Abstract
The human salivary gland (SG) has an elegant architecture of epithelial acini, connecting ductal branching structures, vascular and neuronal networks that together function to produce and secrete saliva. This review focuses on the translation of cell- and tissue-based research toward therapies for patients suffering from SG hypofunction and related dry mouth syndrome (xerostomia), as a consequence of radiation therapy or systemic disease. We will broadly review the recent literature and discuss the clinical prospects of stem/progenitor cell and tissue-based therapies for SG repair and/or regeneration. Thus far, several strategies have been proposed for the purpose of restoring SG function: (1) transplanting autologous SG-derived epithelial stem/progenitor cells; (2) exploiting nonepithelial cells and/or their bioactive lysates; and (3) tissue engineering approaches using 3D (three-dimensional) biomaterials loaded with SG cells and/or bioactive cues to mimic in vivo SGs. We predict that further scientific improvement in each of these areas will translate to effective therapies toward the repair of damaged glands and the development of miniature SG organoids for the fundamental restoration of saliva secretion.
Keywords: Salivary gland, Radiation therapy, Salivary hypofunction, Xerostomia, Regeneration, Transplantation, Stem cells, Organoids
INTRODUCTION
A Place for Cell-Based Therapies
Irreversible SG hypofunction and its associated symptoms, termed xerostomia, are a hallmark of several systemic diseases, such as Sjögren′s syndrome, granulomatous diseases, graft-versus-host disease, cystic fibrosis, uncontrolled diabetes, human immunodeficiency virus infection, thyroid disease, and late-stage liver disease [1]. Hyposalivation is also the most significant long-term complication for more than 550,000 patients that are annually diagnosed with head and neck cancer (HNC) globally and for whom radiation therapy (RT) is the main treatment [2–4]. Saliva is required for digestion, lubrication, oral homeostasis and protection against a variety of microbial and environmental hazards. Thus, a lack in saliva production can cause various life-disrupting pathological events. Rampant caries, painful mucositis, oral fungal infections, taste loss, speech deficits, and difficulty in swallowing are just a few examples of events that greatly impair patients oral and systemic health [3].
Current preventative therapies, such as surgical SG relocation outside the radiation field [5] or use of free radical scavengers [6] are challenging or not always effective. Using advanced SG-sparing intensity-modulated radiation therapy (IMRT) can still result in xerostomia, even though partial improvement of salivary secretion may occur [2, 3, 7]. This functional outcome of IMRT is correlated to each HNC patient′s personalized radiation treatment plan that all or not may affect specific regions harboring epithelial stem/progenitor cells [8] and its unique environment.
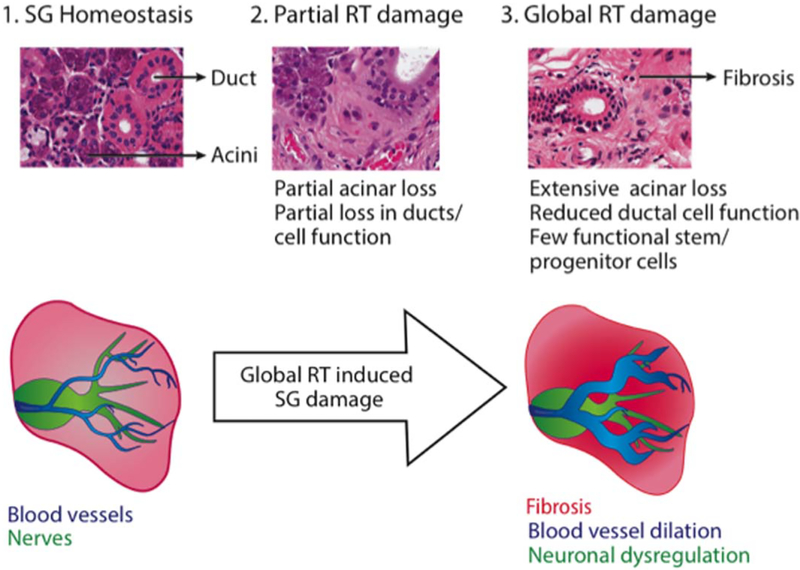
The epithelial compartment of SGs consists of nearly 80% saliva secreting acinar and 20% saliva transporting/modifying ductal cells. When SGs are in the radiation field, radiation damage occurs to these epithelial cells as well as surrounding blood vessels and nerves [4, 9]. While radiation-induced leakage of granules was long considered to be the cause of acute loss of saliva secretion, it couldn′t fully explain why proteolytic enzyme leakage was not accompanied with immediate epithelial cell loss [10]. Main causes of acute radiation damage were later credited to disturbed signal transduction pathways on the cell membrane. Irreversible damage to muscarinic receptor stimulated watery secretion [11] and dysfunction in water channels like Aquaporin 5 [12] more likely explain the high and early radiosensitivity effects. Thereafter, late to very late RT glandular dysfunction responses are due to parenchymal cell loss by apoptosis, and varying degrees of inflammation and fibrosis [10]. Even though most ductal epithelia remain morphologically, it is clear that their cellular function is impaired to some extent after RT, based on the reported decrease in protein expression of signaling receptors and structural cytokeratins [13]. Late-response effects further correlate with damage to the surrounding microenvironment by noticeable blood vessel dilation and function loss [14]. More recently, reduced parasympathetic nervous function was also suggested to be part of late post-RT effects [15, 16]. As nerves and blood vessels aid in epithelial cell repair post-RT, the combined radiation damage to acini, ducts, nerves and blood vessels, and development of fibrosis further obstructs normal gland regeneration (Fig. 1) [4].
Figure 1.
Different stages of damage in salivary glands evoked by radiation therapy (RT). (1) During salivary gland (SG) tissue homeostasis, glands are innervated and vascularized to support the epithelial compartment that consists of ductal and acinar cells. (1) Upon partial RT damage, parts of the gland are mild to moderately affected by RT (depending on the species), including the acinar compartment. (3) When RT damage globally affects the SG, massive fibrosis with varying degrees of inflammation can be observed with extensive loss in acinar and stem/progenitor cells. The irradiated glandular tissue is further marked by reduced endothelial function and neuronal dysregulation. Abbreviations: RT, radiation therapy; SG, salivary gland.
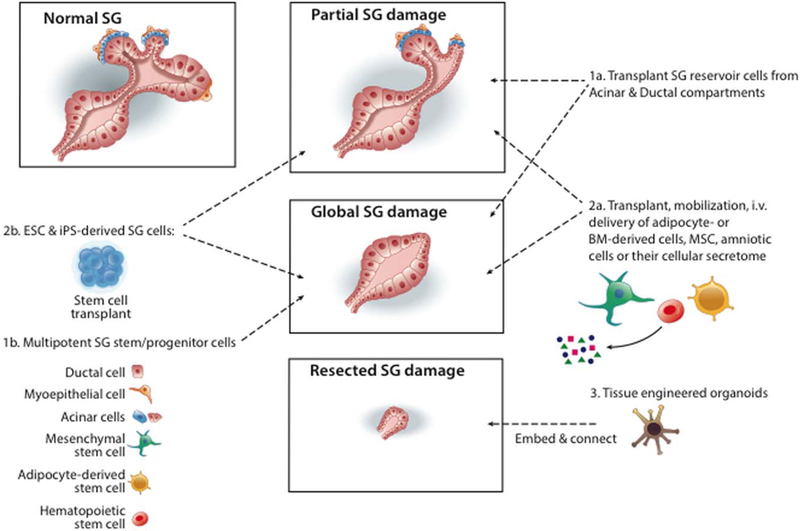
The use of artificial saliva substitutes provides temporary relief of xerostomia [4], and the administration of systemic sialogogues such as Pilocarpine increases saliva secretion, but their efficacy relies on the amount of remaining functional SG cells [18]. As such, HNC patients with extensive SG damage still await treatments to permanently restore salivary function. Due to our improved understanding of tissue morphogenesis and how (partially) damaged cells can be re-activated or replaced, several cellular and tissue-based therapies have been proposed to repair damaged SGs and/or generate new SG tissues (Fig. 2) [19, 20]. Despite cellular differences within the three major SGs (parotid, submandibular, and sublingual) are present, predominantly in the ratio of serous and mucous acini and potentially in their unique set of progenitors, researchers mainly focused their SG regenerative studies on submandibular and parotid glands. However, we propose that the following therapies may be applicable to all major glands. These can be grouped in the following categories:
Figure 2.
Proposed therapies to regenerate radiated salivary glands (SGs). Different epithelial cell types are maintained during homeostasis: ductal (intercalated, striated, granular convoluted tubule, and excretory), myoepithelial, and acinar cells. When glands are partially or globally injured, epithelial cells can undergo apoptosis and/or become functionally damaged. (1a) Reservoir cells of acinar and ductal compartments could then be transplanted post-radiation to locally repair the epithelia. (2a) Similarly, adipocytes, bone marrow (BM)-derived cells, mesenchymal stem cells (MSC) and/or amniotic cells can be transplanted, mobilized or intravenously (i.v.) delivered to aid in repair mechanisms. They can either participate in the formation of glandular cell types or stimulate radiation-surviving cells with their cellular secretome. (1b) After global SG damage, transplantation of multipotent SG specific epithelial stem/progenitors were shown to functionally and morphologically repair the tissue. (2b) Transplants of embryonic stem cells (ESC) and iPS (induced Pluripotent Stem cells) have also been explored to replace lost glandular cell types. (3) When SG are resected, in vitro tissue engineered organoids can be embedded in extracellular matrix and/or biomaterials and placed in the glandular bedding to connect with remaining tissue residues. Abbreviations: BM, bone marrow; MSC, mesenchymal stem cells; ESC, embryonic stem cells.
Autologous epithelial stem/progenitor cell transplantation: prior to RT, cells can be isolated from SG biopsies, potentially in vitro cultured and cryopreserved during RT, and transplanted into the irradiated gland post-RT to replace functionally damaged and/or lost cells.
Application of non-epithelial specific cell types and/or their bioactive lysates: (a) to trigger paracrine regenerative effects on remaining SG cells after SG damage or (b) to generate new SG-specific cells or (c) transplant bioengineered SG tissue into the gland space using cells cultured with biomaterials and/or growth factors.
These proposed therapies have been tested in rodent models and recent outcomes will be highlighted in the following sections. Despite cellular differences are present between the three major SGs, we classified them
A VARIETY OF CELL-BASED THERAPIES TO CHOOSE FROM
Autologous Transplant of SG Epithelial Cells
As mentioned earlier, partial gland loss-of-function can in certain situations be spontaneously recovered post-IMRT [21]. This lead to the hypothesis that endogenous SG cells can participate in organ repair, and thus that cell transplants could potentially be useful to regenerate severe loss-of-function.
The first proof-of-concept study for transplanting autologous SG cells to increase salivary function was carried out in rodents and used epithelial cells expressing the cell surface receptor KIT (c-Kit, CD117). Only as few as 100–300 KIT+ cells were required to generate new acinar and ductal structures and to significantly improve organ function after radiation[19]. This research demonstrated that mouse SGs contain cells with stem/progenitor properties that when transplanted could maintain themselves and differentiate into multiple specialized SG cell types.
Further studies using transplantation of murine KIT+ sub-populations (KIT+ CD24+, KIT+ CD49f+, KIT+ CD24+ CD49f+, KIT+ CD24+ SCA1+), illustrated that KIT+ cells possess different levels of stem/progenitor activity, with KIT1 CD24+ (CD49f+/SCA1+) cells reported to be the most potent [13, 22]. These cells are likely located within the major ducts of the central SG region where the highest stem/progenitor cell number resides [8]. Thus, KIT+ cells have potential for future cell therapy applications, particularly because they are present in human SGs [23] and can be isolated and cultured ex vivo [24]. A very recent ground-breaking study[25] has further supported the clinical use of enriched KIT+ subpopulations. Researchers were capable of rescuing hyposalivation in an in vivo mouse model with at least 500 human KIT+ SG cells per gland [25]. Moreover, regulators of the Wnt pathway were found upregulated in the SG tissues post-transplantation. The same research group showed earlier that the activation of the Wnt pathway is essential to drive the self-renewal of murine SG stem/progenitor cells in vitro [26].
Yet, the use of techniques such as genetic lineage tracing in mice, the application of DNA labels to mark label-retaining quiescent cells, in vitro floating sphere assays (or salispheres), and two-dimensional (2D) or three-dimensional (3D) cultures of both human and rodent SG cells revealed the existence of multiple stem/progenitor-like cells in the SG. These stem/progenitor cells can be identified and isolated based on the expression of a set of proteins and/or enzymes, such as cell surface receptors and cytokeratins (Table 1). Interestingly, these stem/progenitor cells appear at different times during organ development and may compensate for each others cell loss to allow proper organ formation [49]. Even during adult SG homeostasis, multiple reservoir cell types in compartments, such as ducts and acini, harbor high mitotic capacity and the ability to self-duplicate, that is, maintain and/or expand themselves [36, 50, 51]. However, from studies on SGs and other branching organs (reviewed in [52, 53]), it becomes clear that these compartmental reservoirs of stem/progenitor cells that regulate homeostatic maintenance may respond differently to tissue damage and/or become plastic by contributing to a cell population they normally do not form.
Table 1.
Summary list of suggested stem/progenitor cell markers and environmental signaling cues (cytokines, growth factors, enzymes, and hormones) currently studied for salivary gland regenerative therapies
| Stem/progenitor cell markers | Cytokines, growth factors, enzymes, hormones |
|---|---|
| ABCG2 [27] | ALDH3 activator [37] |
| ALDH3 [28] | EDA [38] |
| ASCL3 [29, 30] | EGF [39] |
| CD24 (HSA) [22, 31] | FLX3 [14] |
| CD29 (ITGβ1) [31] | FGF2 [40] |
| CD34 [32] | FGF7 [41] |
| CD44 [33] | G-CSF [14] |
| CD49f (ITGα6) [13, 34] | SHH [42] |
| CD90 (Thy-1) [34] | IL6 [43, 44] |
| CD105 [32] | IGF1 [45] |
| CD117 (KIT) [13, 19, 22, 25] | Melatonin [46] |
| KRT5 [15, 16] | SCF [14] |
| KRT14 [23] | VEGF [43] |
| MUSASHI-1 [19] | WNT [26, 47, 48] |
| SCA-1 [19, 22]p75 [34] | |
| SOX2 [35] | |
| MIST1 [36] |
Thus, even though KIT+ cells as well as CD24+/CD29+ epithelial cells have been shown to restore hyposalivation in vivo ([13, 19, 22, 54], we can not rule out that other cell types are not able and/or are more potent to regenerate SGs. Table 1 summarizes different cell markers that were classified with stem/progenitor potential, but majority were not fully tested yet for their regenerative capacity in RT clinical settings.
Additionally, depending on the location and level of RT-induced damage in the SG, different stem/progenitor cells could potentially be used for repair. A recent study [8] revealed that a specific region within the gland is more sensitive to radiation than others, and that radiation to this area reflects in severe saliva loss and tissue damage. When the 50% of cranial region of the SG was radiated, the entire gland degenerated including the shielded caudal 50% [8]. In contrast, damage remained restricted to the 50% caudal region when only this part was being radiated. This suggests that once multipotent stem/progenitor cells, which are proposed to be located in a cranial sub-volume, are lost other cell types are not able to compensate and repair the gland [8]. However, when cranial stem/progenitor cells remained unaffected they were able to maintain this area of the gland functional. As such, it now becomes speculative whether different cell types could be used in each scenario. For example, while transplantation of multipotent stem/progenitor cells becomes preconditioned when the entire SG is damaged, less potent cells and/or multiple compartmental reservoir cells could be applied for local caudal SG repair [8]. Even acinar cells, which were long assumed to be permanently differentiated and post-mitotic, could now be considered for SG cell therapy as they can self-duplicate after damage in post-duct ligation [36], partial SG excision [55], post-chronic sialadenitis [56] and possibly post-RT conditions to locally repair and maintain the secretory compartment. Most interestingly, SG repair is not only driven by transplanted cells, but also by the remaining endogenous stem/progenitor cells [25]. Radiation can induce stem/progenitor cell dormancy in vivo [41, 57], and thus these cells can be locally activated with the appropriate stimuli. As such, any type of transplanted epithelial cell could enhance local endogenous repair if the appropriate stimuli are produced and a dormant stem/progenitor cell is present nearby.
However, from a clinical standpoint there may be limitations to autologous cell therapy since SGs from aging patients contain fewer stem/progenitor cells [24, 58]. This implies that more stem/progenitor cells (than those obtained in the pre-RT biopsy) may be required for organ repair. Recent efforts to increase the number of KIT+ cells ex vivo using growth factors [59] or Aldehyde dehydrogenase-3 (ALDH3) activator [28] may be useful, although, the absolute cell number required for functional regeneration of the human gland remains unclear. Alternatively, non-SG cells may be considered to address this limitation, as outlined below.
Another caveat in developing SG cell therapies could potentially be the limited lifespan of biopsy-derived cells cultured ex vivo. In such cases, methods to cryopreserve and store these progenitors from biopsies have been developed. Neumann and others [60] established a stem cell banking model where SG CD49f+ CD29+ cells were cryopreserved for up to 3 years without affecting their genetic or functional stability, validating that cryopreservation could be part of a cell therapy option in the near future.
In conclusion, multiple research groups have shown that rodent SG-specific epithelial cell transplantation is a feasible approach to repair irradiated SGs. Future research studies will determine whether human SG cells behave in a similar manner in ex vivo and in vivo assays [25]. Although success has been achieved with epithelial KIT+ cells in rodents, currently, other more multipotent stem/progenitor cell candidates and/or compartmental reservoir cells can be explored. Alternatively, in clinical scenarios where autologous SG cell numbers are low, we may need to take advantage of the regenerative capacity of non-SG cells, as discussed in the next section.
Nonepithelial Cell Types and Bioactive Lysates
There are many reports on the beneficial effects of non-SG and/or non-epithelial cells to regenerate irradiated SGs. These studies include Bone Marrow (BM)-derived cells [14, 61–63], BM-derived mesenchymal stem cells (MSC) [64], human adipose-derived MSCs [65–68], SG-derived MSC-like cells [32,69], amniotic cells [70, 71], embryonic stem cells (ESC) [72], and induced-pluripotent stem cells (iPS) [73].
Despite a proposed differentiation of BM-derived cells and MSCs into SG acinar cells is observed in vitro, their actual contribution to epithelial differentiation in vivo is not clear and disputable. Their beneficial action may primarily occur via paracrine pro-survival/proliferative effects on remaining epithelial stem/progenitor cells and surrounding environmental cells. For example, transplantation of G-CSF/FLT3/SCF-mobilized BM-derived cells [14] not only improved saliva production by inducing epithelial repair but also increased microvessel density, which consequently led to better blood perfusion. Similarly, adipose-derived MSCs diminished acinar cell apoptosis as well as reduced fibrosis [67], and both BMMSC as SG-derived mesenchymal-like cells exerted immunosuppressive activities [69].
The beneficial potential of these paracrine effects led investigators to explore the addition of the bioactive components, also called “soup,” secreted by these adipose and BM-derived cells to repair SGs who underwent RT [43, 74]. The exact content of the bioactive components remains elusive to date, but several potential contributing signaling pathways have been identified. Studies using systemic growth factor delivery or genetic overstimulation of specific signaling pathways suggest that KGF (or FGF7) can increase stem/progenitor cell numbers in vivo post-RT [41, 75]. A similar role was attributed to WNT/β-catenin [47, 48] and Sonic Hedgehog (SHH) signaling [42] in post-RT and post-ductal ligation settings. Also treatment with EGF, IGF1, FGF2 [39, 40, 45], IL6[44], ALDH3 [37], or EDA activators [38] reduced cell apoptosis and promoted proliferation (Table 1). Even post-radiation treatment with hormone Melatonin can decrease oxidative stress and lipid peroxidation in SGs [46]. Another putative activator for inducing acinar differentiation may be the NOTCH signaling pathway [76, 77], even though its beneficial action in vivo post-RT has not been confirmed yet. All these signaling factors are summarized in Table 1.
Since multiple factors (e.g., GM-CSF, VEGF, IL6, and IGF1) are found in “soups,” the anti-apoptotic and pro-proliferative cues can thus aid not only in epithelial but also in microenvironmental repair [43]. Moreover, intravenous “soup” administration may be all that is required to clinically improve saliva production as this delivery route appears to be as effective in rodents [43]. However, it remains to be evaluated whether the “soup” strategy will work as efficiently in every patient. Similar to the clinical efficacy of Pilocarpine administration in RT-induced xerostomia settings [78], the “soup” strategy relies on the amount of remaining SG cells. Thus, clinical successes will depend on the remaining cells that need paracrine stimulation and whether these stimuli are present in the “soup.” While angiogenic factors have been described to be present in certain “soups,” it is not clear yet whether neurotrophic factors are. Neuronal cells, such as the ones from the parasympathetic nervous system, aid in epithelial regeneration post-RT [15, 22] and thus, if required, neurotrophic factors such as Neurturin or Glial cell-Derived Neurotrophic Factor could potentially be (co-)delivered to radiated SGs via retrograde ductal or intraglandular injections.
While BM-derived cells and MSCs might not efficiently differentiate into SG cells, other pluripotent cell types such as ESCs and iPS cells can be explored to provide new pools of SG-specific cells. As such, SG secretory cells were already generated from ESCs [72]. This study used 3D co-culture of mouse ESCs with a human SG-derived fibroblast environment to initiate expression of SG-related markers. While the ESC-derived SG-like cells survived post-RT SG transplantation, it is still unclear whether they functionally regenerate the tissue[72]. If these cells possess genomic stability and lack oncogenic potential, both ESC [72] and iPS-derived SG cells [73] can serve as an additional cell-based therapy.
Tissue Engineering Strategies to Generate Sg Organoids
SG tissue engineering requires three essential components:(1) cell-cell contacts; (2) cell contacts with extracellular matrix (ECM) proteins, and (3) a biocompatible and biodegradable 3D scaffold that can hold these components together [79].
Many scaffolds have been proposed, which are porous and either biologic (e.g., collagen, fibrin, silk, chitosan, alginate, hyaluronic acid (HA)) in origin or synthetic biocompatible biomaterials (e.g., poly-glycolic acid, poly-lactic acid, poly lactic-co-glycolic acid (PLGA), and polyethylene glycol), and/or mixture of both. Depending on its biodegradability, porosity, stiffness and strength, scaffolds promote cell adhesion, migration, and/or differentiation [80]. Ideally, engineered scaffolds should structurally and functionally resemble the native SG ECM architecture (reviewed in [81]).
While there are many new scaffolds being generated, researchers must implement aspects of SG organogenesis, branching morphogenesis and homeostasis to initially form 3D miniature tissues, termed organoids. A summary of currently used human cell-based models with translational potential is presented in Table 2.
Table 2.
Human cell-based therapy models already tested for the development of salivary gland 3D tissue organoids
| Model features | In vivo/in vitro remarks | Limitations | Reference |
|---|---|---|---|
| hSG primary cells in 3D matrix containing Collagen and Matrigel |
|
|
[82] |
| |||
| |||
| hSG progenitor cells in 3D Matrigel-based matrix |
|
|
[24] |
| |||
|
|
||
| hSG primary cells in serum-free conditions in Matrigel-coated dishes |
|
• No in vivo studies | [83] |
| |||
| |||
| hSG primary cells in 3D HA hydrogel |
|
|
[84] |
| |||
| hSG primary cells in a 3D matrix containing Collagen and Matrigel |
|
|
[25] |
| |||
| |||
|
Abbreviation: hSG, human salivary gland.
A long-standing hurdle in the field has been the long-term growth and maintenance of specific acinar cell protein expression, as well as their cell polarity and secretory function. Monolayer cultures, that is, 2D culture, of primary acinar cells cause loss of biological functions including, acinar-specific protein expression (α-amylase, cystatin C, transmembrane protein 16A—TMEM16A, sodium-potassium-chloride cotransporter—NKCC1, and aquaporin 5—AQP5), granule formation, calcium mobilization, transepithelial resistance, and polarized amylase secretion after β-adrenergic receptor stimulation. Gaining control of these biological functions appears to be related to specific media components and ECM products. High calcium concentrations (0.05 mM) provide optimal acinar growth and maintenance of polarization [85], and without addition of ECM proteins the maintenance of acinar cells and formation of organoids will be limited. For example, pure amino acid non-ECM containing PuraMatrix peptide hydrogels hardly maintained SG cells [86], but mucin-secreting cells were easily grown for up to 1 month on natural fibronectin-coated silk fibroin scaffolds. Interestingly, 3D scaffolding itself induced seeded cells to produce significantly more native ECM components than in 2D cultures, which further supports more appropriate cell differentiation and polarization. The observation that parotid cell cultures were better maintained on these silk fibers compared to submandibular cells also indicated that each gland cell-type might require a unique ECM-coated scaffold.
It is also important to note that each ECM differently impacts cell polarization, differentiation, lumenization, and tight junction formation. PLGA nanofibers coupled with laminin-111 and chitosan functional units demonstrated that laminin-111 tends to promote mature SG epithelial tight junctions and apico-basal polarization, but conversely, chitosan antagonizes this process [87]. Encapsulating human SG cells in human-compatible HA hydrogels with recombinant Perlecan IV domain not only induced cell organization into proliferating spheroid structures, but also formed larger acini-like structures with a central lumen that were maintained long-term in vitro [88]. In cases where there is a reduction in the assembly of tight junctions (ZO-1 expression) [89], which are needed for uni-directional flow of saliva, one can overcome this by generating lithographically-based micropatterning curved “craters.” These craters mimic the physical structure of the basement membrane, and thus increased surface area allowed for better apico-basal polarization and differentiation of SG epithelial cells [90].
Apart from generating and maintaining proper cell types, engineered SGs further require formation of branching structures. Chitosan appears to facilitate SG branching by regulating production of basement membrane components[91], and small branching organoids could also be formed in Collagen type I and/or Matrigel [19, 24, 25, 31, 82, 83, 92]. While many positive results were obtained with Matrigel, its components are not xeno-free as it contains basement membrane proteins secreted by mouse sarcoma cells, and therefore, its use is not consistent with current Good Manufacturing Practice regulations by the US Food and Drug Administration (FDA). One alternative is to use the native organ-specific ECM that can be obtained by decellularizing tissues with detergents and then reseeding primary cells onto the gland ECM structure, as accomplished for the rat submandibular SG [93].
Recent advances have also been directed to develop more functional organoids. These efforts include combinations of linked ECM peptides and the development of controlled drug or growth factor releases from scaffolds. These can then be seeded with cells to direct differentiation and branching, with or without various SG cell types. A current challenge remains to let bioengineered tissues grow in size and properly connect with remaining cells in the transplanted area. Efforts toward this goal have recently been initiated in a mouse and rat model [84, 94]. HA-gels with primary human cells were maintained and responded to neurotransmitters when integrated in the area of resected parotid glands in immune compromised rats [95]. An alternative approach showed that fetal SG cells, both epithelium and mesenchyme, within a 3D Collagen environment could be transplanted into the space of completely resected SGs. Interestingly, a suture thread was used to provide guidance for the primary duct to reconnect with the oral cavity. Future efforts will certainly be directed to using a similar approach with adult cells. Whether a similar reconnection with the remaining duct can be obtained in humans remains to be determined.
FUTURE PROSPECTS
Remarkable progression has been made in the last decade, but a definitive therapy for SG hypofunction has not been developed due to intrinsic challenges that come with each approach. An underlying challenge is comparison of the animal models with human SGs. The biological differences between human and rodent SGs and understanding how they respond to RT requires further study but initial important steps have been taken [25]. Moreover, potential differences in development and/or regenerative strategies between the different glands (e.g., parotid, submandibular, sublingual) need to be considered for future clinical translations. Also complicating matters is the variation of RT damage that occurs in individual patients with respect to both the location and dose of RT as well as the patient′s age. However, with each discovery in the future, a range of precision medicine therapies may become available individualized to each patient. An appreciation of the strengths and limitations of each strategy as well as whether the patients have existing RT damage will determine what therapy will be designed and delivered.
Theoretically, there should be no shortage of cell types, as both SG-specific as non-SG specific cells could be used to repair the epithelial compartment and surrounding microenvironment. The paracrine effects of each cell type will aid in the repair process post-RT, and with the development of bio-active scaffolds, we should be able to generate branching SG organoids in the near future.
SIGNIFICANCE STATEMENT.
This review covers recent advances in translating cell-based research toward pre-clinical therapies. We focus on salivary gland (SG) loss-of-function and subsequent dry mouth syndrome as caused by radiation therapy or systemic disease, although the described concepts can be translated to other injured somatic tissues. Proposed therapies include implantation of autologous tissue-specific stem/progenitor cells, non-tissue specific cells and/or their bioactive lysates (secretome); and organoid-like constructs created by cells in the presence or not of bioactive cues and three-dimensional biomaterials. These emerging approaches to repair damaged SGs are discussed herein, and evaluated on their success to restore native tissue architecture, epithelial cell polarization, ductal branching, lumen formation, directionality of secretory flow, and clinically relevant tissue functionality.
ACKNOWLEGMENT
We sincerely apologize for the articles that could not be referenced due to space limitations. This work is supported in part by the National University of Singapore ODPRT start up grant R221-000-072-133 and by the National Medical Research Council grant CNIG/1131/2015. We thank Dr. Matthew Hoffman (National Institutes of Health, MD) for critical reading.
LIST OF ABBREVIATIONS
- SG
salivary gland
- RT
radiation therapy
- HNC
head and neck cancers
- 3D
three-dimensional
- 2D
two-dimensional
- KRT
cytokeratin
- FGFR2b
fibroblast growth factor receptor 2b
- ALDH3
aldehyde dehydrogenase-3
- BM
bone marrow
- MSC
mesenchymal stem cell
- BMSC
bone marrow stem cell
- BM-MSC
bone marrow-derived stem cell
- BM-cMSC
bone marrow clonal mesenchymal stem cell
- SMG
submandibular gland
- FGF
fibroblast growth factor
- GM-CST
Granulocyte-macrophage colony-stimulating factor
- Flt3
Fms-Related Tyrosine Kinase 3
- SCF
stem cell factor
- KGF
keratinocyte growth factor
- VEGF
vascular endothelial growth factor
- IL6
interleukin 6
- IGF1
insulin-like growth factor 1
- ESC
embryonic stem cells
- iPS
induced pluripotent stem cells
- PLGA
poly-(lactic-co-glycolic acid)
- HA
hyaluronic acid
- ECM
extracellular matrix
Footnotes
DISCLOSURE OF POTENTIAL CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interests.
REFERENCES
- 1.von Bultzingslowen I, Sollecito TP, Fox PC et al. Salivary dysfunction associated with systemic diseases: Systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103: S57e51–S15. [DOI] [PubMed] [Google Scholar]
- 2.Jensen SB, Pedersen AM, Vissink A et al. A systematic review of salivary gland hypo-function and xerostomia induced by cancer therapies: Management strategies and economic impact. Support Care Cancer 2010;18: 1061–1079. [DOI] [PubMed] [Google Scholar]
- 3.Wijers OB, Levendag PC, Braaksma MM et al. Patients with head and neck cancer cured by radiation therapy: A survey of the dry mouth syndrome in long-term survivors. Head Neck 2002;24:737–747. [DOI] [PubMed] [Google Scholar]
- 4.Vissink A, Mitchell JB, Baum BJ et al. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: Successes and barriers. Int J Radiat Oncol Biol Phys 2010;78: 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu R, Seikaly H, Jha N. Anatomic study of submandibular gland transfer in an attempt to prevent postradiation xerostomia. J Otolaryngol 2002;31:76–79. [DOI] [PubMed] [Google Scholar]
- 6.Brizel DM, Wasserman TH, Henke M et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol 2000;18:3339–3345. [DOI] [PubMed] [Google Scholar]
- 7.Nutting CM, Morden JP, Harrington KJ et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Luijk P, Pringle S, Deasy JO et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med 2015;7:305ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: From animal models to therapies. J Dent Res 2009;88:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konings AW, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys 2005;62: 1187–1194. [DOI] [PubMed] [Google Scholar]
- 11.Coppes RP, Roffel AF, Zeilstra LJ et al. Early radiation effects on muscarinic receptor-induced secretory responsiveness of the parotid gland in the freely moving rat. Radiat Res 2000;153:339–346. [DOI] [PubMed] [Google Scholar]
- 12.Takagi K, Yamaguchi K, Sakurai T et al. Secretion of saliva in X-irradiated rat submandibular glands. Radiat Res 2003;159:351–360. [DOI] [PubMed] [Google Scholar]
- 13.Nanduri LS, Lombaert IM, van der Zwaag M et al. Salisphere derived c-kit1 cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol 2013;108:458–463. [DOI] [PubMed] [Google Scholar]
- 14.Lombaert IM, Brunsting JF, Wierenga PK et al. Cytokine treatment improves parenchymal and vascular damage of salivary glands after irradiation. Clin Cancer Res 2008;14: 7741–7750. [DOI] [PubMed] [Google Scholar]
- 15.Knox SM, Lombaert IM, Haddox CL et al. Parasympathetic innervation improves epithelial organ regeneration. Nat Commun 2013;4:1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knox SM, Lombaert IM, Reed X et al. Parasympathetic innervation maintains epithelial progenitor cells during salivary organ-ogenesis. Science 2010;329:1645–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lombaert IM, Hoffman MP. Stem cells in salivary gland development and regeneration In: Huang GT-J, Thesleff I, eds. Stem Cells in Craniofacial Development and Regeneration, 1st ed Will, 2013:271–284. [Google Scholar]
- 18.Burlage FR, Faber H, Kampinga HH et al. Enhanced proliferation of acinar and progenitor cells by prophylactic pilocarpine treatment underlies the observed amelioration of radiation injury to parotid glands. Radiother Oncol 2009;90:253–256. [DOI] [PubMed] [Google Scholar]
- 19.Lombaert IM, Brunsting JF, Wierenga PK et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PloS One 2008;3:e2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coppes RP, Stokman MA. Stem cells and the repair of radiation-induced salivary gland damage. Oral Dis 2011;17:143–153. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Taylor JM, Ten Haken RK et al. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys 2007;67:660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao N, Lin Y, Cao H et al. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest 2014;124:3364–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lombaert IM, Abrams SR, Li L et al. Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organ-ogenesis. Stem Cell Rep 2013;1:604–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng J, van der Zwaag M, Stokman MA et al. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol 2009;92:466–471. [DOI] [PubMed] [Google Scholar]
- 25.Pringle S, Maimets M, van der Zwaag M et al. Human salivary gland stem cells functionally restore radiation damaged salivary glands. STEM CELLS 2016;34:640–52. [DOI] [PubMed] [Google Scholar]
- 26.Maimets M, Rocchi C, Bron R et al. Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Rep 2016;6:150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YJ, Kwon HJ, Shinozaki N et al. Comparative analysis of ABCG2-expressing and label-retaining cells in mouse submandibular gland. Cell Tissue Res 2008;334:47–53. [DOI] [PubMed] [Google Scholar]
- 28.Banh A, Xiao N, Cao H et al. A novel aldehyde dehydrogenase-3 activator leads to adult salivary stem cell enrichment in vivo. Clin Cancer Res 2011;17:7265–7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bullard T, Koek L, Roztocil E et al. Ascl3 expression marks a progenitor population of both acinar and ductal cells in mouse salivary glands. Dev Biol 2008;320:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rugel-Stahl A, Elliott ME, Ovitt CE. Ascl3 marks adult progenitor cells of the mouse salivary gland. Stem Cell Res 2012;8:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanduri LS, Baanstra M, Faber H et al. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep 2014;3:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong J, Baek H, Kim YJ et al. Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Exp Mol Med 2013;45:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maria OM, Maria AM, Cai Y et al. Cell surface markers CD44 and CD166 localized specific populations of salivary acinar cells. Oral Dis 2012;18:162–168. [DOI] [PubMed] [Google Scholar]
- 34.Sato A, Okumura K, Matsumoto S et al. Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning Stem Cells 2007;9:191–205. [DOI] [PubMed] [Google Scholar]
- 35.Arnold K, Sarkar A, Yram MA et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011;9:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aure MH, Konieczny SF, Ovitt CE. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell 2015;33:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao N, Cao H, Chen CH et al. A novel aldehyde dehydrogenase-3 activator (Alda-89) protects submandibular gland function from irradiation without accelerating tumor growth. Clin Cancer Res 2013;19:4455–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill G, Headon D, Harris ZI et al. Pharmacological activation of the EDA/EDAR signaling pathway restores salivary gland function following radiation-induced damage. PloS One 2014;9:e112840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohlsson B, Jansen C, Ihse I et al. Epidermal growth factor induces cell proliferation in mouse pancreas and salivary glands. Pancreas 1997;14:94–98. [DOI] [PubMed] [Google Scholar]
- 40.Kojima T, Kanemaru S, Hirano S et al. The protective efficacy of basic fibroblast growth factor in radiation-induced salivary gland dysfunction in mice. Laryngoscope 2011;121:1870–1875. [DOI] [PubMed] [Google Scholar]
- 41.Lombaert IM, Brunsting JF, Wierenga PK et al. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. STEM CELLS 2008;26:2595–2601. [DOI] [PubMed] [Google Scholar]
- 42.Hai B, Qin L, Yang Z et al. Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and para-sympathetic innervation. Clin Cancer Res 2014;20:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.An HY, Shin HS, Choi JS et al. Adipose mesenchymal stem cell secretome modulated in hypoxia for remodeling of radiation-induced salivary gland damage. PloS One 2015;10:e0141862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marmary Y, Adar R, Gaska S et al. Radiation-induced loss of salivary gland function is driven by cellular senescence and prevented by IL-6 modulation. Cancer Res 2016;76: 1170–1180. [DOI] [PubMed] [Google Scholar]
- 45.Limesand KH, Said S, Anderson SM. Suppression of radiation-induced salivary gland dysfunction by IGF-1. PloS One 2009;4: e4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cakmak Karaer I, Simsek G, Yildiz A et al. Melatonins protective effect on the salivary gland against ionized radiation damage in rats. J Oral Pathol Med 2016;45:444–9. [DOI] [PubMed] [Google Scholar]
- 47.Hai B, Yang Z, Shangguan L et al. Concurrent transient activation of Wnt/beta-catenin pathway prevents radiation damage to salivary glands. Int J Radiat Oncol Biol Phys 2012;83:e109–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hai B, Yang Z, Millar SE et al. Wnt/beta-catenin signaling regulates postnatal development and regeneration of the salivary gland. Stem Cells Dev 2010;19:1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arany S, Catalan MA, Roztocil E et al. Ascl3 knockout and cell ablation models reveal complexity of salivary gland maintenance and regeneration. Dev Biol 2011;353: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwak M, Ghazizadeh S. Analysis of his-tone H2BGFP retention in mouse submandibular gland reveals actively dividing stem cell populations. Stem Cells Dev 2015;24:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maruyama EO, Aure MH, Xie X et al. Cell-specific Cre strains for genetic manipulation in salivary glands. PloS One 2016;11: e0146711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pringle S, Van Os R Coppes RP. Concise review: Adult salivary gland stem cells and a potential therapy for xerostomia. STEM CELLS 2013;31:613–619. [DOI] [PubMed] [Google Scholar]
- 53.Hogan BL, Barkauskas CE, Chapman HA et al. Repair and regeneration of the respiratory system: Complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 2014;15:123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nanduri LS, Maimets M, Pringle SA et al. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol 2011;99:367–372. [DOI] [PubMed] [Google Scholar]
- 55.Boshell JL, Pennington C. Histological observations on the effects of isoproterenol on regenerating submandibular glands of the rat. Cell Tissue Res 1980;213:411–416. [DOI] [PubMed] [Google Scholar]
- 56.Ihrler S, Blasenbreu-Vogt S, Sendelhofert A et al. Regeneration in chronic sialadenitis: An analysis of proliferation and apoptosis based on double immunohistochemical labelling. Virchows Arch 2004;444:356–361. [DOI] [PubMed] [Google Scholar]
- 57.Tatsuishi Y, Hirota M, Kishi T et al. Human salivary gland stem/progenitor cells remain dormant even after irradiation. Int J Mol Med 2009;24:361–366. [DOI] [PubMed] [Google Scholar]
- 58.Maimets M, Bron R, de Haan G et al. Similar ex vivo expansion and post-irradiation regenerative potential of juvenile and aged salivary gland stem cells. Radiother Oncol 2015;116:443–448. [DOI] [PubMed] [Google Scholar]
- 59.Patel VN, Lombaert IM, Cowherd SN et al. Hs3st3-modified heparan sulfate controls KIT+ progenitor expansion by regulating 3-O-sulfotransferases. Dev Cell 2014;29:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neumann Y, David R, Stiubea-Cohen R et al. Long-term cryopreservation model of rat salivary gland stem cells for future therapy in irradiated head and neck cancer patients. Tissue Eng Part C 2012;18:710–718. [DOI] [PubMed] [Google Scholar]
- 61.Lombaert IM, Wierenga PK, Kok T et al. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res 2006;12:1804–1812. [DOI] [PubMed] [Google Scholar]
- 62.Sumita Y, Liu Y, Khalili S et al. Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int J Biochem Cell Biol 2011;43:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin CY, Chang FH, Chen CY et al. Cell therapy for salivary gland regeneration. J Dent Res 2011;90:341–346. [DOI] [PubMed] [Google Scholar]
- 64.Lim JY, Yi T, Choi JS et al. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol 2013;49:136–143. [DOI] [PubMed] [Google Scholar]
- 65.Kojima T, Kanemaru S, Hirano S et al. Regeneration of radiation damaged salivary glands with adipose-derived stromal cells. Laryngoscope 2011;121:1864–1869. [DOI] [PubMed] [Google Scholar]
- 66.Lee J, Park S, Roh S. Transdifferentiation of mouse adipose-derived stromal cells into acinar cells of the submandibular gland using a co-culture system. Exp Cell Res 2015;334: 160–172. [DOI] [PubMed] [Google Scholar]
- 67.Lim JY, Ra JC, Shin IS et al. Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PloS One 2013;8: e71167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiong X, Shi X, Chen F. Human adipose tissue derived stem cells alleviate radiation induced xerostomia. Int J Mol Med 2014;34: 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim JY, Yi T, Lee S et al. Establishment and characterization of mesenchymal stem cell-like clonal stem cells from mouse salivary glands. Tissue Eng Part C 2015;21:447–457. [DOI] [PubMed] [Google Scholar]
- 70.Zhang NN, Huang GL, Han QB et al. Functional regeneration of irradiated salivary glands with human amniotic epithelial cells transplantation. Int J Clin Exp Pathol 2013;6: 2039–2047. [PMC free article] [PubMed] [Google Scholar]
- 71.Huang GL, Zhang NN, Wang JS et al. Transdifferentiation of human amniotic epithelial cells into acinar cells using a double-chamber system. Cell Reprogram 2012;14: 377–383. [DOI] [PubMed] [Google Scholar]
- 72.Kawakami M, Ishikawa H, Tachibana T et al. Functional transplantation of salivary gland cells differentiated from mouse early ES cells in vitro. Hum Cell 2013;26:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ono H, Obana A, Usami Y et al. Regenerating salivary glands in the microenvironment of induced pluripotent stem cells. Biomed Res Int 2015;2015:293570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tran SD, Liu Y, Xia D et al. Paracrine effects of bone marrow soup restore organ function, regeneration, and repair in salivary glands damaged by irradiation. PloS One 2013;8:e61632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng C, Cotrim AP, Rowzee A et al. Prevention of radiation-induced salivary hypofunction following hKGF gene delivery to murine submandibular glands. Clin Cancer Res 2011;17:2842–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dang H, Lin AL, Zhang B et al. Role for notch signaling in salivary acinar cell growth and differentiation. Dev Dyn 2009;238:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Gallastegui P, Ibarretxe G, Garcia-Ramirez JJ et al. DLK1 regulates branching morphogenesis and parasympathetic innervation of salivary glands through inhibition of NOTCH signalling. Biol Cell 2014;106:237–253. [DOI] [PubMed] [Google Scholar]
- 78.Burlage FR, Roesink JM, Kampinga HH et al. Protection of salivary function by concomitant pilocarpine during radiotherapy: A double-blind, randomized, placebo-controlled study. Int J Radiat Oncol Biol Phys 2008;70: 14–22. [DOI] [PubMed] [Google Scholar]
- 79.Aframian DJ, Palmon A. Current status of the development of an artificial salivary gland. Tissue Eng Part B 2008;14:187–198. [DOI] [PubMed] [Google Scholar]
- 80.Peters SB, Naim N, Nelson DA et al. Bio-compatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation. Tissue Eng Part A 2014;20: 1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pradhan S, Farach-Carson MC. Mining the extracellular matrix for tissue engineering applications. Regen Med 2010;5: 961–970. [DOI] [PubMed] [Google Scholar]
- 82.Joraku A, Sullivan CA, Yoo J et al. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation 2007;75:318–324. [DOI] [PubMed] [Google Scholar]
- 83.Maria OM, Zeitouni A, Gologan O et al. Matrigel improves functional properties of primary human salivary gland cells. Tissue Eng Part A 2011;17:1229–1238. [DOI] [PubMed] [Google Scholar]
- 84.Pradhan-Bhatt S, Harrington DA, Duncan RL et al. Implantable three-dimensional salivary spheroid assemblies demonstrate fluid and protein secretory responses to neuro-transmitters. Tissue Eng Part A 2013;19: 1610–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jang SI, Ong HL, Gallo A et al. Establishment of functional acinar-like cultures from human salivary glands. J Dent Res 2015;94: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okumura K, Shinohara M, Endo F. Capability of tissue stem cells to organize into salivary rudiments. Stem Cells Int 2012;2012: 502136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cantara SI, Soscia DA, Sequeira SJ et al. Selective functionalization of nanofiber scaffolds to regulate salivary gland epithelial cell proliferation and polarity. Biomaterials 2012; 33:8372–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pradhan S, Liu C, Zhang C et al. Lumen formation in three-dimensional cultures of salivary acinar cells. Otolaryng Head Neck 2010;142:191–195. [DOI] [PubMed] [Google Scholar]
- 89.Jean-Gilles R, Soscia D, Sequeira S et al. Novel modeling approach to generate a polymeric nanofiber scaffold for salivary gland cells. J Nanotechnol Eng Med 2010;1: 31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soscia DA, Sequeira SJ, Schramm RA et al. Salivary gland cell differentiation and organization on micropatterned PLGA nanofiber craters. Biomaterials 2013;34:6773–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang TL, Hsiao YC. Chitosan facilitates structure formation of the salivary gland by regulating the basement membrane components. Biomaterials 2015;66:29–40. [DOI] [PubMed] [Google Scholar]
- 92.Maria OM, Maria O, Liu Y et al. Matrigel improves functional properties of human submandibular salivary gland cell line. Int J Biochem Cell Biol 2011;43:622–631. [DOI] [PubMed] [Google Scholar]
- 93.Gao Z, Wu T, Xu J et al. Generation of bioartificial salivary gland using whole-organ decellularized bioscaffold. Cells Tissues Organs 2014;200:171–180. [DOI] [PubMed] [Google Scholar]
- 94.Ogawa M, Oshima M, Imamura A et al. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun 2013;4:2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pradhan-Bhatt S, Harrington DA, Duncan RL et al. A novel in vivo model for evaluating functional restoration of a tissue-engineered salivary gland. Laryngoscope 2014;124:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]