Abstract
Setting:
Interferon-gamma (IFN-γ) release assays (IGRA) are used to diagnose tuberculosis (TB) but not to measure treatment response.
Objective:
To measure IFN-γ response to active TB treatment.
Design:
Patients from the Henan Provincial Chest Hospital with TB symptom and/or signs were enrolled into this prospective, observational cohort study and followed for 6 months of treatment with blood and sputa collected at 0, 2, 4, 6, 8, 16, and 24 weeks. The Quantiferon TB-Gold assay was run on collected blood samples. Participants received a follow-up phone call at 24 months to determine relapse status.
Results:
152 TB patients were enrolled, with 135 eligible for this analysis, including 118 pulmonary and 17 extrapulmonary TB patients. IFN-γ levels declined significantly over time among all patients (P=0.002), with this decline driven by pulmonary TB patients (P=0.001) largely during the initial 8 weeks of treatment (P=0.019). IFN-γ levels did not change among extrapulmonary TB patients over time or by baseline culture or drug resistance status.
Conclusion:
After 6 months of effective anti-TB treatment, IFN-γ levels decreased significantly in pulmonary TB patients, largely over the initial 8 weeks of treatment. IFN-γ concentrations may offer some value for monitoring TB treatment response for pulmonary TB patients.
Keywords: interferon-gamma release assay, tuberculosis, treatment response
Introduction
Tuberculosis (TB) is a major global health problem with an estimated 9.6 million new cases and 1.5 million deaths in 2014 (1). China has the second highest burden of TB in the world with an estimated 930,000 new cases of TB in 2014. IFN-γ is produced by previously primed effector T-cells when re-exposed to Mycobacterium tuberculosis (Mtb)-specific antigens and measuring this host IFN-γ response is widely used to diagnose latent TB infection (LTBI) (2–4). The value of measuring the change in IFN-γ concentration over time in the treatment of active TB infection is less clear and multiple studies have examined its correlation with treatment response. Some studies showed that IFN-γ levels decreased after treatment (5–9), while others showed an increase or no change depending on the response to treatment (10–13).
Because of these inconsistent results in the literature, we examined the change in IFN-γ response over time to treatment among a cohort of pulmonary and extrapulmonary TB patients in Henan Province, China. We hypothesized that IFN-γ levels would decline over time in response to treatment. We tested this hypothesis within a prospective, natural history study (ClinicalTrials.gov #NCT01071603) whose primary objective was to improve TB diagnosis and testing at the Henan Provincial Chest Hospital by determining the number and proportion of patients admitted with suspected TB who actually had TB.
METHODS
Study population
This study was approved by the Institutional Review Boards of the Henan Provincial Chest Hospital and the National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health. Consecutive patients with TB symptoms and/or signs were approached to enroll into this prospective, observational cohort study from March 2010 to September 2013 and actively followed for 6 months of TB treatment. Inclusion criteria were: 1) age 18–65 years old; 2) if previously treated for TB, having stopped that regimen at least 60 days ago; 3) if presently on anti-TB chemotherapy, started this regimen no more than 14 days before enrollment; 4) willing to adhere to study visits and testing; 5) willing to have samples stored; 6) HIV negative. Enrolled participants were stratified into pulmonary smear-positive, pulmonary smear-negative, and extrapulmonary cohorts, with a target enrollment of 50 patients per cohort. A follow-up phone call around 2 years after enrollment was done to inquire if TB had recurred and to screen for symptoms suggestive of possible recurrence. Twenty-four healthy controls with no signs or symptoms of active TB infection were also enrolled at baseline for a single, cross-sectional visit.
Screening and Procedures
After giving informed consent, each participant provided information regarding their demographics, history of TB, and other related clinical factors. Blood samples and sputa were collected at enrollment (baseline) and after 2, 4, 6, 8, 16, and 24 weeks of TB treatment. Drug sensitivity testing was performed at baseline and after 2 and 6 months of treatment. A high resolution computed tomography (CT) chest scan was also performed at baseline, 2, and 6 months of treatment to monitor response to treatment.
Diagnosis and treatment of TB
All TB cases were diagnosed according to the local standard of care. Pulmonary TB cases included those with sputum culture confirmed Mtb as well as clinically diagnosed cases involving the lung parenchyma or tracheobronchial tree on chest x-ray or CT scan. Extrapulmonary TB referred to cases either culture confirmed Mtb from an extrapulmonary site or clinically diagnosed involving organs other than the lungs. For this analysis, patients with both pulmonary and extrapulmonary TB were classified as pulmonary TB. Cases meeting any one of the following three criteria were defined as confirmed TB: 1) Mtb detected in a culture of sputum or appropriate site clinical sample; or 2) acid-fast bacilli detected from sputum or clinical sample; or 3) caseating granulomas detected on tissue pathology with acid-fast bacilli. Clinically diagnosed pulmonary TB cases did not meet the above criteria for confirmed TB but met all three of the following: 1) had symptoms, signs and radiographic abnormalities consistent with active pulmonary TB; 2) did not respond to a course of broad-spectrum antibiotics; and 3) positive PPD test result. Clinically diagnosed extrapulmonary TB cases also did not meet the above criteria for confirmed TB, had a positive PPD test result, and met any one of the following four criteria: 1) real-time polymerase chain reaction for Mtb positive in pleural fluid or tissue; or 2) caseating granulomas detected on tissue pathology without acid-fast bacilli; or 3) pleural fluid showing lymphocyte predominance, negative tumor cytology, high adenosine deaminase (ADA ≥45 IU/L), and low carcinoembryonic antigen (CEA <5 ng/ml); or 4) typical clinical manifestations that correlated with typical imaging findings and other diseases were excluded.
All patients’ treatment regimens were based on their medical history, drug sensitivity test results, and response to treatment. Standard treatment for treatment-naïve TB patient was 2 months of daily isoniazid, rifampin, pyrazinamide, and ethambutol followed by four months of isoniazid and rifampin. Regimens (drugs or treatment duration) were modified in response to treatment history, disease severity, drug adverse effects, or identified drug resistance.
Interferon-gamma release assay
The Cellestis Quantiferon®-TB Gold interferon-gamma release assay was performed according to the manufacturer’s instructions (http://usa.quantiferon.com/irm/content/PI/QFT/2PK/US.pdf). Three mL of whole blood were collected, with 1 mL transferred into each of three tubes containing nil control, TB antigen, and mitogen control. Tubes were kept between 17–25°C. Immediately after adding blood to the tubes, they were shaken ten times to ensure the inner surface of the tube was coated with blood to solubilize antigens onto the tube walls. After mixing, the tubes were incubated upright at 37°C for 16–24 hours, centrifuged for 15 minutes at 2000 RCF(g), and stored at 2–8°C until tested. The plasma interferon-gamma concentration was determined as negative, positive, or intermediate by the manufacturer’s software (cut-off 0.35 IU/ml). All patients had a positive response to the mitogen tube and a negative response to the nil tube.
Statistical analysis
Baseline IFN-γ levels between TB patients and healthy subjects were compared using a t-test. Among pulmonary smear positive TB patients, baseline IFN-γ levels were compared to baseline CT scan volume of hard disease (defined as disease between −100 to 100 Hounsfield units) and volume of cavity air using Spearman correlation. To determine whether IFN-γ concentration changes over time varied by TB group, generalized estimating equations were used (to account for within-subject correlations). Area under the curve (AUC) was calculated for each subject, and mean values of AUC were compared between PTB and EPTB using the T-test and Wilcoxon rank sum test. Wilcoxon rank sum tests were also used to compare the mean IFN-γ levels at different time points within the same group, with a Bonferroni correction to adjust for multiplicity. Analyses were conducted using SAS version 9.4 and R version 3.2.3.
RESULTS
Characteristics of the Study Patients
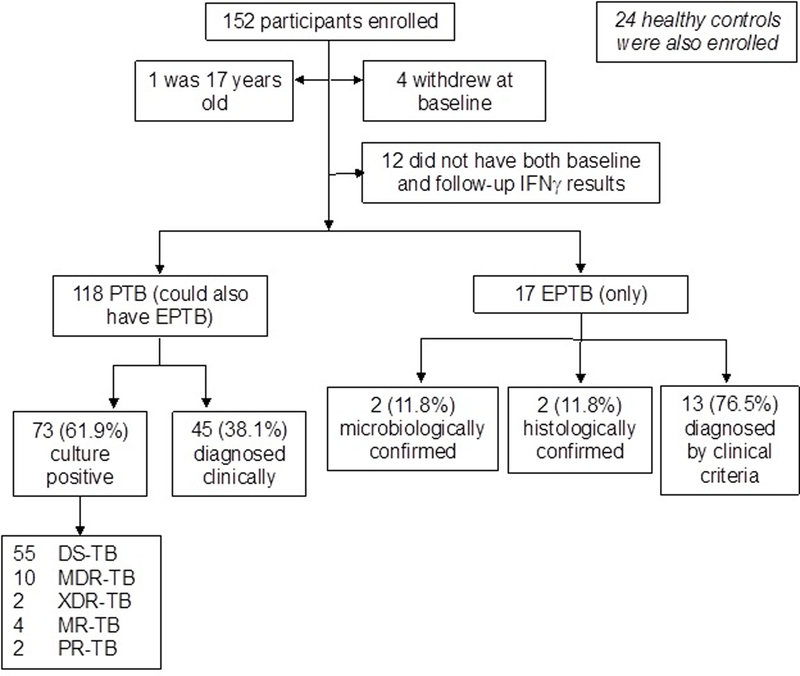
A total of 152 active TB patients were enrolled in this study, of whom 135 had baseline and follow-up IFN-γ data and were included in this analysis (Figure 1). Among these, 118 patients had pulmonary TB (possibly with extrapulmonary TB) and 17 patients had extrapulmonary TB without pulmonary TB. The demographic details of the patients are shown in Table 1. Of the 118 pulmonary TB patients, 73 (61.9%) were Mtb culture positive. The remaining 45 (38.1%) pulmonary TB patients were diagnosed clinically. Of the 17 extrapulmonary TB patients, 2 (11.8%) were microbiologically confirmed and 2 (11.8%) were histologically confirmed. The other 13 (76.5%) extrapulmonary TB patients were diagnosed by clinical criteria. Extrapulmonary TB diagnoses included 13 tuberculous pleurisy, 3 pelvic TB, and 1 chest wall tuberculous abscess. Of the 73 Mtb culture positive patients, 55 (75.3%) were drug-sensitive, 10 (13.7%) were multidrug-resistant (MDR-TB), 2 (2.7%) were extensively drug-resistant (XDR-TB), and 6 (8.2%) had other resistance patterns. Among the 135 TB patients included in the efficacy analysis, 118 (87.4%) successfully completed 6 months treatment and were considered clinical cures. The remaining 17 (12.6%) withdrew from the study during treatment primarily due to first-line treatment failure and/or drug resistance. On follow-up phone calls about 2 years after enrollment, 5 (4.2%) had redeveloped TB symptoms and restarted medications. No follow-up laboratory or microbiological data were available.
Figure 1.
Participants enrolled in this study. DS-TB=drug sensitive tuberculosis. DR-TB=drug resistant tuberculosis. MR-TB=mono-resistant tuberculosis. PR-TB=poly-resistant tuberculosis. MDR-TB= multidrug-resistant tuberculosis. XDR-TB=extensively drug-resistant tuberculosis. NTM=non-tuberculous mycobacteria. PTB=pulmonary tuberculosis. EPTB=extrapulmonary tuberculosis.
Table 1.
Characteristics of TB patients and healthy controls
| Characteristics | PTB | EPTB | Healthy |
|---|---|---|---|
| Number of subjects | 118 | 17 | 24 |
| Mean age, ±SD (years) | 31.9 ±1.12 | 31.5 ±2.86 | 41.1 ±2.49 |
| Sex, N (%) | |||
| Male | 67 (56.8%) | 8 (47.1%) | 10 (41.7%) |
| Female | 51 (43.2%) | 9 (52.9%) | 14 (58.3%) |
| Mean BMI ±SD* | 20.3 ±0.3 | 23.3 ±0.9 | 25.2 ±0.7 |
| Microbiology, N (%) | |||
| Culture positive | 73 (61.9%) | 2 (11.8%) | 0 |
| Culture negative | 45 (38.1%) | 15 (88.2%) | 0 |
| TB symptoms, N (%) | |||
| Chest pain | 42 (35.6%) | 9 (52.9%) | 0 |
| Cough | 71 (60.2%) | 8 (47.1%) | 0 |
| Dyspnea | 28 (23.7%) | 5 (29.4%) | 0 |
| Hemoptysis | 22 (18.6%) | 0 | 0 |
| Night sweats | 41 (34.7%) | 9 (52.9%) | 0 |
| Previous TB history, N (%) | 10 (8.5%) | 1 (5.9%) | 0 |
| Other diseases, N (%) | |||
| Diabetes | 3 (2.5%) | 1 (5.9%) | 0 |
| Hepatitis B | 5 (4.2%) | 0 | 0 |
| Liver disease | 3 (2.5%) | 0 | 0 |
| Lung Disease | 53 (44.9%) | 0 | 0 |
| Smoker, N (%) | 34 (28.8%) | 4 (23.5%) | 3 (12.5%) |
P<0.05
Dynamics of IFN-γ in PTB and EPTB patients
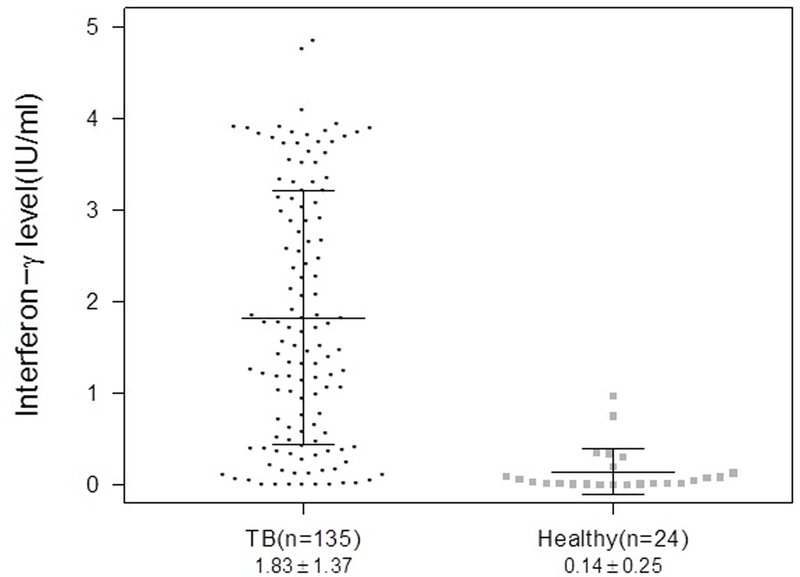
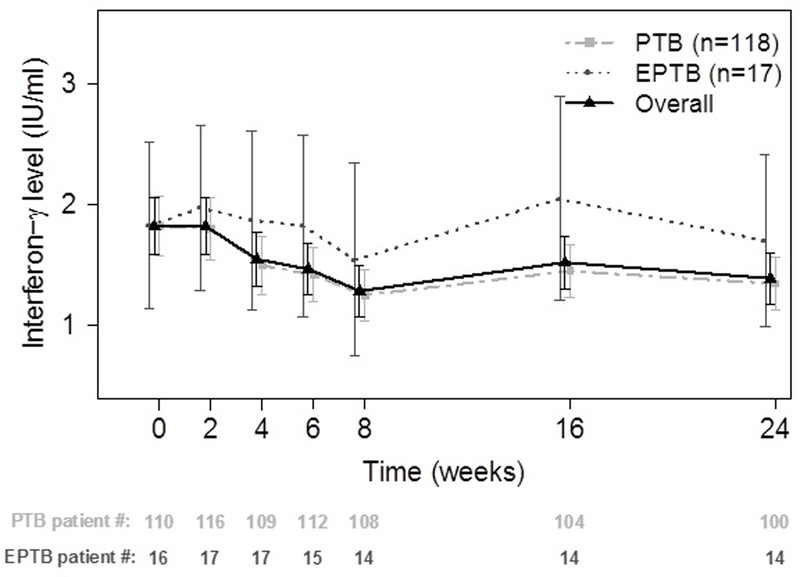
At baseline, the mean (±SD) IFN-γ level among TB patients (1.83 ±1.37 IU/ml) was significantly higher than among healthy controls (0.14 ±0.25 IU/ml; P <0.001; Figure 2). Among pulmonary smear positive TB patients (n=42), the cohort with the biggest range of disease on CT scan at baseline, there was no correlation between baseline IFN-γ levels and CT hard disease volume (p=0.11) or cavity air (p=0.86). Among the 135 patients with at least 2 IFN-γ results, there was a median of 7 (IQR 6–7) IFN-γ results per patient across the 6 months of treatment. Overall, there was a significant decline in IFN-γ levels over time (P=0.002), with this decline driven by the decline in IFN-γ levels among pulmonary TB patients (Figure 3). There was a trend for decline in the mean IFN-γ levels over time (P=0.001) but this decrease occurs largely within the initial 8 weeks of treatment (P=0.019). IFN-γ levels did not change among extrapulmonary TB patients over time. There were no differences in mean AUC values between pulmonary and extra-pulmonary TB patients by T-test (p=0.43) or Wilcoxon rank sum test (p=0.68). There were also no differences in IFN-γ levels over time relative to baseline values between pulmonary and extrapulmonary TB patients (Wilcoxon rank sum test p>0.3). There were no differences in the change in IFN-γ levels over time when comparing baseline drug sensitive (n=55) vs. drug resistant patients (n=18) or baseline culture positive (n=75) vs. culture negative (n=60) patients. A sensitivity analysis was performed excluding subjects with missing values of IFN-γ at any time but this did not change the statistical significance of the results.
Figure 2.
The interferon-γ level in TB patients and healthy subjects at baseline. P<0.0001
Figure 3:
Comparison of interferon-γ level between pulmonary TB and extrapulmonary TB patients. Data are presented as mean ± standard deviation.
DISCUSSION
The Mtb specific CD4+ Th1 cell response is crucial in the immunologic response to Mtb infection by recruiting and activating innate immune cells and producing cytokines such as IFN-γ (14). The importance of IFN-γ is demonstrated by the susceptibility to mycobacterial infections of those with innate or acquired impaired IFN-γ mediated immunity (15, 16). Because IFN-γ production from T-cells increases in response to increased TB antigenic burden, a decline in IFN-γ concentrations may signal a successful treatment response. IFN-γ in response to TB infection is easily measured through the QuantiFERON-TB Gold or T-SPOT.TB IFN-γ release assay kits and thus many studies have used them to assess whether changes in IFN-γ correlate with treatment response. A review of these studies shows a general decline in IFN-γ levels in response to treatment but with considerable variability in individual responses and with most patients still testing positive at the end of treatment (17). Our results are consistent with these findings, with our analysis showing that the decline in IFN-γ concentrations occurs over the initial 8 weeks of treatment in pulmonary TB subjects, followed by generally stable but detectable IFN-γ concentrations subsequently through week 24. Only 12 (10.2%) pulmonary TB patients with an initial positive IFN-γ result converted to negative following treatment. IFN-γ concentrations did not change over time among extrapulmonary TB patients or differ by baseline drug resistance status or baseline culture status. We were not able to assess whether or not this pattern differed among patients with treatment failure as these subjects either died or withdrew during the study and thus did not have a second IFN-γ sample drawn. All subjects who completed 24 weeks of treatment were successfully treated.
The search for a TB biomarker that reflects the true stage of disease, from uninfected to latently infected to active disease to successful vs. unsuccessful treatment to cure vs. relapse, continues to be elusive despite much research in areas ranging from microbiology to radiology to gene expression profiles (18). Our results are consistent with the many previous studies that have shown that the inter-subject variability of the IFN-γ concentration decline over time on treatment does not allow it to serve as a useful biomarker of treatment response on an individual level (17). In addition, in our study IFN-γ levels over time did not differ among extrapulmonary TB subjects or by baseline culture or drug resistance status.
There are some noteworthy limitations of this study. Most patients no longer produced sputum after 1 month of anti-TB treatment so many participants did not have confirmatory negative cultures despite clinical improvement and resolution of TB. Second, 12.3% of patients could not be reached by telephone at 24 months to assess for recurrence. Third, the number of extrapulmonary TB patients and patients with any drug resistance were small and not all extrapulmonary TB patients had invasive procedures to obtain culture confirmation. Finally, there were incomplete data on patients who failed treatment or recurred to compare the longitudinal trend of IFN-γ with those who responded to treatment. These subjects unfortunately either died or withdrew from our study before follow-up IFN-γ data were obtained from them. We were also not able to collect any follow-up samples from the 5 patients who reported clinical recurrence after the end of therapy.
In conclusion, IFN-γ concentrations may offer some value for monitoring TB treatment response for pulmonary TB patients especially over the initial 8 weeks of treatment. More data on patients with poor treatment outcomes are needed to confirm whether or not this drop in IFN-γ levels will differentiate patients with good vs. poor treatment outcomes.
Acknowledgements:
This work was supported by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.World Health Organization. Global Tuberculosis Report 2015. [Available from: http://www.who.int/tb/publications/global_report/en/.]
- 2.Chegou NN, Heyckendorf J, Walzl G, Lange C, Ruhwald M. Beyond the IFN-gamma horizon: biomarkers for immunodiagnosis of infection with Mycobacterium tuberculosis. Eur Respir J 2014;43(5):1472–86. [DOI] [PubMed] [Google Scholar]
- 3.Grant J, Jastrzebski J, Johnston J, Stefanovic A, Jastrabesky J, Elwood K, et al. Interferon-gamma release assays are a better tuberculosis screening test for hemodialysis patients: A study and review of the literature. Can J Infect Dis Med Microbiol 2012;23(3):114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014;27(1):3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauzullo I, Mengoni F, Lichtner M, Massetti AP, Rossi R, Iannetta M, et al. In vivo and in vitro effects of antituberculosis treatment on mycobacterial interferon-gamma T cell response. PLoS One 2009;4(4):e5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis 2004;38(5):754–6. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro S, Dooley K, Hackman J, Loredo C, Efron A, Chaisson RE, et al. T-SPOT.TB responses during treatment of pulmonary tuberculosis. BMC Infect Dis 2009;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SW, Lee CT, Yim JJ. Serial interferon-gamma release assays during treatment of active tuberculosis in young adults. BMC Infect Dis 2010;10:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adetifa IM, Ota MO, Walther B, Hammond AS, Lugos MD, Jeffries DJ, et al. Decay kinetics of an interferon gamma release assay with anti-tuberculosis therapy in newly diagnosed tuberculosis cases. PLoS One 2010;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denkinger CM, Pai M, Patel M, Menzies D. Gamma interferon release assay for monitoring of treatment response for active tuberculosis: an explosion in the spaghetti factory. J Clin Microbiol 2013;51(2):607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theron G, Peter J, Lenders L, van Zyl-Smit R, Meldau R, Govender U, et al. Correlation of mycobacterium tuberculosis specific and non-specific quantitative Th1 T-cell responses with bacillary load in a high burden setting. PLoS One 2012;7(5):e37436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katiyar SK, Sampath A, Bihari S, Mamtani M, Kulkarni H. Use of the QuantiFERON-TB Gold In-Tube test to monitor treatment efficacy in active pulmonary tuberculosis. Int J Tuberc Lung Dis 2008;12(10):1146–52. [PubMed] [Google Scholar]
- 13.Pai M, Joshi R, Bandyopadhyay M, Narang P, Dogra S, Taksande B, et al. Sensitivity of a whole-blood interferon-gamma assay among patients with pulmonary tuberculosis and variations in T-cell responses during anti-tuberculosis treatment. Infection 2007;35(2):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis. Front Immunol 2014;5:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remus N, Reichenbach J, Picard C, Rietschel C, Wood P, Lammas D, et al. Impaired interferon gamma-mediated immunity and susceptibility to mycobacterial infection in childhood. Pediatr Res 2001;50(1):8–13. [DOI] [PubMed] [Google Scholar]
- 16.Xie YL, Rosen LB, Sereti I, Barber DL, Chen RY, Hsu DC, et al. Severe Paradoxical Reaction During Treatment of Disseminated Tuberculosis in a Patient With Neutralizing Anti-IFNgamma Autoantibodies. Clin Infect Dis 2016;62(6):770–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clifford V, He Y, Zufferey C, Connell T, Curtis N. Interferon gamma release assays for monitoring the response to treatment for tuberculosis: A systematic review. Tuberculosis (Edinb) 2015;95(6):639–50. [DOI] [PubMed] [Google Scholar]
- 18.Wallis RS, Maeurer M, Mwaba P, Chakaya J, Rustomjee R, Migliori GB, et al. Tuberculosis-advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect Dis 2016;16(4):e34–46. [DOI] [PubMed] [Google Scholar]