Abstract
The movement of neutrophils between blood and tissues appears to be regulated by chemoattractants and chemorepellents. Compared to neutrophil chemoattractants, relatively little is known about neutrophil chemorepellents. Slit proteins are endogenously cleaved into a variety of N and C terminal fragments, and these fragments are neuronal chemorepellents and inhibit chemoattraction of many cell types, including neutrophils. In this report, we show that the 140 kDa N-terminal Slit2 fragment (Slit2-N) is a chemoattractant and the 110 kDa N-terminal Slit2 fragment (Slit2-S) is a chemorepellent for human neutrophils. The effects of both Slit2 fragments were blocked by antibodies to the Slit2 receptor Robo1 or the Slit2 co-receptor syndecan-4. Slit2-N did not appear to activate Ras, but increased PIP3 levels. Slit2-N induced chemoattraction was unaffected by Ras inhibitors, reversed by PI3 kinase inhibitors, and blocked by cdc42 and Rac inhibitors. In contrast, Slit2-S activated Ras but did not increase PIP3 levels. Slit2-S induced chemorepulsion was blocked by Ras and Rac inhibitors, not affected by PI3 kinase inhibitors, and was reversed by cdc42 inhibitors. Slit2-N but not Slit2-S increased neutrophil adhesion, myosin II light chain phosphorylation, and polarized actin formation and single pseudopods at the leading edge of cells. Slit2-S induced multiple pseudopods. These data suggest that Slit-2 isoforms use similar receptors but different intracellular signaling pathways, and have different effects on the cytoskeleton and pseudopods, to induce neutrophil chemoattraction or chemorepulsion.
Introduction
Chemoattraction is the directed movement of cells toward an attractant and is the mechanism by which, for instance, immune cells move to sites of infection and inflammation (1, 2). Although much is known about chemoattraction in eukaryotic cells, relatively little is known about how eukaryotic cell move away from a repellent (chemorepulsion). Slit was originally identified as an extracellular neuronal chemorepellent protein sensed by Robo receptors in Drosophila, and has since been found in C. elegans and vertebrates (3, 4). Mammals have Slit1, Slit2, and Slit3, which are cleaved into ~110-140 kDa N-terminal and ~55 kDa C-terminal fragments (3, 5). The ~140 kDa Slit2 N-terminal fragment (Slit2-N) contains 4 N-terminal leucine-rich repeats (LRR) domains and 5 EGF-like domains, and shows 96.8% identity (98.8% similarity) between mice and humans. A truncated ~110 kDa Slit2 N-terminal fragment (Slit2-S) containing only the 4 N-terminal LRR domains is 97.2% identical (98.9% similar) between mouse and human. Altered fragmentation patterns of Slit2 have been observed in cancer, inflammation, fibrosis, and obesity (6–11), and we found abnormally low levels of Slit2 fragments in the bronchoalveolar lavage of mice with pulmonary fibrosis (12).
Immune cells such as neutrophils also express Robo receptors (13–15). Slit2 inhibits the chemotaxis of a variety of immune cells toward attractants, but whether Slit can act as a repellent for immune cells is unknown (12–21). In this report, we show that for neutrophils, Slit2-N is a chemoattractant, and Slit2-S is a chemorepellent, and although the two fragments use similar receptors, they use different signal transduction pathways.
Materials and Methods
Cell isolation and culture
Human venous blood was collected from healthy volunteers who gave written consent, and with approval from the Texas A&M University Institutional Review Board. Neutrophils were isolated as previously described (22) with the exceptions that Polymorphprep gradients (Axis-Shield, Oslo, Norway) were used following the manufacturer’s instructions, and centrifugation of gradients was done for 40 minutes. Cells were then resuspended in RPMI-1640 (Lonza, Wakersville, MD) containing 2% BSA (Amresco, Solon, OH) (RPMI-BSA). To check the purity of the neutrophil isolation, cells spots were prepared as described previously (22) and stained with eosin and methylene blue. Isolated cell preparations were 95.6% ± 0.5 neutrophils, 2.2 ± 0.4 monocytes, 1.6 ± 0.4 eosinophils, and 0.6 ± 0.2 lymphocytes (mean ± SEM, n= 16). In addition, cells attached to fibronectin-coated coverslips were air dried, fixed in methanol, and stained with methylene blue and eosin. These were 95.8% ± 1.2 neutrophils, 2.0 ± 1.0 monocytes, 1.8 ± 0.8 eosinophils, and 0.3 ± 0.2 lymphocytes (mean ± SEM, n= 5).
Insall chamber assays
Insall chambers (23) were used to generate gradients of Slit2 and other compounds to observe the movement of neutrophils on glass coverslips coated with 10 μg/ml human plasma fibronectin (Corning, Bedford, MA), as previously described (22, 24, 25). Cells were allowed to adhere to coverslips for 30 minutes before the coverslip was placed onto the Insall chamber. Recombinant mouse Slit2 Gln26-Gln900 ~110 kDa (R&D Systems, Minneapolis, MN), recombinant human Slit2 Gln26-Val1118 120-140 kDa (Peprotech, Rocky Hill, NJ), and recombinant human Slit2-C terminal fragment Thr1122-Ser1529 ~ 50 kDa (R&D Systems) were resuspended in RPMI- BSA. Slit2 proteins were checked for purity and size by PAGE and silver staining, as described previously (12, 26). The neutrophil chemoattractant N-formylmethionine-leucyl-phenylalanine (fMLP) (Alfa-Aesar, Ward Hill, MA) was diluted in RPMI- BSA and used at 10 nM.
Rho GTPase Cdc42 inhibitor ML 141, Ras inhibitory peptide, and Rac inhibitor NSC 23766 (all from Cayman Chemical, Ann Arbor, MI) and the phosphatidylinositol 3 kinase (PI3K) inhibitor LY294002 (BioVision, Milpitas, CA) were reconstituted to 10 mM in DMSO (Amresco) according to the manufacturers’ directions. The DMSO stocks were then diluted in RPMI-BSA and used at 10 μM. Cells were incubated with inhibitors for 30 minutes at 37°C in a humidified 5% CO2 incubator before placing on the coverslip. Where indicated, cells were incubated with 10 μg/ml sheep anti-human Robo1 antibodies (R&D Systems, Minneapolis, MN) or 3 μg/ml sheep anti-human Syndecan-4 antibodies (R&D Systems) for 30 minutes as described above. After placing coverslips with adhered neutrophils on the Insall chamber, we waited 20 minutes for gradients to form before tracking neutrophils as previously described (22, 24). At least 10 neutrophils per experiment were tracked for 40 minutes. Only cells that could be tracked for the full 40 minutes were analyzed. For each donor, neutrophils were tracked in a no-gradient control. Neutrophils were never used past 5 hours from the end of the isolation step. We used two Insall chamber/ microscope/ camera setups in parallel, allowing 6 to 8 experimental conditions to be measured per set of donor neutrophils (see Supplemental videos). The results are expressed as the mean ± SEM of the movement of neutrophils from three or more different donors. We never used the same donor twice for a given experiment.
Isolation of cytoskeletal proteins
Preparation of cytoskeletons and gel electrophoresis to visualize F-actin was done as previously described (27–29). Briefly, 5 × 105 cells in 100 μl RPMI-BSA were incubated at 37°C in the presence or absence of 500 ng/ml Slit2 or 10 nM fMLP. At the indicated time points, 1.4 ml of ice cold PBS was added to the cells and cells were collected by centrifugation at 500 × g for 5 minutes at 4°C. Cells were lysed with 100 μl of 100 mM PIPES (pH 6.8), 1 mM MgCl2, 2.5 mM EGTA, 0.5% Triton X-100 with 4× protease and phosphatase inhibitor (Cell Signaling Technology) on ice for 20 minutes. Triton-insoluble cytoskeletons were collected by centrifugation at 12,000 × g for 5 minutes at 4°C. Cytoskeleton pellets were resuspended in SDS/DTT sample buffer, heated to 98°C for 5 minutes, separated by PAGE, and then stained with Coomassie as described previously (29).
Confocal Microscopy
1 × 106 cells in 200 μl RPMI-BSA were allowed to attach to fibronectin-coated 8 well slides (Corning) for 30 minutes at 37°C. Two microliters of prewarmed RPMI-BSA (control) or prewarmed RPMI-BSA/ 100 μg/μl Slit2-N or Slit2-S, or prewarmed RPMI-BSA/ 200 nM fMLP was then added to the corner of the well, taking care to not to disturb the cells or the medium. After 10 minutes, the cells were fixed by carefully adding 200 μl of prewarmed 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS to the wells for 20 minutes at 37°C. Cells were then washed with PBS and permeabilized with PBS/ 0.1% Triton-X100 (PBS-T) for 5 minutes at room temperature. Cells were then incubated with 1:1000 anti-phospho-AKT substrate (proteins containing phospho-serine or phospho-threonine preceded by arginine at the −3 position; RXXS*/T*) (30) antibody (rabbit IgG, #9614, Cell Signaling Technology, Danvers, MA) in PBS-T containing 2% BSA (PBS-T-BSA) or 5 μg/ml anti-CD11b (mouse IgG, ICRF44, BioLegend, San Diego, CA) in PBS-T-BSA overnight at 4°C. Cells were washed with PBS-T before the addition of either 1 μg/ml goat anti-rabbit IgG Alexa 647 (Invitrogen-Thermo Fisher, Waltham, MA) or donkey anti-mouse IgG Alexa-488 (Jackson ImmunoResearch, West Grove, PA) for 30 minutes at room temperature. Cells were washed with PBS-T and coverslips were mounted with fluorescent mounting medium containing DAPI (Vector Laboratories). Alternatively, after fixation, cells were incubated with 1:2000 phalloidin-Alexa 555 (Abcam, ab176756) in PBS-T for 30 minutes at room temperature. Cells were washed with PBS-T and coverslips were mounted with fluorescent mounting medium containing DAPI (Vector). Immunofluorescence images were captured on an Olympus FV1000 confocal microscope, and analyzed using Olympus Fluoview and ImageJ software as described previously (12, 26). Cells were scored as having polarized phalloidin staining (one region of staining) either towards or away from the corner of the well where material was added, multiple staining (having 2 or more regions of phalloidin staining), or circumferential/uniform staining (where the majority of the cell was stained with phalloidin).
Adhesion assays
96-well polystyrene plates (353072, BD Biosciences) were coated with 10 μg/ml of human plasma fibronectin in PBS for 1 hour at 37°C. The plate was then washed 3 times with PBS and 1 × 105 neutrophils in 100 μl RPMI-BSA were allowed to attach to the coated wells for 30 minutes at 37°C. 100 μl of 10 nM fMLP, 500 ng/ml Slit2-N, 500 ng/ml Slit2-S, or 10 ng/ml TNFα (BioLegend) were then added to the wells and incubated for a further 30 minutes at 37°C. Plates were then washed 3 times with PBS and adherent neutrophils were counted in three 900 μm-diameter fields of view per well as described previously (31). In addition, neutrophils were pre-incubated with 500 ng/ml Slit2-N or Slit2-S for 15 minutes at 37°C before incubation with buffer, fMLP, or TNFα for an additional 15 minutes. Cells were then added to fibronectin-coated wells, and cell adhesion was measured as described above.
PIP2, PIP3, and Ras assays
10 × 106 neutrophils in 0.4 ml RPMI-BSA were incubated at 37°C in the presence or absence of 500 ng/ml Slit2, or 10 nM fMLP. For PIP2 and PIP3 assays, after 5 minutes, 0.4 ml of ice cold 1000 mM trichloroacetic acid (TCA) was added to the cells and then incubated on ice for 5 minutes. Phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-triphosphate (PIP3) were extracted and detected using ELISA assays kits following the manufacturer’s instructions (PIP2 K-4500, PIP3 K-2500s; Echelon, Salt Lake City, UT). For Ras activation, cells were incubated with Slit2 or fMLP for 6 minutes, and then 1.2 ml of ice cold PBS was added to the cells, and the cells were then collected by centrifugation at 500 × g for 5 minutes at 4°C. Cells were then resuspended in 0.2 ml kit lysis buffer and the active GTP-bound form of Ras proteins were isolated and detected following the manufacturer’s instructions (BK008-S; Cytoskeleton Inc., Denver, CO). Briefly, eluate from beads bearing the Ras-binding domain (RBD) of the Ras effector kinase Raf1, which isolates GTP-Ras, was analyzed by western blotting using 4–20% Tris/glycine gels (Lonza, Allendale, NJ) (12, 26). After blotting to PVDF membranes (Immobilon P, MilliporeSigma, Burlington, MA), protein transfer was assessed by Ponceau red staining (32). Blots were blocked and then incubated with anti-Ras antibodies following the manufacturer’s instructions (BK008-S, Cytoskeleton Inc). Cell lysates were also analyzed by western blotting with 200 ng/ml anti-GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) mouse monoclonal antibody (Proteintech, Rosemont, IL), to confirm effective protein transfer.
Statistics
Statistical analyses with t tests, 1-way or 2-way ANOVA with Dunnett’s post-test were done using GraphPad Prism 7 (GraphPad, San Diego, CA). Significance was defined as p < 0.05.
Results
Different isoforms of Slit2 induce chemoattraction or chemorepulsion of neutrophils
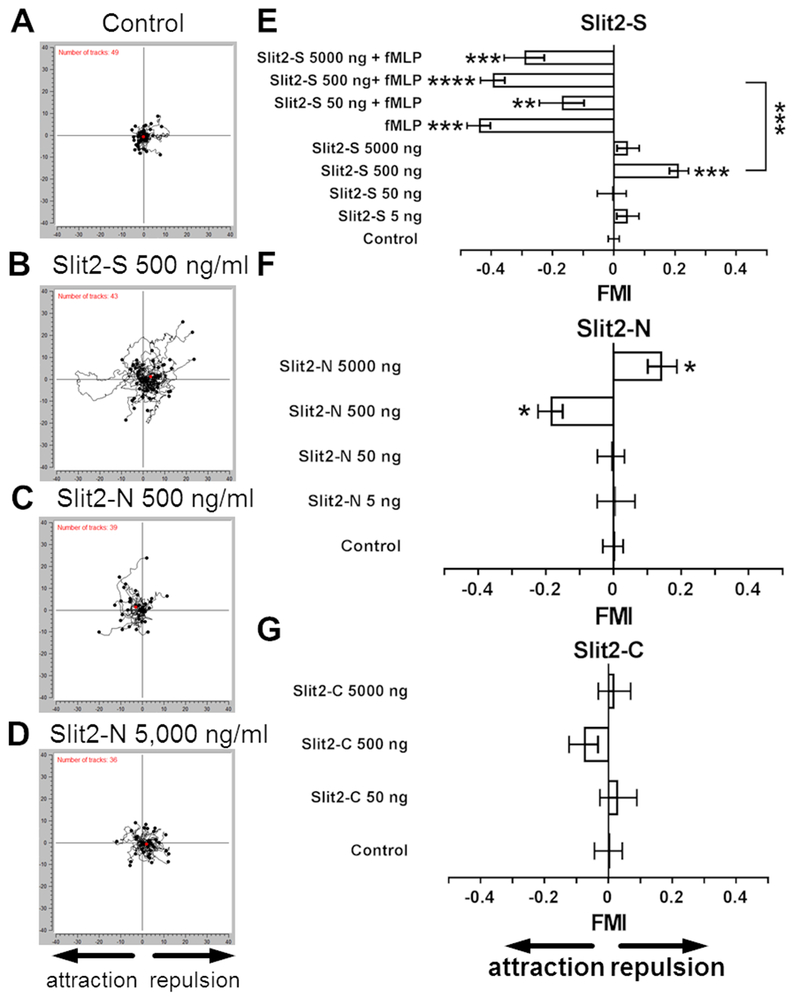
In the developing nervous system, Slit2 acts as a chemorepellent for neurons (3–5). Slit2 concentrations between 400 ng/ml and 100 μg/ml inhibit the chemotaxis of human and murine neutrophils towards the chemoattractants fMLP, CXCL12, IL-8, and the complement component C5a (15, 16, 18). An intriguing possibility is that Slit2 might act directly as a chemorepellent for neutrophils. To test this, we used a gradient chamber to examine the effect of Slit2 gradients on human neutrophil movement. We first examined the effects of a 110 kDa fragment of mouse Slit2, henceforth designated Slit2-S. Compared to cells in no gradient, gradients of 0 – 5 ng/ ml and 0 – 50 ng/ ml Slit2-S did not significantly affect neutrophil movement (Fig. 1A, 1E, Supplemental Fig. 1A-B, 1G, and Supplemental video 1). A gradient of 0 – 500 ng/ml (0 - 5 nM) Slit2-S caused neutrophils to move away from the source of the Slit2-S (Fig. 1B and 1E, Supplemental Fig. 1G, and Supplemental video 2), with a forward migration index (FMI) comparable to the FMI induced by the chemorepellent DPPIV for neutrophils (22) or AprA for Dictyostelium cells (24). We previously observed that approximately 17% of cells in DPPIV or AprA chemorepellent gradients move toward the source of the chemorepellent (22, 24), and we observed that 29 ± 5% of cells (mean ± SEM, n = 6 donors) of the neutrophils in the 0 – 500 ng/ml Slit2-S gradient moved toward the source. A gradient of 0 – 5,000 ng/ml Slit2-S however did not cause chemorepulsion (Fig. 1E). As previously observed (15, 33), 1 nM fMLP caused chemoattraction of neutrophils (Fig. 1E, Supplemental Fig. 1G, and Supplemental video 3). In the fMLP gradient, only 6 ± 4% (mean ± SEM, n = 6 donors) of neutrophils moved away from the fMLP source. This is significantly lower than the percentage of cells going the ‘wrong way’ in the Slit2-S gradient (p < 0.05, t test), suggesting that the fMLP gradient affects more neutrophils than the Slit2-S gradient. An fMLP gradient alongside a 0 – 500 ng/ ml Slit2-S gradient showed a dominance of the fMLP chemoattraction over the Slit2-S chemorepulsion (Fig. 1E and Supplemental Fig. 1G). Some chemoattractants affect the speed and/or directionality (the distance between the starting and ending point of a cell divided by the distance along the cell’s path) of cells (34–36). fMLP with 0 and 50 ng/ml Slit2-S increased the speed of the neutrophils along their tracks (Supplemental Fig. 1A). fMLP and fMLP + 500 ng/ml Slit2-S caused the directionality to increase compared to control (Supplemental Fig. 1B). Other than 0-5 ng/ml Slit2-S decreasing speed for female neutrophils (Supplemental Fig. 2C), there were no significant differences in the response of neutrophils from male and female donors to Slit2-S (Supplemental Fig. 2). Together, these data suggest that Slit2-S can act as a chemorepellent for human male and female neutrophils, but that this can be overridden by an fMLP chemoattraction gradient.
Figure 1. Neutrophils show biased movement away from Slit2-S and towards Slit2-N.
Neutrophils in (A) control, (B) Slit2-S 0-500 ng/ml, (C) Slit2-N 0-500 ng/ml, or (D) Slit2-N 0-5,000 ng/ml gradients were filmed and tracked. Orientation is such that the source of Slit2 is on the left. Graphs are data from one of 10 independent experiments. The number of tracks analyzed is indicated in the top left corner. Red dots represent the average center of mass for the ending positions of all cells. (E-G) Human neutrophils were placed in gradients of the indicated concentrations in ng/ ml of (E) Slit2-S in the presence or absence of 10 nM fMLP, (F) Slit2-N, or (G) Slit2-C using Insall chambers and videomicroscopy was used to record cell movement. A positive forward migration index (FMI) indicates chemorepulsion, and a negative FMI indicates chemoattraction. At least 10 cells per experiment group for each individual donor were tracked for 40 minutes. Values are means ± SEM for neutrophils from at least 5 different donors. * indicates p < 0.05, ** p < 0.01, *** p < 0.001 compared to the no gradient control (1-way ANOVA, Dunnett’s test), or for the indicated comparison between two sets (t test).
We also examined whether gradients of a commercially available ~140 kDa fragment of human Slit2, henceforth designated Slit2-N, can affect neutrophil movement. Gradients of 0 – 5 or 0 – 50 ng/ml Slit2-N did not significantly affect neutrophil movement (Fig. 1F and Supplemental Fig. 1H), 0 - 500 ng/ml (0 - 3.6 nM) gradients caused neutrophils to move towards the Slit2-N (Fig. 1C, 1F, Supplemental Fig. 1H, and Supplemental video 4), and 0 – 5,000 ng/ml gradients caused female but not male cells to move away from the Slit2-N (Fig. 1D, 1F and Supplemental Fig. 2B). In the 0 - 500 ng/ml gradient of Slit2-N, 14 ± 6% of cells (mean ± SEM, n = 6) of the neutrophils moved away from the source; this was not significantly different from the percentage of ‘wrong way’ cells in a fMLP gradient (t test). In the 0- 5,000 ng/ml gradient of Slit2-N, 38 ± 6% (mean ± SEM, n = 6) of the neutrophils moved towards the source; this was significantly higher (p = 0.0042, 1-way ANOVA, Dunnett’s test) than the percentages of ‘wrong way’ cells in fMLP or 0-500 ng/ml Slit2-N gradients (p = 0.0309; 1-way ANOVA, Dunnett’s test). None of the Slit2-N concentrations significantly affected neutrophil speed or directness (Supplemental Fig. 1C and 1D). Other than males not responding to the 0 – 5,000 ng/ ml Slit2-N gradients, there were no significant difference in the response of neutrophils to Slit2-N from male and female donors (Supplemental Fig. 2B, 2D and 2F). The data suggest that 500 ng/ml Slit2-N acts as a chemoattractant.
Although gradients of 0 – 50, 0-500, or 0-5,000 ng/ml of a 55 kDa human Slit2-C fragment did increase neutrophil speed (Supplemental Fig. 1E), they did not significantly affect the direction or directionality of neutrophil movement (Fig. 1G , Supplemental Fig. 1F, and 1I).
Robo and Syndecan-4 antibodies block the effects of Slit2 induced migration
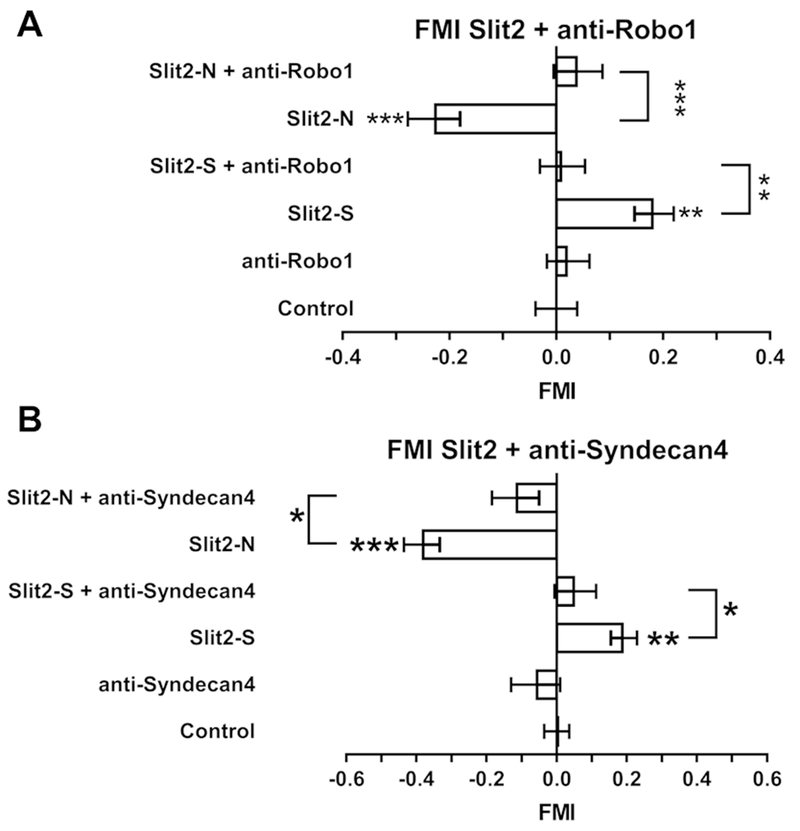
To induce chemorepulsion of growing axons, neurons use the Roundabout homolog 1 (Robo1) receptor to sense Slit2 (3–5). Robo1 also mediates the ability of Slit2 to inhibit neutrophil chemoattraction (15, 18). Neutrophils pre-incubated with anti-Robo1 antibodies did not respond to either a 0 - 500 ng/ml Slit2-S gradient or 0 - 500 ng/ml Slit2-N gradient (Fig. 2A). The anti-Robo1 antibodies did not significantly affect cell speed (Supplemental Fig. 3A), but anti-Robo1 antibody pretreatment with a Slit2-S gradient increased directness (Supplemental Fig. 3B). These data suggest that Robo1 is necessary for Slit2 chemorepulsion or chemoattraction of human neutrophils.
Figure 2. Anti- Robo-1 and Syndecan-4 antibodies inhibit Slit-2 chemotaxis.
Human neutrophils were pre-incubated with Anti- (A) Robo-1 or (B) syndecan-4 antibodies before videomicroscopy in gradients of Slit2 as in Figure 1. At least 10 cells per experiment group for each individual donor were tracked for 40 minutes. Values are means ± SEM for neutrophils from at least 5 different donors. * indicates p < 0.05, ** p < 0.01, *** p < 0.001 compared to the no gradient control (1-way ANOVA, Dunnett’s test), or for the indicated comparison between two sets (t test).
Syndecans are proteoglycans that can act as co-receptors for Robo1 signaling (37, 38). Of the four human syndecans, Syndecan-4 appears to mediate human neutrophil chemotaxis (39). Pre-incubation of human neutrophils with anti-Syndecan-4 antibodies inhibited the response of neutrophils to 0-500 ng/ml gradients of both Slit2-S and Slit2-N (Fig. 2B). In the presence of Slit2-S or Slit2-N gradients, the anti-Syndecan antibodies increased cell speed (Supplemental Fig. 3C), but did not significantly affect directness (Supplemental Fig. 3D). These data suggest that Syndecan-4 is necessary for Slit2 chemorepulsion or chemoattraction of human neutrophils.
Slit2-N generates polarized F-actin in neutrophils
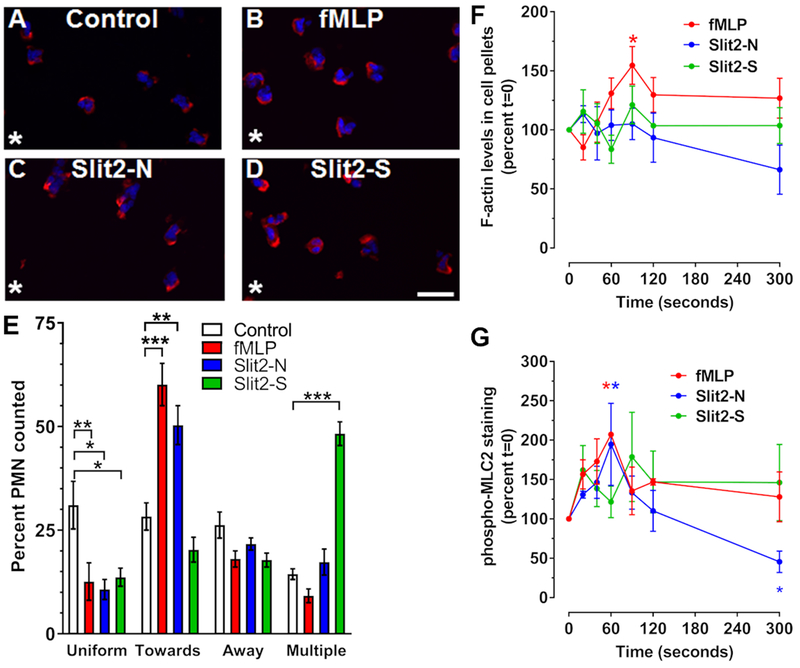
Neutrophil migration to chemoattractants leads to cytoskeletal reorganization and formation of F-actin at the leading edge of the cell, which can be visualized by phalloidin staining (2, 40). To determine if Slit2-induced migration induces F-actin reorganization, neutrophils were allowed to adhere to fibronectin-coated 8-well slides and were then stimulated with a point source of buffer, Slit2, or fMLP for 10 minutes and then fixed, permeabilized, and stained with phalloidin. Cells were then analyzed for the location of the F-actin. The addition of buffer to one corner of the well did not cause any significant asymmetry of F-actin localization toward or away from the source of the buffer, and many cells had F-actin around the periphery of the cell, and some cells had two or more (multiple) spots of F-actin at the edges of cells (Fig. 3A and E). Neutrophils in gradients of fMLP or Slit2-N showed a decrease in the percentage of cells with a uniform distribution of F-actin at the edge of the cell, and an increase in the percent of cells with F-actin localized to the edge of the cell towards the attractant (Fig. 3B, C, and E). Gradients of the chemorepellent Slit2-S also decreased the percentage of cells with F-actin all around the cell, increased the percentage with multiple regions of F-actin at the edge, but did not significantly affect the percent of cells with F-actin at the edge of the cell toward or away from the source of Slit2-S (Fig. 3D and E). As previously observed (27, 28, 41), fMLP transiently increased total F-actin polymerization at 90 seconds (Fig. 3F). Slit2-S and Slit2-N did not cause significant changes in F-actin over 300 seconds (Fig. 3F). Compared to control cells, both fMLP and Slit2-N induced phosphorylation of myosin light chain 2 (MLC2) at 60 seconds, and Slit2-N also led to reduced pMLC2 levels at 5 minutes (Fig. 3G). Slit2-S had no significant effect on pMLC2 levels (Fig. 3G). These data suggest that Slit2-S affects the cytoskeleton to induce chemorepulsion in a manner that is different from how fMLP and Slit2-N affect the cytoskeleton to induce chemoattraction.
Figure 3. Only Slit2-N and fMLP generate polarized F-actin.
Neutrophils were incubated in the presence or absence of a single point source (asterisk) of (A) buffer control, (B) fMLP, (C) Slit2-N, or (D) Slit2-S for 10 minutes. Cells were then fixed and stained for F-actin with phalloidin-Alexa 555 (red) and counterstained with DAPI (blue). Images are from one of 3 different donors. Bar is 20 μm. (E) Quantification of phalloidin staining location, indicating the percent of cells with F-actin at the edge of the cell either towards or away from the stimulus, or cells showing multipolar, or uniform staining. (F-G) Neutrophils were incubated with fMLP, Slit2-N, or Slit2-S, for 20, 40, 60, 90, 120, and 300 seconds. Cells were then lysed in Triton-X 100 buffer to isolate cytoskeletal and cytoplasmic proteins. (F) Triton-X 100 insoluble cytoskeletal proteins were analyzed by PAGE and stained with Coomassie to quantify F-actin. (G) Cytoskeletal proteins were also analyzed for phosphorylated myosin light chain 2 (pMLC2) by western blotting. All values are mean ± SEM for neutrophils from 3-4 different donors. * indicates p < 0.05, ** p < 0.01, *** p < 0.001 compared to the t=0 buffer control (1-way ANOVA, Dunnett’s test).
Slit2 isoforms differentially regulate neutrophil adhesion
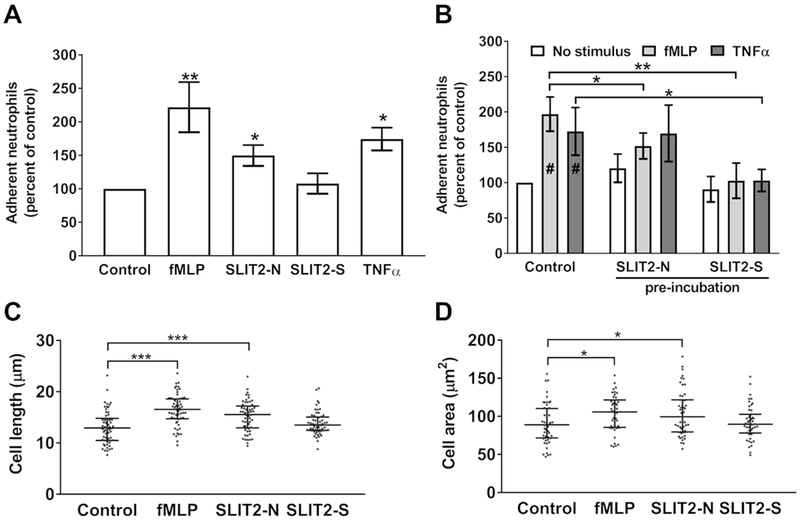
Cell migration requires binding to a surface to allow traction, but not with too low or too high an adhesive force, which prevents migration (34–36). To determine if the Slit2 isoforms affect cell-substratum adhesion, we allowed neutrophils to adhere to fibronectin-coated plates before the addition of Slit2 proteins, the chemoattractant fMLP, or TNFα, a cytokine that promotes neutrophil adhesion (31). Compared to unstimulated neutrophils, cells incubated with fMLP, Slit2-N, or TNFα had significantly increased adhesion (Fig. 4A), while the chemorepellent Slit2-S did not significantly affect adhesion (Fig. 4A). Pre-incubation with Slit2 isoforms inhibit the ability of fMLP or TNFα to increase adhesion of neutrophils to activated endothelial cells (19, 42, 43). Both Slit2-N and Slit2-S significantly inhibited fMLP-induced adhesion to fibronectin, and Slit2-S but not Slit2-N inhibited TNFα-induced adhesion (Fig. 4B). Cell migration leads to a change in cell shape with an elongation of the cell with pseudopods forming at the leading edge of the cell, and a more flattened cell as it attaches to the underlying matrix (1, 2). Compared to unstimulated neutrophils, cells in gradients of fMLP or Slit2-N had an increase in cell length and cell area, whereas cells in Slit2-S gradients were not significantly different from control cells (Fig. 4C and 4D). These data suggest that unlike the chemoattractants fMLP and Slit2-N, which increase adhesion to fibronectin, cell elongation, and cell flattening, the chemorepulsive Slit2-S does not affect these parameters.
Figure 4. Slit2-N and Slit2-S have different effects on cell adhesion and cell area.
(A) Neutrophils were allowed to adhere to fibronectin for 30 minutes, and Slit2 proteins, fMLP, TNFα, or an equal volume of buffer (control) were added for an additional 30 minutes. Plates were then washed, and adherent cells were air-dried, stained, and counted. Values are mean ± SEM, n = 4. (B) Cells were pre-incubated for 15 minutes with either Slit2-N or Slit2-S before the addition of fMLP or TNFα for an additional 15 minutes. Cells were then allowed to adhere to fibronectin for 30 minutes. Adherent cells were air-dried, stained, and counted. Values are mean ± SEM, n = 4. # indicates p < 0.05 compared to the no stimulus control (t test). (C-D) Neutrophils were incubated with a point source of buffer, Slit2 proteins, or fMLP for 10 minutes, then fixed and (C) cell length and (D) cell area were measured with Image J. The results are mean ± interquartile range of 20 cells analyzed from three different donors. * indicates p < 0.05, *** p < 0.001 (1-way ANOVA, Dunnett’s test).
Slit2 isoforms differentially regulate surface receptor and intracellular signaling protein localization
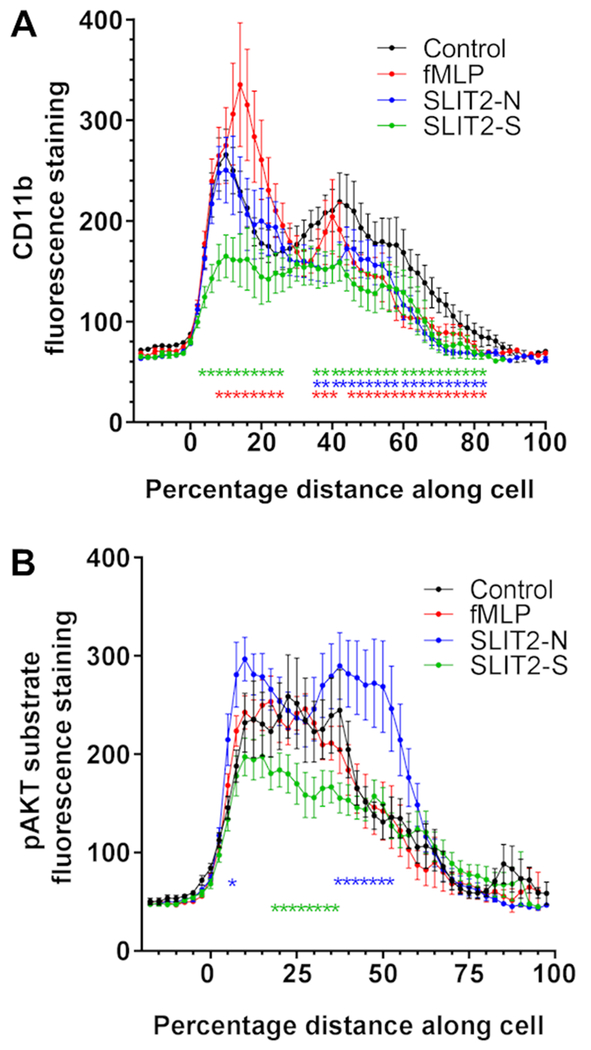
Neutrophil recruitment to an inflamed tissue involves initial attachment to endothelial cells, followed by rolling across the endothelial cell surface, and then firm adhesion to the endothelium, transendothelial migration, and chemotaxis through the tissue to the inflammatory source (1, 44). Adhesion of neutrophils to either endothelial cells or tissue extracellular matrix is mediated by integrins, including CD11b/CD18 (45, 46), which relocate to the front and rear of the cell during adhesion and migration (47, 48). To determine if Slit2 proteins affect integrin relocation, neutrophils were incubated on fibronectin in Slit2 gradients, fixed, stained for CD11b, and the staining intensity was then scanned along an axis running through the middle of the cell parallel to the gradient. Compared to unstimulated cells, which show peaks of CD11b staining at the edges of the cell, fMLP did not significantly alter the staining of CD11b at the edge of the cell closest to the stimulus, increased staining in a region immediately behind the edge, and decreased staining at the rear (Fig. 5A). Cells incubated in a Slit2-S gradient had significantly less CD11b staining at the side of the cell closest to the Slit2-S, and had decreased staining throughout the cell (Fig. 5A). Slit2-N decreased staining of CD11b in the part of the cell furthest from the Slit2-N.
Figure 5. Slit2 proteins regulate CD11b and intracellular signaling proteins redistribution.
Neutrophils were incubated with a point source of Slit2 proteins or fMLP for 10 minutes, then fixed and stained with antibodies to (A) CD11b or (B) phosphorylated AKT substrates. Staining across the cell from the side of the cell closest to the chemo-stimulant (0 on the graph) to the far side of the cell was analyzed by Image J. The results are mean ± SEM of 10 cells analyzed from three different donors (30 cells total). * indicates p < 0.05 compared to the control unstimulated cells (2-way ANOVA, Dunnett’s multiple comparisons test).
Cell migration is driven by the detection of chemostimulants through a diverse number of receptors, including G protein coupled receptors (GPCR) such as the fMLP receptor (49), and single-pass transmembrane receptors, such as Robo (3, 5). Although the intracellular domains of the fMLP and Robo receptors bind different signaling molecules, both receptors appear to regulate pathways downstream from the AKT kinase (14, 50–53). To determine if the isoforms of Slit2 differentially regulate AKT activity, we used an antibody that detects phosphorylation of AKT substrates (30). Compared to unstimulated cells, cells exposed to a gradient of fMLP had no discernable difference is pAKT substrate staining (Fig. 5B). Cells incubated with Slit2-N had increased staining at the front and rear of the cell, whereas cells incubated with Slit2-S had reduced staining in the middle of the cells (Fig. 5B). These data suggest that the Slit2 fragments differentially re-organize cell surface integrins and differentially regulate AKT activity.
PI3 kinase, Cdc42, Rac, and Ras mediate the effect of Slit on neutrophils
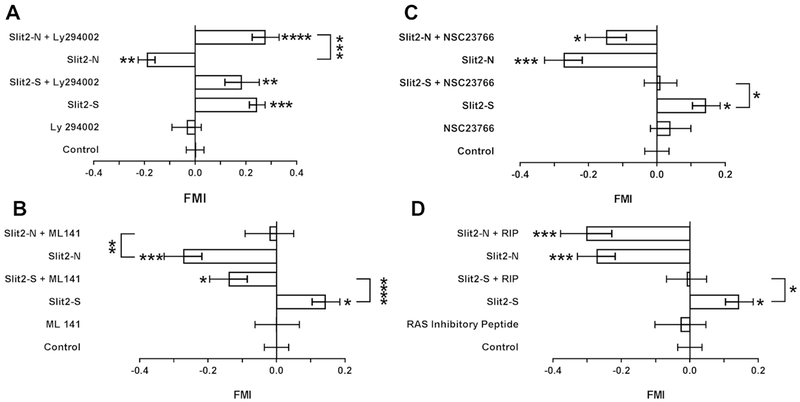
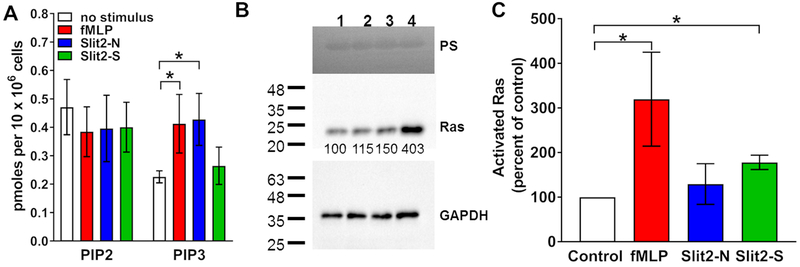
Phosphoinositide 3-kinase (PI3K) mediates the movement of neutrophils towards some chemoattractants (35). LY294002 is an inhibitor of PI3K (54), and has been used at 10-50 μM to inhibit PI3K signaling and migration in human neutrophils and endothelial cells (35, 55). To determine if the effects of Slit2 on neutrophils are mediated by PI3K, neutrophils were pre-incubated with LY294002 and then observed in Slit2 gradients. LY294002 had no significant effect on the ability of a 0-500 ng/ml gradient of Slit2-S to induce chemorepulsion (Fig. 6A). However, LY294002 caused neutrophils in a 0-500 ng/ml Slit2-N gradient to be repelled by the Slit2-N (Fig. 6A and Supplemental video 5). LY294002 caused neutrophil speed to increase in a Slit2-S gradient (Supplemental Fig. 4A), but LY294002 did not significantly affect directness (Supplemental Fig. 4B). To confirm the role of PI3K in Slit2-N mediated chemoattraction, PIP2 and PIP3 levels were measured in neutrophils incubated with Slit2-N, Slit2-S, or fMLP. After 5 minutes incubation, none of the treatments significantly affected PIP2 levels, but compared to unstimulated cells, Slit2-N and fMLP increased levels of PIP3, whereas Slit2-S did not significantly affect PIP3 levels (Fig. 7A). These data suggest that Slit2-S does not increase PIP3 and does not need PI3 kinase to cause chemorepulsion whereas Slit2-N increases PIP3 and needs PI3 kinase to cause chemoattraction.
Figure 6. Signaling inhibitors have different effects on the response of neutrophils to Slit2-N and Slit2-S.
Human neutrophils were pre-incubated for 30 minutes with inhibitors of (A) PI3 kinase (LY294002), (B) Cdc42 (ML141), (C) Rac (NSC23766), or (D) Ras (ras inhibitory peptide-RIP) videomicroscopy in gradients of Slit2 as in Figure 1. A positive forward migration index (FMI) indicates chemorepulsion, and a negative FMI indicates chemoattraction. At least 10 cells per experiment group for each individual donor were tracked for 40 minutes All values are means ± SEM for neutrophils from at least 6 different donors. * indicates p < 0.05, ** p < 0.01, *** p < 0.001 compared to the no gradient control (1-way ANOVA, Dunnett’s test), or for the indicated comparison between two sets (t test).
Figure 7. Slit2-N increases PIP3 and Slit2-S activates Ras.
Neutrophils were incubated with Slit2 proteins or fMLP for A) 5 minutes to measure PIP2 and PIP3 levels, or B and C) 6 minutes to detect Ras activation. B) A western blot of bead lysate from neutrophils incubated with Slit2 proteins or fMLP was stained with Ponceau red (PS) (upper panel), anti-Ras antibodies (middle panel), or anti-GAPDH antibodies (lower panel). The quantification of Ras staining is indicated below the blot (middle panel). The positions of molecular mass standards in kDa are at left. C) Quantification of western blots. Values are means ± SEM for neutrophils from 3-6 different donors. * indicates p < 0.05, compared to the no stimulus control (1-way ANOVA, Dunnett’s test), or for the indicated comparison between two sets (t test).
Cdc42 is a small GTPase that mediates the effects of many chemoattractants on the cytoskeleton (56). In the developing nervous system, Slit2 activation of Robo causes an inactivation of Cdc42, and this inactivation is necessary for the ability of Slit2 to cause chemorepulsion of neuronal cells (57). Cdc42 also mediates the ability of Slit2 to inhibit neutrophil chemotaxis toward stromal cell-derived factor-1α (18). ML141 is an allosteric inhibitor of Cdc42, and has been used at 10 μM to inhibit Cdc42 mediated chemotaxis in human neutrophils (58). ML141 caused 500 ng/ml Slit2-S to act as a chemoattractant rather than as a chemorepellent (Fig.6B and Supplemental video 6), and blocked the ability of 500 ng/ml Slit2-N to act as a chemoattractant (Fig. 6B). For unknown reasons, the 0-500 ng/ml Slit2-S gradient increased the speed of neutrophils from the donors used for this experiment (Supplemental Fig. 4C). In the presence or absence of Slit2, ML141 also increased the speed of cells (Supplemental Fig. 4C), and in the absence of Slit2 slightly increased the directionality of neutrophils (Supplemental Fig. 4D). These results suggest that blocking Cdc42 activity reverses Slit2-S chemorepulsion and inhibits Slit2-N chemoattraction.
Rac is another small GTPase involved in neutrophil chemotaxis (15, 59). The Rac inhibitor NSC23766 inhibits Rac activation (60). Although 10 μM NSC23766 only causes a partial inhibition of Rac in neutrophils (61), we used it at 10 μM, since higher concentrations of this inhibitor have off-target effects (62). NSC23766 blocked the ability of Slit2-S to act as a chemorepellent (Fig. 6C). Although NSC23766 appeared to reduce the ability of Slit2-N to act as a chemoattractant, the effect was not statistically significant. NSC23766 in the presence or absence of Slit increased cell speed (Supplemental Fig. 4E), and the combination of NSC23766 and a Slit2-N gradient increased directionality (Supplemental Fig. 4F). Together, these results suggest that Rac may mediate the ability of Slit2-S to act as a chemorepellent.
Son of sevenless (Sos) is a guanine nucleotide exchange factor that can activate small GTPases such as Ras (63). In Drosophila, Slit activation of Robo in neurons causes recruitment of Sos to the plasma membrane (63). Ras mediates neutrophil chemotaxis (1, 2, 64). Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signaling (65, 66). Ras inhibitory peptide (RIP) corresponds to a region within human Sos1 that interacts with an SH3 domain of Grb2, preventing an interaction that is essential for Ras activation (65) and RIP has been used at 10 μM to inhibit Ras in neutrophils (66). RIP blocked the ability of Slit2-S to act as a chemorepellent, but did not appear to affect the chemoattraction in a 0-500 ng/ml Slit2-N gradient (Fig. 6D). RIP alone or in the presence of Slit2-S, but not in the presence of Slit2-N, increased neutrophil speed (Supplemental Fig. 4G), and RIP had no significant effect on directness (Supplemental Fig. 4H). To confirm the role of Ras in Slit2-S mediated chemorepulsion, GTP-bound Ras was measured in neutrophils incubated with Slit2-N, Slit2-S, or fMLP. After 6 minutes, compared to unstimulated cells, Slit2-S and fMLP increased levels of GTP-bound Ras, whereas Slit2-N did not significantly affect levels of GTP-bound Ras (Fig. 7B and 7C). Together, these data suggest that Slit2-S increases levels of GTP-bound Ras and needs Ras to cause chemorepulsion, whereas Slit2-N does not significantly affect levels of GTP-bound Ras and does not need Ras to cause chemoattraction.
Discussion
We found that two isoforms of the N-terminal fragment of Slit2 differentially regulate neutrophil chemotaxis, with Slit2-N directly acting as a chemoattractant, and Slit2-S directly acting as a chemorepellent. Both Slit2 fragments appeared to require Robo1 and the co-receptor syndecan-4 to direct neutrophil movement. Slit2-N chemoattraction appeared to not require activation of Ras and Rac, was blocked by a cdc42 inhibitor, increased PIP3 levels, and was reversed by a PI3 kinase inhibitor. In contrast, Slit2-S induced chemorepulsion was independent of PI3K, activated Ras, was blocked by Ras and Rac inhibitors, and reversed by a cdc42 inhibitor, indicating that, unexpectedly, the two Slit2 fragments activate different signal transduction pathways. Similar to the canonical neutrophil chemoattractant fMLP, a Slit2-N gradient induced a single zone of F-actin at the leading edge, increased cell adhesion, and altered cell morphology, whereas Slit2-S gradients caused multiple zones of F-actin accumulation and did not increase adhesion or alter cell morphology. Slit2-S thus causes chemorepulsion using an unusual effect on the cytoskeleton. Although Slit2-C did not directly induce chemotaxis, it increased cell speed, indicating that Slit2-C can modulate neutrophil chemotaxis.
Slit2-S induces chemorepulsion using a pathway involving Ras and Rac but not PI3 kinase. In agreement with the observation that Slit2-S does not appear to activate PI3 kinase, Slit2S decreases the phosphorylation of Akt substrates. The ability of a Cdc42 inhibitor to cause Slit2-S to act as a chemoattractant suggests that Slit2-S can activate an unknown chemoattraction pathway, but preferentially activates a chemorepulsion pathway that requires Cdc42. This chemoattraction pathway may be the Ras- and Rac-independent pathway used by Slit-2N. Conversely, the ability of a PI3 kinase inhibitor to cause Slit2-N to act as a chemorepellent suggests that Slit2-N can activate a chemorepulsion pathway, but preferentially activates a chemoattraction pathway that requires PI3 kinase.
The ability of Slit2-N to act as a neutrophil chemoattractant at low concentrations, but act as a chemorepellent at high concentrations (5,000 ng/ml; ~36 nM), is similar to the effects of many other chemotactic factors, which act as chemoattractants at low concentrations but either prevent migration (by inducing increased adhesion) or act as chemorepellents (fugetaxis) at high concentrations (36, 67, 68). 1,000-5,000 ng/ml Slit2 inhibits directed cell migration and activation of intracellular signaling pathways in many cell types (13, 15, 69, 70). An intriguing possibility is that a gradient of Slit2-N that starts at a high concentration may attract cells to tissues but create an exclusion zone near the source of the Slit2-N.
Supplementary Material
Acknowledgements
We thank the volunteers who donated blood, the phlebotomy staff at the Texas A&M Beutel Student Health Center, and Ramesh Rijal for helpful discussions.
Footnotes
This work was supported by National Institutes of Health Grants HL118507 and GM118355.
References
- 1.Nourshargh S, and Alon R. 2014. Leukocyte migration into inflamed tissues. Immunity 41: 694–707. [DOI] [PubMed] [Google Scholar]
- 2.de Oliveira S, Rosowski EE, and Huttenlocher A. 2016. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16: 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hohenester E 2008. Structural insight into Slit-Robo signalling. Biochem Soc Trans 36: 251–256. [DOI] [PubMed] [Google Scholar]
- 4.Blockus H, and Chedotal A. 2016. Slit-Robo signaling. Development 143: 3037–3044. [DOI] [PubMed] [Google Scholar]
- 5.Dickson BJ, and Gilestro GF. 2006. Regulation of Commissural Axon Pathfinding by Slit and its Robo Receptors. Annual Review of Cell and Developmental Biology 22: 651–675. [DOI] [PubMed] [Google Scholar]
- 6.Svensson KJ, Long JZ, Jedrychowski MP, Cohen P, Lo JC, Serag S, Kir S, Shinoda K, Tartaglia JA, Rao RR, Chedotal A, Kajimura S, Gygi SP, and Spiegelman BM. 2016. A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. Cell metabolism 23: 454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y-C, Chen P-N, Wang S-Y, Liao C-Y, Lin Y-Y, Sun S-R, Chiu C-L, Hsieh Y-S, Shieh J-C, and Chang JT. 2015. The differential roles of Slit2-exon 15 splicing variants in angiogenesis and HUVEC permeability. Angiogenesis 18: 301–312. [DOI] [PubMed] [Google Scholar]
- 8.Zakrys L, Ward Richard J., Pediani John D., Godin Antoine G., Graham Gerard J., and Milligan G. 2014. Roundabout 1 exists predominantly as a basal dimeric complex and this is unaffected by binding of the ligand Slit2. Biochemical Journal 461: 61. [DOI] [PubMed] [Google Scholar]
- 9.Delloye-Bourgeois C, Jacquier A, Charoy C, Reynaud F, Nawabi H, Thoinet K, Kindbeiter K, Yoshida Y, Zagar Y, Kong Y, Jones YE, Falk J, Chedotal A, and Castellani V. 2015. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nat Neurosci 18: 36–45. [DOI] [PubMed] [Google Scholar]
- 10.Tang W, Tang J, He J, Zhou Z, Qin Y, Qin J, Li B, Xu X, Geng Q, Jiang W, Wu W, Wang X, and Xia Y. 2015. SLIT2/ROBO1-miR-218–1-RET/PLAG1: a new disease pathway involved in Hirschsprung’s disease. Journal of Cellular and Molecular Medicine 19: 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin X, Shin Y-J, Riew T-R, Choi J-H, and Lee M-Y. 2016. Increased Expression of Slit2 and its Robo Receptors During Astroglial Scar Formation After Transient Focal Cerebral Ischemia in Rats. Neurochemical Research 41: 3373–3385. [DOI] [PubMed] [Google Scholar]
- 12.Pilling D, Zheng Z, Vakil V, and Gomer RH. 2014. Fibroblasts secrete Slit2 to inhibit fibrocyte differentiation and fibrosis. Proceedings of the National Academy of Sciences 111: 18291–18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan H, Zu G, Xie Y, Tang H, Johnson M, Xu X, Kevil C, Xiong W-C, Elmets C, Rao Y, Wu JY, and Xu H. 2003. Neuronal Repellent Slit2 Inhibits Dendritic Cell Migration and the Development of Immune Responses. The Journal of Immunology 171: 6519–6526. [DOI] [PubMed] [Google Scholar]
- 14.Prasad A, Qamri Z, Wu J, and Ganju RK. 2007. Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. Journal of Leukocyte Biology 82: 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tole S, Mukovozov IM, Huang Y-W, Magalhaes MAO, Yan M, Crow MR, Liu GY, Sun CX, Durocher Y, Glogauer M, and Robinson LA. 2009. The axonal repellent, Slit2, inhibits directional migration of circulating neutrophils. Journal of Leukocyte Biology 86: 1403–1415. [DOI] [PubMed] [Google Scholar]
- 16.Wu JY, Feng L, Park H-T, Havlioglu N, Wen L, Tang H, Bacon KB, Jiang Z.-h., Zhang X.-c., and Rao Y. 2001. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 410: 948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanellis J, Garcia GE, Li P, Parra G, Wilson CB, Rao Y, Han S, Smith CW, Johnson RJ, Wu JY, and Feng L. 2004. Modulation of Inflammation by Slit Protein In Vivo in Experimental Crescentic Glomerulonephritis. The American Journal of Pathology 165: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye B-Q, Geng ZH, Ma L, and Geng J-G. 2010. Slit2 Regulates Attractive Eosinophil and Repulsive Neutrophil Chemotaxis through Differential srGAP1 Expression during Lung Inflammation. The Journal of Immunology 185: 6294–6305. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Xiao Y, Subramanian RR, Okamoto E, Wilcox JN, Anderson L, and De Leon H. 2016. Potential Role of Axonal Chemorepellent Slit2 in Modulating Adventitial Inflammation in a Rat Carotid Artery Balloon Injury Model. Journal of cardiovascular pharmacology 67: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuen DA, Huang Y-W, Liu G-Y, Patel S, Fang F, Zhou J, Thai K, Sidiqi A, Szeto SG, Chan L, Lu M, He X, John R, Gilbert RE, Scholey JW, and Robinson LA. 2016. Recombinant N–Terminal Slit2 Inhibits TGF-β–Induced Fibroblast Activation and Renal Fibrosis. Journal of the American Society of Nephrology 27: 2609–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherchan P, Huang L, Wang Y, Akyol O, Tang J, and Zhang JH. 2016. Recombinant Slit2 attenuates neuroinflammation after surgical brain injury by inhibiting peripheral immune cell infiltration via Robo1-srGAP1 pathway in a rat model. Neurobiology of disease 85: 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herlihy SE, Pilling D, Maharjan AS, and Gomer RH. 2013. Dipeptidyl Peptidase IV Is a Human and Murine Neutrophil Chemorepellent. The Journal of Immunology 190: 6468–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muinonen-Martin AJ, Veltman DM, Kalna G, and Insall RH. 2010. An Improved Chamber for Direct Visualisation of Chemotaxis. PLoS One 5: e15309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips JE, and Gomer RH. 2012. A secreted protein is an endogenous chemorepellant in Dictyostelium discoideum. Proc Natl Acad Sci U S A 109: 10990–10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White MJV, Chinea LE, Pilling D, and Gomer RH. 2018. Protease activated-receptor 2 is necessary for neutrophil chemorepulsion induced by trypsin, tryptase, or dipeptidyl peptidase IV. Journal of Leukocyte Biology 103: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilling D, Vakil V, Cox N, amd Gomer RH 2015. TNF-α-stimulated fibroblasts secrete lumican to promote fibrocyte differentiation. Proceedings of the National Academy of Sciences 112: 11929–11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White JR, Naccache PH, and Sha’afi RI. 1983. Stimulation by chemotactic factor of actin association with the cytoskeleton in rabbit neutrophils. Effects of calcium and cytochalasin B. J Biol Chem 258: 14041–14047. [PubMed] [Google Scholar]
- 28.Sheikh S, Gratzer WB, Pinder JC, and Nash GB. 1997. Actin polymerisation regulates integrin-mediated adhesion as well as rigidity of neutrophils. Biochem Biophys Res Commun 238: 910–915. [DOI] [PubMed] [Google Scholar]
- 29.Tang L, Gao T, McCollum C, Jang W, Vicker MG, Ammann RR, and Gomer RH. 2002. A cell number-counting factor regulates the cytoskeleton and cell motility in Dictyostelium. Proc.Natl.Acad.Sci.U.S.A 99: 1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, and Cohen P. 1996. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett 399: 333–338. [DOI] [PubMed] [Google Scholar]
- 31.Maharjan AS, Roife D, Brazill D, and Gomer RH. 2013. Serum amyloid P inhibits granulocyte adhesion. Fibrogenesis Tissue Repair 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harlow E, and Lane D. 2006. Staining Immunoblots for Total Protein Using Ponceau S. Cold Spring Harbor Protocols 2006: pdb.prot4269. [DOI] [PubMed] [Google Scholar]
- 33.Williams LT, Snyderman R, Pike MC, and Lefkowitz RJ. 1977. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proceedings of the National Academy of Sciences of the United States of America 74: 1204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiMilla PA, Barbee K, and Lauffenburger DA. 1991. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophysical Journal 60: 15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heit B, Tavener S, Raharjo E, and Kubes P. 2002. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol 159: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell JJ, Foxman EF, and Butcher EC. 1997. Chemoattractant receptor cross talk as a regulatory mechanism in leukocyte adhesion and migration. Eur.J.Immunol 27: 2571–2578. [DOI] [PubMed] [Google Scholar]
- 37.Steigemann P, Molitor A, Fellert S, Jackle H, and Vorbruggen G. 2004. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Current biology : CB 14: 225–230. [DOI] [PubMed] [Google Scholar]
- 38.Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, and Van Vactor D. 2004. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Current biology : CB 14: 499–504. [DOI] [PubMed] [Google Scholar]
- 39.Dunzendorfer S, Kaneider N, Rabensteiner A, Meierhofer C, Reinisch C, Romisch J, and Wiedermann CJ. 2001. Cell-surface heparan sulfate proteoglycan-mediated regulation of human neutrophil migration by the serpin antithrombin III. Blood 97: 1079–1085. [DOI] [PubMed] [Google Scholar]
- 40.Insall RH, and Machesky LM. 2009. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell 17: 310–322. [DOI] [PubMed] [Google Scholar]
- 41.Ku CJ, Wang Y, Weiner OD, Altschuler SJ, and Wu LF. 2012. Network crosstalk dynamically changes during neutrophil polarization. Cell 149: 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altay T, McLaughlin B, Wu JY, Park TS, and Gidday JM. 2007. Slit modulates cerebrovascular inflammation and mediates neuroprotection against global cerebral ischemia. Exp Neurol 207: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaturvedi S, Yuen DA, Bajwa A, Huang Y-W, Sokollik C, Huang L, Lam GY, Tole S, Liu G-Y, Pan J, Chan L, Sokolskyy Y, Puthia M, Godaly G, John R, Wang C, Lee WL, Brumell JH, Okusa MD, and Robinson LA. 2013. Slit2 Prevents Neutrophil Recruitment and Renal Ischemia-Reperfusion Injury. Journal of the American Society of Nephrology 24: 1274–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell D, Tauzin S, Hind LE, Deng Q, Beebe DJ, and Huttenlocher A. 2017. Chemokine Signaling and the Regulation of Bidirectional Leukocyte Migration in Interstitial Tissues. Cell reports 19: 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diamond MS, Garciaaguilar J, Bickford JK, Corbi AL, and Springer TA. 1993. The i-domain is a major recognition site on the leukocyte integrin mac-1 (cd11b/cd18) for 4 distinct adhesion ligands. Journal Of Cell Biology 120: 1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Berg JM, Mul FPJ, Schippers E, Weening JJ, Roos D, and Kuijpers TW. 2001. β1 integrin activation on human neutrophils promotes β2 integrin-mediated adhesion to fibronectin. European Journal of Immunology 31: 276–284. [DOI] [PubMed] [Google Scholar]
- 47.Rochon YP, Kavanagh TJ, and Harlan JM. 2000. Analysis of integrin (CD11b/CD18) movement during neutrophil adhesion and migration on endothelial cells. Journal of microscopy 197: 15–24. [DOI] [PubMed] [Google Scholar]
- 48.Szczur K, Zheng Y, and Filippi M-D. 2009. The small Rho GTPase Cdc42 regulates neutrophil polarity via CD11b integrin signaling. Blood 114: 4527–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas KM, Pyun HY, and Navarro J. 1990. Molecular cloning of the fMet-Leu-Phe receptor from neutrophils. Journal of Biological Chemistry 265: 20061–20064. [PubMed] [Google Scholar]
- 50.Tseng RC, Lee SH, Hsu HS, Chen BH, Tsai WC, Tzao C, and Wang YC. 2010. SLIT2 attenuation during lung cancer progression deregulates beta-catenin and E-cadherin and associates with poor prognosis. Cancer research 70: 543–551. [DOI] [PubMed] [Google Scholar]
- 51.Chang PH, Hwang-Verslues WW, Chang YC, Chen CC, Hsiao M, Jeng YM, Chang KJ, Lee EY, Shew JY, and Lee WH. 2012. Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/beta-catenin pathway. Cancer Res 72: 4652–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi R, Yang Z, Liu W, Liu B, Xu Z, and Zhang Z. 2014. Knockdown of Slit2 promotes growth and motility in gastric cancer cells via activation of AKT/beta-catenin. Oncology reports 31: 812–818. [DOI] [PubMed] [Google Scholar]
- 53.Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, Meek S, Smith AJ, Okkenhaug K, and Vanhaesebroeck B. 2008. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci U S A 105: 8292–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fruman DA, Meyers RE, and Cantley LC. 1998. Phosphoinositide kinases. Annual review of biochemistry 67: 481–507. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, Xiao Y, Ding B-B, Zhang N, Yuan X.-b., Gui L, Qian K-X, Duan S, Chen Z, Rao Y, and Geng J-G. 2003. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer cell 4: 19–29. [DOI] [PubMed] [Google Scholar]
- 56.McCormick B, Chu JY, and Vermeren S. 2017. Cross-talk between Rho GTPases and PI3K in the neutrophil. Small GTPases 22: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong K, Ren X-R, Huang Y-Z, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, Wu JY, Xiong W-C, and Rao Y. 2001. Signal Transduction in Neuronal Migration: Roles of GTPase Activating Proteins and the Small GTPase Cdc42 in the Slit-Robo Pathway. Cell 107: 209–221. [DOI] [PubMed] [Google Scholar]
- 58.Surviladze Z, Waller A, Strouse JJ, Bologa C, Ursu O, Salas V, Parkinson JF, Phillips GK, Romero E, Wandinger-Ness A, Sklar LA, Schroeder C, Simpson D, Noth J, Wang J, Golden J, and Aube J. 2010. A Potent and Selective Inhibitor of Cdc42 GTPase In Probe Reports from the NIH Molecular Libraries Program. National Center for Biotechnology Information (US), Bethesda (MD). [PubMed] [Google Scholar]
- 59.Sun CX, Downey GP, Zhu F, Koh AL, Thang H, and Glogauer M. 2004. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood 104: 3758–3765. [DOI] [PubMed] [Google Scholar]
- 60.Akbar H, Cancelas J, Williams DA, Zheng J, and Zheng Y. 2006. Rational Design and Applications of a Rac GTPase–Specific Small Molecule Inhibitor. Methods Enzymol 406: 554–565. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell T, Lo A, Logan MR, Lacy P, and Eitzen G. 2008. Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. American journal of physiology. Cell physiology 295: C1354–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dutting S, Heidenreich J, Cherpokova D, Amin E, Zhang SC, Ahmadian MR, Brakebusch C, and Nieswandt B. 2015. Critical off-target effects of the widely used Rac1 inhibitors NSC23766 and EHT1864 in mouse platelets. J Thromb Haemost 13: 827–838. [DOI] [PubMed] [Google Scholar]
- 63.Yang L, and Bashaw GJ. 2006. Son of sevenless directly links the Robo receptor to rac activation to control axon repulsion at the midline. Neuron 52: 595–607. [DOI] [PubMed] [Google Scholar]
- 64.Yang HW, Collins SR, and Meyer T. 2015. Locally excitable Cdc42 signals steer cells during chemotaxis. Nature Cell Biology 18: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, and Schlessinger J. 1993. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature 363: 85–88. [DOI] [PubMed] [Google Scholar]
- 66.Filina YV, Safronova VG, and Gabdoulkhakova AG. 2012. Small G-proteins Ras, Rac and Rho in the regulation of the neutrophil respiratory burst induced by formyl peptide. Biochemistry (Moscow) Supplement Series A: Membrane and Cell Biology 6: 67–74. [Google Scholar]
- 67.Poznansky MC, Olszak IT, Foxall R, Evans RH, Luster AD, and Scadden DT. 2000. Active movement of T cells away from a chemokine. Nat Med 6: 543–548. [DOI] [PubMed] [Google Scholar]
- 68.Tharp WG, Yadav R, Irimia D, Upadhyaya A, Samadani A, Hurtado O, Liu SY, Munisamy S, Brainard DM, Mahon MJ, Nourshargh S, van Oudenaarden A, Toner MG, and Poznansky MC. 2006. Neutrophil chemorepulsion in defined interleukin-8 gradients in vitro and in vivo. Journal of Leukocyte Biology 79: 539–554. [DOI] [PubMed] [Google Scholar]
- 69.Prasad A, Kuzontkoski PM, Shrivastava A, Zhu W, Li DY, and Groopman JE. 2012. Slit2N/Robo1 Inhibit HIV-gp120-Induced Migration and Podosome Formation in Immature Dendritic Cells by Sequestering LSP1 and WASp. PLOS ONE 7: e48854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan X, Yang H, Kumar S, Tumelty KE, Pisarek-Horowitz A, Rasouly HM, Sharma R, Chan S, Tyminski E, Shamashkin M, Belghasem M, Henderson JM, Coyle AJ, Salant DJ, Berasi SP, and Lu W. 2016. SLIT2/ROBO2 signaling pathway inhibits nonmuscle myosin IIA activity and destabilizes kidney podocyte adhesion. JCI Insight 1: e86934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.