Abstract
Mesenchymal stem cells (MSCs) are capable of secreting exosomes, extracellular vesicles, and cytokines to regulate cell and tissue homeostasis. However, it is unknown whether MSCs use a specific exocytotic fusion mechanism to secrete exosomes and cytokines. We show that Fas binds with Fas-associated phosphatase–1 (Fap-1) and caveolin-1 (Cav-1) to activate a common soluble N-ethylmaleimide–sensitive factor (NSF) attachment protein receptor (SNARE)–mediated membrane fusion mechanism to release small extracellular vesicles (sEVs) in MSCs. Moreover, we reveal that MSCs produce and secrete interleukin-1 receptor antagonist (IL-1RA) associated with sEVs to maintain rapid wound healing in the gingiva via the Fas/Fap-1/Cav-1 cascade. Tumor necrosis factor–α (TNF-α) serves as an activator to up-regulate Fas and Fap-1 expression via the nuclear factor κB pathway to promote IL-1RA release. This study identifies a previously unknown Fas/Fap-1/Cav-1 axis that regulates SNARE-mediated sEV and IL-1RA secretion in stem cells, which contributes to accelerated wound healing.
INTRODUCTION
Mesenchymal stem cells (MSCs) are capable of self-renewal and differentiation into mesenchymal and non-mesenchymal lineages (1, 2). MSCs have been used therapeutically for tissue regeneration and auto-immune disease treatment (3–12). Recent emerging evidence shows that multiple mechanisms contribute to MSC-based therapies in which secretion of cytokines, growth factors, and small extracellular vesicles (sEVs) such as exosomes may serve as paracrine or autocrine mediators to regulate immune responses and tissue regeneration (13–15). MSCs have been identified to secrete at least three types of exosomes (16); these exosomes represent an important mode of intercellular communication. It is well known that a variety of cells, including neuroendocrine, endocrine, exocrine, and immune cells, use different mechanisms to secrete EVs and that soluble N-ethylmaleimide–sensitive factor (NSF) attachment protein receptor (SNARE)–dependent exocytotic fusion plays a major role in the exocytosis of these EVs (17, 18). Dysfunction of the secretion process may lead to various human diseases, such as schizophrenia, Alzheimer’s disease, diabetes, and albinism (19–22). However, it is unknown whether and, if so, how stem cells use an exocytotic fusion mechanism to secrete sEVs.
Mammalian adult cutaneous wound healing can be regulated by numerous secreted factors including cytokines, growth factors, chemokines, and EVs (23, 24). MSC-derived exosomes play an important role in cutaneous wound healing, by reducing heat stress–induced apoptosis (25) and promoting collagen synthesis and angiogenesis (26). Compared to adult cutaneous wounds, fetal wounds heal faster, are more akin to a scarless regeneration, and display mild inflammation with fewer inflammatory cells and lower expression of proinflammatory factors (27). Like fetal wounds, oral gingival/mucosal wounds heal faster than cutaneous wounds and exhibit minimal scar formation (28), which may be due to reduced inflammatory cell infiltration and proinflammatory factor production in the gingiva (29–31). Thus, the secretion profile of MSCs may control accelerated wound healing in the gingiva. Gingiva-derived MSCs (GMSCs) have a distinct neural crest origin and show characteristics of self-renewal, multipotent differentiation, and immunomodulatory capacities both in vitro and in vivo (32, 33). However, it is unknown whether GMSCs have a unique secretory mechanism to regulate wound healing in the gingiva. Here, we show that GMSCs use the Fas/Fas-associated phosphatase–1 (Fap-1)/caveolin-1 (Cav-1) complex to activate SNARE-mediated membrane fusion to secrete higher amounts of interleukin-1 receptor antagonist (IL-1RA)–expressing sEVs to accelerate wound healing in the gingiva.
RESULTS
GMSCs produce and secrete higher amounts of IL-1RA–expressing sEVs than skin MSCs
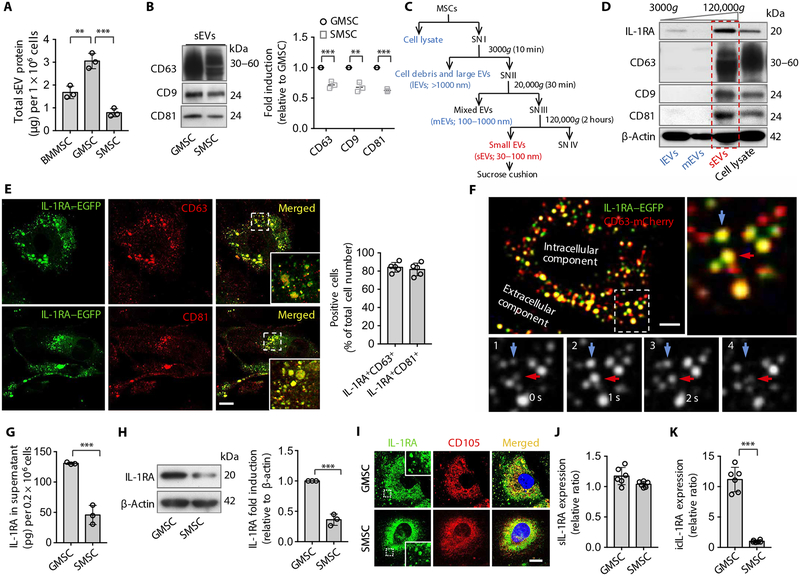
To explore whether MSCs use a unique mechanism to secrete sEVs, we first isolated exosome-like sEVs from the culture supernatant of several MSCs, including mouse and human bone marrow MSCs (BMMSCs), GMSCs, and skin MSCs (SMSCs). We found that both mouse and human GMSCs secreted higher amounts of sEV-associated proteins, and SMSCs secreted lower amounts of sEV-associated proteins in the culture supernatant compared to GMSCs (Fig. 1A and fig. S1A). We verified that these exosome-like sEVs showed standard exosome microstructure and expressed exosome-associated proteins CD63, CD9, and CD81 (Fig. 1B and fig. S1B). Cytokine array analysis showed that GMSC-derived sEVs contained higher amounts of IL-1RA, a natural inhibitor of the proinflammatory cytokine IL-1β (34), compared to sEVs from SMSCs (fig. S1C). Because wound healing in the gingiva is faster than that in the skin (28) and MSC-derived exosomes play an important role in cutaneous wound healing (25), we hypothesized that secretion of higher amounts of sEVs and IL-1RA by GMSCs may contribute to a quick wound healing process in the gingiva. To further confirm that sEVs contain IL-1RA, a differential centrifugation approach, followed by sucrose cushion to isolate sEVs at a higher purity grade (35, 36), was used to isolate different sizes of EVs in the culture supernatant of GMSCs and SMSCs (Fig. 1C). We showed that cell lysate, cell debris and large EVs, and sEVs from GMSCs and SMSCs expressed IL-1RA; however, IL-1RA was not expressed in mixed EVs (Fig. 1D and fig. S1D), suggesting that IL-1RA might be released via sEVs. To rule out potential contributions from nonvesicular particles, we loaded the crude EV fraction from differential centrifugation on a sucrose gradient (36). We found that IL-1RA mainly appeared in 3 light fractions out of the 10 high-speed ultra-centrifugation fractions and matched the expression with CD63, CD9, and CD81 (fig. S1E), which was consistent with a previous report that MSC-derived sEVs usually float in gradients with 1.10 to 1.18 g/ml (37). To demonstrate that the sEV-associated secretion is the major pathway of IL-1RA secretion, we collected the supernatant from each step of differential centrifugation as described (fig. S1F). After the initial steps of centrifugation, we found that the supernatants collected from SN I, SN II, and SN III sections contained similar amounts of IL-1RA. However, the amount of IL-1RA reduced from 134.07 pg/ml in SN III to 12.22 pg/ml in SN IV after ultracentrifugation (fig. S1G). We next showed that the isolated sEVs expressed equal amounts of IL-1RA compared with the concentrated supernatant collected before ultracentrifugation (SN III), but the concentrated proteins from the ultracentrifuged supernatant (SN IV) showed a markedly decreased amount of IL-1RA (fig. S1H). We further performed immunogold electron microscopy to show that IL-1RA was detected on the surface of purified exosome-like EVs (fig. S1I). These results support our conclusion that IL-1RA is secreted by sEVs.
Fig. 1. Murine gingival MSCs and skin MSCs produce and secrete IL-1RA–EV.
(A) Total protein contained within small extracellular vesicles (sEVs) isolated from the culture supernatant of 1 × 106 murine human bone marrow mesenchymal stem cells (BMMSCs), gingiva-derived MSCs (GMSCs), and skin MSCs (SMSCs) (n = 3). (B) Western blotting and semi-quantification analysis of CD63, CD9, and CD81 expression from sEVs isolated from GMSCs and SMSCs. (C) Differential centrifugation and sucrose cushion procedure for the isolation of EVs from MSC culture supernatants (SN). (D) Interleukin-1 receptor antagonist (IL-1RA), CD63, CD9, and CD81 expression in lysates from fractions corresponding to (C). (E) Super-resolution stimulated emission depletion staining and quantification for IL-1RA–enhanced green fluorescent protein (EGFP) (green), CD63 (red), and CD81 (red) in GMSCs transfected with plasmids containing IL-1RA–EGFP fusion protein. The lower right box is a higher magnification of the boxed region in the merged image; colocalization of IL-1RA with CD63 or CD81 is shown in yellow (n = 5). Scale bar, 10 μm. (F) Total internal reflection fluorescence (TIRF) microscopy images from GMSCs cotransfected with plasmids expressing IL-1RA–EGFP (green) and CD63-mCherry (red). The top right panel is a higher magnification of the boxed region in the left image; colocalization of IL-1RA–EGFP and CD63-mCherry is shown in yellow. The bottom panels (1 to 4) show sequential images from live-cell imaging. Arrows indicate two individual IL-1RA–positive vesicle fusion events. Scale bar, 10 μm. (G) Enzyme-linked immunosorbent assay (ELISA) of IL-1RA from the culture supernatant of GMSCs and SMSCs (n = 3). (H) Western blotting and semi-quantification analysis of IL-1RA expressed by GMSCs and SMSCs. (I) Immunocytofluorescence staining of IL-1RA (green) and the MSC marker CD105 (red) in GMSCs and SMSCs. Scale bar, 20 μm. (J and K) Real-time polymerase chain reaction analysis of soluble IL-1RA (sIL-1RA) mRNA (J) and intracellular IL-1RA (icIL-1RA) mRNA (K) in GMSCs and SMSCs. All results are representative of data generated in at least three independent experiments (J and K) (n = 6). **P < 0.01, ***P < 0.001. Error bars are means ± SD. Data were analyzed using one-way analysis of variance (ANOVA) with Bonferroni correction (A), or independent un-paired two-tailed Student’s t tests (B, G, H, J, and K).
To further confirm the presence of IL-1RA–positive sEVs, we transfected GMSCs with IL-1RA–enhanced green fluorescent protein (EGFP) plasmids and then used super-resolution stimulated emission depletion (STED) microscopy to show colocalization of IL-1RA with CD63 and CD81 (Fig. 1E). To verify EV–IL-1RA exocytosis, GMSCs were cotransfected with plasmids expressing IL-1RA–EGFP fusion protein and CD63-mCherry fluorescent protein, and colocalization was observed by total internal reflection fluorescence (TIRF) microscopy (Fig. 1F). The sequential fluorescent images displayed fusion of individual IL-1RA–EGFP/CD63-mCherry double-positive exosome-like EVs with the plasma membrane (Fig. 1F). Moreover, we found that IL-1RA–EGFP/CD63-mCherry double-positive exosome-like EVs fused with the plasma membrane in living GMSCs (movie S1).
Next, we showed that GMSCs secreted a higher amount of IL-1RA in the culture supernatant compared to SMSCs, as assessed by enzyme-linked immunosorbent assay (ELISA) (Fig. 1G). Western blotting showed that both human and mouse GMSCs expressed elevated IL-1RA relative to SMSCs (Fig. 1H and fig. S1J). IL-1RA was coexpressed with MSC markers CD105, CD44, and CD90 in GMSCs and SMSCs (Fig. 1I and fig. S1K). There are four isotypes of IL-1RA: One isoform is secreted (sIL-1RA), whereas the three others lack a consensus leader peptide and remain intracellular (icIL-1RA1, icIL-1RA2, and icIL-1RA3) (34). GMSCs express a similar amount of sIL-1RA mRNA, but a significantly higher amount of icIL-1RA mRNA compared to SMSCs (Fig. 1, J and K), suggesting that altered expression of IL-1RA is mainly caused by icIL-1RA. Because icIL-1RA does not have a signaling peptide that marks it for transport outside of the cells, it is possible that icIL-1RA is instead packaged into sEVs and transported to the extracellular microenvironment.
Fas controls IL-1RA–sEV release in MSCs
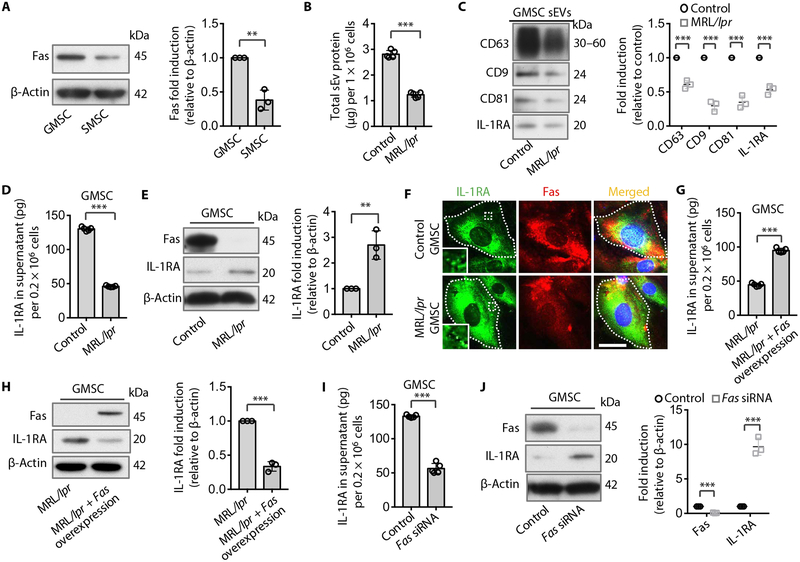
Our previous study showed that Fas controls monocyte chemoattractant protein–1 (MCP-1) secretion to regulate MSC-based immune therapies (4). We therefore hypothesized that Fas may control IL-1RA–sEV release to modulate wound healing. We showed that GMSCs expressed elevated Fas in the cytoplasm compared to SMSCs (Fig. 2A). GMSCs and SMSCs from Fas-deficient MRL/lpr mice showed reduced capacity to secrete sEV protein to the culture supernatant (Fig. 2B and fig. S2A). EVs from MRL/lpr GMSCs and SMSCs expressed reduced CD63, CD9, CD81, and IL-1RA compared to wild-type (WT) control GMSCs (Fig. 2C and fig. S2B). We further confirmed that GMSCs from MRL/lpr mice showed reduced IL-1RA secretion into the culture supernatant, along with elevated cytoplasmic accumulation of IL-1RA when compared to WT control GMSCs (Fig. 2, D and E). In addition, immunostaining showed IL-1RA colocalized with Fas where it accumulated in Fas-deficient MRL/lpr GMSCs (Fig. 2F). Overexpression of Fas in MRL/lpr GMSCs rescued IL-1RA secretion into the culture supernatant and reduced the cytoplasmic expression of IL-1RA (Fig. 2, G and H). When Fas expression was knocked down in GMSCs by small interfering RNA (siRNA), IL-1RA secretion into the culture supernatant was reduced; however, cytoplasmic IL-1RA was increased (Fig. 2, I and J), which was also observed in MRL/lpr GMSCs. These data suggest that Fas may control IL-1RA–sEV release in GMSCs.
Fig. 2. Fas controls IL-1RA–sEV secretion in murine MSCs.
(A) Western blotting and semi-quantification of Fas expression in GMSCs and SMSCs (n = 3). (B) Secreted sEV-associated protein quantification from Fas-deficient MRL/lpr and wild-type (WT) control GMSCs (n = 5). (C) Western blotting and semi-quantification analysis of CD63, CD9, CD81, and IL-1RA from sEV from Fas-deficient MRL/lpr and WT control GMSCs. sEV-associated proteins from culture supernatants of equal numbers of cells in control and MRL/lpr GMSC groups were loaded (n = 3). (D) ELISA analysis of secreted IL-1RA from the culture supernatant from WT control and Fas-deficient MRL/lpr GMSCs (n = 3). (E) Western blotting and semi-quantification analysis of cytoplasmic IL-1RA from WT control and Fas-deficient MRL/lpr GMSCs (n = 3). (F) Immunocytofluorescent double staining of IL-1RA (green) and Fas (red) in WT control and Fas-deficient MRL/lpr GMSCs. Dashed lines indicate the cell edge. Scale bar, 20 μm. (G) ELISA analysis of IL-1RA secretion in the culture supernatant of MRL/lpr and Fas-overexpressing MRL/lpr GMSCs (n = 5). (H) Western blotting and semi-quantification analysis of cytoplasmic IL-1RA from MRL/lpr and Fas-overexpressing MRL/lpr GMSCs (n = 3). (I) ELISA analysis of IL-1RA secretion in the culture supernatant from WT control GMSCs treated with and without Fas small interfering (siRNA) (n = 5). (J) Western blotting and semi-quantification of cytoplasmic IL-1RA and Fas from WT control GMSCs treated with or without Fas siRNA (n = 3). All results are representative of data generated from at least three independent experiments. **P < 0.01, ***P < 0.001. Error bars are means ± SD. All data were analyzed using independent unpaired two-tailed Student’s t tests.
Fas bound with Fap-1 and Cav-1 controls IL-1RA–sEV release
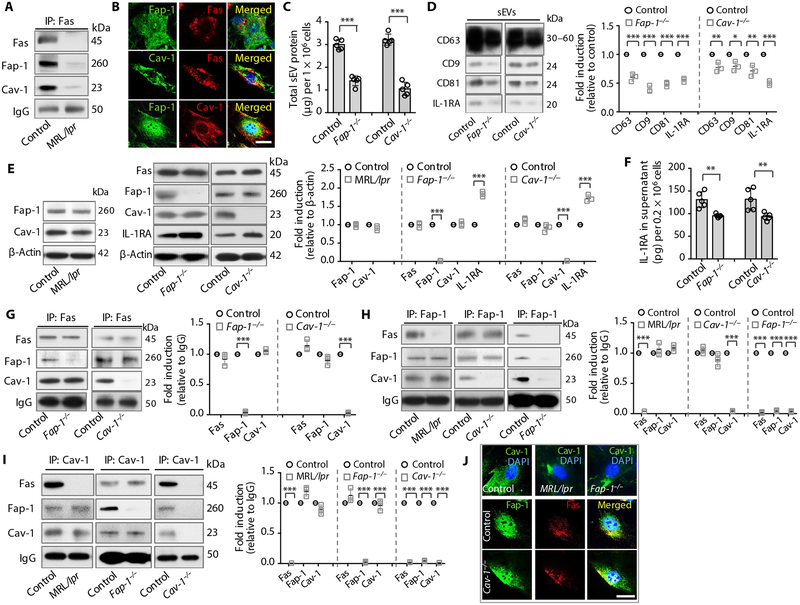
Fap-1 is a protein tyrosine phosphatase capable of binding to the cytosolic domain of Fas (38), and Cav-1 can also bind to Fas to regulate apoptosis (39). However, it is unknown whether Fap-1 and Cav-1 are involved in Fas-controlled sEV/cytokine release. SNARE family proteins mediate the exocytosis of cellular transport vesicles with the cell membrane; some of the key molecules in this family are synaptosome-associated protein of 25 kDa (SNAP25) and vesicle-associated membrane protein (VAMP) (18). We speculated that Fas might control IL-1RA–sEV release through interaction with Fap-1, Cav-1, and SNARE proteins. We saw that Fas coimmunoprecipitated with Fap-1, Cav-1, SNAP25, and VAMP5; this coimmunoprecipitation (co-IP) was absent in Fas-deficient MRL/lpr GMSCs (Fig. 3A and fig. S3A). Immunostaining confirmed that Fas colocalized with Fap-1, Cav-1, SNAP25, and VAMP5, and that Fap-1 colocalized with Cav-1 in WT GMSCs (Fig. 3B and fig. S3B). To assess the functional role of Fap-1/Cav-1 in IL-1RA–sEV release, we examined Fap-1 and Cav-1 knockout GMSCs and SMSCs. Fap-1 and Cav-1 knockout GMSCs and SMSCs showed reduced sEV secretion into the culture supernatant (Fig. 3C and fig. S3C). sEVs from Fap-1 and Cav-1 knockout GMSCs and SMSCs expressed reduced CD63, CD9, CD81, and IL-1RA compared to control GMSCs and SMSCs, respectively (Fig. 3D and fig. S3D). To further dissect the functional role of Fas/Fap-1/Cav-1, we showed that there was no difference in the expression of Fap-1 and Cav-1 in Fas-deficient MRL/lpr GMSCs (Fig. 3E), but there was reduced secretion of exosome-associated proteins and IL-1RA into the culture supernatant and there were elevated amounts of IL-1RA in the cytoplasm (Fig. 2, B to E). Knockout of Fap-1 or Cav-1 in GMSCs reduced IL-1RA secretion into the culture supernatant and increased cytoplasmic IL-1RA, but failed to affect the expression of Fas/Cav-1 or Fas/Fap-1 (Fig. 3, E and F). Knockdown of Fas, Fap-1, or Cav-1 by siRNAs in GMSCs failed to affect the expression of the other two members, but decreased IL-1RA secretion into the culture supernatant with increased cytoplasmic accumulation of IL-1RA (fig. S3, E and F), which is the same as that observed in MRL/lpr, Fap-1, and Cav-1 knockout GMSCs. These data suggest that Fas/Fap-1/Cav-1 control IL-1RA release. In addition, knockdown of Fas, Fap-1, or Cav-1 failed to affect the expression of SNAP25 and VAMP5 (fig. S3E). To test whether SNAP25 and VAMP5 are also involved in Fas/Fap-1/Cav-1–controlled IL-1RA release, we showed that knockdown of SNAP25 or VAMP5 by siRNA failed to affect the expression of Fas, Fap-1, and Cav-1 (fig. S3E), but reduced IL-1RA secretion into the culture supernatant along with increased cytoplasmic accumulation of IL-1RA (fig. S3, E and F). These data suggest that Fas/Fap-1/Cav-1 machinery may control IL-1RA release via SNAP25/VAMP5.
Fig. 3. Fas binds with Fap-1 and Cav-1 to regulate IL-1RA–sEV release in murine GMSCs.
(A) Fas co-IP of WT control and MRL/lpr GMSC lysate. (B) Immunocytofluorescence double staining for Fas, Fap-1, and Cav-1 in WT GMSCs. (C) Secreted sEV-associated protein quantification from WT control, Fap-1, and Cav-1 knockout GMSCs (n = 3). (D) Western blotting and semi-quantification of CD63, CD9, CD81, and IL-1RA in sEVs from WT control, Fap-1, and Cav-1 knockout GMSCs. Culture supernatants from equal numbers of cells from control and knockout GMSCs were loaded for Western blotting analysis (n = 3). (E) Western blotting and semi-quantification of Fas, Fap-1, Cav-1, and IL-1RA expression in WT control, MRL/lpr, Fas-deficient, Fap-1 knockout, and Cav-1 knockout GMSCs (n = 3). (F) ELISA analysis of secretion of IL-1RA in the culture supernatant from WT control, Fap-1–deficient, and Cav-1–deficient GMSCs (n = 5). (G) Immunoprecipitation (IP) and semi-quantification analysis of Fas from WT control, Fap-1–deficient, and Cav-1 knockout GMSC lysates (n = 3). (H) IP and semi-quantification analysis of Fap-1 from WT control, MRL/lpr, Cav-1 knockout, and Fap-1 knockout GMSC lysates (n = 3). (I) IP and semi-quantification analysis of Cav-1 from WT control, MRL/lpr, Fap-1 knockout, and Cav-1 knockout GMSCs (n = 3). (J) Immunocytofluorescence staining of Cav-1 in WT, Fas-deficient MRL/lpr, and Fap-1 knockout GMSCs, and double-staining of Fap-1 and Fas in WT and Cav-1 knockout GMSCs. For A-P, Fas-deficient GMSCs from MRL/lpr mice, GMSCs with Fap-1 knocked out using a CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 (CRISPR-associated protein 9) plasmid, and Cav-1 knockout GMSCs from Cav-1−/− mice were used, and WT GMSCs served as a control. For IP, whole-cell lysates from indicated GMSCs were immunoprecipitated with corresponding antibodies, and the immunocomplexes were subjected to Western blotting with antibodies against Fas, Fap-1, and Cav-1. All results are representative of data generated from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars are means ± SD. Scale bars, 20 μm. All data were analyzed using independent unpaired two-tailed Student’s t tests. DAPI, 4′,6-diamidino-2-phenylindole; IgG, immunoglobulin G.
We next determined how Fas binds to the downstream complex SNAP25/VAMP5 and how Fas/Fap-1/Cav-1 bind together to control exosome/IL-1RA release. We performed IP using Fas-deficient MRL/lpr, Fap-1 knockout, and Cav-1 knockout GMSCs to show that knocking out Fap-1 failed to reduce the co-IP of Fas with Cav-1, SNAP25, and VAMP5, whereas knockout of Cav-1 failed to affect the co-IP of Fas with Fap-1 but resulted in the reduction of Fas co-IP with SNAP25 and VAMP5 (Fig. 3G and fig. S3G). These data suggest that the binding of Fas with SNAP25/VAMP5 is mediated by Cav-1. To test how Fap-1 binds with the other proteins, we showed that Fas-deficient MRL/lpr GMSCs failed to affect the co-IP of Fap-1 with Cav-1, SNAP25, and VAMP5, whereas knocking out Cav-1 expression failed to affect the co-IP of Fap-1 with Fas, but resulted in the reduction of Fap-1 co-IP with SNAP25 and VAMP5 (Fig. 3H and fig. S3H). We next used immunocytofluorescence staining to confirm that Fap-1 was colocalized with SNAP25 and VAMP5 (fig. S3I). These data suggest that the binding of Fap-1 with SNAP25/VAMP5 is also mediated by Cav-1.
To examine how Cav-1 binds with the other proteins, we showed that MRL/lpr or Fap-1 knockout GMSCs failed to affect the co-IP of Cav-1 with Fap-1 or Fas and also failed to affect the co-IP of Cav-1 with SNAP25 and VAMP5 (Fig. 3I and fig. S3J). Immunocytofluorescence staining further confirmed that Cav-1 was colocalized with SNAP25 and VAMP5 (fig. S3K). Together, these data suggest that Fas/Fap-1/Cav-1 bind together, but these events occur independently of one another; that the binding between Cav-1 and SNAP25/VAMP5 is independent of Cav-1 binding with Fas/Fap-1; and that the binding of Fas/Fap-1 with SNAP25/VAMP5 is mediated by Cav-1. Fas-deficient MRL/lpr and Fap-1 knockout GMSCs had marked clustering of Cav-1 expression in the cytoplasm, but Cav-1 knockout failed to affect the colocalization or the distribution of Fas and Fap-1 (Fig. 3J). These data imply that Fas/Fap-1 may bind to Cav-1 and control its trans-location, thereby controlling SNAP25/VAMP5-related IL-1RA–sEV secretion (fig. S3L).
Tumor necrosis factor–α activated IL-1RA–sEV release via up-regulation of Fas/Fap-1 expression in MSCs
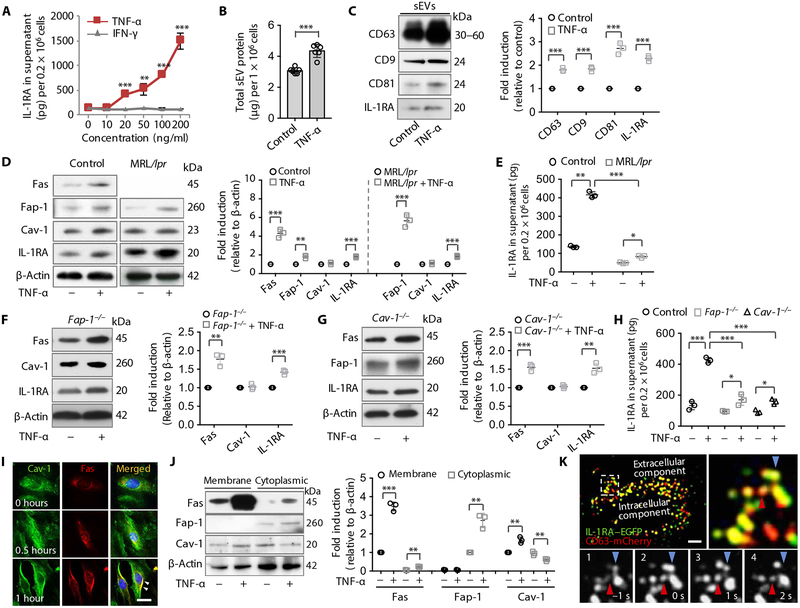
Because tumor necrosis factor–α (TNF-α) can increase the production of MSC-secreted growth factors (40), we used TNF-α and interferon-γ (IFN-γ) to treat GMSCs and found that only TNF-α significantly enhanced the secretion of IL-1RA in the culture supernatant in a dose-dependent manner (P < 0.01) (Fig. 4A). Moreover, we found that TNF-α–treated GMSCs and SMSCs showed increased capacity to secrete sEV-associated protein to the culture supernatant (Fig. 4B and fig. S4A). sEV-associated protein from TNF-α–treated GMSCs and SMSCs expressed elevated amounts of CD63, CD9, CD81, and IL-1RA compared to control GMSCs (Fig. 4C and fig. S4B). To determine whether TNF-α affects Fas/Fap-1–controlled sEV/IL-1RA release, we showed that TNF-α treatment up-regulated the expression of Fas, Fap-1, and IL-1RA, but not Cav-1 in control and MRL/lpr GMSCs (Fig. 4D). TNF-α treatment–induced secretion of IL-1RA and sEV-associated protein was significantly reduced in MRL/lpr GMSCs when compared to control GMSCs (P < 0.001) (Fig. 4E and fig. S4C). These data suggest that TNF-α may serve as an activator to enhance IL-1RA–EV release through up-regulation of Fas and Fap-1. To confirm that TNF-α regulates Fas, we used siRNA to knock down Fas expression in GMSCs and showed that TNF-α treatment resulted in reduced secretion of IL-1RA into the culture supernatant with elevated intracellular IL-1RA compared to control GMSCs (fig. S4, D and E). Fap-1 and Cav-1 knockout GMSCs showed that TNF-α treatment resulted in elevated intracellular Fas and IL-1RA in Fap-1 knockout GMSCs and elevated intracellular Fas, Fap-1, and IL-1RA in Cav-1 knockout GMSCs (Fig. 4, F and G). TNF-α treatment–induced secretion of IL-1RA and exosome protein in the culture supernatant was significantly reduced in Fap-1 and Cav-1 knockout GMSCs when compared to control GMSCs (P < 0.001) (Fig. 4H and fig. S4F). Fas and Cav-1 translocated to the cell membrane region at 0.5 to 1 hour after TNF-α treatment (Fig. 4I). Fap-1 was also distributed near the membrane region along with Cav-1 at 0.5 to 1 hour after TNF-α treatment (fig. S4G). Western blotting showed that TNF-α treatment increased the expression of Fas and Cav-1 in the cell membrane as well as Fas and Fap-1 in the cytoplasm in GMSCs, but decreased the expression of Cav-1 in the cytoplasm (Fig. 4J). These data indicate that TNF-α treatment induces the membrane translocation of Fas and Cav-1 to control Fas/Fap-1/Cav-1–mediated IL-1RA release. Because TNF-α is able to activate the nuclear factor κB (NF-κB) pathway (41) and NF-κB is capable of regulating the transcription of Fas and Fap-1 (42), we further showed that treatment with an NF-κB inhibitor, pyrrolidinedithiocarbamate (PDTC), was able to block TNF-α–induced IL-1RA release in GMSCs (fig. S4H).
Fig. 4. TNF-α up-regulates Fas/Fap-1 expression to promote IL-1RA–sEV release in murine MSCs.
(A) ELISA analysis of IL-1RA secretion into the culture supernatant from GMSCs treated with tumor necrosis factor–α (TNF-α) or interferon-γ (IFN-γ) (n = 3). (B) Secreted sEV-associated proteins from control or TNF-α (20 ng/ml)–treated GMSCs (n = 6). (C) Western blotting and semi-quantification of CD63, CD9, CD81, and IL-1RA expression in WT control GMSCs with or without TNF-α (20 ng/ml) treatment. sEV-associated proteins from culture supernatants of equal numbers of cells were loaded for Western blotting analysis (n = 3). (D) Western blotting and semi-quantification analysis of Fas, Fap-1, Cav-1, and IL-1RA expression in WT GMSCs (left) and MRL/lpr GMSCs (right) treated with or without TNF-α (n = 3). (E) ELISA analysis of secretion of IL-1RA in the culture supernatant in control or MRL/lpr GMSCs treated with and without TNF-α (20 ng/ml) (n = 3). (F) Western blotting and semi-quantification of Fas, Cav-1, and IL-1RA in Fap-1 knockout GMSCs with and without TNF-α (20 ng/ml) treatment (n = 3). (G) Western blotting and semi-quantification of Fas, Fap-1, and IL-1RA in Cav-1 knockout GMSCs with and without TNF-α (20 ng/ml) treatment (n = 3). (H) ELISA analysis of IL-1RA in the culture supernatant of WT control, Fap-1, and Cav-1 knockout GMSCs treated with and without TNF-α (20 ng/ml) (n = 3). (I) Immunocytofluorescence staining of GMSCs at various time points after TNF-α (20 ng/ml) treatment. Scale bar, 20 μm. (J) Western blotting and semi-quantification analysis of Fas, Fap-1, and Cav-1 in membrane and cytoplasmic fractions of GMSCs treated with and without TNF-α (20 ng/ml) (n = 3). (K) TIRF microscopy images of IL-1RA–EGFP (green) and CD63-mCherry (red) cotransfected into WT GMSCs treated with TNF-α (20 ng/ml) for 0.5 hours. The top left panel is a higher magnification of the boxed region in the left image to show colocalization (yellow); the bottom panels show sequential images (1 to 4). Arrows indicate two individual IL-1RA–positive vesicle fusion events. Scale bar, 10 μm. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars are means ± SD. Data were analyzed using independent unpaired two-tailed Student’s t tests (A to D, F, G, and J), or one-way ANOVA with Bonferroni correction (E and H).
We next determined whether TNF-α could activate IL-1RA–EV release. When GMSCs were transfected with plasmids expressing IL-1RA–EGFP and the vesicular organelles were stained with LysoTracker TIRF microscopy showed that IL-1RA–EGFP overlapped with LysoTracker-positive EVs (fig. S4I). After 0.5 hours of TNF-α treatment, IL-1RA–EGFP exosome-like EVs fused with the plasma membrane in living GMSCs (movie S2). Sequential fluorescent images displayed fusion of individual IL-1RA–EGFP–positive exosome-like EVs to the plasma membrane in response to TNF-α treatment (fig. S4I). To confirm TNF-α–activated IL-1RA–positive exosome-like EV release, GMSCs were cotransfected with plasmids expressing IL-1RA–EGFP fusion protein and CD63-mCherry fluorescent protein (Fig. 4K). After 0.5 hours of TNF-α treatment, colocalization of IL-1RA–EGFP and CD63-mCherry exosome-like EVs was observed by TIRF microscopy (Fig. 4K). Moreover, we found intensive IL-1RA–EGFP/CD63-mCherry double-positive exosome-like EVs fused with the plasma membrane in living GMSCs (movie S3), and sequential fluorescent images displayed fusion of individual IL-1RA–EGFP/CD63-mCherry double-positive exosome-like EVs to the plasma membrane after TNF-α treatment (Fig. 4K). These data indicate that TNF-α up-regulates Fas/Fap-1 expression via the NF-κB pathway to induce the membrane translocation of Cav-1, thereby enhancing IL-1RA release in GMSCs (fig. S4J).
GMSCs produce high amounts of IL-1RA to facilitate wound healing in the gingiva
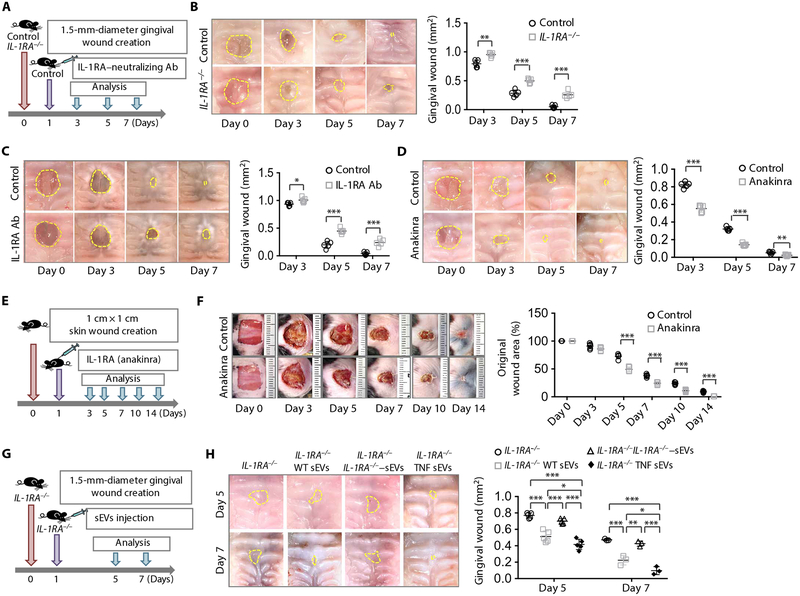
Wound healing in the gingiva is faster than that in the skin (28), and we showed that GMSCs and gingival wound tissue have higher expression of IL-1RA than SMSCs and skin wound tissue, respectively (Fig. 1, G and H, and fig. S5A). We examined whether the IL-1RA produced by GMSCs contributed to accelerated wound healing in the gingiva using immunohistofluorescence staining and found that GMSCs expressed IL-1RA at the margins of wound areas (fig. S5B). Staining showed that IL-1RA was coexpressed with neural crest–derived MSCs in gingival wound tissue in Wnt1-Cre-tdTomato mice, further confirming that GMSCs produce IL-1RA (fig. S5C). A previous study reported that IL-1RA knockout mice showed impaired cutaneous wound healing (43). We found that gingival healing was significantly delayed in IL-1RA knockout mice at 3, 5, and 7 days post-wounding compared to WT control mice (3 days, P < 0.01; 5 days, P < 0.001; 7 days, P < 0.001) (Fig. 5, A and B, and fig. S5D). Moreover, injection of IL-1RA neutralizing antibody significantly inhibited gingival wound healing at 3, 5, and 7 days after wound creation compared to the control mice (3 days, P < 0.05; 5 days, P < 0.001; 7 days, P < 0.001) (Fig. 5C and fig. S5E). Because IL-1RA was also detected in SMSCs near the margin of cutaneous wound areas (fig. S5F), we showed that IL-1RA knockout mice have delayed wound healing in a full-thickness square cutaneous wound model at 5, 7, 10, and 14 days after wound creation when compared to WT control mice (fig. S5, G to I). This effect was confirmed in a circular cutaneous wound model (fig. S5J). These data suggest that MSC-secreted IL-1RA plays a crucial role in gingival and cutaneous wound healing.
Fig. 5. GMSCs produce IL-1RA, which contributes to gingival wound healing in mice.
(A) Scheme illustrating the gingival wound procedure and treatment with IL-1RA neutralizing antibody. Full-thickness circular wounds were made in the palates of WT control mice and IL-1RA−/− mice and submucosally injected one time with placebo (0.9% saline) or IL-1RA neutralizing antibody (IL-1RA Ab, 10 μg per mouse) 1 day after wound creation. (B) Representative macroscopic images and quantification of gingival wound area in WT control and IL-1RA−/− mice. All the gingival wound is outlined in a dashed line (n = 5). (C) Representative macroscopic images and quantification of gingival wound area in WT mice after treatment with or without IL-1RA Ab (n = 5). (D) Representative macroscopic images and quantification of gingival wound area in WT mice after treatment with or without IL-1RA drug (n = 5). (E) Scheme illustrating cutaneous wound procedure and treatment with IL-1RA drug anakinra. Full-thickness excision cutaneous wounds (1 cm × 1 cm) were created in the mid-backs of WT mice. One day after wound creation, the mice were subcutaneously injected with either placebo (0.9% saline) or the IL-1RA drug anakinra (500 μg per mouse). (F) Representative macroscopic images and quantification of closure of full-thickness cutaneous wounds in WT mice after treatment with or without anakinra (n = 5). (G) Scheme illustrating the gingival wound procedure and administration of sEVs. IL-1RA knockout mice were submucosally injected with placebo (0.9% saline) or WT GMSC–derived, IL-1RA knockout GMSC–derived, or TNF-α–activated GMSC-derived sEVs 1 day after wound creation. (H) Representative macroscopic images and quantification of gingival wound area in IL-1RA knockout mice after treatment with or without sEVs (n = 3 for day 7; n = 5 for day 5). *P < 0.05, **P < 0.01, ***P < 0.001. Error bars are means ± SD. Data were analyzed using independent unpaired two-tailed Student’s t tests (B to F) or one-way ANOVA with Bonferroni correction (H).
To further examine the role of IL-1RA in wound healing, anakinra, a Food and Drug Administration (FDA)–approved form of recombinant IL-1RA, was locally injected into wounds. We found that gingival wound healing was significantly accelerated by administration of anakinra at 3, 5, and 7 days after wound creation compared to the control group (3 days, P < 0.001; 5 days, P < 0.001; 7 days, P < 0.01) (Fig. 5D and fig. S5, K and L). Moreover, administration of anakinra significantly promoted cutaneous wound healing at 5, 7, 10, and 14 days after wound creation compared to the control group (P < 0.001) (Fig. 5, E and F, and fig. S5M). This effect was also confirmed in a circular cutaneous wound model (fig. S5N).
To confirm the effect of GMSC-secreted IL-1RA–sEV on wound healing, we isolated sEVs from WT and IL-1RA knockout GMSCs and locally injected them into the gingival wounds in IL-1RA knockout mice. We found that administration of WT control GMSC-derived sEVs significantly promoted gingival wound healing (P < 0.001), whereas injection of IL-1RA knockout GMSC–derived sEVs failed to accelerate the wound healing (Fig. 5, G and H, and fig. S5O). To confirm the effect of TNF-α on GMSC sEV secretion, we isolated sEVs from TNF-α–treated GMSCs and used these sEVs to treat gingival wounds. We found that local injection of TNF-α–treated GMSC-derived sEVs promoted the closure of wound healing at 7 days after wound creation in IL-1RA knockout mice as compared to injection of WT control sEVs (Fig. 5, G and H). Similar to gingival wound healing, we isolated sEVs from WT and IL-1RA knockout SMSCs and locally injected these sEVs into the cutaneous wounds in IL-1RA knockout mice. We found that administration of WT SMSC–derived sEVs significantly promoted cutaneous wound healing at 3, 5, 7, 10, and 14 days after wounding in IL-1RA knockout mice (P < 0.05), whereas injection of IL-1RA knockout SMSC-derived sEVs failed to accelerate the wound healing (fig. S5, P to R). To confirm the effect of TNF-α on SMSC sEVs, we isolated sEVs from TNF-α–treated SMSCs and locally injected these sEVs into cutaneous wounds. We found that administration of TNF-α–treated SMSC-derived sEVs further potentiated wound healing at 7, 10, and 14 days after wound creation in IL-1RA knockout mice as compared to injection of sEVs from WT SMSCs (fig. S5, P to R). These results suggest that MSC-derived IL-1RA–EVs can serve as a therapeutic agent for wound healing.
Previous studies reported that diabetic mice showed a delayed wound healing process (44, 45). It would therefore be interesting to know whether diabetic GMSCs have similar characteristics to normal GMSCs. We showed that GMSCs from diabetic mice had reduced capacity to secrete sEV proteins to the culture supernatant (fig. S6A). Moreover, GMSCs from diabetic mice showed reduced IL-1RA secretion into the culture supernatant, along with elevated cytoplasmic accumulation of IL-1RA and decreased Fas expression when compared to WT GMSCs (fig. S6, B and C). Streptozotocin-induced type 1 diabetic mice exhibited significantly delayed gingival and skin wound healing compared to WT mice (gingival, P < 0.001; skin, P < 0.05) (fig. S6, D to I). Injection of WT GMSC–derived sEVs partially rescued the delayed wound healing; however, injection of IL-1RA knockout GMSC–derived sEVs failed to rescue the delayed wound healing in diabetic mice (fig. S6, D to I). These results suggest that sEVs containing IL-1RA can partially ameliorate the delayed wound healing process in diabetic mice.
Fas-controlled IL-1RA secretion regulates wound healing in the gingiva and skin
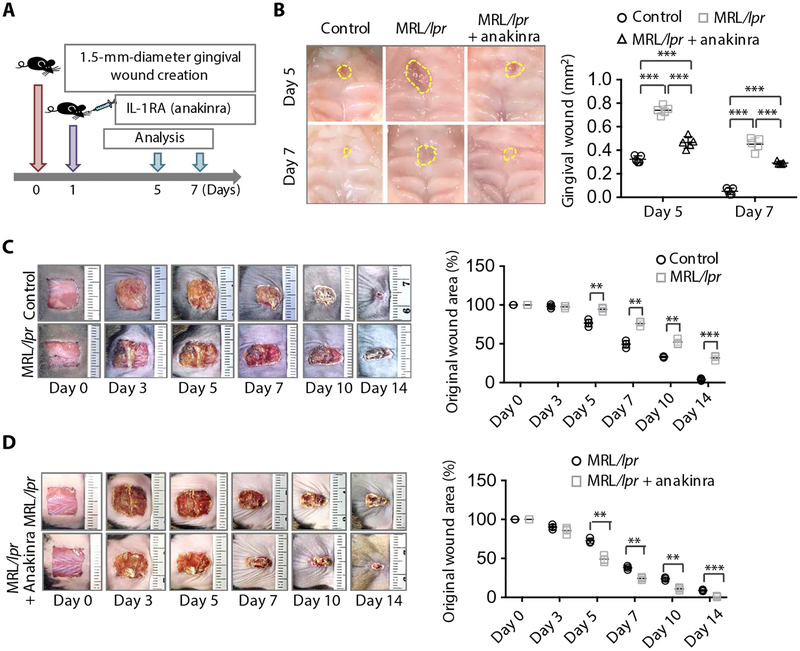
Next, we examined whether Fas-controlled IL-1RA release governs the wound healing process. Immunohistofluorescence staining showed that Fas was coexpressed with IL-1RA in both gingival and cutaneous wound sites in WT mice (fig. S7, A and B). Fas-deficient MRL/lpr mice exhibited significantly delayed gingival wound healing at 5 and 7 days after wounding compared to WT control mice (P < 0.001), and injection of anakinra partially rescued the delayed wound healing (Fig. 6, A and B, and fig. S7C). In addition, MRL/lpr mice exhibited significantly delayed cutaneous wound healing at 5, 7, 10, and 14 days after wounding compared to the WT control mice (5 days, P < 0.01; 7 days, P < 0.01; 14 days, P < 0.001), and injection of anakinra partially rescued the delayed wound healing in MRL/lpr mice (Fig. 6, C and D, and fig. S7D). These results suggest that Fas may control the IL-1RA release in MSCs and, thus, govern the wound healing process. In summary, these results suggest that MSCs use the Fas/Fap-1/Cav-1 axis to regulate SNARE-mediated IL-1RA–sEV release to regulate wound healing in the gingiva. TNF-α serves as an activator to up-regulate Fas and Fap-1 to enhance IL-1RA–sEV release (fig. S8).
Fig. 6. Fas-controlled IL-1RA secretion regulates wound healing in mice.
(A) Scheme illustrating the gingival wound procedure in MRL/lpr mice and treatment with the IL-1RA drug anakinra. Full-thickness circular wounds were made in the palates of WT and MRL/lpr mice with a biopsy punch, and MRL/lpr mice were submucosally injected with either placebo (0.9% saline) or the IL-1RA drug anakinra (100 μg per mouse) 1 day after wound creation. (B) Representative macroscopic images and quantification of gingival wound area in WT and MRL/lpr mice after treatment with and without anakinra (n = 5). (C) Representative macroscopic images and quantification of dermal wound area in WT and MRL/lpr mice over time (n = 3). (D) Representative macroscopic images and quantification of dermal wound area in MRL/lpr mice treated with placebo (0.9% saline) or anakinra (500 μg per mice) injected 1 day after wound creation as in (A) (n = 3). **P < 0.01, ***P < 0.001. Error bars are means ± SD. Data were analyzed using one-way ANOVA with Bonferroni correction (B) or independent unpaired two-tailed Student’s t tests (C and D).
DISCUSSION
The trafficking of secretory vesicles to the plasma membrane of eukaryotic cells is essential for intercellular communication through the release of a variety of extracellularly acting molecules. Secretory vesicles and some extracellular molecules (plasma proteins, antibodies, and extracellular matrix components) are also secreted by a constitutive exocytotic pathway (17, 46). Regulation of exocytosis has been studied in a wide range of cell types specialized to secrete large amounts of secretory products, including neuroendocrine, endocrine, and exocrine cells. Most exocytotic secretory processes are mediated through mechanisms with SNARE protein–dependent exocytotic fusion (17, 18). Cells that use this mechanism include astrocytes, which are cells of the central nervous system that secrete chemical mediators (47), and β cells, which are cells of the endocrine system that secrete insulin (48). However, it is unknown whether and how stem cells use exocytotic fusion mechanisms to secrete EVs.
The secretion of cytokines by immune cells in response to inflammatory or infectious stimuli plays an important role in the regulation of immune response. The trafficking machinery and secretory (exocytotic) pathways are complex and highly regulated in immune cells. The majority of secretory cytokines contain N-terminal or internal signal peptides that direct their sorting into the endoplasmic reticulum (ER) for synthesis as either soluble or transmembrane precursors and are then transported to the extracellular space or the plasma membrane through the ER-Golgi secretory pathway, which is called the conventional secretory pathway (49). Secretion of TNF by macrophages is an example of the conventional secretory pathway that proceeds via granule-mediated and constitutive routes. MSCs secrete a variety of growth factors, immunomodulatory cytokines, and exosomes (13–15, 24), serving as paracrine regulators. However, whether MSCs serve as secretory cells, using a unique mechanism to release cytokines and EVs, is largely unknown.
Here, we used MSCs as a model to dissect a distinctive cytokine secretion mechanism in stem cells in which Fas/Fap1/Cav1 machinery controls SNAP25/VAMP5–associated IL-1RA–sEV exocytosis. Cav-1 is the main component of lipid rafts that dynamically participate in a number of cellular processes, including signal transduction, lipid regulation, and membrane trafficking (50). Cav-1 is known to bind to Fas and is perhaps involved in the apoptotic process (39). As expected, Cav-1 knockout mice showed impaired cutaneous wound healing (51). Cav-1 also binds with SNAP25 and is associated with neurosecretion (52). It is known that Fap-1, a protein tyrosine phosphatase, binds with Fas (38). We found that Fap-1 also binds with Cav-1. Recent studies reveal that interactions between synaptotagmin-1 and the SNARE complex can promote neuronal exocytotic fusion (53). Rab27a and Rab27b control multivesicular endosomes docking at the plasma membrane to regulate exosome secretion in HeLa cells (54). Our findings identify a role for the Fas/Fap-1/Cav-1 complex in MSC-mediated IL-1RA–sEV secretion.
The surrounding microenvironment may stimulate paracrine factor production to promote MSC-mediated tissue homeostasis. Once MSCs have encountered the microenvironment of injured tissues, many factors, including inflammatory cytokines (such as TNF-α, IFN-γ, and IL-1), toxins of infectious agents, and hypoxia, can stimulate the release of various regulatory molecules (14). Elevation of internal Ca2+ concentrations can activate astrocytes to initiate inter-cellular communication by SNARE-dependent glutamate vesicle release (55), and glucose stimulates SNARE-dependent insulin vesicle exocytosis in β cells (48). Components of the microenvironment, including the wound milieu, may stimulate paracrine factor production to promote MSC-mediated tissue repair and immune regulation. We found that TNF-α is able to activate sEV/IL-1RA release via up-regulating Fas/Fap-1 expression. It is known that the interaction of TNF-α with TNF-αR may activate the NF-κB pathway and induce the expression of prosurvival genes including B cell lymphoma 2 (Bcl-2), X-linked inhibitor of apoptosis protein (XIAP), and the FLICE-inhibitory protein (FLIP); furthermore, FLIP can directly inhibit caspase-mediated cell apoptosis (41, 56). Here, we found that TNF-α activates IL-1RA release in MSCs via the NF-κB pathway to promote wound healing. Supporting evidence for this conclusion came from a previous study that also showed that TNF-α could promote human MSCs to secrete growth factors such as vascular endothelial growth factor (40).
Oral gingival/mucosal wounds heal faster than cutaneous ones, with minimal scar formation (28) and reduced inflammatory cell infiltration (29–31). A previous study reported the beneficial effects of MSCs on wound healing, by promoting M2 macrophage polarization (57). Recent studies showed that MSC-derived exosomes are capable of transporting active proteins and microRNAs to communicate with the extracellular environment (13, 16, 58, 59). We also showed that sEVs from GMSCs and SMSCs contain many cytokines, among which GMSC-derived sEVs contain a significantly higher amount of IL-1RA. Given that IL-1RA is a natural inhibitor of the proinflammatory cytokine IL-1β, it modulates a variety of IL-1–related immune and inflammatory responses (60–62), thus contributing to the rapid wound healing of the gingiva. MSC-produced IL-1RA mediates the anti-inflammatory and antifibrotic effect during lung injury in mice (63). Also, MSC-derived IL-1RA promotes macrophage polarization and inhibits B cell differentiation (64). IL-1RA–deficient mice show delayed cutaneous wound healing, spontaneous and lethal arteritis, and destructive arthritis (43, 65, 66). Delivery of anakinra, a commercially available form of IL-1RA approved by the FDA for treating rheumatoid arthritis and neonatal-onset multisystem inflammatory disease (67, 68), into the wound area can reduce fibrotic invasion (69).
It is well known that, unlike human skin wounds, mouse cutaneous wounds heal largely via contraction, due to the nature of their loose skin with dense hair follicles. This might explain why we only observed significant differences of wound closure at the later stages (5 days after surgery). We found that IL-1RA knockout mice exhibited delayed wound healing in both the gingiva and skin. Blockage of IL-1RA by submucosal injection of IL-1RA neutralizing antibody at wound sites resulted in impaired gingival wound healing. Conversely, injection of anakinra accelerated gingival and cutaneous wound healing, thus highlighting the role of IL-1RA in promoting wound healing. MSC-derived exosomes may also affect the wound healing process by other mechanisms, such as by delivery of Wnt4 to promote proliferation of skin cells and by promoting collagen synthesis and angiogenesis in the wounded area (25, 26). We found that WT MSC–derived, but not IL-1RA knockout MSC–derived, EVs promoted delayed wound healing in IL-1RA knockout or diabetic mice. These results suggest that MSC-derived IL-1RA–sEV plays an important role in gingiva and cutaneous wound healing. Exosome membrane proteins can interact with receptors in a target cell or can be cleaved by proteases in the extracellular space to act as soluble ligands that bind to the target cell surface (70). A previous study showed that exosomes carry active Wnt proteins on their surface to induce Wnt signaling (71). Here, we found that IL-1RA was detected on exosome-like EV membranes. This might explain why IL-1RA–sEV effectively communicated with the extracellular environment in wound areas. In addition, sEVs may contain the consensus leader peptide-containing isoform of IL-1RA, which may bind to a receptor on sEV surface after targeting the luminal side of ER-Golgi secretory vesicles. Further research is required to dissect the detailed mechanism(s) by which intracellular isoforms of IL-1RA are shuttled to the membrane of exosome-like EVs. Other oral cavity factors such as saliva and the microbiome may also affect gingival wound healing, and the effect of epidermal stem cells is particularly important in the process of epithelialization during cutaneous wound healing. Nevertheless, these studies support the notion that IL-1RA–sEV plays a crucial role in regulating wound healing in both the gingiva and the skin and suggest that anakinra and IL-1RA–positive sEVs can be used to improve cutaneous wound healing.
Here, we found that MSCs release IL-1RA using a mechanism by which Fas/Fap-1/Cav-1 regulate dynamic exocytosis of IL-1RA–EV. TNF-α serves as an activator to promote IL-1RA–sEV exocytosis from MSCs. Our previous study showed that MSC-derived sEVs are able to rescue Fas-deficient MSCs through a Fas reuse mechanism to improve miR-29b release in recipient MSCs (58). These pieces of evidence suggest that sEVs play a crucial role in biological cross-talk between MSCs and surrounding cells or recipient cells. This study identifies an sEV/cytokine secretion mechanism used by MSCs that may play a crucial role in MSC-based wound healing therapies.
MATERIALS AND METHODS
Study design
Here, we used MSCs as a model to examine whether stem cells have a unique exocytic fusion mechanism to release EVs and whether gingival MSCs secrete a high amount of EVs with IL-1RA to contribute to accelerated wound healing. Three experimental studies were designed: (i) We identified GMSC production and secretion of IL-1RA–expressing sEVs using ultracentrifugation and sucrose gradient, immunoelectron microscopy, colocalization of IL-1RA with EV markers observed by STED microscopy, living cell exocytosis of IL-1RA–EGFP/CD63-mCherry double-positive exosome-like EVs observed by TIRF microscopy, ELISA, and Western blotting; (ii) we used Western blotting, ELISA, immunocytofluorescence staining, and co-IP in WT, gene knockout, and siRNA-treated MSCs to reveal that MSCs use the Fas/Fap-1/Cav-1 axis to regulate SNARE-mediated EVs and IL-1RA secretion; and (iii) we determined the therapeutic effect of recombinant IL-1RA or IL-1RA/sEVs in accelerating gingival and cutaneous wound healing in WT, IL-1RA knockout, MRL/lpr, and diabetic mouse models. The sample sizes for the in vivo studies were based on the resource equation because the effect size was unknown. Exact numbers for each experiment are included in the figure legends. The investigators were not blinded when conducting or evaluating the experiments. Mice were randomly assigned to the treatment and control groups. Individual subject-level data for experiments where n < 20 are included in table S1.
Animals
Female C57BL/6J, C3H/HeJ, B6.129S-Il1rntm1Dih/J (IL-1RA knockout), C3MRL-Faslpr/J (MRL/lpr), and Cav1tm1Mls/J (Cav-1 knockout) mice were purchased from the Jackson Laboratory. Age-matched 8- to 10-week female mice from the same background were used in all experiments. All animal experiments were performed under institutionally approved protocols for the use of animal research (University of Pennsylvania Institutional Animal Care and Use Committee, #805478).
Antibodies and reagents
Anti–IL-1RA and VAMP5 antibodies were purchased from Abcam. Anti-Fas antibody was purchased from Millipore. Anti–Fap-1, Cav-1, SNAP25, CD105, CD90, CD44, CD63, CD9, and CD81 antibodies were purchased from Santa Cruz Biotechnology. Anti–p-NF-κB p65 and NF-κB p65 antibodies were purchased from Cell Signaling. Anti–β-actin antibody was purchased from Sigma-Aldrich. Alexa Fluor 488 and Alexa Fluor 568 secondary antibodies were purchased from Invitrogen. Protein A/G PLUS-Agarose was purchased from Santa Cruz Biotechnology. NF-κB inhibitor ammonium PDTC was purchased from Sigma-Aldrich.
Isolation of mouse and human bone marrow, gingival, and skin MSCs
Stem cells from mouse and human tissue were isolated and cultured as reported by other groups (72, 73) and our previous studies (4, 9, 32, 33, 74–80). Briefly, gingival and skin tissues from mice were gently separated, minced, and digested with solution containing collagenase type I (2 mg/ml) (Worthington Biochemical) and dispase II (4 mg/ml) (Roche Diagnostics) in phosphate-buffered saline (PBS) for 1 hour at 37°C. Bone marrow cells were flushed out from the bone cavities of femurs and tibias with 2% heat-inactivated fetal bovine serum (FBS; Equitech-Bio) in PBS. Single-cell suspensions from the gingiva, skin, or bone marrow were obtained by passing the cells through a 70-μm strainer (BD Biosciences). All nucleated cells were seeded on 100-mm culture dishes (Corning) in complete media containing α-minimum essential medium (α-MEM) (Invitrogen) supplemented with 20% FBS, 2 mM l-glutamine (Invitrogen), 55 μM 2-mercaptoethanol (Invitrogen), penicillin (100 U ml−1), and streptomycin (100 μg ml−1) (Invitrogen), followed by an initial incubation for 48 hours at 37°C and 5% CO2. The cultures were washed with PBS twice to eliminate the nonadherent cells. Attached cells were cultured for another 12 days under the same conditions in the complete medium mentioned above. Skin MSCs were isolated and cultured using the same procedure described for gingival MSCs. These single colonies were passaged with frequent medium changes to eliminate potential hematopoietic cell contamination (72, 73). We further identified these MSCs and demonstrated that BMMSCs, GMSCs, and SMSCs showed the capacity of self-renewal, assessed by fibroblast colony-forming units and high rate of proliferation (fig. S9). Flow cytometric analysis confirmed that these MSCs were positive for the MSC surface markers CD44, CD90, CD105, and Sca-1, but were negative for the hematological markers CD34 and CD45 (fig. S9). These MSCs also showed the capacity for multipotent differentiation, including osteogenic, adipogenic, chondrogenic, and neurogenic differentiation (fig. S9).
Human bone marrow aspirates from healthy human adult volunteers (20 to 35 years of age) were purchased from AllCells LLC. Human GMSCs and SMSCs were isolated from the gingival and skin tissues obtained as remnants of discarded human tissues under an approved Institutional Review Board protocol at the University of Pennsylvania. Human gingival and skin tissues were treated aseptically and incubated overnight at 4°C with dispase (2 mg/ml) to separate the epithelial and lower spinous layers. The tissues were minced into 1- to 3-mm2 fragments and digested at 37°C for 2 hours in sterile PBS containing collagenase IV (4 mg/ml) (Worthington Biochemical). The dissociated cell suspension was filtered through a 70-μm cell strainer; plated in 100-mm culture dishes with α-MEM containing 10% FBS, penicillin (100 U ml−1), streptomycin (100 μg ml−1), 2 mM l-glutamine, and 10 mM l-ascorbic acid phosphate; and cultured at 37°C in a humidified tissue culture incubator with 5% CO2. After 48 hours, the nonadherent cells were removed. The plastic-adherent confluent cells were passaged with 0.05% trypsin containing 1 mM EDTA and continuously subcultured and maintained in the complete growth medium.
Isolation and characterization of EVs
Cells were cultured in exosome-depleted medium (complete medium depleted of FBS-derived exosomes by overnight centrifugation at 100,000g) for 48 hours. EVs from culture supernatants of 12 × 106 MSCs were isolated by differential centrifugation, as described in the literature (35), at 300g for 10 min, 3000g for 10 min, 20,000g for 30 min, and 120,000g for 70 min. After differential centrifugation, we used a sucrose cushion to purify sEVs as described in previous literature (35, 36, 81, 82). Briefly, a crude sEV pellet from 12 × 106 cells was resuspended in PBS, underlain by a single cushion composed of 30% sucrose prepared in tris/D2O, and centrifuged at 110,000g for 3 hours at 4°C. The ability of D2O to readily diffuse with H2O across the discontinuous interphase allowed for the formation of a continuous minigradient between 1.10 and 1.18 g/cm3 (82), which matched the ideal gradients for MSC exosome isolation (37). The sEVs captured within the sucrose layer were collected, washed with PBS twice, and centrifuged at 110,000g for 90 min at 4°C. The purified sEVs were further verified by transmission electron microscopy and Western blotting analysis. For analysis of exosome protein secretion, we used a Bradford protein assay (Bio-Rad Laboratories) to measure the amount of total protein in purified exosomes from 12 × 106 MSCs. Total exosome protein was normalized to 1 × 106 cells to show the amount as micrograms per 1 × 106 cells. To further confirm exosomes containing IL-1RA, sucrose gradient–purified exosomes were collected as previously described (37, 83). Briefly, a crude exosome pellet from 24 × 106 cells was mixed with 2.5 M sucrose, loaded at the bottom of an ultracentrifuge tube, and overlaid with a gradient of decreasing concentrations of sucrose (2.0 to 0.4 M). Tubes were centrifuged for 16 hours at 4°C at 200,000g. After centrifugation, 10 fractions were collected from the top of the tube. Total proteins from the concentrated fractions were loaded for Western blotting analysis to detect IL-1RA.
Wound healing in mice
Full-thickness circular gingival wounds with a diameter of 1.5 mm were made in the palates of the mice with a biopsy punch. Full-thickness square excision wounds (1 cm × 1 cm) were created by marking the area of the wound on the mid-backs of the mice with a fine marker and a ruler, lifting the skin with a pair of a forceps, and excising the full-thickness skin along the lines with a pair of surgical scissors. To create circular cutaneous wounds, 4-mm-diameter full-thickness wounds were made in the mid-backs of the mice with a biopsy punch. After the surgery on day 1, the wounds were topically submucosally injected with placebo (0.9% saline), IL-1RA neutralizing antibody (10 μg per mouse), or IL-1RA drug (anakinra, 100 μg per mouse for gingival wounds and 500 μg per mouse for cutaneous wounds). For in vivo sEV treatments of gingival wounds, the wounds were submucosally injected with either placebo or sEVs (40 μg) suspended in PBS (40 μl). For cutaneous wounds, the wounds were subcutaneously injected with either placebo or sEVs (200 μg) suspended in PBS (200 μl) on the basis of previous reports (25, 26). The gingival wound images were taken via a stereoscope after sacrifice of the animal at the indicated time point. A series of digital photographs of the cutaneous wounds was taken, including a ruler for scale. At the indicated time points, the wound areas (for gingival wounds) or percentage of wound closure (for cutaneous wounds) was quantified on photographs using Adobe Photoshop 7.0.1 software (Adobe Systems). Changes in the area of the cutaneous wounds were expressed as a percentage of the initial wound area.
Western blotting
Cells and purified exosomes were lysed in M-PER mammalian protein extraction reagent (Thermo Fisher Scientific) with protease and phosphatase inhibitors (Roche), and proteins were quantified using a protein concentration assay (Bio-Rad Laboratories). For Western blotting of cell lysis, 20 μg of proteins was separated by SDS–polyacrylamide gel electrophoresis and transferred to 0.2-μm nitrocellulose membranes (Millipore). For Western blotting analysis of exosome, exosome protein was isolated from the culture supernatant of 4 × 106 cells, and 10 μg of total exosome proteins was loaded. The membranes were blocked with 5% nonfat dry milk and 0.1% Tween 20 for 1 hour, followed by incubation overnight with the primary antibodies diluted in blocking solution according to the manufacturer’s instructions. Antibodies to mouse Fas (05–351) were purchased from Millipore. Antibodies to mouse Fap-1 (sc-15356), Cav-1 (sc-894), and SNAP25 (sc-7538) were purchased from Santa Cruz Biotechnology. Antibodies to mouse IL-1RA (ab124962) and VAMP5 (ab85581) were purchased from Abcam. Antibody to mouse β-actin (A5441) was purchased from Sigma-Aldrich. The membranes were then incubated under room temperature for 1 hour in species-related horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology) diluted at 1:10,000 in blocking solution. Immunoreactive proteins were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) and Biomax film (Kodak); the sensitivity of this substrate can be used to detect low-picogram amounts of protein in polyvinylidene difluoride membrane. The relative density was measured using ImageJ 1.49v software (Wayne Rasband). The quantification of Western blotting for total EV protein was normalized against the control group or GMSC group, and the quantification of the other Western blotting experiments was normalized against loading control β-actin. Western blotting films corresponding to Figs. 1 to 4 are shown in database S1.
Immunoprecipitation
Cells were lysed in M-PER mammalian protein extraction reagent (Thermo Fisher Scientific) with protease and phosphatase inhibitors (Roche), and proteins were quantified using a protein concentration assay (Bio-Rad Laboratories). One microgram of the appropriate control immunoglobulin G, together with 20 μl of resuspended protein A/G PLUS-Agarose, was added to precleared lysate and incubated at 4°C for 30 min. Pellet beads were centrifuged at 2500 rpm for 5 min at 4°C, and the supernatant with total cellular protein (300 μg) was transferred to a fresh centrifuge tube on ice. Primary antibody was added and incubated overnight at 4°C. Twenty microliters of resuspended protein A/G PLUS-Agarose was added at 4°C. After 2 hours, the tubes were centrifuged at 2500 rpm for 5 min at 4°C and the immunoprecipitates were collected. The beads were pelleted and washed with radioimmunoprecipitation assay buffer. Beads were then pelleted, washed, and resuspended in 40 μl of electrophoresis sample buffer. The samples were boiled at 90°C for 5 min, and 20-μl aliquots were subjected to Western blotting analysis.
siRNA knockdown, CRISPR/Cas9 knockout, and cytokine treatments
For siRNA knockdown, GMSCs (0.2 × 106) were seeded on a six-well culture plate. Fas, FAP-1, Cav-1, SNAP25, and VAMP5 siRNAs (Santa Cruz Biotechnology) were used to treat the GMSCs according to the manufacturer’s instructions. Nontargeting control siRNAs (Santa Cruz Biotechnology) were used as negative controls. Fap-1 CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 (CRISPR-associated protein 9) knockout plasmid (sc-422505, Santa Cruz Biotechnology) was used to knock out Fap-1 expression in GMSCs according to the manufacturer’s instructions. Briefly, GMSCs (0.2 × 106) were seeded on a six-well culture plate. The cells were allowed to grow to 40 to 80% confluence and then transfected with Fap-1 CRISPR/Cas9 knockout plasmids using Lipofectamine LTX with Plus reagent (Life Technologies) according to the manufacturer’s instructions. Scrambled guide RNA CRISPR/Cas9 plasmids were used as a negative control. The efficiency of siRNA knockdown and CRISPR/Cas9 knockout was confirmed by Western blot analysis. For cytokine treatments, GMSCs were treated with different concentrations of IFN-γ and TNF-α (Peprotech; 0, 20, 50, 100, and 200 ng/ml) or TNF-α (20 ng/ml) for 24 hours. After transfection or cytokine treatment, cells were used for protein extraction for Western immunoblotting, and the culture supernatants were used for ELISA.
Plasmid transfection, TIRF, and STED microscopy
For protein tracing, IL-1RA–EGFP fusion protein expression plasmids (System Biosciences) and CD63-mCherry fusion protein expression plasmids (a gift from C.G., University of Pennsylvania) were used. Empty plasmids with the same backbone were used as a control. The cells were transfected with plasmids using Lipofectamine LTX with Plus reagent (Life Technologies) according to the manufacturer’s instructions. For IL-1RA–positive vesicle colocalization studies, WT GMSCs were transfected with plasmids expressing IL-1RA–EGFP fusion protein and fixed with 4% paraformaldehyde. Next, the cells were incubated with anti-CD63 or anti-CD81 antibodies and imaged using a super-resolution STED microscope (Leica Microsystems). To visualize IL-1RA–positive vesicle dynamics, WT GMSCs were transfected with plasmids expressing IL-1RA–EGFP fusion protein, and the vesicular organelles were stained with LysoTracker. To visualize IL-1RA–positive exosome dynamics, WT GMSCs were cotransfected with plasmids expressing IL-1RA–EGFP fusion protein and CD63-mCherry fluorescent protein. Forty-eight hours after transfection, the GMSCs were treated with TNF-α (20 ng/ml), and the dynamics of IL-1RA–positive microvesicle exocytosis were monitored in living GMSCs using TIRF microscopy. Briefly, about 3.0 × 104 transfected cells were plated on an eight-well chambered cover glass (Lab-Tek II, Nunc) in the imaging medium Dulbecco’s modified Eagle’s medium–Hepes without phenol red (Thermo Fisher Scientific). Cells were imaged at a rate of 1 frame/s using a Leica TIRF microscope equipped with adaptive focus control and an environmental control system set to 5% CO2 and 37°C. Fluorophores were excited with solid-state 488- and 561-nm lasers, TIRF angle was set to 90 nm depth, and images were acquired using a Photometrics Evolve EMCCD camera. Images were analyzed and processed using FIJI software.
Statistics
Comparisons between two groups were analyzed using independent unpaired two-tailed Student’s t tests, and comparisons between more than two groups were analyzed using one-way analysis of variance (ANOVA) with Bonferroni correction. P values less than 0.05 were considered statistically significant.
Supplementary Material
Database S1. Western blotting films corresponding to Figs. 1 to 4.
Movie S1. GMSCs secrete IL-1RA-positive exosome-like EVs.
Movie S2. Exocytotic fusions of IL-1RA-positive vesicles in living GMSCs.
Movie S3. TNF-α-activated GMSCs release IL-1RA-positive exosome-like EVs.
Fig. S1. GMSCs secrete higher amounts of sEVs and cytokines.
Fig. S2. Fas controls IL-1RA-sEV secretion in murine SMSCs.
Fig. S3. Fas/Fap-1 binds with Cav-1 to control SNAP25/VAMP5-mediated IL-1RA release in murine MSCs.
Fig. S4. TNF-α promotes sEV and IL-1RA release in murine MSCs.
Fig. S5. Histomorphology of IL-1RA in wound healing in mice.
Fig. S6. sEVs containing IL-1RA ameliorate delayed wound healing in diabetic mice.
Fig. S7. Histomorphology of Fas in wound healing in mice.
Fig. S8. Schematic drawing of Fas/Fap-1/Cav-1-controlled IL-1RA-sEV secretion in MSCs.
Fig. S9. Characterization of BMMSCs, GMSCs, and SMSCs.
Table S1. Individual subject-level data.
Acknowledgments
Funding: This work was supported by grants from the National Institute of Dental and Craniofacial Research, NIH, Department of Health and Human Services (R01DE017449 and R01DE019932 to S.S. and K99E025915 to C.C.) and a Schoenleber Pilot Research Grant (to S.S.) from the University of Pennsylvania School of Dental Medicine. This work was also supported by grants from the Pew Biomedical Scholars Award, the American Association of Immunologists, and the NIH (R01GM123020) awarded to C.G.G.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data reported in the paper are included in the manuscript or are available in the Supplementary Materials.
REFERENCES AND NOTES
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR, Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV, Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 17, 331–340 (1974). [DOI] [PubMed] [Google Scholar]
- 3.Liang J, Zhang H, Hua B, Wang H, Wang J, Han Z, Sun L, Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult. Scler 15, 644–646 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S, Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 10, 544–555 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G, Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 56, 1175–1186 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Huang GT-J, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S, Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng. Part A 16, 605–615 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O; Developmental Committee of the European Group for Blood and Marrow Transplantation., Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 371, 1579–1586 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O, Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S, Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat. Med 17, 1594–1601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S, Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 26, 1065–1073 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S, Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 27, 1421–1432 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal S, Pittenger MF, Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DWH, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA, Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun 6, 8472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardo ME, Fibbe WE, Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 13, 392–402 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Caplan AI, Dennis JE, Mesenchymal stem cells as trophic mediators. J. Cell. Biochem 98, 1076–1084 (2006).16619257 [Google Scholar]
- 16.Lai RC, Tan SS, Yeo RWY, Choo ABH, Reiner AT, Su Y, Shen Y, Fu Z, Alexander L, Sze SK, Lim SK, MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 5, 29828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombo M, Raposo G, Théry C, Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol 30, 255–289 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Jahn R, Scheller RH, SNAREs—Engines for membrane fusion. Nat. Rev. Mol. Cell Biol 7, 631–643 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Lian H, Yang L, Cole A, Sun L, Chiang AC-A, Fowler SW, Shim DJ, Rodriguez-Rivera J, Taglialatela G, Jankowsky JL, Lu H-C, Zheng H, NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 85, 101–115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wulff K, Gatti S, Wettstein JG, Foster RG, Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci 11, 589–599 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Weyer C, Bogardus C, Mott DM, Pratley RE, The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest 104, 787–794 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stinchcombe J, Bossi G, Griffiths GM, Linking albinism and immunity: The secrets of secretory lysosomes. Science 305, 55–59 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Forbes SJ, Rosenthal N, Preparing the ground for tissue regeneration: From mechanism to therapy. Nat. Med 20, 857–869 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Rani S, Ryan AE, Griffin MD, Ritter T, Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol. Ther 23, 812–823 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W, HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 33, 2158–2168 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y, Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med 13, 49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu MS, Maan ZN, Wu J-C, Rennert RC, Hong WX, Lai TS, Cheung ATM, Walmsley GG, Chung MT, McArdle A, Longaker MT, Lorenz HP, Tissue engineering and regenerative repair in wound healing. Ann. Biomed. Eng 42, 1494–1507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Häkkinen L, Uitto V-J, Larjava H, Cell biology of gingival wound healing. Periodontol. 2000 24, 127–152 (2000). [PubMed] [Google Scholar]
- 29.Szpaderska AM, Zuckerman JD, DiPietro LA, Differential injury responses in oral mucosal and cutaneous wounds. J. Dent. Res 82, 621–626 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Schrementi ME, Ferreira AM, Zender C, DiPietro LA, Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen. 16, 80–86 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Larjava HS, Wiebe CB, Gallant-Behm C, Hart DA, Heino J, Häkkinen L, Exploring scarless healing of oral soft tissues. J. Can. Dent. Assoc 77, b18 (2011). [PubMed] [Google Scholar]
- 32.Xu X, Chen C, Akiyama K, Chai Y, Le AD, Wang Z, Shi S, Gingivae contain neural-crest-and mesoderm-derived mesenchymal stem cells. J. Dent. Res 92, 825–832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD, Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol 183, 7787–7798 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muzio M, Polentarutti N, Sironi M, Poli G, De Gioia L, Introna M, Mantovani A, Colotta F, Cloning and characterization of a new isoform of the interleukin 1 receptor antagonist. J. Exp. Med 182, 623–628 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thery C, Amigorena S, Raposo G, Clayton A, Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol Chapter 3, Unit 3.22 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F, Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2, 20360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DPV, Lim SK, Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 4, 214–222 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Sato T, Irie S, Kitada S, Reed JC, FAP-1: A protein tyrosine phosphatase that associates with Fas. Science 268, 411–415 (1995). [DOI] [PubMed] [Google Scholar]
- 39.Chen Z-H, Lam HC, Jin Y, Kim H-P, Cao J, Lee S-J, Ifedigbo E, Parameswaran H, Ryter SW, Choi AMK, Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc. Natl. Acad. Sci. U.S.A 107, 18880–18885 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR, Human mesenchymal stem cells stimulated by TNF-α, LPS, or hypoxia produce growth factors by an NFκB-but not JNK-dependent mechanism. Am. J. Physiol. Cell Physiol 294, C675–C682 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Hsu H, Xiong J, Goeddel DV, The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell 81, 495–504 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Ivanov VN, Ronai Z, Hei TK, Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas ligand-mediated apoptosis. J. Biol. Chem 281, 1840–1852 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishida Y, Kondo T, Kimura A, Matsushima K, Mukaida N, Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-κB activation and a reciprocal suppression of TGF-β signal pathway. J. Immunol 176, 5598–5606 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Luo J-D, Wang Y-Y, Fu W-L, Wu J, Chen AF, Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation 110, 2484–2493 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Falanga V, Wound healing and its impairment in the diabetic foot. Lancet 366, 1736–1743 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Raposo G, Stoorvogel W, Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol 200, 373–383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verkhratsky A, Matteoli M, Parpura V, Mothet J-P, Zorec R, Astrocytes as secretory cells of the central nervous system: Idiosyncrasies of vesicular secretion. EMBO J. 35, 239–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi N, Sawada W, Noguchi J, Watanabe S, Ucar H, Hayashi-Takagi A, Yagishita S, Ohno M, Tokumaru H, Kasai H, Two-photon fluorescence lifetime imaging of primed SNARE complexes in presynaptic terminals and β cells. Nat. Commun 6, 8531 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stow JL, Murray RZ, Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 24, 227–239 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Parton RG, del Pozo MA, Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol 14, 98–112 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Grande-García A,, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA, Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J. Cell Biol 177, 683–694 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun JEA, Madison DV, A novel SNAP25–caveolin complex correlates with the onset of persistent synaptic potentiation. J. Neurosci 20, 5997–6006 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Q, Lai Y, Bacaj T, Zhao M, Lyubimov AY, Uervirojnangkoorn M, Zeldin OB, Brewster AS, Sauter NK, Cohen AE, Soltis SM, Alonso-Mori R, Chollet M, Lemke HT, Pfuetzner RA, Choi UB, Weis WI, Diao J, Südhof TC, Brunger AT, Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature 525, 62–67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C, Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol 12, 19–30 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A, Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci 7, 613–620 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Moon D-O, Kim M-O, Kang S-H, Choi YH, Kim G-Y, Sulforaphane suppresses TNF-α-mediated activation of NF-κB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett. 274, 132–142 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Zhang Q-Z, Su W-R, Shi S-H, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD, Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 28, 1856–1868 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu S, Liu D, Chen C, Hamamura K, Moshaverinia A, Yang R, Liu Y, Jin Y, Shi S, MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab. 22, 606–618 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tkach M, Théry C, Communication by extracellular vesicles: Where we are and where we need to go. Cell 164, 1226–1232 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Jesus AA, Goldbach-Mansky R, IL-1 blockade in autoinflammatory syndromes. Annu. Rev. Med 65, 223–244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garlanda C, Dinarello CA, Mantovani A, The interleukin-1 family: Back to the future. Immunity 39, 1003–1018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dinarello CA, Anti-inflammatory agents: Present and future. Cell 140, 935–950 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ortiz LA, DuTreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG, Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. U.S.A 104, 11002–11007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, Jorgensen C, Noël D, Mesenchymal stem cell-derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells 34, 483–492 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y, Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J. Exp. Med 191, 313–320 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicklin MJH, Hughes DE, Barton JL, Ure JM, Duff GW, Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J. Exp. Med 191, 303–312 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, Hill S, Turner ML, Karp BI, Aksentijevich I, Pucino F, Penzak SR, Haverkamp MH, Stein L, Adams BS, Moore TL, Fuhlbrigge RC, Shaham B, Jarvis JN, O’Neil K, Vehe RK, Beitz LO, Gardner G, Hannan WP, Warren RW, Horn W, Cole JL, Paul SM, Hawkins PN, Pham TH, Snyder C, Wesley RA, Hoffmann SC, Holland SM, Butman JA, Kastner DL, Neonatal-onset multisystem inflammatory disease responsive to interleukin-1β inhibition. N. Engl. J. Med 355, 581–592 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen SB, Moreland LW, Cush JJ, Greenwald MW, Block S, Shergy WJ, Hanrahan PS, Kraishi MM, Patel A, Sun G, Bear MB; 990145 Study Group., A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann. Rheum. Dis 63, 1062–1068 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW, Reichner JS, Albina JE, Disruption of interleukin-1 signaling improves the quality of wound healing. Am. J. Pathol 174, 2129–2136 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathivanan S, Ji H, Simpson RJ, Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics 73, 1907–1920 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Gross JC, Chaudhary V, Bartscherer K, Boutros M, Active Wnt proteins are secreted on exosomes. Nat. Cell Biol 14, 1036–1045 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Soleimani M, Nadri S, A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc 4, 102–106 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Zhu H, Guo Z-K, Jiang X-X, Li H, Wang X-Y, Yao H-Y, Zhang Y, Mao N, A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc 5, 550–560 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Chen C, Wang D, Moshaverinia A, Liu D, Kou X, Yu W, Yang R, Sun L, Shi S, Mesenchymal stem cell transplantation in tight-skin mice identifies miR-151–5p as a therapeutic target for systemic sclerosis. Cell Res. 27, 559–577 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen C, Akiyama K, Wang D, Xu X, Li B, Moshaverinia A, Brombacher F, Sun L, Shi S, mTOR inhibition rescues osteopenia in mice with systemic sclerosis. J. Exp. Med 212, 73–91 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, Wang S, Zandi E, Du J, Ambudkar IS, Shi S, Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca2+ channel sulfhydration. Cell Stem Cell 15, 66–78 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seo B-M, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S, Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149–155 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S, SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. U.S.A 100, 5807–5812 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang C-Y, Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat. Biotechnol 20, 587–591 (2002). [DOI] [PubMed] [Google Scholar]
- 80.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S, Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A 97, 13625–13630 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raj DAA, Fiume I, Capasso G, Pocsfalvi G, A multiplex quantitative proteomics strategy for protein biomarker studies in urinary exosomes. Kidney Int. 81, 1263–1272 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Lamparski HG, Metha-Damani A, Yao J-Y, Patel S, Hsu D-H, Ruegg C, Le Pecq J-B, Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods 270, 211–226 (2002). [DOI] [PubMed] [Google Scholar]
- 83.Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C, Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J. Extracell. Vesicles 1, 18397 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Database S1. Western blotting films corresponding to Figs. 1 to 4.
Movie S1. GMSCs secrete IL-1RA-positive exosome-like EVs.
Movie S2. Exocytotic fusions of IL-1RA-positive vesicles in living GMSCs.
Movie S3. TNF-α-activated GMSCs release IL-1RA-positive exosome-like EVs.
Fig. S1. GMSCs secrete higher amounts of sEVs and cytokines.
Fig. S2. Fas controls IL-1RA-sEV secretion in murine SMSCs.
Fig. S3. Fas/Fap-1 binds with Cav-1 to control SNAP25/VAMP5-mediated IL-1RA release in murine MSCs.
Fig. S4. TNF-α promotes sEV and IL-1RA release in murine MSCs.
Fig. S5. Histomorphology of IL-1RA in wound healing in mice.
Fig. S6. sEVs containing IL-1RA ameliorate delayed wound healing in diabetic mice.
Fig. S7. Histomorphology of Fas in wound healing in mice.
Fig. S8. Schematic drawing of Fas/Fap-1/Cav-1-controlled IL-1RA-sEV secretion in MSCs.
Fig. S9. Characterization of BMMSCs, GMSCs, and SMSCs.
Table S1. Individual subject-level data.