus of post-transplant care is catch-up growth. Previous studies suggest that most children catch up to their peers in weight by 1 year post-transplant2, 3 but lag in height even 5 years post-transplant.4 This imbalance in weight and height catch-up may predispose children to overweight and obesity.
United Network for Organ Sharing (UNOS) data demonstrates that 5 to 10 years post-liver transplant, 20–50% of children are overweight or obese, with most obesity developing within 2 years post-transplant.5 Childhood obesity is a strong risk factor for adult obesity and obesity-related morbidity; it may thus have an important impact on pediatric liver transplant patients’ long-term outcomes.6 The identification of children at risk for persistent overweight/obesity may help with targeted interventions to prevent long-term morbidities in this population.
Post-transplant care has traditionally focused on supporting rapid catch-up growth because pre-transplant failure-to-thrive is so common. As cholestasis and ascites resolve, absorption and intake of calories improves. Metabolic demands decrease. Many children receive corticosteroids post-transplant, which increase appetite and adiposity. Parents and providers are thrilled that the child can finally gain weight successfully.
But if catch-up growth is over-zealous, short-term gains could have long-term risks. In children without liver transplant, rapid weight gain in infancy is a strong predictor of childhood obesity.7,8,9,10,11 Compared to slow gain, rapid weight gain in infancy is also associated with significantly elevated risk of obesity, higher total body fat, and higher percent abdominal fat in mid-adulthood.12
We hypothesized that rapid weight gain in the early post-transplant period, within the first 6 months, would be associated with later overweight/obesity in the pediatric liver transplant population. Since the post-transplant period is a time of intensive monitoring and counseling for families and children, it may be a feasible time to intervene for steady but not excessive weight gain—to optimize long-term outcomes.
METHODS
After obtaining approval from the UCSF Committee on Human Research (IRB approval 12-10290,10-01363), we conducted a retrospective review of all pediatric liver transplant recipients, ages 0–18 years at transplant, who received transplants between at UCSF. The cohort was limited to children less than six years of age at transplant and those receiving their first liver transplant between 01/01/1994 and 6/30/2013 (FIGURE S1, Supplemental Digital Content 1). Data on demographics, transplant, and post-transplant anthropometrics were extracted from clinical records and stored in a REDCap database, a secure, web-based application.13
BMI, weight, and height adjusted for age and gender were calculated at each timepoint of interest using World Health Organization (WHO) growth parameters and reported as percentiles. 14,15 Due to missing data on height at several timepoints, the denominators for height and BMI sometimes differed from the denominator used for weight at a given timepoint. WHO guidelines were used because they allow for BMI percentile calculation at less than 2 years of age. The WHO guidelines thus allowed consistency in our outcome reporting across timepoints. Children were categorized as underweight if their BMI percentile was less than 5th percentile for age and gender, normal weight if it at least 5th percentile but lower than 85th percentile, overweight if it was at least 85th but less than 95th percentile, and obese if it was 95th percentile or higher.16
We examined post-transplant BMI and weight gain trajectories as predictors of later overweight/obesity. Our primary outcomes were overweight/obesity at 3 years and 5 years post-transplant. We also evaluated 10 years post-transplant.
We examined growth trajectories by failure to thrive (FTT) status at transplant since these children are especially encouraged to gain weight rapidly after transplant. Though there is no consensus definition of FTT,17 we used the strictest definition of FTT – a weight percentile less than 3% – as a conservative measure.
Rapid weight gain was also examined a predictor of obesity. Rapid weight gain was defined as a change in weight-for-age Z score ≥ 0.67 within the first 6 months post-transplant. A change in weight-for-age Z score ≥ 0.67 is commonly used in the literature as this corresponds to crossing one major percentile line on a pediatric growth chart.9,10,18 No consensus exists on the optimal time interval over which to measure weight gain –prior studies use intervals ranging from 3–24 months18 – therefore we selected 6 months as a conservative interval which has been shown to predict future metabolic risk.19
Since the cohort spanned two decades, we divided the cohort into two eras: children who received their transplant between 1994–2004, and children transplanted between 2005 – 2013. We compared prevalence of post-transplant overweight/obesity between the two groups.
For post-transplant management, all subjects received methylprednisolone induction followed by prednisone taper after transplant. Mycophenolate mofetil was started at transplant and weaned off as tolerated, usually within 1–2 years. All were maintained on a calcineurin-inhibitor as primary maintenance immunosuppression. There were no protocolized changes in dose or duration during the study period.
Statistical analysis
Data on demographics and potential confounders were reported with descriptive statistics. BMI, weight, and height percentiles, as well as other continuous variables, are reported as median with interquartile range (25th–75th percentiles) to avoid skewing by outliers. Non-parametric Kruskal-Wallis testing was used to compare anthropometric percentiles by weight status. Chi-squared testing was used for descriptive statistics on categorical variables, and Mann-Whitney U-tests or t-tests for continuous variables depending on variable skew. Logistic regression was used to evaluate the association between rapid weight gain and other predictors with categorical outcomes: (1) overweight/obesity at 3 years, and (2) overweight/obesity at 5 years. Variables with p<0.05 in univariate analysis were considered for multivariate modeling. Final multivariate models were refined using backward stepwise selection. Stata 14 (College Park, TX) was used for statistical analysis.
RESULTS
The study cohort included 70 children aged 0–5 years at liver transplant. Most were ≤ 1 year of age at transplant. Biliary atresia was the most common diagnosis. (TABLE 1).
Table 1.
Characteristics of pediatric liver transplant recipient cohort (n=70)
| Gender | |
| Female | 43% |
| Male | 57% |
| Age at Transplant | |
| < 1 year | 57% |
| 1–2 years | 21% |
| 2–5 years | 21% |
| Race | |
| Caucasian | 39% |
| African-American | 4% |
| Asian | 23% |
| Other/Unknown | 34% |
| Ethnicity | |
| Latino | 31% |
| Diagnosis category* | |
| Biliary atresia | 46% |
| Metabolic disease | 21% |
| Cholestatic conditions | 13% |
| Acute liver failure | 11% |
| Tumor | 6% |
| Other | 3% |
| Donor Type | |
| Deceased-donor | 69% |
| Living-related | 31% |
| Organ transplant type | |
| Whole liver | 34% |
| Split liver (deceased donor) | 34% |
| Partial liver (living-related) | 31% |
| Ascites at transplant | |
| Yes, on exam | 33% |
| Yes, on imaging | 1% |
| No | 11% |
| Not recorded | 17% |
| Unknown | 37% |
| On diuretics at discharge from transplant admission | |
| Yes | 31% |
| No | 66% |
Metabolic liver disease includes alpha-1-antitrypsin deficiency, Crigler-Najjar syndrome, cystic fibrosis, glycogen storage disease, inborn errors in bile acid metabolism, neonatal hemochromatosis, primary hyperoxaluria, tyrosinemia, urea cycle defects, Wilson’s disease. Cholestatic conditions include Alagille syndrome, Byler disease, progressive intrahepatic cholestatic syndromes, total parenteral nutrition cholestasis, sclerosing cholangitis, and idiopathic cholestasis. Other liver disease includes congenital hepatic fibrosis, Budd-Chiari syndrome, autoimmune hepatitis cirrhosis, drug toxicity, hepatitis C cirrhosis, and unknown cirrhosis.
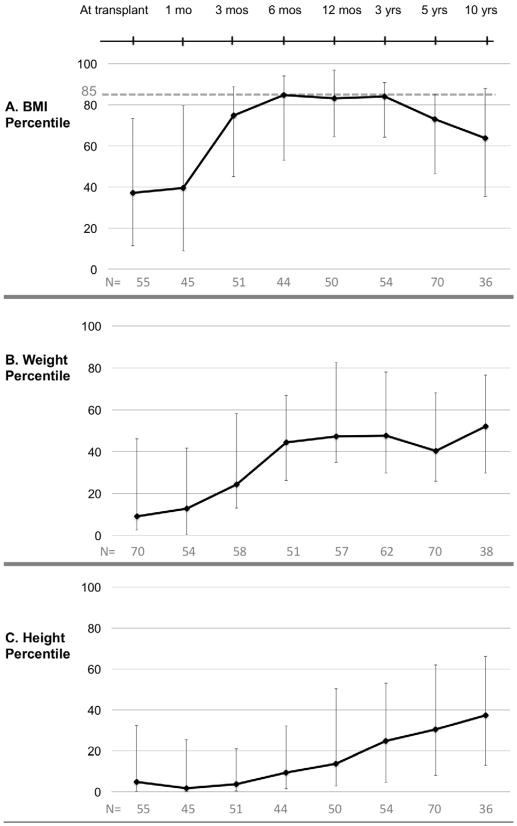
At transplant, the cohort’s median BMI was 37th percentile (IQR: 12 – 73), median weight 9th percentile (IQR: 3–46), and median height 5th percentile (IQR: 0.3–32) (FIGURE 1). By 6 months post-transplant, the cohort’s median BMI was 85th percentile (IQR: 53–94). Median weight was 44th percentile (IQR: 26–67), but median height was only 9th percentile (IQR: 2–32). Median BMI remained above the 80th percentile at 12 months, 1 year, and 3 years but dropped to 73rd percentile by 5 years. Median weight rose to 48th percentile (IQR: 30–78) at 3 years post-transplant, then peaked at 52nd percentile (IQR: 30–77) at 10 years. Meanwhile, median height percentile steadily increased beginning at 3 months and continuing throughout the duration of the study, reaching a maximum of 37th percentile at 10 years. (FIGURE 1C).
Figure 1.
BMI, weight and height percentile trajectories for pediatric liver transplant recipients (N=70). (A) BMI percentile (B) Weight percentile (C) Height percentile. Data points indicate medians; error bars indicate 25th to 75th percentile (interquartile range).
The prevalence of overweight/obesity peaked at 3 years post-transplant; 44% of children were overweight or obese (N=54 with 3 year BMI). At 5 years post-transplant, 26% of children (N=70 with 5 year BMI) were overweight or obese. Overweight/obesity at 3 and 5 years was not associated with age, gender, race, diagnosis, failure to thrive at transplant (weight <3rd percentile), liver donor (living vs. deceased), or liver transplant type (whole vs. split vs. partial) (data not shown). Overweight/obesity prevalence did not vary by era of transplant, although our sample was small. Latino ethnicity was significantly associated with overweight/obesity at 5 years (OR: 3.0 p=0.05) but not at 3 years. Children who were overweight/obese at transplant were more likely to be overweight/obese at 3 years (OR: 9.4, p=0.02) but not at 5 years.
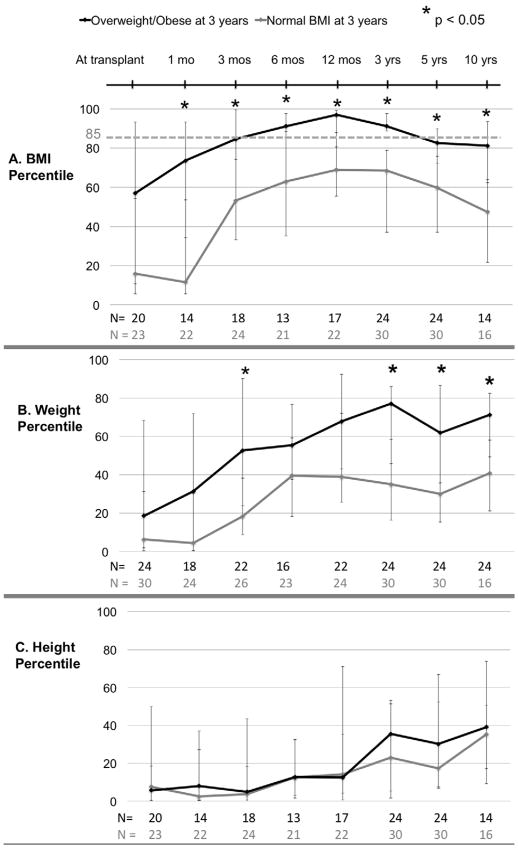
The next analysis focused only on the children in the cohort who had BMI data available at 3 years (N=54) and grouped children by whether they were overweight/obese or normal weight at 3 years post-transplant. For both those overweight/obese and those with normal BMI at 3 years, BMI percentiles increased steadily over the first year post-transplant and then gradually decreased (FIGURE 2A). Overweight/obesity at 6 months and 12 months did predict overweight/obesity at 3 years (OR: 6.7, p = 0.013 and OR: 6.4, p=0.007, respectively). The two groups had similarly steady increases in median weight percentiles for the first 6 months. After 6 months, the children with normal BMI percentile at 3 years had a plateau in BMI percentile, while the group that was overweight/obese at 3 years had a continued rise from 6 months to 3 years. (FIGURE 2B) The height percentile trajectories of the two groups were similar, and the median remained below 40th percentile through follow-up. (FIGURE 2C).
Figure 2.
BMI, weight and height percentile trajectories for pediatric liver transplant recipients by weight status at 3 years post-transplant (N= 54 with BMI available at 3 years post-transplant). (A) BMI percentile (B) Weight percentile (C) Height percentile. Data points indicate medians; error bars indicate 25th to 75th interquartile range. Asterisks (*) indicate p < 0.05.
However, of children who were overweight/obese at 3 years post-transplant, 58% had normal BMI percentiles at 5 years and 64% did at 10 years. Ten children were persistently overweight/obese at 3 and 5 years. Interestingly, 40% were overweight/obese at transplant and 50% were failure to thrive at transplant. Of the children who were FTT at transplant and then persistently overweight/obese post-transplant, 67% become overweight or obese by 12 months post-transplant. Compared to children who were overweight/obese at 3 years but not at 5 years, the persistently overweight/obese children had a lower median BMI at transplant (33th percentile vs. 57th) and their median BMI percentile increased more rapidly (65th vs. 39th) over the first year post-transplant.
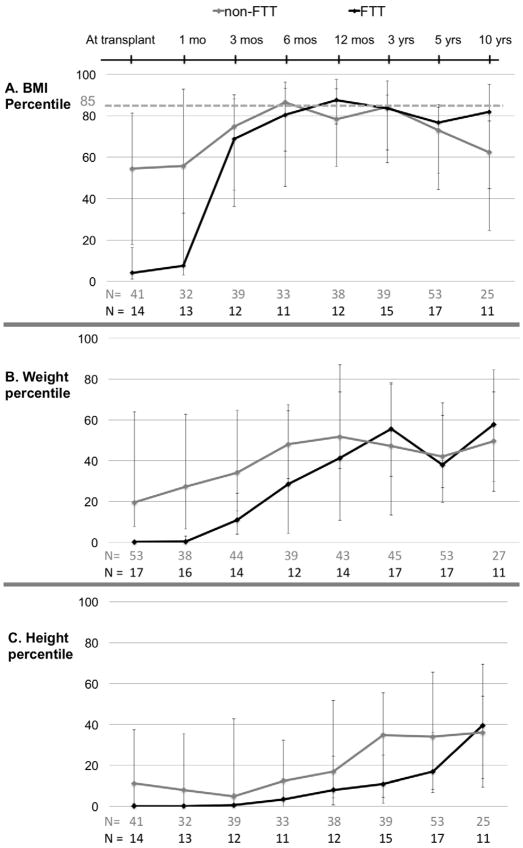
Of the 70 children studied, 27% were FTT at transplant (data not shown). Of those FTT at transplant with BMI data available, 44% were overweight or obese at 3 years post-transplant (n=16) and 32% were overweight or obese at 5 years (n=19). FTT at transplant was not significantly associated with obesity at 3 or 5 years in univariate analysis (data not shown). Over half (54%) of FTT children achieved a BMI above the 50th percentile within 3 months post-transplant. (FIGURE 3) From 3 months to 5 years post-transplant, the BMI percentile trajectories of FTT and non-FTT children were similar. Both groups had a peak median BMI above the 85th percentile, at 6 months for non-FTT children and 12 months for the FTT group (Figure 3A). At 10 years post-transplant, however, the FTT group’s median BMI percentile remained at the 82nd percentile. The median height percentile of the FTT group did not catch up to the non-FTT group until 10 years post-transplant. Median height percentiles of both groups remained below the 50th percentile throughout study. (FIGURE 3C)
Figure 3.
BMI, weight and height percentile trajectories for pediatric liver transplant recipients by failure-to-thrive (FTT) status at transplant (N=70). (A) BMI percentile (B) Weight percentile (C) Height percentile. Data points indicate medians; error bars indicate 25th to 75th interquartile range.
We considered rapid weight gain as a predictor of later overweight and obesity in univariate and multivariate regression. In this relatively small sample of children, early rapid weight gain was not a significant predictor of being overweight/obese 3–5 years post-transplant after controlling for age, gender, race, ethnicity, diagnosis, liver donor or transplant type or when stratifying by age at transplant (age <1 year at transplant compared to 1–5 years at transplant). (Data not shown)
DISCUSSION
In this retrospective analysis, almost half of pediatric liver transplant recipients were overweight/obese at 3 years post-transplant, but that the prevalence decreased by five years. The children overweight/obese at 3 years were likely to have a BMI percentile exceeding the 85th percentile by 6–12 months post-transplant. However, early post-transplant obesity was not persistent for all children. Persistent overweight/obesity may also be modulated by factors including FTT status and rate of weight gain post-transplant. Importantly, linear growth catch-up remained a challenge for all children post-liver transplant, regardless of FTT status at transplant, rate of weight gain and BMI percentile post-transplant.
While the findings of obesity prevalence and poor linear catch-up growth have been reported in prior studies, 20,21,22 this study further shows that BMI and weight percentiles rise dramatically in the post-transplant period but typically plateau within the first year post-transplant. BMI percentile peaked in our cohort around 1 year post-transplant. However, the weight percentile trajectories did not plateau at the same time for all children. Children who were normal/underweight at 3 years tended to plateau in weight percentile by 6 months post-transplant while children who were overweight/obese by 3 years post-transplant continued to rise. These findings highlight that the first and second years post-transplant may be an important time to identify children at risk for later overweight/obesity – and provide counseling to families on obesity prevention strategies during this time of still-intensive follow-up.
Concern about catch-up growth is particularly pertinent for children who are FTT at transplant. We found that these children tend to catch up to non-FTT children in BMI percentile rapidly. But their catch-up growth in height was slower. Consequently, FTT children were still at risk for long-term and persistent overweight/obesity despite their low weight at transplant. These data underscore the importance of examining BMI for age and gender percentiles separately from weight and height percentile trajectory curves.
This study was limited by its retrospective nature and relatively small sample size. Missing data on heights or weights prevented calculation of BMI percentile for all patients at every timepoint, further limiting sample size. We could not extract exact stopping dates for corticosteroids accurately from the medical record to examine its role in weight gain or stabilization, although our standard practice is to stop steroids within 3 months after transplant and there were not protocolized changes in dose or duration during the study period.
In conclusion, most children gain weight rapidly post-transplant. Those who become overweight/obese tend to so within the first year post-transplant. Because weight gain outpaces linear growth for most children and overweight/obesity in the early post-transplant period is common, careful nutritional counseling and close follow-up is critical. Providers should track BMI percentiles and trajectories, in addition to weight and height charts in the years post-liver transplant. Early detection of overweight and obesity within the first few years post-transplant may allow for effective intervention.
Because childhood overweight/obesity can have long-term implications for these children,23 further information is needed to understand both the risk factors and the implications of transient and persistent childhood overweight/obesity in pediatric liver transplant patients. Future studies should seek to better characterize metabolic changes (e.g., impaired glucose tolerance, dyslipidemia) in children with post-transplant overweight/obesity compared to children with normal BMI. Future research is also needed to examine whether variations in dose or duration of steroids are associated with post-transplant obesity. Larger, multicenter studies to allow more detailed investigation of rapid weight gain post-transplant would help optimize long-term outcomes for pediatric liver transplant recipients.
Supplementary Material
Supplemental Figure S1: Selection of study cohort
What is known?
For most children, post-transplant weight gain outpaces linear growth.
Childhood obesity is a strong risk factor for adult obesity and obesity-related morbidity.
What is new?
Early post-transplant obesity is common but not necessarily persistent for all children.
Children who are overweight/obese 3 years after transplant have higher BMI percentiles at 1 year post-transplant.
Children who are failure-to-thrive at transplant remain at risk of overweight/obesity after transplant.
Footnotes
Conflicts of Interests: none
Disclosure of funding: This work was supported by grants from the University of California San Francisco Resource Allocation Program for Trainees (RAPTr, Swenson) and by the NIH-NIDDK (K23 DK0990253-A101, Perito).
LITERATURE CITED
- 1.Ng VL, Fecteau A, Shepherd R, Magee J, Bucuvalas J, Alonso E, et al. Outcomes of 5-year survivors of pediatric liver transplantation: Report on 461 children from a North American multicenter registry. Pediatrics. 2008 Dec;122(6):e1128–35. doi: 10.1542/peds.2008-1363. [DOI] [PubMed] [Google Scholar]
- 2.Ng VL, Alonso EM, Bucuvalas JC, Cohen G, Limbers CA, Varni JW, et al. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: Report of the studies of pediatric liver transplantation experience. J Pediatr. 2011 Dec 20; doi: 10.1016/j.jpeds.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saito T, Mizuta K, Hishikawa S, Kawano Y, Sanada Y, Fujiwara T, et al. Growth curves of pediatric patients with biliary atresia following living donor liver transplantation: Factors that influence post-transplantation growth. Pediatric Transplantation. 2007;11:764–770. doi: 10.1111/j.1399-3046.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 4.McDiarmid SV, Gornbein JA, DeSilva PJ, et al. Factors affecting growth after pediatric liver transplantation. Transplantation. 1999 Feb 15;67(3):404–411. doi: 10.1097/00007890-199902150-00011. [DOI] [PubMed] [Google Scholar]
- 5.Perito ER, Glidden D, Roberts JP, Rosenthal P. Overweight and obesity in pediatric liver transplant recipients: Prevalence and predictors before and after transplant, United Network for Organ Sharing data, 1987–2010. Pediatr Transplant. 2012;16(1):41–9. doi: 10.1111/j.1399-3046.2011.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 7.Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Davey Smith G, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatric and Perinatal Epidemiology. 2012;26:19–26. doi: 10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 8.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Arch Dis Child. 2012;97(12):1019–1026. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monteiro POA, Victora CG. Rapid growth in infancy and childhood and obesity in later life – a systematic review. Obesity Reviews. 2005;6:143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 10.Ong KK, Loos RJF. Rapid infancy weight gain and subsequent obesity: Systematic reviews and hopeful suggestions. Acta Paediatrica. 2006;95:904–8. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 11.Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, Campbell K. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obesity Reviews. 2018;19:321–332. doi: 10.1111/obr.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demerath EW, Reed D, Choh AC, Soloway L, Lee M, Czerwinski SA, et al. Rapid Postnatal Weight Gain and Visceral Adiposity in Adulthood: The Fels Longitudinal Study. Obesity. 2009;17:2060–2066. doi: 10.1038/oby.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 15.Vidmar SI, Cole TJ, Pan H. Standardizing anthropometric measures in children and adolescents with functions for egen: Update. Stata Journal. 2013 Jan 1;13(2):366–78. [Google Scholar]
- 16.Barlow SE Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 17.Olsen EM. Failure to thrive: still a problem of definition. Clinical pediatrics. 2006 Jan;45(1):1–6. doi: 10.1177/000992280604500101. [DOI] [PubMed] [Google Scholar]
- 18.Eckhardt CL, Eng H, Dills JL, Wisner KL. The prevalence of rapid weight gain in infancy differs by the growth reference and age interval used for evaluation. Annals of human biology. 2016;43(1):85–90. doi: 10.3109/03014460.2014.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekelund U, Ong KK, Linné Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rössner S. Association of weight gain in infancy and early childhood with metabolic risk in young adults. The Journal of Clinical Endocrinology & Metabolism. 2007 Jan 1;92(1):98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- 20.Saito T, Mizuta K, Hishikawa S, Kawano Y, Sanada Y, Fujiwara T, et al. Growth curves of pediatric patients with biliary atresia following living donor liver transplantation: Factors that influence post-transplantation growth. Pediatric Transplantation. 2007;11:764–770. doi: 10.1111/j.1399-3046.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 21.McDiarmid SV, Gornbein JA, DeSilva PJ, et al. Factors affecting growth after pediatric liver transplantation. Transplantation. 1999 Feb 15;67(3):404–411. doi: 10.1097/00007890-199902150-00011. [DOI] [PubMed] [Google Scholar]
- 22.Ng VL, Fecteau A, Shepherd R, Magee J, Bucuvalas J, Alonso E, et al. Outcomes of 5-year survivors of pediatric liver transplantation: Report on 461 children from a North American multicenter registry. Pediatrics. 2008 Dec;122(6):e1128–35. doi: 10.1542/peds.2008-1363. [DOI] [PubMed] [Google Scholar]
- 23.Perito ER, Lustig RH, Rosenthal P. Metabolic Syndrome Components After Pediatric Liver Transplantation: Prevalence and the Impact of Obesity and Immunosuppression. Am J Transplant. 2016 Jun;16(6):1909–16. doi: 10.1111/ajt.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: Selection of study cohort