Abstract
BACKGROUND:
Cyclopropyl-methoxycarbonyl metomidate (CPMM) is a “soft” etomidate analogue currently being developed as a propofol alternative for anesthetic induction and maintenance.
METHODS:
We compared the potencies of CPMM and propofol by assessing their abilities to directly activate α1(L264T)β3γ2 gamma-aminobutyric acid type A (GABAA) receptors and induce loss of righting reflexes in tadpoles. We also measured the rates of encephalographic recovery in rats after CPMM and propofol infusions ranging in duration from 5 to 120 minutes.
RESULTS:
CPMM and propofol activate GABAA receptors and induce loss of righting reflexes in tadpoles with respective 50% effective concentrations (EC50s) of 3.8 ± 0.4 and 3.9 ± 0.2 μM (GABAA receptor) and 2.6 ± 0.19 and 1.3 ± 0.04 μM (tadpole). Encephalographic recovery after prolonged infusion was faster with CPMM and lacked propofol’s context sensitivity.
CONCLUSION:
CPMM and propofol have similar potencies in GABAA receptors and tadpoles; however, CPMM provides more rapid and predictable recovery than propofol, particularly after prolonged infusion. (Anesth Analg 2014;118:563–7)
Propofol and etomidate are commonly administered as bolus injections to induce anesthesia at the start of surgery. Propofol is also often continuously infused in combination with opiates, benzodiazepines, or other sedative hypnotics to maintain anesthesia during surgery or to provide sedation during nonoperative procedures such as endoscopies and radiological imaging. Although etomidate was once similarly infused for anesthetic and sedative maintenance,1–3 this practice has been abandoned because it produces profound and persistent adrenocortical suppression.4–7
As a strategy for reducing the duration of adrenocortical suppression after single bolus injection or continuous infusion of etomidate, we recently developed a series of “soft” etomidate analogues.8–11 Similar to remifentanil and esmolol, these analogues contain a metabolically labile ester moiety that facilitates rapid metabolism of the drug by esterases. Among these soft etomidate analogues, cyclopropyl-methoxycarbonyl metomidate (CPMM) is currently the most promising for clinical development because its hypnotic potency is highest and it does not produce persistent adrenocortical suppression even after prolonged continuous infusion.12–14 Consequently, CPMM may emerge as an alternative to propofol, particularly for patients who are elderly or critically ill. The purpose of this study was to characterize and compare the pharmacology of CPMM and propofol at the molecular and whole animal levels to assess their actions in models of ranging complexity.
METHODS
All studies were conducted with the approval of and in accordance with rules and regulations of the Institutional Animal Care and Use Committee at the Massachusetts General Hospital, Boston, Massachusetts. Xenopus laevis tadpoles and adult female Xenopus laevis frogs were purchased from Xenopus One (Ann Arbor, MI). Adult male Sprague-Dawley rats (300–450 gm) were purchased from Charles River Laboratories (Wilmington, MA). For gamma-aminobutyric acid type A (GABAA) receptor and tadpole studies, propofol (2,6-diisopropylphenol) was purchased from SAFC Supply Solutions (Milwaukee, WI). For rat studies, propofol (10 mg/mL emulsion clinical formulation) was obtained from APP Pharmaceuticals (Lake Zurich, IL). CPMM was synthesized by Aberjona Laboratories (Beverly, MA) as previously described.8
GABAA Receptor Direct Activation Assay
Oocytes (stage 4 and 5) were obtained as previously reported11 and injected with messenger RNA encoding the α1(L264T), β3, and γ2 subunits of the human GABAA receptor (5 ng messenger RNA total at a subunit ratio of 1:1:3). We chose to study GABAA receptors harboring a mutation that enhances channel-gating efficacy because it increases anesthetic sensitivity, allowing complete concentration-response curves for direct activation to be generated for an anesthetic by using concentrations that are below its aqueous solubility limit.15 After injection, oocytes were incubated for at least 18 hours at 17°C in ND96 buffer (96 mΜ NaCl, 2 mΜ KCl, 1 mΜ CaCl2, 0.8 mΜ MgCl2, 10 mM HEPES, pH = 7.4) containing 0.1 mg/mL of ciprofloxicin, 0.1 mg/mL of amikacin, and 0.05 mg/mL of gentamicin before electrophysiological study.
All electrophysiological recordings were performed by using the whole cell 2 -electrode voltage-clamp technique with oocytes voltage clamped at −50 mV by using an Oocyte Clamp OC-725C amplifier (Warner Instruments, Hamden, CT) and perfused with ND96 buffer with 1 mM EGTA at a rate of 4 to 6 mL/min.15 Capillary electrodes were filled with 3M KCl. Buffer perfusion was controlled by using an 8-channel valve controller (Warner Instruments), interfaced with a Digidata 1322A data acquisition system (Molecular Devices, Sunnyvale, CA), and driven by a Dell personal computer (Round Rock, TX). Current responses were recorded by using Clampex 9.2 software (Molecular Devices) and processed by using a Bessel (8-pole) low-pass filter with a cutoff at 50 Hz by using Clampfit 9.2 software (Molecular Devices). Peak current amplitudes elicited by application of propofol or CPMM (in ND96 buffer containing 1 mM EGTA) were normalized to control currents elicited with 100 μM GABA in the same oocyte. Fifty percent effective concentrations (EC50s) for direct activation were calculated by fitting the concentration-mean response data to a Hill equation with minima and maxima constrained to 0% and 100%, respectively. To assess the effect of picrotoxin (PTX) on hypnotic-activated currents, oocytes were preequilibrated with buffer containing PTX for 30 seconds before and then during hypnotic application. Control studies (i.e., no PTX) were also performed in the same oocyte. The percent inhibition by PTX was calculated as the peak hypnotic-activated current obtained in the presence of PTX normalized to the control peak hypnotic-activated current obtained in the absence of PTX.
Tadpole Loss of Righting Reflexes Assay
Group of 5 tadpoles were placed in 50 mL water buffered with 2.5 mΜ Tris HCl (pH = 7.4) containing the desired concentration of propofol or CPMM added from stock solutions in dimethyl sulfoxide. The final dimethyl sulfoxide concentration (0.01%) was at least 60 times lower than that which produces loss of righting reflex (LoRR) in tadpoles.16 With the use of an observer who was blinded to hypnotic identity and concentration, tadpoles were tipped every 5 minutes with a flame-polished pipette until the response stabilized (typically 20–30 minutes) and determined to have LoRR if it failed to right itself within 5 seconds after being turned supine. Each drug’s EC50 for LoRR was determined from the concentration-dependence of LoRR by using a Hill equation with minima and maxima constrained to 0% and 100%, respectively.
Electroencephalographic Burst Suppression Ratio Recovery After Closed-loop Infusions to Rats
Propofol (10 mg/mL emulsion) and CPMM (5 mg/mL in 10% propylene glycol) were administered IV (through either a central venous catheter preimplanted by the vendor or a catheter placed in a tail vein) in a background of 1% isoflurane by using a closed-loop infusion system with burst suppression ratio (BSR) feedback as previously described.10,13,14 In this approach, the BSR is measured during each 6-second epoch and the infusion rate automatically adjusted by the computer software to maintain a target BSR value. In the current studies, we used a target BSR value of 80%. To prevent inadvertent overdosing, the maximum infusion rate was limited to 4 mg/kg/min for propofol and 3 mg/kg/min for CPMM. A minimal infusion rate of 0.1 mg/kg/min was also used for both hypnotic agents. The BSR was recorded for 5 minutes before beginning closed-loop hypnotic infusion and then until the BSR recovered to the baseline value after the infusion was completed. The time-dependent increase in BSR on infusion initiation and its subsequent decrease on infusion termination was fit to a biphasic sigmoidal equation.14 From the fit, we calculated the 90% BSR recovery time, which was defined as the time from infusion termination until the time when the BSR decreased 90% toward the postinfusion baseline value.
Statistical Analysis
All data are reported as mean ± SD. Curve fitting and statistical analyses were performed by using Igor Pro 6.1 (Wavemetrics, Lake Oswego, OR) and Prism (Graphpad Software, La Jolla, CA). For multiple comparisons, we performed a 2-way analysis of variance followed by a Tukey multiple comparisons test.
RESULTS
Direct Activation of GABAA Receptors
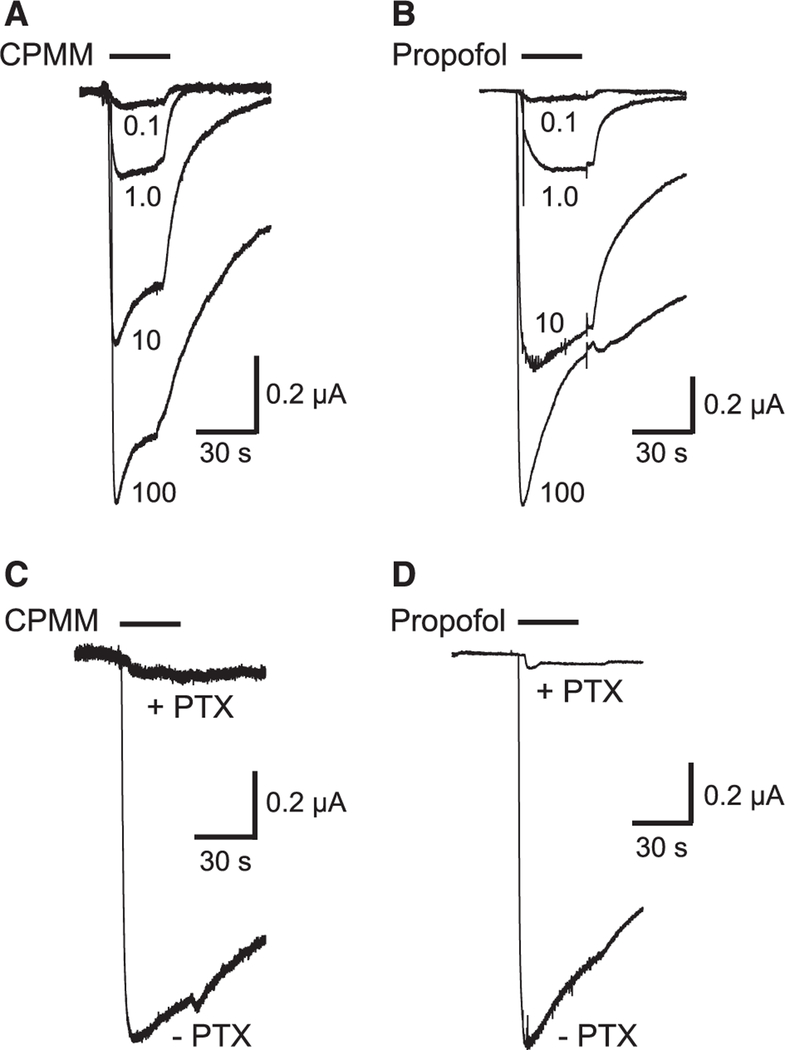
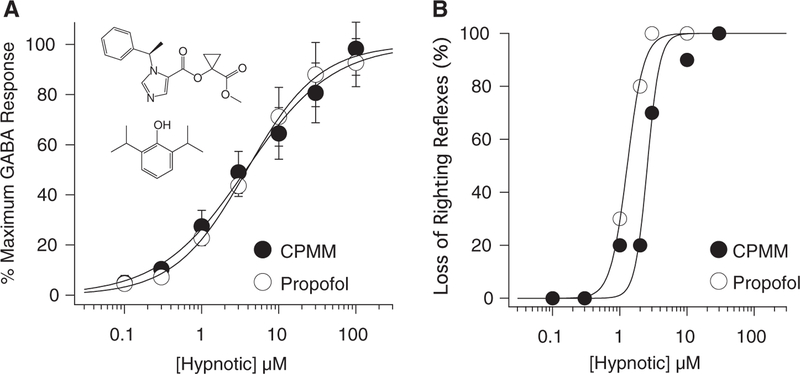
CPMM and propofol directly activated α1(L264T)β3γ2 GABAA receptors in a concentration-dependent manner and over a similar concentration range (Fig. 1, A and B). At the highest concentration studied (100 μM), both hypnotics elicited currents that had amplitudes that approximated those elicited by a maximally activating concentration of GABA (100 μM). The GABAA receptor antagonist PTX (2 mM) inhibited currents activated by 100 μM CPMM and propofol by 95.6% ± 2.7% and 97.4% ± 1.7%, respectively (Fig. 1, C and D, N 6 oocytes/hypnotic), confirming that hypnotic-activated currents were mediated by GABAA receptors. A fit of the CPMM and propofol concentration-response curves for direct activation to a Hill equation yielded EC50s of 3.8 ± 0.4 and 3.9 ± 0.2 μM (P = 0.8222) and Hill coefficients of 0.77 ± 0.06 and 0.93 ± 0.03, respectively (Fig. 2A).
Figure 1.
Hypnotic activation of α1(L264T)β3γ2 gamma-aminobutyric acid (GABA) type A receptors. A, Representative traces obtained by using a single oocyte on application of cyclopropyl-methoxycarbonyl metomidate (CPMM) at concentrations ranging from 0.1 to 100 μM. B, Representative traces obtained by using a single oocyte on application of propofol at concentrations ranging from 0.1 to 100 μM. C, Representative traces obtained by using a single oocyte on application of 100 μM CPMM in the absence or presence of 2 mM picrotoxin (PTX). D, Representative traces obtained by using a single oocyte on application of 100 μM propofol in the absence or presence of 2 mM PTX.
Figure 2.
A, Hypnotic concentration-response curves for direct activation of α1(L264T)β3γ2 gamma-aminobutyric acid (GABA) type A receptors. The amplitudes of currents activated by hypnotics were normalized to those activated by 100 μM GABA. Each data point is the mean (± SD) of 6 experiments. The curves are fits of the cyclopropyl-methoxycarbonyl metomidate (CPMM) and propofol datasets to a Hill equation yielding EC50s of 3.8 ± 0.4 and 3.9 ± 0.2 μM, respectively. The inset shows the structures of CPMM (top) and propofol (bottom). B, Hypnotic concentration-response curves for loss of righting reflexes in tadpoles. Each data point was obtained by using 10 tadpoles. The curves are fits of the CPMM and propofol datasets by using a Hill equation yielding EC50s of 2.6 ± 0.19 and 1.3 ± 0.04 μM, respectively.
LoRR in Tadpoles
CPMM and propofol produced LoRR in tadpoles in a concentration-dependent manner and over a similar concentration range (Fig. 2B). From the CPMM and propofol concentration-response curves, the EC50s for LoRR were calculated to be 2.6 ± 0.19 and 1.3 ± 0.04 μM (P = 0.0002) and the Hill coefficients were 4.7 ± 1.7 and 3.5 ± 0.3, respectively.
Encephalographic Recovery After Continuous Infusion
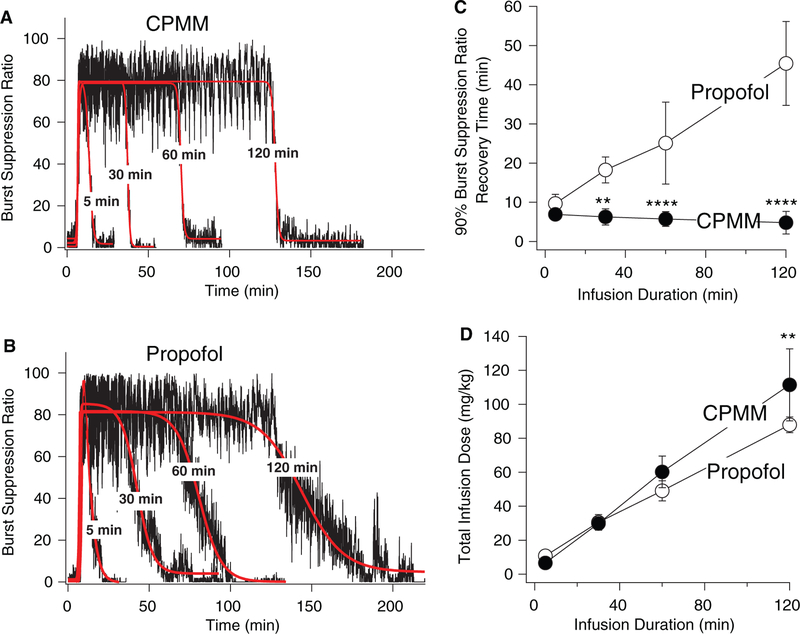
On initiating a closed-loop infusion of CPMM or propofol to rats, the BSR increased from a near-zero baseline value toward the target value of 80% and remained near that target value throughout the hypnotic infusion period. After the infusion was complete, the BSR decreased toward the baseline value. Figure 3, A and B show this hypnotic infusion-dependent change in the BSR from a series of representative experiments in which CPMM (A) or propofol (B) was infused for the indicated durations. In the individual rat experiments shown in those figure panels, the 90% BSR recovery times on termination of 5-, 30-, 60-, and 120-minute durations were 6.1, 3.6, 6.8, and 5.1 minutes with CPMM and 9.4, 19, 31, and 49 minutes with propofol. For the 2 hypnotics, Figure 3C plots the mean (± SD) 90% BSR recovery time as a function of hypnotic infusion duration (n = 5 rats/data point). With propofol infusion, the 90% BSR recovery time increased with infusion duration from 9.7 ± 2.3 minutes after 5-minute infusions and reached 45 ± 11 minutes after 120-minute infusions. In contrast, the 90% BSR recovery time failed to increase with CPMM infusion duration. For infusion durations that were 30 minutes or longer, 90% BSR recovery times were significantly shorter with CPMM than with propofol. Figure 3D shows that for each infusion duration the total hypnotic dose delivered by the closed-loop infusion system was similar for the 2 hypnotics, with a statistical difference identified only for infusions lasting 120 minutes in duration (112 ± 21 mg/kg for CPMM vs 88 ± 4 mg/kg for propofol; P < 0.01).
Figure 3.
Representative data from individual experiments showing the change in the burst suppression ratio (BSR) during closed-loop infusion of either cyclopropyl-methoxycarbonyl metomidate (CPMM) (A) or propofol (B) to rats. In each panel, 4 separate experiments having different infusion durations (5, 30, 60, and 120 minutes) are overlayed. After a 5-minute baseline period, the closed-loop infusion was started and continued for the indicated durations. The target BSR was 80% (achieved in a background of 1% isoflurane). The red curves are fits of the time-dependent BSR data to a biphasic sigmoidal equation. These fits were used to calculate the 90% BSR recovery time, which is defined as the time required for the BSR to decrease 90% toward the final baseline after infusion termination. C, BSR recovery time of 90% as a function of hypnotic infusion duration. Each data point is the mean value (± SD) from 5 experiments that were performed by using 5 different rats. Different time points were obtained by using different rats. D, Total closed-loop infusion dosing as a function of infusion duration. Each data point is the mean value (± SD) from 5 experiments that were performed by using 5 different rats. Different time points were obtained by using different rats. **, P < 0.01 vs propofol group; ****, P < 0.0001 vs propofol group.
DISCUSSION
In the current studies, we determined that CPMM directly activates GABAA receptors and induces LoRR in tadpoles. Although its GABAA receptor potency was similar to that of propofol, its tadpole potency was 2-fold lower. This difference in vivo hypnotic potency between the 2 anesthetics in spite of having similar GABAA receptor potency may reflect differences in their abilities to affect other targets (e.g., the N-methyl-d-aspartate receptor) that contribute to the hypnotic state. In previous studies, we determined that CPMM’s potency for inducing LoRR in rats after IV bolus administration was 6-fold higher than that of propofol (ED50: 0.69 ± 0.04 and 4.1 ± 0.3 mg/kg for CPMM and propofol, respectively).8,11 This apparent discrepancy with the current studies may be explained, at least in part, by differences between the 2 hypnotics in their plasma protein binding; propofol is approximately 98% protein-bound in blood, whereas etomidate (and presumably its similarly lipophilic analog CPMM) is only 75% to 80% protein-bound.17–19 Such protein binding is expected to significantly increase ED50 potency measurements in rats (but not EC50 potency measurements in tadpoles or GABAA receptors) because it decreases the free-aqueous anesthetic concentration. In addition, there may be differences between the 2 anesthetics in the contributions that GABAA receptors play in mediating hypnosis. We also found that dosing requirements in closed-loop infusion studies were similar for the 2 hypnotics. Such requirements are highly dependent on a drug’s potency and its elimination rate; anesthetics with lower potencies and faster elimination rates require higher infusion rates to maintain a desired anesthetic depth. As CPMM is significantly more potent than propofol in rats (as reflected by its lower ED50 for LoRR), it is likely that its maintenance dosing is similar to propofol because its in vivo elimination in rats is significantly faster. Electroencephalographic recovery after infusion termination was also significantly faster with CPMM than with propofol (for infusions durations 30 minutes or longer) and lacked propofol’s context sensitivity. These findings are also consistent with more rapid in vivo elimination of CPMM as compared with propofol. If similar pharmacological behavior is confirmed in humans, then CPMM may be a suitable replacement for propofol when more rapid and predictable hypnotic recovery is desired.
Acknowledgments
This research was supported by grants to DER (R01-GM087316 from the National Institutes of Health, Bethesda, MD) and JFC (K08-GM083216), and by the Department of Anesthesia, Critical Care & Pain Medicine at the Massachusetts General Hospital.
Footnotes
Name: Rile Ge, MD, PhD.
Conflict of Interest: The author has no conflicts of interest to declare.
Name: Ervin Pejo.
Conflict of Interest: The author has no conflicts of interest to declare.
Name: Hilary Gallin.
Conflict of Interest: The author has no conflicts of interest to declare.
Name: Spencer Jeffrey.
Conflict of Interest: The author has no conflicts of interest to declare.
Name: Joseph F. Cotten, MD, PhD.
Conflict of Interest: Joseph F. Cotten is an inventor on patent applications submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of cyclopropyl-methoxycarbonyl metomidate or related analogues.
Name: Douglas E. Raines, MD.
Conflict of Interest: Douglas E. Raines is an inventor on patent applications submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of cyclopropyl-methoxycarbonyl metomidate or related analogues. Douglas E. Raines is a consultant for and holds an equity position in Annovation BioPharma, a pharmaceutical company that seeks to develop technologies covered by those patents.
REFERENCES
- 1.Edbrooke DL, Newby DM, Mather SJ, Dixon AM, Hebron BS. Safer sedation for ventilated patients. A new application for etomidate. Anesthesia 1982;37:765–71 [DOI] [PubMed] [Google Scholar]
- 2.Lindeburg T, Spotoft H, Bredgaard Sørensen M, Skovsted P. Cardiovascular effects of etomidate used for induction and in combination with fentanyl-pancuronium for maintenance of anesthesia in patients with valvular heart disease. Acta Anesthesiol Scand 1982;26:205–8 [DOI] [PubMed] [Google Scholar]
- 3.Lees NW, Glasser J, McGroarty FJ, Miller BM. Etomidate and fentanyl for maintenance of anesthesia. Br J Anesth 1981;53:959–61 [DOI] [PubMed] [Google Scholar]
- 4.Watt I, Ledingham IM. Mortality amongst multiple trauma patients admitted to an intensive therapy unit. Anesthesia 1984;39:973–81 [DOI] [PubMed] [Google Scholar]
- 5.Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med 1984;310:1415–21 [DOI] [PubMed] [Google Scholar]
- 6.de Jong FH, Mallios C, Jansen C, Scheck PA, Lamberts SW. Etomidate suppresses adrenocortical function by inhibition of 11 beta-hydroxylation. J Clin Endocrinol Metab 1984;59:1143–7 [DOI] [PubMed] [Google Scholar]
- 7.Ledingham IM, Watt I. Influence of sedation on mortality in critically ill multiple trauma patients. Lancet 1983;1:1270. [DOI] [PubMed] [Google Scholar]
- 8.Husain SS, Pejo E, Ge R, Raines DE. Modifying methoxycarbonyl etomidate interester spacer optimizes in vitro metabolic stability and in vivo hypnotic potency and duration of action. Anesthesiology 2012;117:1027–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pejo E, Cotten JF, Kelly EW, Le Ge R, Cuny GD, Laha JK, Liu J, Lin XJ, Raines DE. In vivo and in vitro pharmacological studies of methoxycarbonyl-carboetomidate. Anesth Analg 2012;115:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotten JF, Le Ge R, Banacos N, Pejo E, Husain SS, Williams JH, Raines DE. Closed-loop continuous infusions of etomidate and etomidate analogs in rats: a comparative study of dosing and the impact on adrenocortical function. Anesthesiology 2011;115:764–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotten JF, Husain SS, Forman SA, Miller KW, Kelly EW, Nguyen HH, Raines DE. Methoxycarbonyl-etomidate: a novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology 2009;111:240–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge R, Pejo E, Cotten JF, Raines DE. Adrenocortical suppression and recovery after continuous hypnotic infusion: etomidate versus its soft analogue cyclopropyl-methoxycarbonyl metomidate. Crit Care 2013;17:R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pejo E, Ge R, Banacos N, Cotten JF, Husain SS, Raines DE. Electroencephalographic recovery, hypnotic emergence, and the effects of metabolite after continuous infusions of a rapidly metabolized etomidate analog in rats. Anesthesiology 2012;116:1057–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge R, Pejo E, Husain SS, Cotten JF, Raines DE. Electroencephalographic and hypnotic recoveries after brief and prolonged infusions of etomidate and optimized soft etomidate analogs. Anesthesiology 2012;117:1037–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge RL, Pejo E, Haburcak M, Husain SS, Forman SA, Raines DE. Pharmacological studies of methoxycarbonyl etomidate’s carboxylic acid metabolite. Anesth Analg 2012;115:305–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Enantioselectivity of steroid-induced gamma-aminobutyric acidA receptor modulation and anesthesia. Mol Pharmacol 1996;50:1581–6 [PubMed] [Google Scholar]
- 17.Servin F, Desmonts JM, Haberer JP, Cockshott ID, Plummer GF, Farinotti R. Pharmacokinetics and protein binding of propofol in patients with cirrhosis. Anesthesiology 1988;69:887–91 [DOI] [PubMed] [Google Scholar]
- 18.Gin T, Yau G, Jong W, Tan P, Leung RK, Chan K. Disposition of propofol at cesarean section and in the postpartum period. Br J Anesth 1991;67:49–53 [DOI] [PubMed] [Google Scholar]
- 19.Meuldermans WE, Heykants JJ. The plasma protein binding and distribution of etomidate in dog, rat and human blood. Arch Int Pharmacodyn Ther 1976;221:150–62 [PubMed] [Google Scholar]