SUMMARY
The primary causative agent of eosinophilic meningoencephalitis (EoM) in endemic regions is the nematode Angiostrongylus cantonensis. The occurrence of EoM was previously restricted to countries in Southeast Asia and the Pacific Islands; however, more recently, it has been reported from other regions, including Brazil. The commonly used diagnosis is detection of specific antibody reactivity to the 31 kDa antigen, which is derived from female worm somatic extracts. Here we report the occurrence of cross-reactivity to this antigen in sera from other parasitic infections, especially those that may cause EoM, such as gnathostomiasis, toxocariasis, hydatidosis and strongyloidiasis. We also demonstrated that the cross-reactivity, in part, is dependent of the concentration of antigen used in Western blot assays. We discuss the importance of these findings on the interpretation of this test.
Keywords: Angiostrongyliasis diagnosis, 31 kDa antigen, cross-reactivity, eosinophilic meningoencephalitis
INTRODUCTION
Eosinophilic meningoencephalitis (EoM) is an acute illness defined by the presence of at least 10% eosinophils in the cerebrospinal fluid (CSF) total leukocyte count (Sawanyawisuth et al. 2009). Different conditions may cause EoM, including neoplastic diseases, drug use, as well as infectious agents such as fungi, bacteria and helminths (Graeff-Teixeira et al. 2009). However, the most common causative agent in endemic regions is the foodborne nematode Angiostrongylus cantonensis.
Humans can acquire the infection by ingesting raw or undercooked infected snails/slugs and/or paratenic hosts containing infective third stage larvae (L3) and/or eating contaminated foods, particularly leafy green vegetables containing L3 (Wang et al. 2008). Diagnosis of EoM in endemic areas is based on a history of eating raw mollusks, or exposure to live mollusks, clinical symptoms such as severe headache, neck stiffness and fever, and CSF eosinophilia. Definitive laboratory diagnosis is made when larvae are found in CSF examination, but this finding is very rare (Sawanyawisuth et al. 2009).
Clinical symptoms and CSF eosinophilia are not specific to A. cantonensis infection, immunodiagnosis can play a crucial role in diagnosing A. cantonensis EoM (Morassutti et al. 2014). There are no WHO recommendations for the immunodiagnosis of A. cantonensis infection, therefore immunodiagnostic methods employed vary. Most of the reported clinical and outbreak associated cases have based the diagnosis on a test detecting a circulating antibody reactive to a protein with an estimated molecular weight of 31 kDa (Slom et al. 2002; Lindo et al. 2004; Lima et al. 2009; Espírito-Santo et al. 2013).
In the last few years the epidemiology and the geographic distribution of the infection has changed. Once angiostrongyliasis was known to be restricted to some Pacific Islands and Southeast Asian countries, it has recently been described in several other parts of the world (Aguiar Prieto et al. 1981; Kim et al. 2002; Lima et al. 2009), using the 31 kDa antigen serological test (Morassutti et al. 2014). Here we report an evaluation of the specificity of 31 kDa antigen using sera from cases with other parasitic infections, including other infectious causes of EoM.
MATERIAL AND METHODS
Defined sera and pooled serum samples
Four sets of defined sera were used: (1) 25 sera from cases of A. cantonensis infection from CDC. These cases were defined based on the presence of clinical symptoms and signs and immunoreactivity with the 31 kDa antigen; (2) 19 sera from cases of A. costaricensis infection based on the presence of clinical symptoms and histopathological signs; (3) a set of control sera, consisting of 47 sera from US residents with no known illness at the time of serum collection; and (4) a convenience panel for evaluating cross-reactivity, consisting of infection sera from confirmed cases of neurocysticercosis (n = 2); filariasis (n = 1), hydatidosis (n = 3); schistosomiasis (n = 4); toxocariasis (n = 8); baylisascariasis (n = 2); gnathostomiasis (n = 4); strongyloidiasis (n = 4); toxoplasmosis (n = 4); trichinellosis (n = 4), which were all confirmed by a combination of clinical and serological findings. Sera from cases of ascariasis (n = 4); trichuriasis (n = 3); and hookworm infection (n = 8), all defined by microscopic detection of eggs in stool were also used.
Pooled serum reagents: A pool of ‘normal’ human serum (NHS) was prepared from 20 serum samples collected from people with no travel history outside the USA. An A. cantonensis-positive serum pool was prepared by combining in equal volumes of 20 serum samples from cases of angiostrongyliasis, defined by clinical and serological data (reactivity with 31 kDa antigen). A Toxocara positive serum pool was prepared using eight sera that were collected from cases with clinical toxocariasis and positive reactivity in an enzyme-linked immunosorbent assay (ELISA) developed to diagnose that infection (de Savigny et al. 1979).
Antigen preparation
A somatic A. cantonensis extract was obtained from female worms collected from rats. Forty adult worms were macerated in liquid nitrogen and homogenized in phosphate buffered saline (PBS), pH 7·4, containing protease inhibitors (Sigma-Aldrich, P-2714). The suspension was centrifuged at 12 000 × g for 1 h at 4 °C and the supernatants used as the antigen preparation. Protein concentrations were determined using the Qubit® Protein Assay Kit (Invitrogen). Antigen titration was performed by loading different concentrations of the antigen extract in 12 polyacrylamide gels with 100 mM dithiothreitol (DTT) as a reducing agent (R) or without DTT (NR) and separated using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The purified 31 kDa antigen provided by Dr P. Eamsobhana was used as a control.
Immunoblot assay
The A. cantonensis somatic worm extract was resolved using SDS-PAGE and then electro-transferred onto nitrocellulose membranes. The membranes were washed 3 times with PBS-T (0·05% Tween) and blocked with 5% skim-milk or bovine serum albumin (BSA) 0·5% for 1 h at room temperature. For specificity analysis the nitrocellulose membranes were cut into 2 mm width strips. Nitrocellulose membranes were incubated for 1 h with different sera (diluted 1:200 in PBS-T). After the membranes were washed as described above, they were probed with a secondary peroxidase-conjugated goat anti-human IgG antibody (Sigma, St. Louis, MO) (diluted 1:8000 in PBS-T) for 1 h at room temperature. The precipitating peroxidase substrate, Diaminobenzidine (DAB)/H2O2 (Sigma) (0·05% DAB – 0·015% H2O2 in PBS, pH 7·4) was added as a developer reagent to visualize the immune complexes.
RESULTS
Optimization of Western blot (WB) antigen concentration
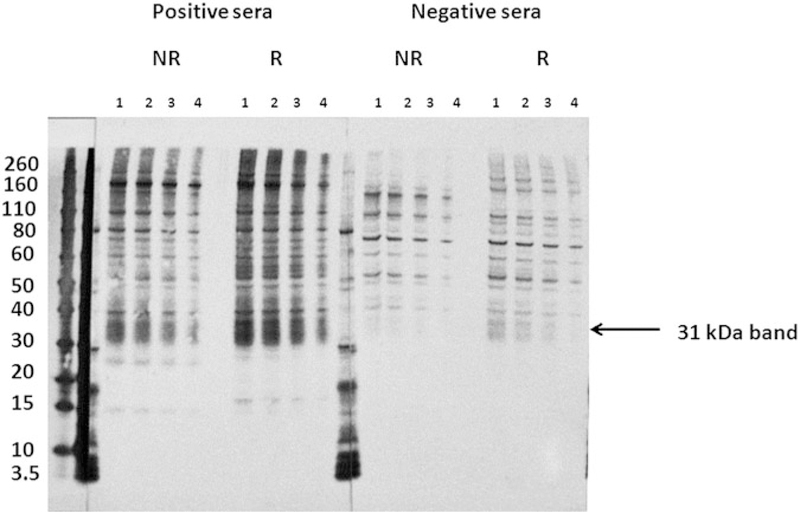
Different concentrations of the adult worm extract were loaded to determine the optimal protein concentration using a pools of negative (the NHS pool) and positive (the A. cantonensis infection pool) sera under reducing or non-reducing conditions. The 31 kDa antigen was recognized specifically by the A. cantonensis infection serum pool (Fig. 1) and not by the NHS only at a concentration of 800 ng per well under non-reducing conditions. The 31 kDa antigen was strongly recognized by NHS at all concentrations tested when the reducing agent was employed.
Fig. 1.
Antigen titration and the effect of DTT as the reducing agent. Western blot analysis of the somatic antigen extract of A. cantonensis worms. Lanes 1–4 represent a titration of the antigen extract 1–3·2 µg per well; 2–2·4 µg per well; 3–1·6 µg per well, and 4–0·8 µg per well) and was treated with SDS and with DTT (R) or without DTT (NR). The blots were probed with positive (A. cantonensis) and negative (uninfected) pooled serum samples. DTT, dithiothreitol; SDS, sodium dodecyl sulphate.
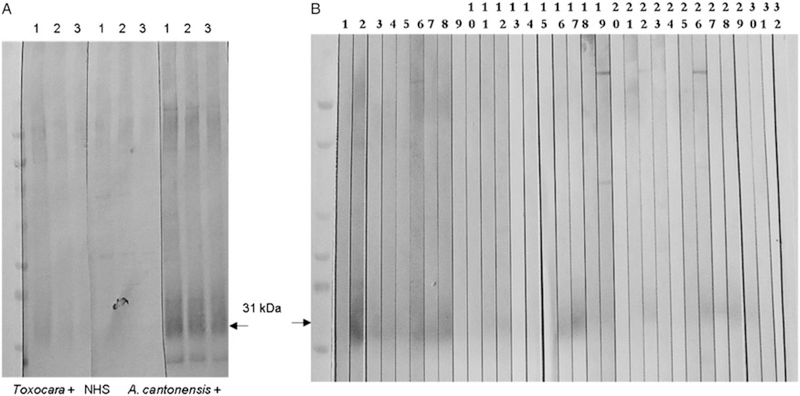
After establishing the optimal antigen concentration for the positive and negative serum pools, we looked at the reactivity of the Toxocara positive serum pool. The 31 kDa band was recognized by a pool of Toxocara positive sera at concentrations of 100 ng per well and higher and was negative only at 50 ng per well (Fig. 2A). We tested the Toxocara positive sera individually and one out of eight sera was reactive with the 31 kDa protein even after the antigen concentration was decreased to 35 ng per well (Fig. 2B). Based on this observation, we chose a concentration of 35 ng per well of the antigen extract for a more in depth analysis of test specificity.
Fig. 2.
Western blot analysis using sera with potential cross-reactivity. (A) The crude A. cantonensis worms antigen extract was loaded at a concentration of: 100 ng per well (lane 1), 75 ng per well (lane 2) and 50 ng per well (lane 3). The blots were incubated with pooled serum samples prepared from cases with Toxocara infection, NHS and A. cantonensis infection. (B) Individual Western blot strips were prepared from the crude antigen extract loaded at a concentration of 35 ng per well. The strips were tested against the following sera: 1-NHS; 2–8 cases of Echinococcus infection; 9–12 Strongyloides infection; 13–16 Toxocara infection; 17–32 Angiostrongylus infection. NHS, normal human sera.
Cross-reactivity analysis
To perform a more rigorous evaluation of specificity, we evaluated a panel of sera from cases with heterologous parasitic infections using 35 ng per well of antigen. Cross reactions occurred with sera from cases of Gnathostoma spp. infections (25%, 1/4); hydatid disease (100%, 3/3); hookworm (13%, 1/8); strongyloidiasis (50%, 2/4); trichinellosis (75%, 3/4); of Trichuris trichiura infections (33%, 1/3) and with normal human sera (2%, 1/47) (Table 1). The sensitivity of the 31 kDa antigen was affected at this concentration (35 ng per well); only 24% (6/25) of sera from cases with A. cantonensis infections sera were positive and the sensitivity was 63% (12/19) when sera from A. costaricensis infections were examined.
Table 1.
31 kDa antigen (35 ng per well) cross-reactivity with different sera
| Serum sample | 31 kDa positive/N |
|---|---|
| Cerebral Angiostrongyliasis | 6/25 |
| Abdominal Angiostrongyliasis | 12/19 |
| Ascariasis | 0/4 |
| Baylisascariasis | 0/2 |
| Cysticercosis | 0/2 |
| Filariasis | 0/1 |
| Gnathostomiasis | 1/4 |
| Hydatidosis | 3/3 |
| Hookworm infection | 1/8 |
| Normal human sera | 1/47 |
| Schistosomiasis | 0/4 |
| Strongyloidiasis | 2/4 |
| Toxocariasis | 1/8 |
| Toxoplasmosis | 0/4 |
| Trichinellosis | 3/4 |
| Trichuriasis | 1/3 |
DISCUSSION
The 31 kDa antigen test is very cross reactive, even at low protein concentrations and results of testing for angiostrongyliasis as a cause of EoM must be interpreted in light of that fact. The 31 kDa molecule has been in use in different forms for over two decades, with varying sensitivities and specificities reported. Nuamtanong (1996) tested crude worm antigens of A. cantonensis by ELISA and WB and found that the 31 kDa band was 100% sensitive and 66·8% specific. The authors observed cross-reactivity with sera from several helminth infections, including Gnathostoma spp., Toxocara spp., Echinococcus granulosus, and hookworm. This cross-reactivity could be due to the concentration of protein loaded in the immunoblot assay (20 µg per well). As observed here, specificity of the 31 kDa was greatly dependent on the amount of protein used in the WB assay (Figs 1 and 2). Only after decreasing the antigen to 0·8 µg per well were reactions with normal human sera eliminated in our study.
Eamsobhana et al. (2001) purified the 31 kDa component by electroelution from polyacrylamide gels. The sensitivity and specificity observed was 100% when loading 5 µg per well of purified protein was used in a larger gel system (16 cm long) in immuno-blotting and 1 µg mL−1 in ELISA. These authors did not detect cross-reactivity with infection sera from other parasitic infections such as gnathostomiasis, toxocariasis, filariasis, paragonimiasis, cysticercosis and malaria. In our study, using a mini-gel system (7 cm long), we found that even after decreasing the antigen concentration to 35 ng per well, cross-reactivity with other helminth infected sera still occurred and consequently, at this concentration the sensitivity to A. cantonensis and to A. costaricensis positive sera decreased to 24 and 63%, respectively (Table 1).
The discrepancy between the previous data and that presented here could be explained because of genetic variation among different geographic isolates and by consequence variation on the antigenicity of molecules as observed with the antigen of Echinococcus multilocularis, Em18, in which cross-reactivity is dependent on the epitope composition (Jiang et al. 2004).
A frequent problem encountered in assay development studies is the pedigree of the clinical samples used. For instance, a panel of sera initially identified as reactive with Angiostrongylus antigens may also contain immunoglobulins reactive to Toxocara spp., as the patient could have been exposed to or co-infected by the nematodes, either currently or in the past. Antibodies are known to persist in many helminthic infections even if symptoms are no longer present. In our study we cannot rule our concomitant or past infections with soil transmitted helminths and other parasitic infections.
Another important variable affecting the outcome of an antibody detecting method is the use of reducing agents in antigen preparation, as observed here (Fig. 1). There was a stronger recognition of the 31 kDa antigen by both true positive sera (sera from A. cantonensis infections) and NHS, when the antigen extract was pre-treated with reducing agent (DDT). Reduction of disulphide bonds by DTT may reveal cross-reactive epitopes, not accessible in non-reduced antigens.
A proteomic investigation of the 31 kDa band revealed that it is actually seen as 4 acidic spots by 2D electrophoresis and it is composed of at least 2 proteins: a 14–3-3 protein and a nascent polypeptide-associated complex (NAC) domain-containing protein (Morassutti et al. 2012). Those two identified proteins were expressed in a prokaryotic system but the recombinant proteins were not recognized specifically by sera from cases of Angiostrongylus infection (Morassutti et al. 2013). In fact the recognition of the 31 kDa antigen has been shown to be dependent of carbohydrate moieties (Veríssimo et al. 2016). In contrast, previous findings showed that antibody recognition of the 31 kDa antigen is not dependent on the presence of carbohydrates (Eamsobhana et al. 1998). In that study, a large amount of antigen was used (10 µg per well) in WB analysis, so the in-gel m-periodate treatment may not have been able to oxidize all of the glycan moieties in the sample.
Many of the sera that were cross-reactive with 31 kDa antigen, are from infections that have the potential to cause eosinophilic meningoencephalitis, such as toxocariasis, hydatidosis, trichinellosis, gnathostomiasis, and strongyloidiasis (Graeff-Teixeira et al. 2009). A reliable diagnosis can be made based on the clinical manifestations of some of these infections, which are somewhat different and are distinguishable from angiostrongyliasis, one example is gnathostomiasis causing haemorrhagic manifestations in the CSF. However, cases where no diagnostic feature is present, differential diagnosis should always include primary tests for toxocariasis, trichinellosis and strongyloidiasis. Also, other kind of exams may help elucidate the infective agent, image analysis of the brain, for instance, would disclose cysts formation caused by cysticercosis and hydatidosis. Image analysis of EoM due to Angiostrongylus is not well distinguishable from other causes, some alterations may be found using gadolinium chelate in magnetic resonance, but it would be present in 45% of patients (Jin et al. 2005, 2008).
The most cases of angiostrongyliasis have included extensive investigation to other possible causes of EoM and field examination of mollusks,combined to molecular methods for confirmation of A. cantonensis infection (Morassutti et al. 2014). The real time polymerase chain reaction (PCR) developed by CDC has been shown to be able to detect specific DNA of A. cantonensis of less than one larva in CSF examinations (Qvarnstrom et al. 2016). However, some infected patients may have negative PCR and 31 kDa antigen. This is important to note that serologic confirmation responses during acute infection may be negative or only minimally apparent, but are often positive or clearly apparent several weeks after acute infection, as observed by Espírito-Santo et al. (2013) in a patient only positive for 31 kDa antigen after a while of infection.
In conclusion, these data draw attention to the fact that the serological diagnosis of EoM should not be based on only one or a few known antigenic molecules. Physicians should raise suspicion of angiostrongyliasis when consumption of mollusks or travel to endemic areas is known. The 31 kDa antigen should be used complemented with other tests, which should include the other causes of EoM. To preserve sensitivity of the 31 kDa antigen, 50 ng per well should be used for screening and once observed positivity, confirmation may be achieved by CSF examination for specific DNA of A. cantonensis. Future efforts should focus incorporating a panel of antigens that represent different potential causes of EoM.
ACKNOWLEDGEMENTS
We thank Dr Praphathip Eamsobhana, Department of Parasitology, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand, for careful revision of this manuscript and help regard the 31 kDa antigen.
FINANCIALSUPPORT
Financial support was provided by Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico do Brasil (CNPq, grant number 307005/2014-3 to C.G.T.) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Edital 32 (Parasitologia Básica), to C. G.T.
REFERENCES
- Aguiar Prieto PH, Pascual Gispert J, Dumenigo B, Perera De Puga G and Gálvez Oviedo MD (1981). Angiostrongylus cantonensis. Intermediate hosts in the 2 Havana provinces. Revista Cubana de Medicina Tropical 33, 173–177. [PubMed] [Google Scholar]
- de Savigny DH, Voller A and Woodruff AW (1979). Toxocariasis: serological diagnosis by enzyme immunoassay. Journal of Clinical Pathology 32(3), 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamsobhana P, Tungtrongchitr A, Wanachiwanawin D, Yong HS and Mak JW (1998). Characterization of 31 kda specific Antigen from Parastrongylus cantonensis (Nematoda: Metastrongylidae). Journal of International Medical Research 2(1), 9–12. [Google Scholar]
- Eamsobhana P, Yoolek A and Suvouttho S (2001). Purification of a specific immunodiagnostic Parastrongylus cantonensis antigen by electroelution from SDS-polyacrylamide gels. Southeast Asian Journal of Tropical Medicine and Public Health 32, 308–313. [PubMed] [Google Scholar]
- Espírito-Santo MC, Pinto PL, Mota DJ and Gryschek RC (2013). The first case of Angiostrongylus cantonensis eosinophilic meningitis diagnosed in the city of São Paulo, Brazil. Revista do Instituto de Medicina Tropical de São Paulo 55, 129–132. [DOI] [PubMed] [Google Scholar]
- Graeff-Teixeira C, Da Silva AC and Yoshimura K (2009). Update on eosinophilic meningoencephalitis and its clinical relevance. Clinical and Microbiology Reviews 22, 322–348. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Stewart TB, Bauer RW and Mitchell M (2002). Parastrongylus (=Angiostrongylus) cantonensis now endemic in Louisiana wildlife. Journal for Parasitology 88, 1024–1026. [DOI] [PubMed] [Google Scholar]
- Jiang L, Xu XN, Li X, Xue HC and Feng Z (2004). Identification of the immunodominant regions of the Em18 antigen and improved serodiagnostic specificity for alveolar echinococcosis. Parasite Immunology 10, 377–385. [DOI] [PubMed] [Google Scholar]
- Jin E, Ma D, Liang Y, Ji A and Gan S (2005). MRI findings of eosinophilic myelomeningoencephalitis due to Angiostrongylus cantonensis. Clinical Radiology 60, 242–250. [DOI] [PubMed] [Google Scholar]
- Jin EH, Ma Q, Ma DQ, He W, Ji AP and Yin CH (2008). Magnetic resonance imaging of eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis following eating freshwater snails. Chinese Medical Jounal 121, 67–72. [PubMed] [Google Scholar]
- Lima AR, Mesquita SD, Santos SS, Aquino ER, Rosa LAR, Duarte FS, Teixeira AO, Costa ZR and Ferreira ML (2009). Alicata disease: neuroinfestation by Angiostrongylus cantonensis in Recife, Pernambuco, Brazil. Arquivos de Neuro-Psiquiatria 67, 1093–1096. [DOI] [PubMed] [Google Scholar]
- Lindo JF, Escoffery CT, Reid B, Codrington G, Cunningham-Myrie C and Eberhard ML (2004). Fatal autochthonous eosinophilic meningitis in a Jamaican child caused by Angiostrongylus cantonensis. American Journal of Tropical Medicine and Hygiene 70, 425–428. [PubMed] [Google Scholar]
- Morassutti AL, Levert K, Perelygin A, Da Silva AJ, Wilkins P and Graeff-Teixeira C (2012). The 31-kDa antigen of Angiostrongylus cantonensis comprises distinct antigenic glycoproteins. Vector Borne and Zoonotic Diseases 12, 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morassutti AL, Perelygin A, Levert K, Lin SC, Lee YM, Da Silva AJ, Wilkins PP and Graeff-Teixeira C (2013). Expression of recombinant antigenic proteins from Angiostrongylus cantonensis: a brief report. Hawai’i Journal of Medicine & Public Health 72, 58–62. [PMC free article] [PubMed] [Google Scholar]
- Morassutti Al Thiengo Sc, Fernandez M, Sawanyawisuth K and Graeff-Teixeira C (2014). Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Memorias do Instituto Oswaldo Cruz 109(4), 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuamtanong S (1996). The evaluation of the 29 and 31 kDa antigens in female A. cantonensis for serodiagnosis of human angiostrongyliasis. Southeast Asian Journal of Tropical Medicine and Public Health 27, 291–296. [PubMed] [Google Scholar]
- Qvarnstrom Y, Xayavong M, da Silva AC, Park SY, Whelen AC, Calimlim PS, Sciulli RH, Honda SA, Higa K, Kitsutani P, Chea N, Heng S, Johnson S, Graeff-Teixeira C, Fox LM and da Silva AJ (2016). Real-Time polymerase chain reaction detection of Angiostrongylus cantonensis DNA in cerebrospinal fluid from patients with eosinophilic meningitis. American Journal of Tropical Medicine and Hygiene 94(1), 176–181. doi: 10.4269/ajtmh.15-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawanyawisuth K, Takahashi K, Hoshuyama T, Senthong V, Limpawattana P, Intapan PM, Wilson D, Tiamkao S, Jitpimolmard S and Chotmongkol V (2009). Clinical factors predictive of encephalitis caused by Angiostrongylus cantonensis. American Journal of Tropical Medicine and Hygiene 81, 698–701. [DOI] [PubMed] [Google Scholar]
- Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, Lopez AS, Zambrano CH, Suftt RL, Sakolvaree Y, Chaicumpa W, Herwaldt BL and Johnson S (2002). An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. New England Journal of Medicine 346, 668–675. [DOI] [PubMed] [Google Scholar]
- Veríssimo CM, Morassutti AL, von Itzstein M, Sutov G, Hartley-Tassell L, McAtamney S, Dell A, Haslam SM and Graeff-Teixeira C (2016). Characterization of the N-glycans of female Angiostrongylus cantonensis worms. Experimental Parasitology 166, 137–143. doi: 10.1016/j.exppara.2016.04.012 [DOI] [PubMed] [Google Scholar]
- Wang QP, Lai DH, Zhu XQ, Chen XG and Lun ZR (2008). Human angiostrongyliasis. Lancet Infectious Diseases 8, 621–630. [DOI] [PubMed] [Google Scholar]