Abstract
To best promote animal wellbeing and the efficacy of biomedical models, scientific, husbandry, and veterinary professionals must consider the mechanisms, influences, and outcomes of rodent thermoregulation in contemporary research environments. Over the last 2 decades, numerous studies have shown that laboratory mice and rats prefer temperatures that are several degrees warmer than the environments in which they typically are housed within biomedical facilities. Physiologic changes to rodents that are cage-housed under standard temperatures (20 to 26 °C) are attributed to ‘cold stress’ and include alterations in metabolism, cardiovascular parameters, respiration, and immunologic function. This review article describes common behavioral and physiologic adaptations of laboratory mice and rats to cold stress within modern vivaria, with emphasis on environmental enrichment and effects of anesthesia and procedural support efforts. In addition, potential interventions and outcomes for rodents are presented, relative to the importance of repeating and reproducing experiments involving laboratory rodent research models of human disease.
Abbreviations: BAT, brown adipose tissue; LCT, lower critical temperature; NST, nonshivering thermogenesis; TNZ, thermoneutral zone
Thermal Biology and Thermoregulation in Laboratory Mice
The thermal biology of laboratory mice encompasses a robust, dynamic, and multifaceted mixture of behavior and physiology. Physical and physiologic adaptations provide the remarkable capacity for mice to survive in temperatures as low as 4 °C and as high as 43 °C.54,89 Comprehension of these complex systems necessitates a clear definition and solid understanding of the murine thermoneutral zone (TNZ), which is the range of temperatures across which the resting metabolic rate of heat production is at equilibrium with the animal's evaporative heat loss to the surrounding environment.14,54
Within the TNZ, animals can maintain stable core body temperatures by responsive behaviors, peripheral vessel diameter, and body postures.54 The overall mouse TNZ is bound by the lower and upper critical temperature limits, beyond which mice must engage in heating or cooling adjustments, respectively; further definition of these critical temperatures is provided in a glossary of terms for thermal physiology.22 TNZ is determined by body size and weight, morphology, condition, and resting metabolic rate and is particularly narrow in mice, spanning just 1 to 3 °C, because of a large surface-to-volume ratio and meager body insulation (for example, body hair).54,74,120 These responses to the ambient environment lead to dramatic increases in metabolic rate and alterations in thermal profiles (Figure 1).14,54 Long-term (chronic) cold-induced exposures for mice often alter experimental results, described across multiple disciplines.8,10,27,92,118,129 As a result, the biomedical scientific community has asserted the need to account for and better support the thermal biology of mice,35,40,65,75,92 although dissenting opinions on this matter have been expressed.127
Figure 1.
Calculated resting metabolic rates as a function of body weight and an assumed constant core temperature of 36 °C. Arrows represent the corresponding lower critical limit at various body weights. The slope below the lower critical temperatures (outlined in dashed colored lines) are a function of whole-body thermal conductance that is directly proportional to the animal‘s surface area:mass ratio and inversely proportional to insulation. Note that the metabolic rate increases at colder temperatures and the spread between body weights narrows at warmer temperatures. Dotted horizontal lines continue beyond the calculated upper critical limit. Reprinted with permission from reference 54.
Unlike many large endotherms, mice do not have stable core temperatures. Their body temperature oscillates over short bursts of approximately 1 °C even within the TNZ. Mice also show circadian fluctuations in their core temperatures and sleep patterns at standard housing temperatures: mice in barren caging conditions at 23.5 °C maintain a core body temperature of 36.2 °C during the light cycle and 37.5 °C during the dark cycle.48,72 When provided with deep bedding for nesting, light cycle core temperatures increase to an average 37.2 °C, while the dark cycle temperatures remain at 37.5 °C.48 The mouse's core temperature and related physiologic state should not be attributed to a static number but instead should be viewed as a dynamic value dependent on environmental context. Over many generations of exposure to particular conditions, mice acclimate through the development of anatomic differences based on their rearing temperatures. Mice raised in colder environments grow significantly shorter tails55 and ears,4 have longer fur for increased insulation,4,60 develop larger livers and kidneys55 and bones,3 and have larger deposits of brown adipose tissue (BAT) with increased thermogenic capacity.63,89 The evolutionary strategy of energy conservation through environmental responsiveness and dynamic oscillation in core temperature has earned mice the description of being ‘opportunistic’ endotherms rather than ‘true’ endotherms.54 Compared with its cold adaption, the mouse's ability to adjust to excessive heat stress is quite limited. Murine hyperthermic housing conditions tend to be less relevant to contemporary vivarium conditions; therefore, this review focuses on hypothermic adaptations of laboratory mice and rats.
Behavioral Thermoregulation in Mice
Behavior is the preferred thermal adaption of mice 37,54 and is generally geared to minimize energy expenditures.49 Typically, behavioral adaptations precede various physiologic responses that serve to increase body heat (for example, thermogenesis.)54 Behavioral thermoregulation centers around sustaining metabolic heat through mechanisms of thermotaxis, nest building, and postural changes, like huddling.37,54 Thermotaxis is the action of moving toward a warmer environment; mice spend the majority of their time in the warmest environment available, up to the upper critical temperature.37,38,40 Thermal preference of mice varies greatly over the circadian cycle: during the light phase, a period dominated by sedentary behaviors (for example, a predominance of sleep), mice prefer 30 to 32 °C; during the dark phase, when physical activity peaks, mice select ambient temperatures as low as 26 °C. For mice, the average preferred temperature range over a 24-h cycle is 27.7 to 28.6 °C.49,50,56
Spontaneous activity within the cage is temperature dependent, with physical activity increasing as temperatures decrease. This pattern suggests that increased activity, especially during a normally less-active period like the light phase, serves as an additional mechanism of heat production.130 Shelters (for example, plastic domes, paper huts) and nesting materials (pressed-cotton pads, paper strips) provide insulation that allows mice to behaviorally maintain warmer environments (Figure 2) and to manipulate those environments to achieve the desired ambient temperature.34,36,37 In addition, shelters and nests reduce the biologic energy costs of maintaining physiologic homeostasis. For example, providing nesting material (to support a warmer environment) reduces food consumption,102 increases body weight,73 blunts thermotaxis,38 and improves breeding performance.41
Figure 2.
Mean radiated temperature is plotted against the distance from the center of a nest. Significant differences between treatments are indicated by filled circles for BALB/c mice and open triangles for CD1 mice. Reprinted with permission from reference 38.
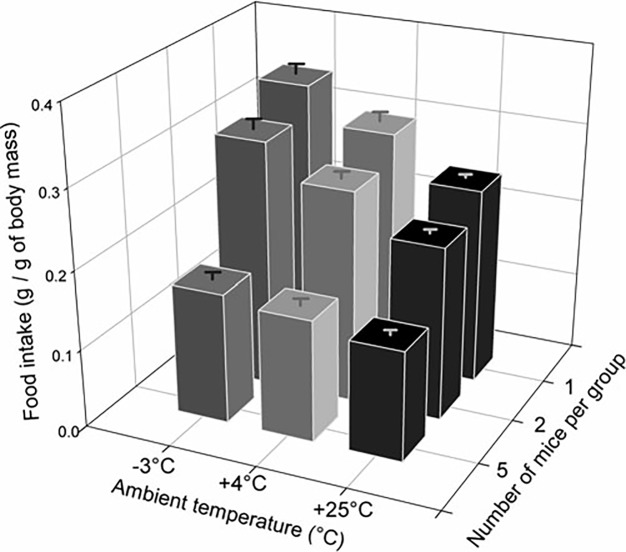
When faced with a colder environment or aversive drafts from air change cycles within ventilated housing cages, mice often adjust their posture into a hunched spheroid shape to effectively limit exposed body surface area and may demonstrate piloerection, the muscular contraction of the skin that leads to protrusion of hairs.18 Additional postural alterations are considered to be part of socially huddling, that is, “active and close aggregation of animals.”42 Huddling is a well-preserved thermoregulatory behavior seen in small mammals that serves the dual purpose of reducing the individual's exposed surface area by approximately 35% while maximizing heat-sharing for the grouped animals.11,40,56 When housed together over prolonged periods, groups of mice show reduced BAT mass, energy expenditure, and feed consumption as environmental temperatures rise (Figure 3).42,64 In time-budget studies, social huddling is the most common thermal activity of mice; unsurprisingly, time spent in the huddle and size of the huddle increases in cooler environmental temperatures, whereas social huddling is nearly absent at TNZ temperatures.6,11,64
Figure 3.
Food intake (g of feed intake/g of body mass; mean ± SEM) of mice housed individually or in a group and exposed to ambient temperatures of –3 °C, +4 °C, or +25 °C. The modified figure is reprinted with permission from reference 42.
Physiologic Responses: Peripheral Vasoconstriction and Thermogenesis in Mice
Heat loss occurs primarily at the animal's extremities (legs, tail), which have a high surface area-to-volume ratio.64 When mice are exposed to cold, peripheral blood flow to the tail and paws is reduced as a mechanism to diminish body heat loss to the environment (Figure 4).24,30 The ultimate benefits of peripheral vasoconstriction in mice, when compared with larger species, are limited due to their low body mass.109
Figure 4.
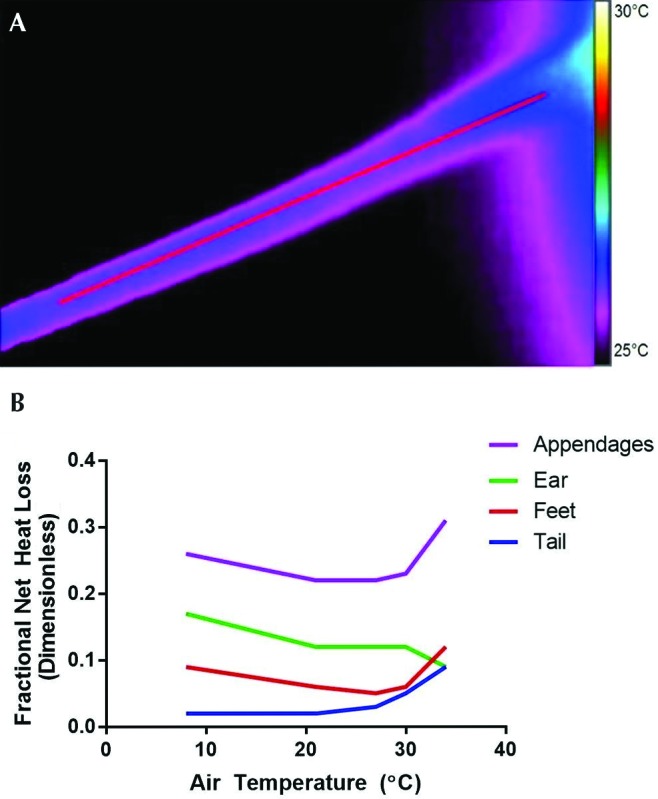
(A) Thermal image of a mouse tail at an ambient temperature of 21 °C, with a colored scale of temperature. The tail base is to the right of the image. (B) Fractional net heat loss as a function of air temperature; this illustration demonstrates the central role of temperature-dependent vasoconstriction and dilation in the tail and paws—but not ears—in thermal conservation by a similarly sized mouse species (Peromyscus maniculatus). Modified with permission from reference 24.
When other energy-conserving adaptations are overwhelmed, endotherms increase metabolic heat production through thermogenesis—the physiologic process of generating additional heat above the basal metabolic rate.69 Thermogenesis can be divided into 2 subtypes: shivering and nonshivering. Shivering thermogenesis is the product of rhythmic contraction of skeletal muscle; it plays an important thermoregulatory role in large adult mammals.104 In theory, adult mice use shivering thermogenesis only when abruptly exposed to extreme cold; even then, their small muscle mass makes shivering thermogenesis relatively ineffective, and mortality rates may be high.89 Therefore, neonatal mammals and adult small rodents primarily depend on nonshivering thermogenesis (NST) for maintaining core body temperatures.14,50,53,54 NST takes place in BAT, also known as brown fat.82,103 The capacity for NST can be extended through recruitment of white adipocytes, which when stimulated can develop a BAT-like phenotype referred to as beige fat, recruited BAT, or Brite fat.45
Brown fat is a misnomer, given that BAT is closely related to skeletal muscle tissue.123 BAT is rich in mitochondria with a high capacity for oxidative metabolism,18 due to high concentrations of uncoupling protein 1 (UCP1), a mitochondrial protein that dissipates the proton gradient, thus generating heat.121 The largest deposit of BAT in mice is located in the intrascapular region. NST is under the control of thyroid hormone, adrenergic receptors,17,79 catecholamine-producing macrophages,101 the CNS,103 and direct sympathetic neuron innervation.99 Under cold conditions, catecholamines stimulate BAT cells to produce NST.79 Simultaneously, with cold exposure, bloodflow to BAT increases and can account for as much as 40% of the rodent total cardiac ejection fraction.30 The blood supplied to BAT subsequently is warmed and returned to the general circulation for redistribution to organs to maintain core temperature; warmed blood is minimally returned to peripheral sites, such as the tail, due to simultaneous peripheral vasoconstriction.110 BAT exhibits the capacity for adaptive and inducible thermogenesis, with the ability to increase thermogenic capacity over time through mitochondrial expansion and increased UCP1 levels. With just 2 to 8 wk of conditioning, mice can be maintained in 4 °C experimental housing conditions, an accomplishment that can be attributed to both the facultative and adaptive properties of BAT.15
Thermal Biology and Thermoregulation in Laboratory Rats: Comparison with Mice
Rats continue to be used in a variety of physiologic, pharmacologic, and toxicologic studies in which changes in thermoregulation are the main point of interest.113 Fortunately, a long-standing and well-detailed body of literature exists on the thermal physiology of laboratory rats.50,53,57 Although numerous aspects of thermal biology are similar among various species of rodents, researchers should be cognizant of the key differences in thermoregulatory responses between laboratory mice and rats.
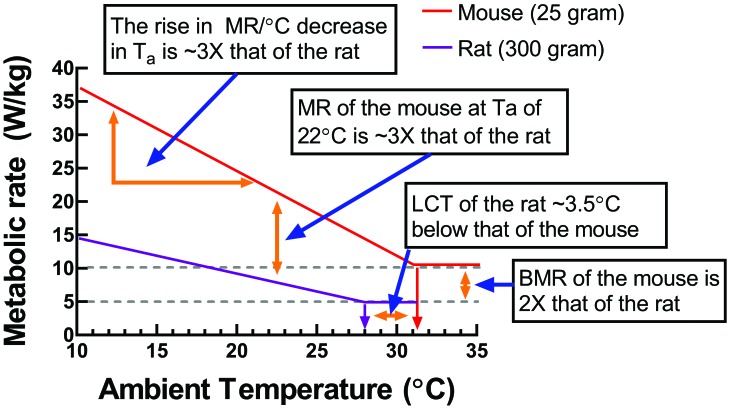
The thermoneutral profile—the relationship between ambient temperature and metabolic rate—is determined by measuring rates over a wide range of temperatures and is one of the most conventional methods used to study the thermoregulatory sensitivity of endotherms. To illustrate the salient differences in thermoregulatory sensitivity between laboratory mice and rats, thermoregulatory data for a typical 25-g mouse and 300-g rat were compiled and demonstrate the effects of changes in ambient temperature on the metabolic rate at temperatures within and below the TNZ of these species (Figure 5). This figure illustrates differences in metabolic sensitivity, given that rats—primarily due to their larger body mass, reduced thermal conductance, and greater insulation—have a lower basal metabolic rate and smaller rise in metabolic rate per 1 °C decrease in ambient temperature compared with mice. It is important to note that basal metabolic rate in Figure 5 is normalized to body mass (that is, W/kg). When metabolic rate is not normalized to body mass (that is, W), the metabolic rate of rats is approximately 50 times higher than that of mice. In rodent thermoregulatory studies, basal metabolic rate and resting metabolic rate typically are normalized to body mass.50,69
Figure 5.
A comparison of the metabolic-ambient temperature of a typical laboratory rat (body weight, 300 g) and laboratory mouse (25 g) using a summary of data (see references 50, 53, and 54). A general regression analysis of ambient temperature compared with metabolic rate (MR) normalized to body mass is presented. Four salient features of the metabolic sensitivity to changes in ambient temperature are illustrated (see text boxes). The lowest text box shows that the basal (or minimal) metabolic rate at thermoneutrality of the mouse is approximately double that of the rat. The second box shows that the lower critical temperature (LCT) at which metabolic rate (MR) must increase above basal levels to maintain a balance between heat loss and heat production, which is 31.5 °C for mice and 28 °C for rats (under conventional calorimeter conditions). The third text box shows that the slope of the line below the LCT for the mouse is approximately 3 times greater than for rats, consistent with mice being less insulated than rats. The uppermost text box shows that, considering the differences in basal metabolic rate (BMR) and slope of the lines below the LCT, at a housing room temperature of 22 °C, the metabolic rate of mice (normalized to body mass) is approximately 3 times that of rats.
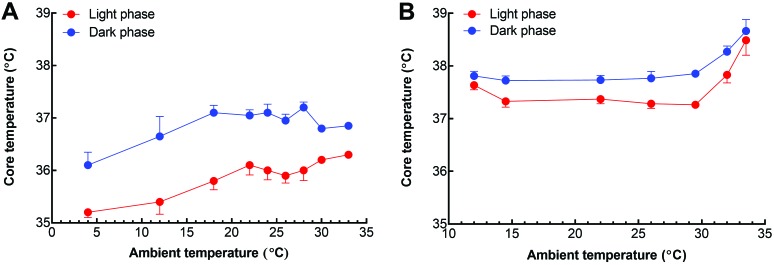
In addition, the temperature limits of normothermia are an important means for comparing and contrasting the thermoregulatory efficacy of different species. The limit of normothermia is a measure of how effectively endotherms maintain a stable core temperature during a decrease or increase in ambient temperature.50 Comparing the limits of normothermia by using radiotelemetry is an ideal method for measurement of core body temperature (Figure 6) across species and strains and over prolonged time periods; after surgical implantation of transmitters, this technique is fairly noninvasive and does not require animal restraint for data collections.1,139 In one study, C57BL/6 mice were notably better at maintaining a stable core temperature at ambient temperatures above the lower critical temperature (LCT) than were Long–Evans rats.1 More precisely, when ambient temperature rose above the LCT of rats (equivalent to 28 °C), rats showed an abrupt elevation in core temperature during both day and night cycles. However, mice began to show signs of hypothermia at ambient temperatures below 18 °C.1 In contrast, the core temperature of rats remains relatively stable and can increase with prolonged exposure at an ambient temperature of approximately 12 °C.139 Overall, the simple physical differences between mice and rats explain some of the variability in their limits of normothermia. The 10-fold increase in body mass, which is associated with lower thermal conductance, increased thermal inertia, and improved insulation, is likely a key mechanism that enables rats to maintain a normal core temperature when exposed to cold. However, these same physical differences may allow mice to thermoregulate more effectively at temperatures above the LCT.
Figure 6.
Comparison of the ambient temperature limits of normothermia during the day and night cycles for laboratory rats and mice, as monitored by radiotelemetry. Both (A) C57BL/6 mice and (B) Long–Evans rats exhibit higher core temperatures during the night, but the overall temperature of the rats is approximately 1 °C higher than that of the mice. Mice are unable to maintain a stable core temperature below an ambient temperature of 18 °C, whereas rats are notably better adapted to cold and overcompensate with a hyperthermic response at 12 °C. Interestingly, mice are better suited to stable core temperatures with exposure to warmer ambient temperatures, only until a clearly delineated point of thermoregulatory failure in rats beginning at approximately 30 °C. Data for mice are from reference 1; data for rats are from reference 139.
Behavioral Thermoregulation in Rats
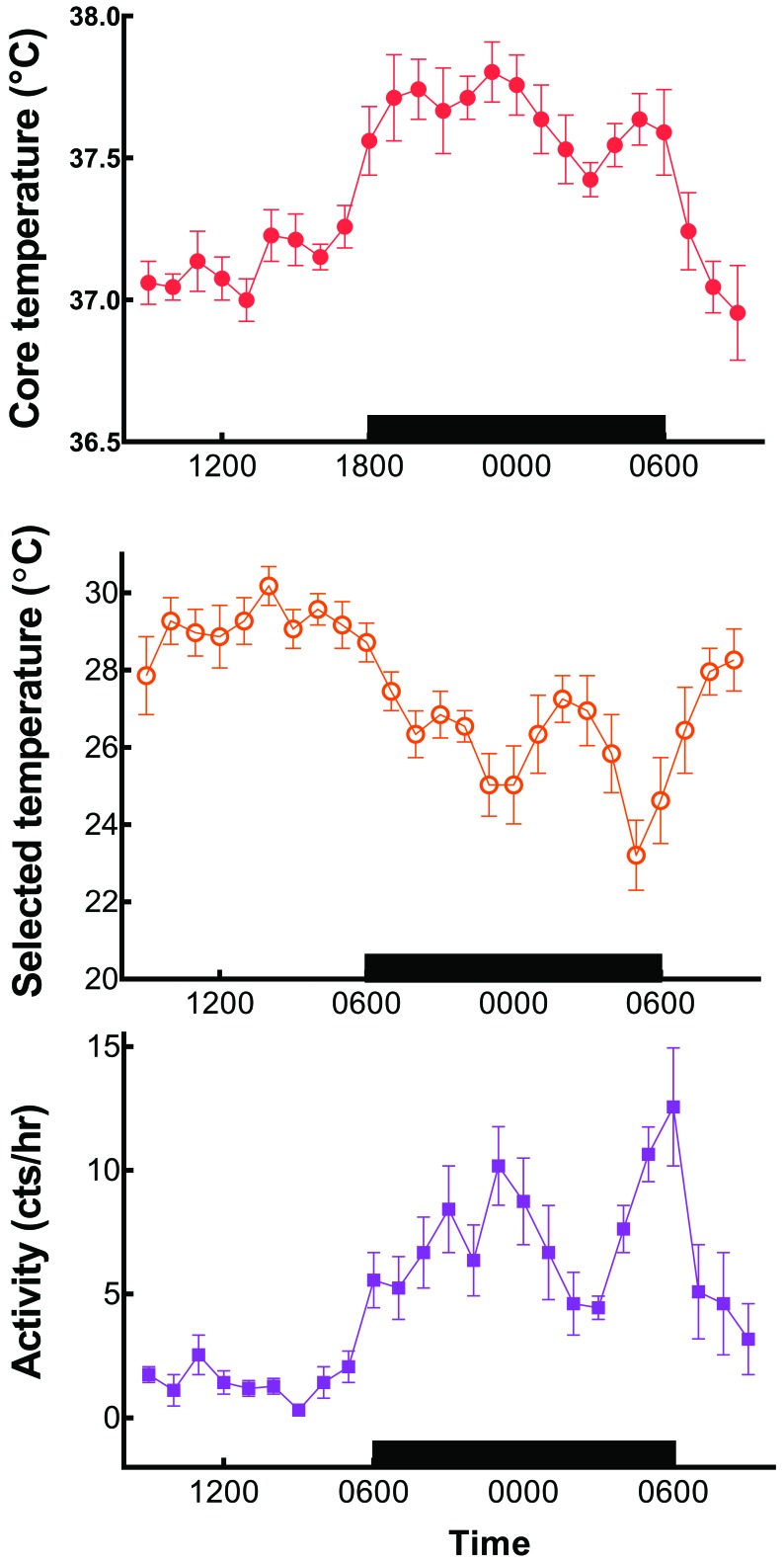
Compared with mice, rats placed in a temperature gradient require much longer to adapt to the conditions of the novel environment.57 However, when fully adapted overnight in a temperature gradient, individual rats will select a light-phase temperature of 28 to 30 °C, which is closely associated with their LCT (Figure 7). Toward the end of the light phase, rats show a slight anticipatory decrease in the selected ambient temperature and then a marked reduction during the dark phase. As that preferred temperature is decreasing, core temperature and motor activity are increasing. The preferred ambient temperature of rats reaches a nadir of 22 °C during the last hour of the dark phase, coinciding with a secondary peak of increased core temperature and motor activity.51
Figure 7.
Time course of core temperatures monitored by radiotelemetry. Selected ambient temperature and motor activity (mean ± SEM) of individual Long–Evans rats housed in a temperature gradient. Note that selection of a warm ambient temperatures during the daytime correlates with a low core temperature and minimal motor activity. Increased activity and elevated core temperature at night (black bar) are associated with preference of much cooler selected temperatures on the gradient. Modified from reference 51.
Rats prefer an ambient temperature that is approximately 6 °C above the standard temperature (20 to 26 °C) of the modern vivarium; however, these gradient studies were performed with individual rats in cages without bedding material.51 Furthermore, rearing neonatal rats at temperatures of 18 to 23 °C induces permanent developmental alterations in the capacity for stimulation of BAT.99 Rat preferences for ambient temperatures ultimately will depend on the type of caging, type of bedding, cage density, and other microenvironmental factors.
Physiologic Responses: Peripheral Vasoconstriction and Thermogenesis in Rats
As stated previously, the extremities of rodents, including the tail, assist with thermoregulation through the dissipation of excess body heat.50,53,54 In both mice and rats, tails are well-vascularized and lack insulating fur, thus providing an avenue for regulating dry heat exchange to the environment by shunting blood flow to or away from the tail. The surface area of the rat tail makes up approximately 7% of total body surface area; 50,53 under ideal conditions of thermoneutrality, rats can dissipate approximately 25% of their total heat production through the tail.140
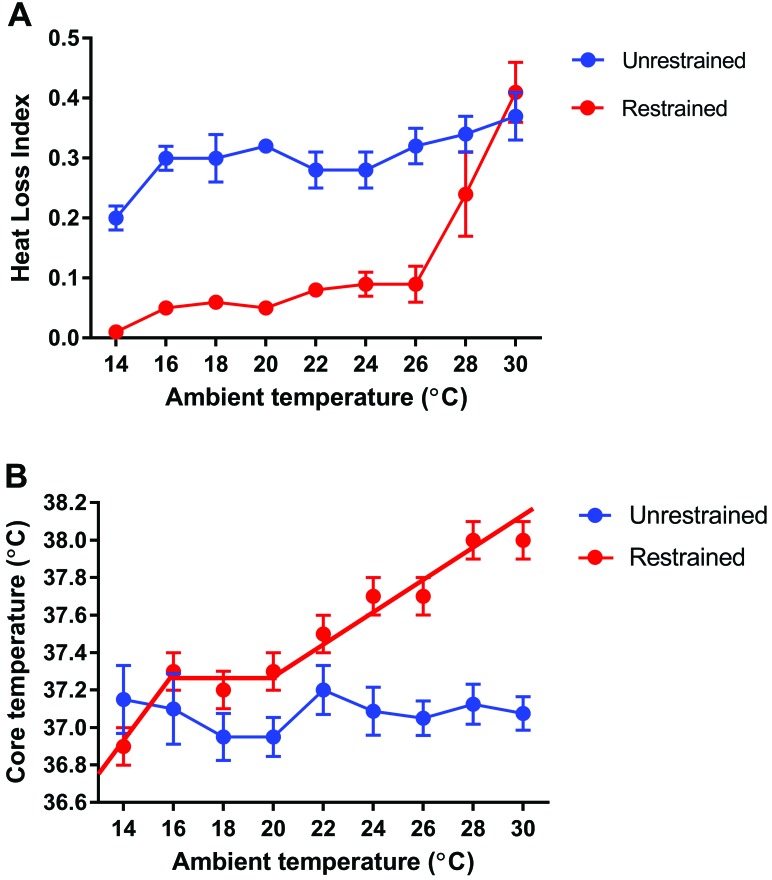
An important consideration relevant to heat dissipation is that the majority of tail vasomotor studies have been performed in restrained rodents. Early studies showed an abrupt point of tail vasodilation at an ambient temperature of approximately 26 °C in restrained rats. Physical restraint can induce stress, thus likely affecting thermoregulation and vasomotor control. A study in Brown Norway rats found that even in animals that were well adapted to a restraint device, tail vasomotor control was nonetheless compromised by the restraint procedure (Figure 8).7 Restraint led to a marked tail vasoconstriction over a wide range of ambient temperatures. Restrained rats have diminished ability to shiver when cold and to groom saliva onto their fur as means of increasing heat loss by evaporation when overheated. The net result of these effects appears to be a narrowing of the ambient temperature limits of normothermia.7
Figure 8.
Influence of 90 min of physical restraint on the (A) heat loss index (mean ± SEM) of the tail and (B) the core temperatures (mean ± SEM) of Brown Norway rats maintained at ambient temperatures of 14 to 30 °C. Note the overall reduction in heat loss, indicative of vasocontriction of blood flow to the tail around 26 °C, and the narrow limits of normothermia at ambient temperatures of 16 to 20 °C. Both graphs modified from reference 7.
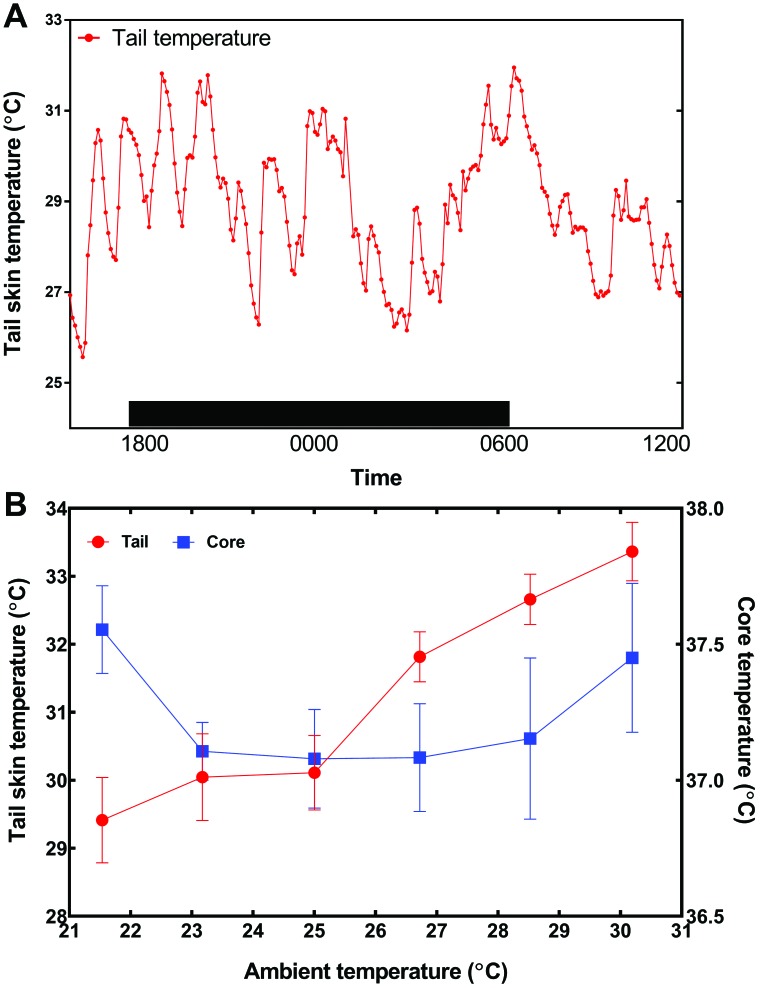
The effects of restraint on thermoregulation led to the development of a device to monitor the surface temperature of rat tails over a minimal period of 24 h.58 The device held a small telemetry transmitter over the dorsal surface of the rat tail and had a protective cap to prevent the rat from disturbing the device. When rats adapt to the device under standard vivarium conditions in a cage with provided bedding, their tail skin temperature displayed relatively large fluctuations. When the ambient temperature of the cage was gradually raised from 21.5 °C to 30.5 °C, a threshold of 25 °C was defined the point at which tail skin temperature increased abruptly, indicating vasodilation of blood flow to the tail (Figure 9).58
Figure 9.
(A) Time course of tail skin temperature (mean ± SEM) as measured remotely over 18 h at tail base by using a telemetry device. The black box indicates dark phase. (B) Effect of gradual increase in ambient temperature on the tail and core temperatures (mean ± SEM) of laboratory rats as measured by radiotelemetry. Ambient temperature increased in 2 °C increments every 2 h. Note the abrupt rise in tail temperature at 25 °C, representing peripheral vasodilation of tail. Graphs modified from reference 58.
To summarize the salient similarities and differences in thermoregulation between mice and rats, both species select a relatively warm temperature during the light phase when given the option to behaviorally thermoregulate. Both mice and rats display homeothermic patterns, with the limits of thermal stability somewhat narrower for mice than for rats. Core temperatures for rats are consistently 1 to 2 °C above that of mice, and these temperatures are subject to striking fluctuations throughout the typical photocycle, even when animals are housed under ‘ideal’ environmental conditions.54,93
Current State of Vivarium Operations
The Guide for the Care and Use of Laboratory Animals (the Guide) sets the expectations for domestic and international research animal care and is used as the primary resource for animal programs by both the NIH Office of Laboratory Animal Welfare and AAALAC.66 The Guide undergoes regular updating, with the most recent revision including substantial changes to expectations regarding ambient temperature. The 1972 and 1978 editions recommended a temperature range of 18 to 29 °C with little regard to the special needs of rodents. The 1985 edition decreased the upper limit to 26 °C for rodents but did not provide a rationale for the change beyond a reference to “reduce thermal loads caused by animals,” likely in recognition of overheating risks. The standard of 18 to 26 °C for laboratory rodents was maintained through the 1996 edition and revised in the 2011 version, raising the lower limit by 2 °C to the current range of 20 to 26 °C.66 As a practical matter, rodent vivaria typically are maintained at a subTNZ ‘room temperature’ of 20 to 23 °C, primarily for human comfort.40 Equally important to ambient temperature range, the Guide states that “nesting material and deep bedding allow mice to control their temperature and avoid cold stress during resting and sleeping,” yet this statement falls short of recommending (as a ‘should’ or ‘must’) the addition of nesting material or deeper bedding to routine housing (Figure 10).66
Figure 10.
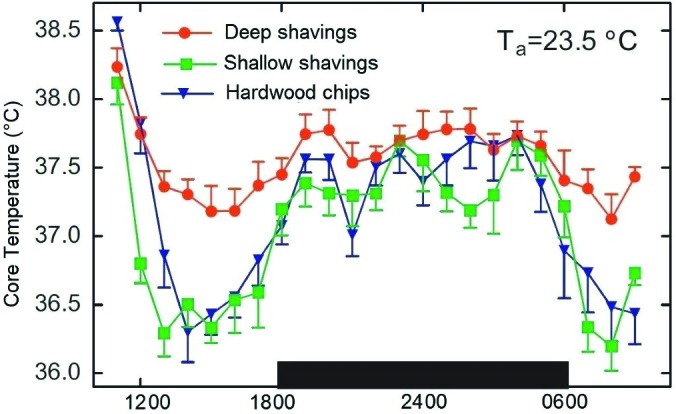
Core temperature (mean ± SEM) of telemeterized group-housed mice maintained in static cages containing hardwood chips, wood shavings, or deep wood shavings. The black box indicates dark phase. Reprinted with permission from reference 54.
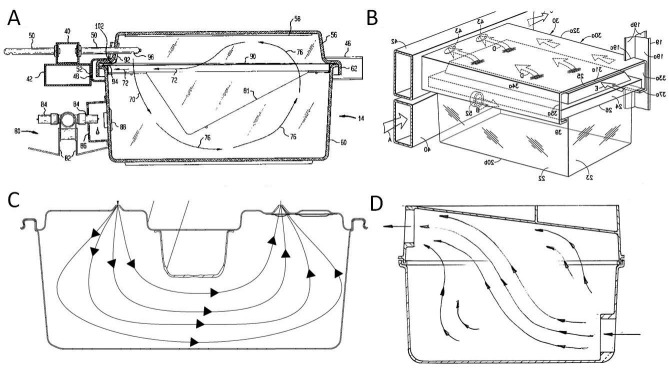
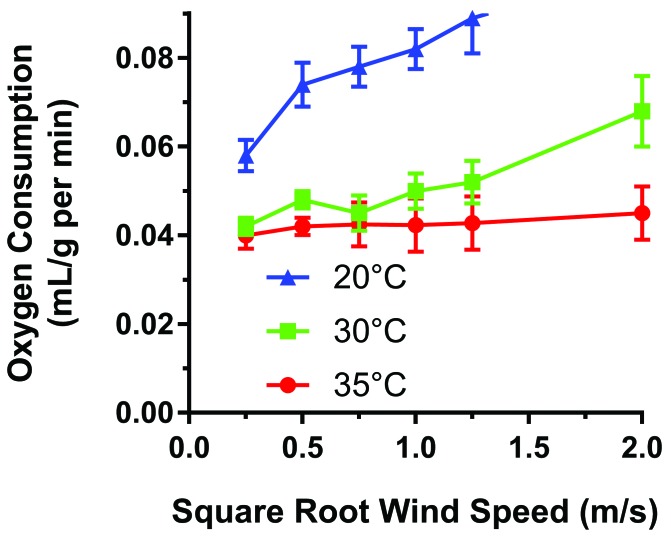
Beyond the housing room (the macroenvironment), the microenvironment inside cages can have a significant influence on thermoregulation. Cage types typically are either static (open or filter-topped), in which air exchange depends on room air exchanges, or IVC that force air exchange. Animal exposure to drafts in IVC varies depending on design features, including rack type, air changes per hour, and airflow geometry (Figure 11).20,23,68,114 Published comparative studies of the influence of IVC design on mouse physiology are rare, but a study of the influence of wind on metabolism in deer mice (Peromyscus spp.) demonstrated significant cold stress at wind speeds similar to those measured as IVC drafts (Figure 12).18 Mice have demonstrated avoidance of ventilation in preference testing; this avoidance behavior can partially be reduced by provision of nesting material.12,73 Therefore, it can be extrapolated that cold stress due to IVC drafts on laboratory rodents varies between IVC designs.
Figure 11.
Examples of (A) lengthwise cross-section of a high-supply, high-exhaust circular ventilation geometry, (B) isotropic view of low supply at the animal level with high exhaust in a cone shape, (C) cross-section high-supply, high-exhaust U-shaped design, and (D) cross-section of low-supply, high-exhaust linear supply. Fluid dynamics of ventilation are represented by arrows (not to scale between images).
Figure 12.
Oxygen consumption (mL/g/min) of deer mice at different combinations of wind speed at 3 different ambient temperatures. Vertical lines indicate 2 SE. Recreated from reference 18.
The microenvironmental cage temperature is influenced further by a diversity of available husbandry components (Figure 13) that include opaque or clear plastic, bedding substrates (for example, paper, wood, corn cob), enrichment devices and materials, number and size (age and weight) of cagemates, room light exposure, expression of phenotypes (for example, diabetic animals with increased urination may increase cage humidity levels), and cage size relative to cage density. Researchers can select the combination of these housing parameters that they believe is best for a particular model without realizing that the husbandry combinations may directly affect the consistency of core body temperatures and the expression of disease phenotypes.
Figure 13.
Husbandry and environmental parameters that can alter rodent housing temperatures and potentially influence rodent thermoregulation.
Uniformity of environmental exposures is virtually impossible for decentralized animal care organizations to accomplish; even under the best attempts to control for rodent housing conditions, unanticipated facility ‘events’ can lead to variations in environmental stability. Within carefully designed and constructed vivaria, animal housing areas nonetheless remain susceptible to seasonal weather fluctuations in which central controls of supply chillers or boilers are unable to align exactly with weather patterns and therefore inadvertently may overheat or overcool animal rooms. Due to this occasional unpredictability in HVAC functionality, room temperatures and humidity ranges may undergo swings outside of Guide parameters66 until centralized control is restored. Power outages may be caused by planned (for example, system checks) and unplanned events (extreme weather-, wind-, accident-related events), all of which are beyond the control of the animal program and contribute to inconsistent delivery of in-range vivarium temperatures, permissible humidity levels, and reliably constant housing conditions to animals.
Rodent thermoneutrality and housing conditions are increasingly recognized as contributing factors to variations in successful biomedical modeling. The management of environmental influences needs to be nimble to identify thermal ranges that will benefit specific rodent models and disease phenotypes. Providing a gradient of environmental temperatures for rodent housing is ideal, thereby allowing animals to self-thermoregulate by choosing their preferred conditions, depending on breeding status, number of cagemates, time of day, and expression of species-specific behaviors and activities.39 To date, efforts are being made in the laboratory animal industry to accommodate thermoneutrality demands, for example by raising animal room temperatures to a higher baseline and by manufacturing racks and cages that contain gradient heat sources.59
Effects of Anesthesia on Rodent Thermoregulation
The challenge of thermoregulation in rodents is complicated by the complexity of applied laboratory practices and procedures, such as anesthesia. The influence of anesthetics can result in rodents experiencing hypothermia for many reasons, including increased heat loss, decreased sensing of hypothermia by the CNS, and inhibition of compensatory thermogenic responses, with potentially dire consequences.16,124,131 Heat is primarily lost through radiation and evaporation from the skin. Anesthetic drugs commonly used in rodents, including isoflurane, result in peripheral dilation of the blood vessels, which increases heat loss that can be further exacerbated when a body cavity, such as the abdomen or thorax, is opened and exposed to the environment.28 Even the presumed innocuous act of applying surgical scrub to aseptically prepare skin for a surgical incision can have significant cooling effects in mice.125 The most common consequence of hypothermia under anesthesia is a delayed recovery to consciousness.16,34,88
The hypothalamus is the main moderator of thermoregulation in the brain, receiving and integrating afferent input from peripheral body sites. Anesthetics impart a dose-dependent suppression of hypothalamic activity, lowering the temperature at which the hypothalamus responds to hypothermia.83,96,138 Not only is loss of heat increased and detection of cold inhibited by anesthetics, but the compensatory responses to generate or preserve heat are impaired. Cerebral suppression from anesthesia inhibits sympathetic responses, resulting in decreased heart and respiratory rates and inhibition of typical catecholamine induced increases in metabolic rate and heat production through BAT stimulation. These effects are the opposite response of conscious animals placed in a cold environment, which normally experience increased metabolic, heart, and respiratory rates to maintain body temperature,16 as described previously in this review. Lastly, as hypothermic animals recover normal physiologic functions and consciousness after anesthesia, peripheral vasoconstriction, in response to low body temperature, may slow the delivery of ambient heat from external warming devices to assist with raising core temperature.
In most species tested, including humans, anesthesia-associated hypothermia causes increased risk of infection (due to decreased circulating WBC and altered immune function at the surgical site), cardiac arrhythmias (due to abnormal cardiac conduction and increased sympathetic activity on recovery), and coagulopathies (due to abnormal platelet function).31,84,112,133 Although these effects have not specifically been assessed in mice, the consistent demonstration of these signs in other species supports the critical need to maintain body temperature while rodents are anesthetized. Postanesthetic hypothermia in humans has been described as profoundly distressful25 and therefore is likely a source of distress for research animal patients as well.
Body temperature has a direct effect on the animal's response to anesthetizing agents, with hypothermia resulting in a deepened plane of anesthesia.5,71,91 The hypothermic effect is due to decreases in the rate of CNS metabolism and a potential and related decrease in the ability to metabolize particular anesthetics. The minimum alveolar concentration is the concentration of gas vapor in lungs needed to prevent movement in 50% of subjects when given a surgical stimulus; minimum alveolar concentration, as a measure, is used to compare the potency of gas anesthesia. In hypothermic animals, additional drugs (for example, opiates) that might otherwise decrease the need for gas anesthesia are less effective at reducing minimum alveolar concentration than they would be in normothermic animals.108,135
Monitoring Body Temperature and Thermogenic Support Devices
Many rodent studies have demonstrated different experimental outcomes due to differences in body temperature under anesthesia, including studies of the heart, brain, liver, and urogenital system.16,77,85,95,111,119,137 The technique used to measure body temperature is critical to the interpretation of an experiment, due to the heterogeneity of temperature in different locations of the animal's body, ranging from the temperature of the tail to the animal's true core temperature. The most traditional measurement of the core temperature is the use of direct rectal thermography by either a thermocouple or thermistor.98 An important key to the accuracy of this measurement in rodents is that the device is inserted as far as 2 cm into the rectum, such that the device is located in the colon. Inadequate insertion can result in wide variability in temperature measurements, which could misrepresent core temperatures. Consider that taking repeated rectal temperatures from the same animal will likely induce stress and potentially increase resting body temperatures. This added stressor might be ameliorated by the use of intraperitoneal telemetry devices, which noninvasively provide core body temperatures, although telemetry equipment is comparatively expensive and requires surgical placement for use. Some telemetry transponders (microchips or chips) are designed to be placed in the subcutaneous space, usually inserted between the scapula. Despite the fact that subcutaneous chips cannot measure the exact core temperature, several studies have shown a strong correlation between the core temperature and subcutaneous temperature, with readings that vary by approximately 3 °C.16,52,61,81 Placement of microchips in the interscapular area may be influenced by proximity to the location of active BAT.26 Another option for noninvasive thermometry is the use of infrared thermography, which captures the surface temperature to which the laser is directed.115 The tail temperature can be assessed as a measure of vasoconstriction and dilation,98 and the skin surface at the interscapular area can be assessed to measure BAT metabolism.26 When interpreting thermometric data, it is important to remember that the skin temperature can be distinctly different from the animal's core temperature and that the presence of hair can alter temperature readings.115
Maintaining normal body temperature in anesthetized mice presents a number of challenges. Several techniques and devices used for large animals—including forced air heaters, heating pads, and warmed intravenous fluids—are impractical or unsafe for use with rodents. The most effective techniques are the use of circulating warm-water blankets, warming lamps, and infrared heating devices. In addition, research animal patients can be supplemented with warmed fluids, delivered subcutaneously or intraperitoneally but with caution to avoid overheating or burning the animal inadvertently.16,131 The surface temperature of devices should be kept well below 45 °C and should be insulated to protect animals from direct placement on the devices and avoid the risk of thermal burns to the skin.29 Other precautions with heat provision include prevention of dessication of viscera caused by heating lamps. Furthermore, even modest elevations in body temperature can cause marked alterations in the metabolism and function of abdominal organs, potentially dramatically altering experimental outcomes.28,95 For surface heating devices, maintaining the temperature of the heating device at 37.5 °C sustains the core body temperature of an isoflurane-anesthetized mouse at 36 to 37 °C for as long as 30 min.16 Future work is necessary to test for the best methods to consistently keep the body temperature elevated in mice undergoing laparotomy or thoracotomy for an extended period of time. Several other devices have been marketed that purport to keep mice warm while anesthetized. However, reflective foil sleeves have been shown to provide no benefit unless combined with an additional device, such as thermogenic gels.16 Thermogenic gel packs can reach surface temperatures sufficiently high to cause thermal burns unless several protective layers of material are used as insulation.16 In addition, new devices and techniques of thermal support, including preoperative warming, may further improve our ability to keep rodents warm during anesthesia.116,122
Due to the variable response of mice to anesthesia, it is important—even with supplemental heat—to monitor the temperature of each individual animal during surgeries lasting longer than 10 min. When heat supplementation is either excessive or inadequate, mice can gain or lose as much as 3 °C within 10 min.16 Devices with a feedback loop, measuring the animal's temperature and the output of the warming device, are of great value in rodent surgeries, which are often performed by a single surgeon–anesthetist.
Anesthesia has similar effects on the ability to thermoregulate in rats as in mice. Furthermore, similar side effects of anesthesia can be expected in rats as occur in other species, including coagulopathies,62,70 cardiac arrhythmias,128,134 and infection.132 An important difference from mice, however, is that the larger body mass of rats lessens the rate of heat loss during anesthesia.131 Ultimately, as with all species, rats that will be anesthetized for more than a few minutes need to receive thermal support, given the loss of thermal control. The devices and mechanisms for monitoring and maintaining warmth in mice are applicable to rats as well.
Effects of Temperature on Rodent Models of Disease
Variability in model outcomes is widespread across biomedical disciplines. Recent review articles regarding how cold stress affects phenotyping,105 mouse models,75 and model translation94 have heightened discussions of housing temperatures and their effects on scientific data. Therefore, one can postulate that the phenotype of laboratory mice or rats raised at conventional housing temperatures(19 to 22 °C) is not the same—metabolically or thermally—as that of otherwise identical mice or rats raised at thermoneutrality (30 to 32 °C).94 In normotensive rats and mice, small incremental changes in ambient temperature, within a range of 18 to 30 °C, result in statistically different cardiovascular parameters for blood pressure, heart rate, pulse pressure, heart rate variability, and metabolic rate.106,129,130 As a brief example, the heart rate of a cold-stressed mouse (approximately 600 bpm) is twice that of a mouse housed at thermoneutrality129 (approximately 300 bpm), and the metabolic rate of a cold-stressed mouse is approximately 50% to 70% greater than that of a mouse housed within the TNZ.15,35,50,78,94,129
In several scientific disciplines, variability in data outcomes and blunting of disease phenotypes have been linked to effects of environmental temperatures. The presence of an intact immune response, which is known to be altered in cold-stressed mice, plays a major role in disease and treatment responses. Because providing an exhaustive review of models and environmental effects in this article is not feasible, we direct readers to the reference list for further exploration of this subject. Salient examples of thermal effects on representative research models are briefly outlined in the following paragraphs.
Graft-versus-host disease (GVHD).
Dampened immune responses at 22 °C suppress the ability of T cells to mediate GVHD, a major complication of cell transplantations. The historical conclusion has been that mice are resistant to GVHD development; however, animals studied at thermoneutral temperatures (approximately 30 °C) do show evidence of GVHD, thus refuting this conclusion.87
Tumor development.
Regardless of the cell lines being studied, tumors grow rapidly in mice housed at 20 to 26 °C (standard housing room temperatures). In contrast, for mice housed in the TNZ at 30 to 31 °C, a significant reduction in tumor growth rate occurs.80,97
To address whether the presence of tumors affects the thermal preference of mice, clinically normal nontumor-bearing mice were placed in a thermal preference apparatus that permitted individual animals to move along a gradient of chambers at 22 °C to 38 °C. These clinically healthy mice spent the majority of time in the 30 °C chamber, confirming their preferred thermoneutral environment. However, tumor-bearing mice spent most of their time in the warmest chamber available, at 38 °C. This shift in temperature preference indicated that tumor-bearing mice sought an environment approximately 16 °C warmer than the temperature at which they were routinely housed.80
Uptake of contrast media for imaging.
When mice are housed below the LCT, their brown fat and skeletal muscles are highly active to sustain body temperature. In addition, these active tissues tend to take up great amounts of molecular probes used for tumor visualization (for example, 18F-FDG), which then overshadows the imaging of tumors. However, in one study, once animals were warmed (by placing the cage on a heating pad at 30 °C), contrast uptake by BAT was reduced significantly, thus markedly improving visualization of tumor xenografts.33
Atherosclerosis.
Wildtype C57BL/6 mice were thought to be highly resistant to atherosclerosis, even when fed an obesogenic diet.44,75 However, when the mice were housed within their TNZ, allowing the immune system to function more effectively and to respond to inflammation, they developed atherosclerotic disease. In addition, obesity developed after provision of a high-fat diet.43,44
Microbiome.
The gastrointestinal microbiome (the collection of microorganisms like bacteria, viruses, fungi that live in the gastrointestinal tract) plays a critical role in numerous energy balance and health processes. In fact, soon after the arrival of rodents to their receiving institution, their vendor-established microbiome shifts due to differences in water, food, and other husbandry interventions from the originating site.32 Given that TNZ housing influences immune responses, food intake, and weight gain in rodents, it is logical to presume that environmental temperature can alter the intestinal microbiome as well.136 Cold exposure dramatically alters energy balance (as described throughout this review) and subsequent responses by the microbiome.19,47,136
LPS.
The development of hypothermia compared with fever during severe forms of inflammatory disease (induced by LPS and Escherichia coli) differs between mice and rats and impacts mortality, with hypothermia improving survival rates in rats.90,118 Hypothermic conditions were thought to be of benefit in these rat models, as cooler animals had a drop in arterial pressure, leading to increased hypotension, decreased tissue perfusion, and less damage to abdominal organ function.90
Responses to infectious pathogens.
Compared with mice housed at 22 °C, mice maintained at 28 °C displayed elevated antigen-specific T-cell responses to Francisella tularensis and survived intranasal challenge with live vaccine that was fatal to immunized mice at 22 °C. In addition, mice housed at the higher temperature were 6.8% lighter than cold-stressed mice, a difference attributed to the increased amount of food ingested at cooler housing temperatures.117 In another study, mice housed at 22° and 26 °C developed hypothermia and showed reduced locomotor activity after inoculation with influenza virus, compared with mice housed and inoculated at 30 °C.72 Monitoring body temperatures to identify hypothermia in inoculated rodents may serve as a useful humane endpoint in infectious disease studies.61
Concluding Comments
Investigators and advocacy organizations have challenged research teams to improve the details of experimental design and reporting, including published descriptions of procedures, adverse events, protocols, and unexpected variations, as well as housing, cage density, and husbandry conditions.2,9,13,21, 46,67,76,86, 100,107,126 Ultimately, a perceived ‘lack of reproducibility’ in rodent research may have much less to do with experimental failure than with variables in housing, husbandry, and thermoregulation of animals. Additional recommendations to foster reproducibility include transparency regarding communication of emergency events (for example, power outages and loss of HVAC) and outcomes, along with strategic facility planning, to support animal models that require higher environmental temperatures to fully recapitulate the human condition. With continued discussion, innovation, and creativity, resolving rodent thermoneutrality issues within housing facilities may well be the next major change in the practice of laboratory animal medicine and science.
Acknowledgements
Disclaimer: The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, US Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
References
- 1.Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML. 2015. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab 4:461–470. 10.1016/j.molmet.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhtar A. 2015. The flaws and human harms of animal experimentation. Camb Q Healthc Ethics 24:407–419. 10.1017/S0963180115000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Hilli F, Wright EA. 1983. The effects of changes in the environmental temperature on the growth of bone in the mouse. Radiological and morphological study. Br J Exp Pathol 64:43–52. [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hilli F, Wright EA. 1988. The effects of environmental temperature on the body temperature and ear morphology of the mouse. J Therm Biol 13:197–199. 10.1016/0306-4565(88)90034-4. [DOI] [Google Scholar]
- 5.Antognini JF. 1993. Hypothermia eliminates isoflurane requirements at 20 °C. Anesthesiology 78:1152–1156. 10.1097/00000542-199306000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Arakawa H, Blanchard DC, Blanchard RJ. 2007. Colony formation of C57BL/6J mice in visible burrow system: Identification of eusocial behaviors in a background strain for genetic animal models of autism. Behav Brain Res 176:27–39. 10.1016/j.bbr.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aydin C, Grace CE, Gordon CJ. 2011. Effect of physical restraint on the limits of thermoregulation in telemetered rats. Exp Physiol 96:1218–1227. 10.1113/expphysiol.2011.060301. [DOI] [PubMed] [Google Scholar]
- 8.Baccan GC, Sesti-Costa R, Chedraoui-Silva S, Mantovani B. 2010. Effects of cold stress, corticosterone, and catecholamines on phagocytosis in mice: differences between resting and activated macrophages. Neuroimmunomodulation 17:379–385. 10.1159/000292058. [DOI] [PubMed] [Google Scholar]
- 9.Baker M. 2015. Reproducibility crisis: blame it on the antibodies. Nature 521:274–276. 10.1038/521274a. [DOI] [PubMed] [Google Scholar]
- 10.Bartelt A, Bruns O, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul M, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. 2011. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17:200–205. 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 11.Batchelder P, Lynch CB, Schneider JE. 1982. The effects of age and experience on strain differences for nesting behavior in Mus musculus. Behav Genet 12:149–159. 10.1007/BF01065762. [DOI] [PubMed] [Google Scholar]
- 12.Baumans V, Schlingmann F, Vonck MA, van Lith H. 2002. Individually ventilated cages: beneficial for mice and men? Contemp Top Lab Anim Sci 41:13–19. [PubMed] [Google Scholar]
- 13.Begley CG, Ellis LM. 2012. Raise standards for preclinical cancer research. Nature 483:531–533. 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 14.Cannon B, Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359. 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 15.Cannon B, Nedergaard J. 2011. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214:242–253. 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 16.Caro AC, Hankenson FC, Marx JO. 2013. Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. J Am Assoc Lab Anim Sci 52:577–583. [PMC free article] [PubMed] [Google Scholar]
- 17.Celi FS. 2009. Brown adipose tissue—when it pays to be inefficient. N Engl J Med 360:1553–1556. 10.1056/NEJMe0900466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell MA, Holsclaw DS. 1984. Effects of wind on thermoregulation and energy balance in deer mice (Peromyscus maniculatus). J Comp Physiol B 154:619–625. 10.1007/BF00684416. [DOI] [Google Scholar]
- 19.Chevalier C, Stojanovic O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, Rigo D, Fabbiano S, Stevanovic A, Hagemann S, Montet X, Seimbille Y, Zamboni N, Hapfelmeier S, Trajkovski M. 2015. Gut microbiota orchestrates energy homeostasis during cold. Cell 163:1360–1374. 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Coiro MA, Murray DR, Lastowski PA. [Internet]. 1994. Ventilated rack and animal cage system. Google patents. [Cited 30 Sept 2018]. Available at: https://patents.google.com/patent/US5307757
- 21.Collins FS, Tabak LA. 2014. Policy: NIH plans to enhance reproducibility. Nature 505:612–613. 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Commission for Thermal Physiology of the International Union of Physiological Sciences. 2001. Glossary of terms for thermal physiology, 3rd ed. Jpn J Physiol 51:245–280. [Google Scholar]
- 23.Conger DL, Perazzo TM, Cannell A. [Internet]. 2011. Method for adjusting airflow in a rodent containment cage. Google patents. [Cited 30 Sept 2018]. Available at: https://patents.google.com/patent/US7874268B2/en
- 24.Conley KE, Porter WP. 1985. Heat loss regulation: role of appendages and torso in the deer mouse and the white rabbit. J Comp Physiol B 155:423–431. 10.1007/BF00684671. [DOI] [PubMed] [Google Scholar]
- 25.Cory M, Fossum S, Donaldson K, Francis D, Davis J. 1998. Constant temperature monitoring: a study of temperature patterns in the postanesthesia care unit. J Perianesth Nurs 13:292–300. 10.1016/S1089-9472(98)80033-6. [DOI] [PubMed] [Google Scholar]
- 26.Crane JD, Mottillo EP, Farncombe TH, Morrison KM, Steinberg GR. 2014. A standardized infrared imaging technique that specifically detects UCP1-mediated thermogenesis in vivo. Mol Metab 3:490–494. 10.1016/j.molmet.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David JM, Knowles S, Lamkin DM, Stout DB. 2013. Individually ventilated cages impose cold stress on laboratory mice: a source of systemic experimental variability. J Am Assoc Lab Anim Sci 52:738–744. [PMC free article] [PubMed] [Google Scholar]
- 28.Devey L, Festing MF, Wigmore SJ. 2008. Effect of temperature control upon a mouse model of partial hepatic ischaemia– reperfusion injury. Lab Anim 42:12–18. 10.1258/la.2007.06009e. [DOI] [PubMed] [Google Scholar]
- 29.Dunlop CI, Daunt DA, Haskins SC. 1989. Thermal burns in 4 dogs during anesthesia. Vet Surg 18:242–246. 10.1111/j.1532-950X.1989.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 30.Foster DO, Frydman ML. 1979. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol 57:257–270. 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 31.Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, Beattie C. 1997. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 277:1127–1134. 10.1001/jama.1997.03540380041029. [DOI] [PubMed] [Google Scholar]
- 32.Franklin CL, Ericsson AC. 2017. Microbiota and reproducibility of rodent models. Lab Anim (NY) 46:114–122. 10.1038/laban.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fueger BJ, Czernin J, Hildebrandt I, Tran CS, Halpern BS, Stout D, Phelps ME, Weber WA. 2006. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med 47:999–1006. [PubMed] [Google Scholar]
- 34.Fuhrman FA. 1947. The effect of body temperature on the duration of barbiturate anesthesia in mice. Science 105:387–388. 10.1126/science.105.2728.387. [DOI] [PubMed] [Google Scholar]
- 35.Ganeshan K, Chawla A. 2017. Warming the mouse to model human diseases. Nat Rev Endocrinol 13:458–465. 10.1038/nrendo.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garner JP, Gaskill BN, Weber EM, Ahloy-Dallaire J, Pritchett-Corning KR. 2017. Introducing therioepistemology: the study of how knowledge is gained from animal research. Lab Anim (NY) 46:103–113. 10.1038/laban.1224. [DOI] [PubMed] [Google Scholar]
- 37.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. 2012. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One 7:1–11. 10.1371/journal.pone.0032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaskill BN, Gordon CJ, Pajor EE, Lucas JR, Davis JK, Garner JP. 2013. Impact of nesting material on mouse body temperature and physiology. Physiol Behav 110–111:87–95. 10.1016/j.physbeh.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Gaskill BN, Garner JP. 2017. Stressed out: providing laboratory animals with behavioral control to reduce the physiological effects of stress. Lab Anim (NY) 46:142–145. 10.1038/laban.1218. [DOI] [PubMed] [Google Scholar]
- 40.Gaskill BN, Rohr SA, Pajor EA, Lucas JR, Garner JP. 2009. Some like it hot: mouse temperature preferences in laboratory housing. Appl Anim Behav Sci 116:279–285. 10.1016/j.applanim.2008.10.002. [DOI] [Google Scholar]
- 41.Gaskill BN, Winnicker C, Garner JP, Pritchett-Corning KR. 2013. The naked truth: breeding performance in nude mice with and without nesting material. Appl Anim Behav Sci 143:110–116. 10.1016/j.applanim.2012.10.009. [DOI] [Google Scholar]
- 42.Gilbert C, McCafferty D, Le Maho Y, Martrette JM, Giroud S, Blanc S, Ancel A. 2010. One for all and all for one: the energetic benefits of huddling in endotherms. Biol Rev Camb Philos Soc 85:545–569. [DOI] [PubMed] [Google Scholar]
- 43.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Graspeuntner S, Cappelletti M, Wu D, Mukherjee R, Chan CC, Lawson MJ, Klarquist J, Sunderhauf A, Softic S, Kahn CR, Stemmer K, Iwakura Y, Aronow BJ, Karns R, Steinbrecher KA, Karp CL, Sheridan R, Shanmukhappa SK, Reynaud D, Haslam DB, Sina C, Rupp J, Hogan SP, Divanovic S. 2017. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med 23:829–838. 10.1038/nm.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giles DA, Ramkhelawon B, Donelan EM, Stankiewicz TE, Hutchison SB, Mukherjee R, Cappelletti M, Karns R, Karp CL, Moore KJ, Divanovic S. 2016. Modulation of ambient temperature promotes inflammation and initiates atherosclerosis in wildtype C57BL/6 mice. Mol Metab 5:1121–1130. 10.1016/j.molmet.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giralt M, Villarroya F. 2013. White, brown, beige/Brite: different adipose cells for different functions? Endocrinology 154:2992–3000. 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- 46.Godlee F. 2014. How predictive and productive is animal research? BMJ 348:g3719 10.1136/bmj.g3719 [DOI] [Google Scholar]
- 47.Gomez de la Torre Canny S, Rawls J F. 2015. Baby, it's cold outside: host-microbiota relationships drive temperature adaptations. Cell Host Microbe 18:635–636. 10.1016/j.chom.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Gordon CJ. 2004. Effect of cage bedding on temperature regulation and metabolism of group-housed female mice. Comp Med 54:63–68. [PubMed] [Google Scholar]
- 49.Gordon CJ. 1985. Relationship between autonomic and behavioral thermoregulation in the mouse. Physiol Behav 34:687–690. 10.1016/0031-9384(85)90365-8. [DOI] [PubMed] [Google Scholar]
- 50.Gordon CJ. 1993. Temperature regulation in laboratory rodents. New York (NY): Cambridge University Press; 10.1017/CBO9780511565595 [DOI] [Google Scholar]
- 51.Gordon CJ. 1994. 24-hour control of body temperature in rats. I. Integration of behavioral and autonomic effectors. Am J Physiol 267:R71–R77. [DOI] [PubMed] [Google Scholar]
- 52.Gordon CJ. 2017. The mouse thermoregulatory system: its impact on translating biomedical data to humans. Physiol Behav 179:55–66. 10.1016/j.physbeh.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon CJ. 1990. Thermal biology of the laboratory rat. Physiol Behav 47:963–991. 10.1016/0031-9384(90)90025-Y. [DOI] [PubMed] [Google Scholar]
- 54.Gordon CJ. 2012. Thermal physiology of laboratory mice: defining thermoneutrality. J Therm Biol 37:654–685. 10.1016/j.jtherbio.2012.08.004. [DOI] [Google Scholar]
- 55.Gordon CJ, Aydin C, Repasky EA, Kokolus KM, Dheyongera G, Johnstone AF. 2014. Behaviorally mediated, warm adaptation: a physiological strategy when mice behaviorally thermoregulate. J Therm Biol 44:41–46. 10.1016/j.jtherbio.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Gordon CJ, Becker P, Ali JS. 1998. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol Behav 65:255–262. 10.1016/S0031-9384(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 57.Gordon CJ, Lee KL, Chen TL, Killough P, Ali JS. 1991. Dynamics of behavioral thermoregulation in the rat. Am J Physiol 261:R705–R711. [DOI] [PubMed] [Google Scholar]
- 58.Gordon CJ, Puckett E, Padnos B. 2002. Rat tail skin temperature monitored noninvasively by radiotelemetry: characterization by examination of vasomotor responses to thermomodulatory agents. J Pharmacol Toxicol Methods 47:107–114. 10.1016/S1056-8719(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 59.Gordon CJ, Puckett ET, Repasky ES, Johnstone AF. 2017. A device that allows rodents to behaviorally thermoregulate when housed in vivariums. J Am Assoc Lab Anim Sci 56:173–176. [PMC free article] [PubMed] [Google Scholar]
- 60.Hammel HT. 1955. Thermal properties of fur. Am J Physiol 182:369–376. [DOI] [PubMed] [Google Scholar]
- 61.Hankenson FC, Ruskoski N, van Saun M, Ying GS, Oh J, Fraser NW. 2013. Weight loss and reduced body temperature determine humane endpoints in a mouse model of ocular herpesvirus infection. J Am Assoc Lab Anim Sci 52:277–285. [PMC free article] [PubMed] [Google Scholar]
- 62.Heinius G, Hahn RG, Sonden A. 2011. Hypothermia increases rebleeding during uncontrolled hemorrhage in the rat. Shock 36:60–66. 10.1097/SHK.0b013e3182116143. [DOI] [PubMed] [Google Scholar]
- 63.Heldmaier G. 1974. Temperature adaptation and brown adipose tissue in hairless and albino mice. J Comp Physiol 92:281–292. 10.1007/BF00696616. [DOI] [Google Scholar]
- 64.Heldmaier G. 1975. The influence of the social thermoregulation on the cold-adaptive growth of BAT in hairless and furred mice. Pflugers Arch 355:261–266. 10.1007/BF00583688. [DOI] [PubMed] [Google Scholar]
- 65.Hylander BL, Repasky EA. 2016. Thermoneutrality, mice, and cancer: a heated opinion. Trends Cancer 2:166–175. 10.1016/j.trecan.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th 8th ed. Washington (DC): The National Academies Press. [Google Scholar]
- 67.Ioannidis JP. 2005. Why most published research findings are false. PLoS Med 2:696–701. 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Irwin L, Park C, Gerringer R, Murray D, Huss J, Campbell NE. [Internet]. 2002. Cage and rack system for housing animals in cages having different widths. Google patents.[Cited 30 Sept 2018]. Available at: https://patents.google.com/patent/US7320294
- 69.IUPS Thermal Commission. 1987. Glossary of terms for thermal physiology. Second edition. Revised by The Commission for Thermal Physiology of the International Union of Physiological Sciences (IUPS Thermal Commission). Pflugers Arch 410:567–587. [PubMed] [Google Scholar]
- 70.Iwamoto S, Takasu A, Sakamoto T. 2010. Therapeutic mild hypothermia: effects on coagulopathy and survival in a rat hemorrhagic shock model. J Trauma 68:669–675. 10.1097/TA.0b013e3181a0fbb3. [DOI] [PubMed] [Google Scholar]
- 71.Janus C, Golde T. 2014. The effect of brief neonatal cryoanesthesia on physical development and adult cognitive function in mice. Behav Brain Res 259:253–260. 10.1016/j.bbr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jhaveri KA, Trammell RA, Toth LA. 2007. Effect of environmental temperature on sleep, locomotor activity, core body temperature, and immune responses of C57BL/6J mice. Brain Behav Immun 21:975–987. 10.1016/j.bbi.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson JS, Taylor DJ, Green AR, Gaskill BN. 2017. Effects of nesting material on energy homeostasis in BALB/cAnNCrl, C57BL/6NCrl, and Crl:CD1(ICR) mice housed at 20 °C. J Am Assoc Lab Anim Sci 56:254–259. [PMC free article] [PubMed] [Google Scholar]
- 74.Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. 2010. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59:1657–1666. 10.2337/db09-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karp CL. 2012. Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med 209:1069–1074. 10.1084/jem.20120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:1–5. 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiryu S, Inoue Y, Watanabe M, Ohtomo K. 2011. Effect of isoflurane anesthesia and hypothermia on the hepatic kinetics of Gd-EOB-DTPA: evaluation using MRI of conscious mice. J Magn Reson Imaging 34:354–360. 10.1002/jmri.22650. [DOI] [PubMed] [Google Scholar]
- 78.Klaus S, Munzberg H, Truloff C, Heldmaier G. 1998. Physiology of transgenic mice with brown fat ablation: obesity is due to lowered body temperature. Am J Physiol 274:R287–R293. [DOI] [PubMed] [Google Scholar]
- 79.Koivisto A, Siemen D, Nedergaard J. 2000. Norepinephrine-induced sustained inward current in brown fat cells: α1-mediated by nonselective cation channels. Am J Physiol Endocrinol Metab 279:E963–E977. 10.1152/ajpendo.2000.279.5.E963. [DOI] [PubMed] [Google Scholar]
- 80.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, Repasky EA. 2013. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA 110:20176–20181. 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kort WJ, Hekking-Weijma JM, TenKate MT, Sorm V, VanStrik R. 1998. A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Lab Anim 32:260–269. 10.1258/002367798780559329. [DOI] [PubMed] [Google Scholar]
- 82.Krinke GJ. 2004. Normative histology of the organs, chapter 9. p 133–166. In: Hedrich H. The laboratory mouse. Chapel Hill (NC): Academic Press. [Google Scholar]
- 83.Kurz A, Go JC, Sessler DI, Kaer K, Larson MD, Bjorksten AR. 1995. Alfentanil slightly increases the sweating threshold and markedly reduces the vasoconstriction and shivering thresholds. Anesthesiology 83:293–299. 10.1097/00000542-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 84.Kurz A, Sessler DI, Lenhardt R. 1996. Perioperative normothermia to reduce the incidence of surgical wound infection and shorten hospitalization. Study of wound infection and temperature group. N Engl J Med 334:1209–1216. 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 85.Kushikata T, Hirota K, Kotani N, Yoshida H, Kudo M, Matsuki A. 2005. Isoflurane increases norepinephrine release in the rat preoptic area and the posterior hypothalamus in vivo and in vitro: relevance to thermoregulation during anesthesia. Neuroscience 131:79–86. 10.1016/j.neuroscience.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. 2012. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490:187–191. 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leigh ND, Kokolus KM, O'Neill RE, Du W, Eng JW-L, Qiu J, Chen GL, McCarthy PL, Farrar JD, Cao X, Repasky EA. 2015. Housing temperature–induced stress is suppressing murine graft-versus-host disease through β2-adrenergic receptor signaling. J Immunol 195:5045–5054. 10.4049/jimmunol.1500700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lessin AW, Parkes MW. 1957. The relation between sedation and body temperature in the mouse. Br J Pharmacol Chemother 12:245–250. 10.1111/j.1476-5381.1957.tb00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lim S, Honek J, Xue Y, Seki T, Cao Z, Andersson P, Yang X, Hosaka K, Cao Y. 2012. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat Protoc 7:606–615. 10.1038/nprot.2012.013. [DOI] [PubMed] [Google Scholar]
- 90.Liu E, Lewis K, Al-Saffar H, Krall CM, Singh A, Kulchitsky VA, Corrigan JJ, Simons CT, Petersen SR, Musteata FM, Bakshi CS, Romanovsky AA, Sellati TJ, Steiner AA. 2012. Naturally occurring hypothermia is more advantageous than fever in severe forms of lipopolysaccharide- and Escherichia coli-induced systemic inflammation. Am J Physiol Regul Integr Comp Physiol 302:R1372–R1383. 10.1152/ajpregu.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu M, Hu X, Liu J. 2001. The effect of hypothermia on isoflurane MAC in children. Anesthesiology 94:429–432. 10.1097/00000542-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 92.Lodhi IJ, Semenkovich CF. 2009. Why we should put clothes on mice. Cell Metab 9:111–112. 10.1016/j.cmet.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 93.Lujan HL, Janbaih H, Feng HZ, Jin JP, Dicarlo SE. 2012. Ventricular function during exercise in mice and rats. Am J Physiol Regul Integr Comp Physiol 302:R68–R74. 10.1152/ajpregu.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. 2014. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 29:413–420. [DOI] [PubMed] [Google Scholar]
- 95.Marschner JA, Schafer H, Holderied A, Anders HJ. 2016. Optimizing mouse surgery with online rectal temperature monitoring and preoperative heat supply. effects on postischemic acute kidney Injury. PLoS One 11:1–16. 10.1371/journal.pone.0149489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsukawa T, Kurz A, Sessler DI, Bjorksten AR, Merrifield B, Cheng C. 1995. Propofol linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology 82:1169–1180. 10.1097/00000542-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 97.Messmer MN, Kokolus KM, Eng JWL, Abrams SI, Repasky EA. 2014. Mild cold stress depresses immune responses: implications for cancer models involving laboratory mice. BioEssays 36:884–891. 10.1002/bies.201400066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meyer CW, Ootsuka Y, Romanovsky AA. 2017. Body temperature measurements for metabolic phenotyping in mice. Front Physiol 8:1–13. 10.3389/fphys.2017.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morrison SF, Madden CJ, Tupone D. 2012. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 3:1–19. 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.National Research Council. 2011. Guidance for the description of animal research in scientific publications. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 101.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. 2011. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480:104–108. 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olsson IA, Dahlborn K. 2002. Improving housing conditions for laboratory mice: a review of ‘environmental enrichment’. Lab Anim 36:243–270. 10.1258/002367702320162379. [DOI] [PubMed] [Google Scholar]
- 103.Ootsuka Y, de Menezes RC, Zaretsky DV, Alimoradian A, Hunt J, Stefanidis A, Oldfield BJ, Blessing WW. 2009. Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest–activity cycle. Neuroscience 164:849–861. 10.1016/j.neuroscience.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oufara S, Barre H, Rouanet JL, Chatonnet J. 1987. Adaptation to extreme ambient temperatures in cold-acclimated gerbils and mice. Am J Physiol 253:R39–R45. [DOI] [PubMed] [Google Scholar]
- 105.Overton JM. 2010. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int J Obes (Lond) 34 Suppl 2:S53–S58. 10.1038/ijo.2010.240. [DOI] [PubMed] [Google Scholar]
- 106.Overton JM, Williams TD, Chambers JB, Rashotte ME. 2001. Cardiovascular and metabolic responses to fasting and thermoneutrality are conserved in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 280:R1007–R1015. 10.1152/ajpregu.2001.280.4.R1007. [DOI] [PubMed] [Google Scholar]
- 107.Perrin S. 2014. Preclinical research: make mouse studies work. Nature 507:423–425. 10.1038/507423a. [DOI] [PubMed] [Google Scholar]
- 108.Pettifer GR, Hosgood G. 2003. The effect of rectal temperature on perianesthetic serum concentrations of transdermally administered fentanyl in cats anesthetized with isoflurane. Am J Vet Res 64:1557–1561. 10.2460/ajvr.2003.64.1557. [DOI] [PubMed] [Google Scholar]
- 109.Phillips PK, Heath JE. 1995. Dependency of surface temperature regulation on body size in terrestrial mammals. J Therm Biol 20:281–289. 10.1016/0306-4565(94)00061-M. [DOI] [Google Scholar]
- 110.Rauch JC, Hayward JS. 1969. Topography and vascularization of brown fat in a small nonhibernator (deer mouse, Peromyscus maniculatus). Can J Zool 47:1301–1314. 10.1139/z69-203. [DOI] [PubMed] [Google Scholar]
- 111.Redfors B, Shao Y, Omerovic E. 2013. Influence of anesthetic agent, depth of anesthesia, and body temperature on cardiovascular functional parameters in the rat. Lab Anim 48:6–14. 10.1177/0023677213502015. [DOI] [PubMed] [Google Scholar]
- 112.Reed RL, 2nd, Johnson TD, Hudson JD, Fischer RP. 1992. The disparity between hypothermic coagulopathy and clotting studies. J Trauma 33:465–470. 10.1097/00005373-199209000-00022. [DOI] [PubMed] [Google Scholar]
- 113.Rider CV, Boekelheide K, Catlin N, Gordon CJ, Morata T, Selgrade MK, Sexton K, Simmons JE. 2013. Cumulative risk: toxicity and interactions of physical and chemical stressors. Toxicol Sci 137:3–11. 10.1093/toxsci/kft228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rivard GF. [Internet]. 2001. Animal caging and biological storage systems. Google patents. [Cited 30 Sept 2018]. Available at: https://patents.google.com/patent/US6257171
- 115.Romanovsky AA. 2014. Skin temperature: its role in thermoregulation. Acta Physiol (Oxf) 210:498–507. 10.1111/apha.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosenkilde C, Vamosi M, Lauridsen JT, Hasfeldt D. 2017. Efficacy of prewarming with a selfwarming blanket for the prevention of unintended perioperative hypothermia in patients undergoing hip or knee arthroplasty. J Perianesth Nurs 32:419–428. 10.1016/j.jopan.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 117.Rubin RL. 2017. Mice housed at elevated vivarium temperatures display enhanced T-cell response and survival to Francisella tularensis. Comp Med 67:491–497. [PMC free article] [PubMed] [Google Scholar]
- 118.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. 2005. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 289:R1244–R1252. 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- 119.Sadler KE, Stratton JM, DeBerry JJ, Kolber BJ. 2013. Optimization of a pain model: effects of body temperature and anesthesia on bladder nociception in mice. PLoS One 8:1–8. 10.1371/journal.pone.0079617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Scholander PF, Hock R, Walters V, Johnson F, Irving L. 1950. Heat regulation in some arctic and tropical mammals and birds. Biol Bull 99:237–258. 10.2307/1538741. [DOI] [PubMed] [Google Scholar]
- 121.Schulz TJ, Tseng YH. 2013. Brown adipose tissue: development, metabolism, and beyond. Biochem J 453:167–178. 10.1042/BJ20130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schuster CJ, Pang DSJ. 2017. Forced-air prewarming prevents perianaesthetic hypothermia and shortens recovery in adult rats. Lab Anim 52:142–151. 10.1177/0023677217712539. [DOI] [PubMed] [Google Scholar]
- 123.Seale P, Bjork B, Yang W, Kajimura S, Kuang S, Scime A, Devarakonda S, Chin S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. 2008. PRDM16 controls a brown fat–skeletal muscle switch. Nature 454:961–967. 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shirey MJ, Smith JB, Kudlik DE, Huo BX, Greene SE, Drew PJ. 2015. Brief anesthesia, but not voluntary locomotion, significantly alters cortical temperature. J Neurophysiol 114:309–322. 10.1152/jn.00046.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Skorupski AM, Zhang J, Ferguson D, Lawrence F, Hankenson FC. 2017. Quantification of induced hypothermia from aseptic scrub applications during rodent surgery preparation. J Am Assoc Lab Anim Sci 56:562–569. [PMC free article] [PubMed] [Google Scholar]
- 126.Smith AJ, Clutton RE, Lilley E, Hansen KEA, Brattelid T. 2018. PREPARE: guidelines for planning animal research and testing. Lab Anim 52:135–141. 10.1177/0023677217724823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Speakman JR, Keijer J. 2013. Not so hot: optimal housing temperatures for mice to mimic the thermal environment of humans. Mol Metab 2:5–9. 10.1016/j.molmet.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sprung J, Cheng EY, Gamulin S, Kampine JP, Bosnjak ZJ. 1992. The effect of acute hypothermia and serum potassium concentration on potassium cardiotoxicity in anesthetized rats. Acta Anaesthesiol Scand 36:825–830. 10.1111/j.1399-6576.1992.tb03572.x. [DOI] [PubMed] [Google Scholar]
- 129.Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton J. 2008. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol 294:H1581–H1588. 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- 130.Swoap SJ, Overton JM, Garber G. 2004. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol 287:R391–R396. 10.1152/ajpregu.00731.2003. [DOI] [PubMed] [Google Scholar]
- 131.Taylor DK. 2007. Study of 2 devices used to maintain normothermia in rats and mice during general anesthesia. J Am Assoc Lab Anim Sci 46:37–41. [PubMed] [Google Scholar]
- 132.Torossian A, Ruehlmann S, Middeke M, Sessler DI, Lorenz W, Wulf HF, Bauhofer A. 2004. Mild preseptic hypothermia is detrimental in rats. Crit Care Med 32:1899–1903. 10.1097/01.CCM.0000139608.34486.FD. [DOI] [PubMed] [Google Scholar]
- 133.Tranquilli WJ, Thurmon JC, Grimm KA, Lumb WV. 2007. Lumb and Jones’ veterinary anesthesia and analgesia, 4th ed. Ames (IA): Blackwell Publishing [Google Scholar]
- 134.Vidrio H, Garcia-Marquez F. 1987. Reversal by hypothermia of vasodilator-induced tachycardia in anesthetized rats. Arch Int Pharmacodyn Ther 288:217–228. [PubMed] [Google Scholar]
- 135.Wilson D, Pettifer GR, Hosgood G. 2006. Effect of transdermally administered fentanyl on minimum alveolar concentration of isoflurane in normothermic and hypothermic dogs. J Am Vet Med Assoc 228:1042–1046. 10.2460/javma.228.7.1042. [DOI] [PubMed] [Google Scholar]
- 136.Worthmann A, John C, Ruhlemann MC, Baguhl M, Heinsen FA, Schaltenberg N, Heine M, Schlein C, Evangelakos I, Mineo C, Fischer M, Dandri M, Kremoser C, Scheja L, Franke A, Shaul PW, Heeren J. 2017. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med 23:839–849. 10.1038/nm.4357. [DOI] [PubMed] [Google Scholar]
- 137.Xiao H, Run X, Cao X, Su Y, Sun Z, Tian C, Sun S, Liang Z. 2013. Temperature control can abolish anesthesia-induced tau hyperphosphorylation and partly reverse anesthesia-induced cognitive impairment in old mice. Psychiatry Clin Neurosci 67:493–500. 10.1111/pcn.12091. [DOI] [PubMed] [Google Scholar]
- 138.Xiong J, Kurz A, Sessler DI, Plattner O, Christensen R, Dechert M, Ikeda T. 1996. Isoflurane produces marked and nonlinear decreases in the vasoconstriction and shivering thresholds. Anesthesiology 85:240–245. 10.1097/00000542-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 139.Yang Y, Gordon CJ. 1996. Ambient temperature limits and stability of temperature regulation in telemetered male and female rats. J Therm Biol 21:353–363. 10.1016/S0306-4565(96)00021-6. [DOI] [Google Scholar]
- 140.Young AA, Dawson NJ. 1982. Evidence for on–off control of heat dissipation from the tail of the rat. Can J Physiol Pharmacol 60:392–398. 10.1139/y82-057. [DOI] [PubMed] [Google Scholar]