Abstract
An adult rhesus macaque developed seizures after the induction of ischemic stroke. Initially, on the day of surgery, a focal ischemic lesion was present exclusively in the right caudate nucleus. By 48 h after stroke induction, the lesion had extended into the putamen, when a seizure was observed. Our report highlights the temporal changes in infarction of unilateral basal ganglia after acute stroke and the accompanying clinical symptoms. This unusual case may provide additional information regarding the involvement of the basal ganglia in seizures, given that prior case reports and studies usually have not described the temporal and spatial evolution of the lesion before clinical symptoms emerge.
Abbreviation: MCA, middle cerebral artery
The basal ganglia is a group of subcortical nuclei that are involved in many neuronal pathways associated with emotional, motivational, associative, and cognitive functions. Injury to the basal ganglia can result in serious complication in movement, perception, or judgement.53 Stroke in the basal ganglia is not as common as in the cortex.5,9,15,44,50 Patients with injury to the basal ganglia and internal capsule may have hypotonia, flaccid paralysis, or persistently impaired balance and ambulation and therefore benefit less from therapy.31 In addition, the basal ganglia can be involved in epileptic seizures46 as a remote inhibitory control circuit;17 consequently the basal ganglia has been suggested as a potential target for neuromodulatory and pharmacologic treatment of temporal lobe epilepsy.27,36
Compared with those of rodents, the brains of NHP are structurally and functionally more similar to human brain and therefore are excellent models for stroke research.12,13,39 NHP models of stroke have been developed by occluding the middle cerebral artery (MCA); in these models, the ischemic lesion generally targets the cortical areas of the MCA territory. In our experimental exploration of NHP stroke model, we performed MCA occlusion in 12 macaques (8 animals received permanent MCA occlusion; the remaining 4 animals underwent transient occlusion). One of the macaques with permanent MCA occlusion initially developed a focal infarct in the right basal ganglion and later exhibited seizure symptoms at 48 h after stroke. MRI is a robust means to examine stroke lesions in both clinical and preclinical studies.3,4,41,51,56 In the present study, we used MRI to document the longitudinal evolution of the basal ganglia infarction in the rhesus macaque that developed seizures.
Materials and Methods
The subject described here is an adult Indian-origin rhesus macaque (Macaca mulatta; ID no. Rpf6; sex, female; weight, 11 kg; age, 14 y) that was born and reared at the Yerkes National Primate Research Center, an AAALAC-accredited facility. The animal received the interventional stroke surgical procedure as reported previously.14,45,55 Briefly, to induce permanent infarction within the MCA territory in the right cerebral hemisphere, a microcatheter was navigated through a parent catheter to the targeted M2 and M3 branches of the MCA, which were occluded by using 4-0 silk suture segments (5 to 10 mm). After the occlusion was confirmed angiographically by using fluoroscopy, the catheters were removed from the femoral artery before moving the animal to MRI.
Animal care during MRI and surgery.
The animal was initially anesthetized with 3 to 5 mg/kg of tiletamine–zolazepam and then intubated and maintained under approximately 2.0% isoflurane anesthesia for the stroke surgery and 1% to 1.5% isoflurane during MRI scans. The subject was immobilized during surgery and MRI by using custom-built head holders. All major physiologic parameters were monitored and remained within normal ranges.29 Occlusion of the MCA was identified by using fluoroscopy before moving the animal to MRI. The macaque was euthanized without recovery from anesthesia through fatal barbiturate overdose immediately after the 48-h MRI scan.
Animal care of pre and post stroke.
Animal care before and after stroke was described previously.54 Briefly, the macaque was housed in an appropriately sized indoor cage where the environment was maintained on a 12:12-h light:dark cycle at 60 to 75 °F (22.6 to 23.9 °C) and relative humidity of 30% to 70%. The macaque was fed a commercial diet (Jumbo Monkey Diet 5037, Purina Mills, St Louis, MO) free choice and supplemented with fresh produce. After stroke surgery and each MRI scan, the macaque was under close supervision by a staff veterinarian until full recovery from anesthesia. Then the animal was returned to her home cage, where she was housed individually; checked routinely by veterinary staff; and monitored trough continuous closed-circuit video recording. A liquid nutritional supplement (Boost, Nestlé Nutrition, Highland Park, MI) and a combination of regular or fruit-juice softened biscuits, applesauce, soft fruits, yogurt, and peanut butter were offered at least every 2 h. Analgesics were provided for the duration of the survival period.
MRI.
All images were acquired by using a 3T clinical scanner (Siemens Medical Solutions, Malvern, PA) and an 8-channel phase-array knee coil (Invivo, Gainesville, FL). The imaging setting and protocols were as reported previously.55 In the present study, anatomic structural images including MR angiography, T2-weighted images, and diffusion-weighted images with 30 gradient directions were collected. Bolus gadolinium injection (0.02 mg/kg) was administrated near the end of each scan session. Pre- and postcontrast T1-weighted structural images were obtained through 3D magnetization-prepared gradient-echo sequence to evaluate for possible hemorrhage. Diffusion tensor imaging was repeated hourly during the day 0 (surgical day) scan. In addition, susceptibility-weighted imaging was applied to detect complications due to hemorrhage (data not shown). MRI was performed for screening at 7 d before stroke induction; within 7 h after stroke surgery (day 0); and at 48 h (day 2) after occlusion.
Data processing.
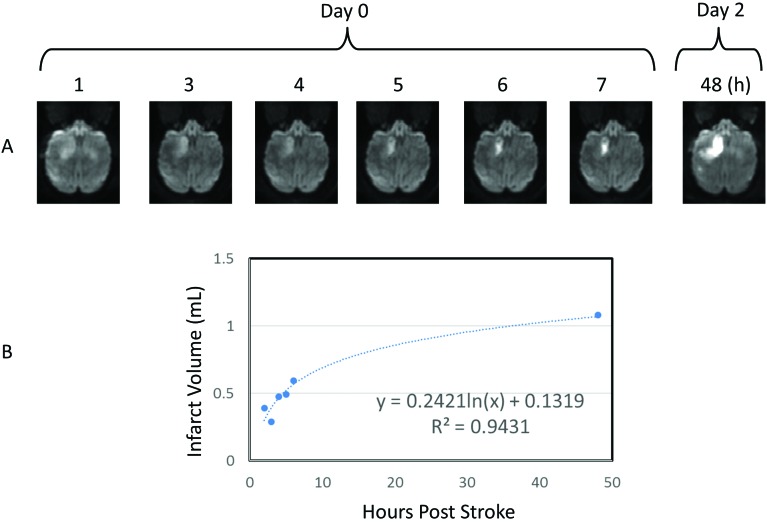
Diffusion-weighted images were preprocessed, coregistered, and averaged at each time point by using FSL (www.fmrib.ox.ac.uk/fsl/). Lesion volumes from diffusion-weighted images were derived by using the threshold (mean + 2 SD) of the diffusion-weighted intensity on the contralateral side. Lesion volumes from T2-weighted images were calculated through manual tracing. The infarct volumes on diffusion-weighted images were estimated by using inhouse-built MatLab (MathWorks, Natick, MA) scripts. Lesion volumes were calculated as the sum of the lesion region in each slice multiplied by the slice thickness. The temporal evolution of stroke infarct was fitted by using the natural logarithm.52,54
Neurologic examination.
Neurologic assessments were performed before stroke induction day (baseline) and on days 1 and 2 after stroke induction by using the previously reported grading scale for stroke in NHP.43
Histology.
The macaque was euthanized immediately after the 48-h MRI scan by pentobarbital overdose and immediately perfused intracardially with saline followed by 10% buffered formalin, according to well-established protocols approved by the Emory IACUC. The whole brain was removed and immersed in 10% buffered formalin. The brain then was blocked and sectioned at 50 µm by using a freezing microtome. Selected sections were stained with hematoxylin and eosin according to standard procedures.
All procedures were approved by the IACUC of Emory University and in compliance with the Animal Welfare Act,1 the Public Health Service Policy on Humane Care and Use of Laboratory Animals,33 and the Guide for the Care and Use of Laboratory Animals.24
Results
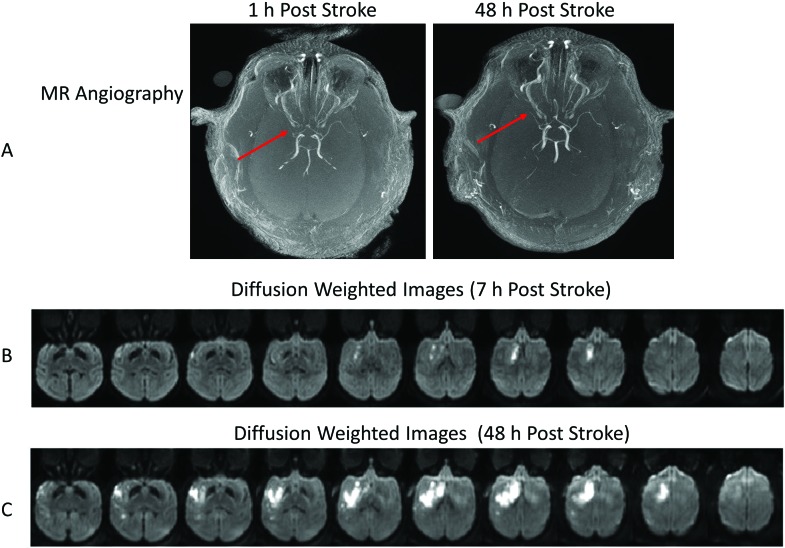
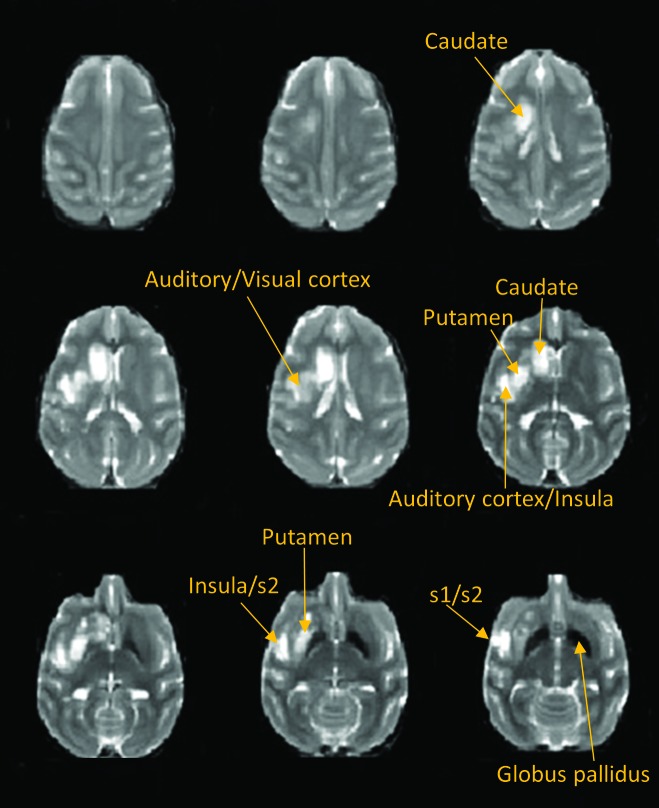
MCA occlusion was validated through MR angiology (Figure 1 A). Occlusion of the main stem (M1) segment of the MCA was apparent on the day of surgery. On diffusion-weighted images, ischemic infarction was largely in the right caudate nucleus at 7 h after surgery (Figure 1 B) and dramatically extended to most basal ganglia areas (caudate, putamen) and some cortical regions (primary auditory cortex, insula, and primary and secondary somatosensory cortexes) at 48 h (Figure 1 C). Edema was seen in the caudate nucleus only on T2-weighted images at 7 h after stroke (data not shown) and extended into regions including the putamen, insula, and primary and secondary somatosensory cortexes at 48 h after stroke (Figure 2).
Figure 1.
MR angiography and diffusion-weighted images of the rhesus macaque that developed seizures after stroke (animal ID RPF6). (A) Axial MR angiographic images at 1 and 48 h after stroke show the occluded MCA (arrows). Diffusion-weighted imaging highlights the evolution of the infarct at (B) 7 h and (C) 48 h after MCA occlusion.
Figure 2.
These T2-weighted images at 48 h after stroke illustrate the presence of edema after the stroke insult in our macaque.
The temporal evolution of stroke infarct volumes (Figure 3 A) fitted a natural logarithmic pattern (Figure 3 B), indicating significant infarction growth from day 0 to day 1. No hemorrhagic transformation was observed. In addition, heavy iron deposition was seen in the globus pallidus (Figures 2 and 4).
Figure 3.
Temporal and spatial evolution of the stroke lesion in the basal ganglia. Single panels show the development of infarction from 1 to 48 h after surgery (upper panel). The progressive changes of entire infarction volumes fit a natural logarithmic pattern (bottom panel).
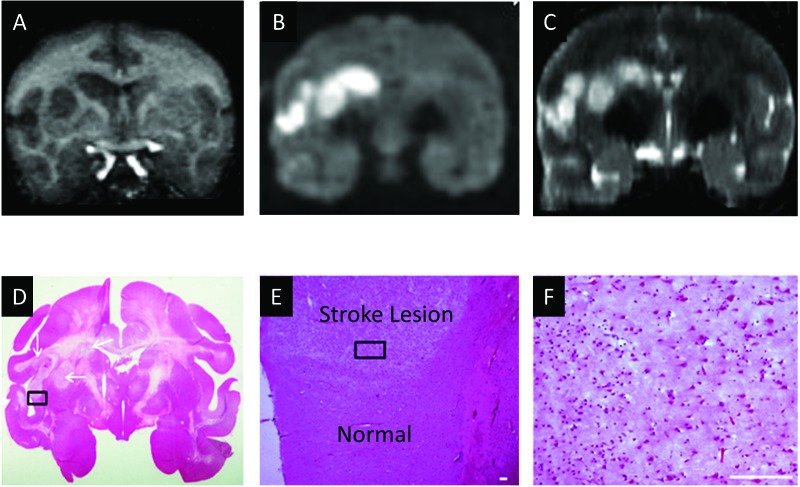
Figure 4.
Demonstration of the stroke lesion in the macaque's brain by using MRI and neuropathology. (A) T1-weighted coronal image. (B) Diffusion-weighted coronal images. (C) T2-weighted coronal image. (D) Hematoxylin and eosin staining. (E) Magnification of the box in panel D. Scale bar, 100 µm. (F) Magnification of the box in panel E. Scale bar, 100 µm.
Clinical evaluation.
On day 1 (24 h after stroke), the animal was quiet, alert, and responsive. Hemiparesis of the left forelimb and left hindlimb was present. The motor strength of the right extremities was generally weakened but not paretic. In addition, the animal was reluctant to ambulate and was only able to move to the right. On day 2 (48 h after stroke), the macaque was bright, alert, and responsive. Hemiparesis of the left extremities was less apparent. Muscle strength of the right extremities had returned to normal. The macaque engaged in species-appropriate threat behavior consisting of an open-mouth stare, small upper-body lunge-type movements toward the observer, and head bobs. However, she remained reluctant to move freely about the enclosure. In contrast to the previous day, she was able to move her body to the left as well as the right.
Shortly after behavioral scoring was completed on day 2, the macaque began having seizures. She was anesthetized immediately as planned for the scan at 48 h after stroke. In light of the marked changes in the animal's clinical condition on the morning of the follow-up scan and because of the new basal ganglia lesions discovered during MRI, euthanasia was elected in keeping with the IACUC protocol.
The neurologic score of the macaque was 100 at baseline (measured 3 times before stroke surgery), 50 at day 1 (24 h after stroke), and 55 on day 2 (48 h after stroke; Table 1).
Table 1.
Findings from neurologic examination of the macaque that developed seizures after MCA occlusion
| Points | Baseline 1 | Baseline 2 | Baseline 3 | Day 1 | Day 2 | |
| Motor function | ||||||
| Severe hemiparesis | 10 | |||||
| Mild hemiparesis | 25 | 25 | 25 | |||
| Normal strength but favors opposite extremity | 55 | |||||
| Normal strength and normal function | 70 | 70 | 70 | 70 | ||
| Behavior | ||||||
| Death | 0 | |||||
| Coma | 1 | |||||
| Aware of surroundings but not active | 5 | |||||
| Aware of surroundings and moves in response to examiner | 15 | 15 | ||||
| Normal aggression | 20 | 20 | 20 | 20 | 20 | |
| Ocular and cranial nerve function | ||||||
| Facial movement paretic | 5 | 5 | 5 | |||
| Facial movement normal | 5 | 5 | 5 | 5 | ||
| Visual field hemianoptic | 1 | |||||
| Visual field normal | 5 | 5 | 5 | 5 | 5 | 5 |
| Total score | 100 | 100 | 100 | 50 | 55 | |
Neuropathology.
The results from hematoxylin and eosin staining demonstrated ischemia-induced neural and glial cell damage in the MRI-identified regions (Figure 4).
Discussion
In the present study, we report a case of right basal ganglia infarction in an adult rhesus macaque monkey due to experimental MCA occlusion, and seizures occurred 48 h after stroke as a consequence. Seizures did not occur during the first 24 h after ischemic occlusion, when the lesion was mainly limited to the caudate nucleus, but appeared at approximately 48 h after surgery, when the lesion involved most of the putamen.
The basal ganglia comprises the subcortical nuclei, including the striatum (caudate, putamen) and globus pallidus. These structures receive input from the cerebral cortex, which they process in a highly specific manner, and then send information back to the cerebral cortex by way of the thalamus. Specifically, the basal ganglia–thalamocortical circuitry is essential for higher level behavioral control, and injury to the basal ganglia can cause serious deficits in movement, perception, or judgement.16
Basal ganglia infarction is an uncommon type of lacunar stroke, with lesions usually confined to the caudate, putamen, or globus pallidus (lentiform nucleus). Abulia and abnormalities of speech, memory, and motor or sensory function are typically seen.8,21,49 Acute bilateral, left, or right caudate infarction has been seen in human patients. As reported previously,26 caudate infarction—usually caused by small artery disease—can cause abulia, psychic akinesia, frontal system abnormalities, speech deficits (left-sided lesion), and neglect syndromes (right-sided lesion). In addition, 4 patients who had large infarcts in the right caudate nucleus showed prominent faciobrachiocrural paresis and behavioral abnormalities (in particular, one patient had severe impairment of motor and mental activity and could not move unless asked to eat or to stand). None of these patients had seizures, similar to what we observed neurologically in our subject on day 1.
Early human clinical case reports describe putamen infarction or lesions and their correlation with symptomatic dystonia.2,11,20 Schizophrenia-like psychosis (with inconclusive seizure activity) occurred in a patient with a left putamen infarct.19 In addition, a patient with bilateral putamen nuclei ischemic infarctions demonstrated acute aphonia.40
The involvement of the basal ganglia in epileptic seizures has been demonstrated in previous clinical studies (for a review, see reference 46), and the role of the basal ganglia in seizures has been studied as well.36,47 Specifically, the striatum and pallidum are involved in temporal lobe epilepsy, and the basal ganglia has been suggested to play an inhibitory role during temporal lobe seizures.36 Significant volumetric loss in subcortical structures (including the putamen) has been demonstrated in patients with unilateral temporal lobe epilepsy.35 Progressive microstructural alterations in the putamen have been reported in a patient with epilepsy,23 and patients with negative symptoms in temporal lobe epilepsy showed significant volume reduction in the putamen and globus pallidus.22 In addition, a recent MRI study of patients revealed significant changes in the resting-state functional connectivity between cortical and subcortical structures, indicating the important role of the basal ganglia and thalamus in focal epilepsies.48
In comparison to the other macaques with permanent MCA occlusion in the same cohort, our macaque with seizures lacked prominent ischemic infarction in the cortex, due to the abundant collateral blood supply in NHP.45 Instead, ischemic infarction was confined to the caudate on the day of surgery and extended to the putamen at 48 h after stroke. Because the infarction volume evolves in a logarithm pattern during acute stroke,52,54 it may be reasonable to deduce that the infarct in the putamen was already present on day 1 (24 h after stroke), even though seizures did not develop until 48 h after stroke. These findings reveal the possible relationship between infraction evolution in the putamen and seizure onset and thus indicate a specific role of the putamen in seizure activity. In addition, this result is in agreement with a recent functional MRI finding in which the functional connectivity between the putamen and cortex is disturbed in patients with epilepsy37 and with a case report of a patient with microstructural alterations in the putamen.23
Stroke is one of the most frequent causes of seizures in patients, and subcortical infarcts have been associated with poststroke epileptic seizures.6,7,28,42 As we reported previously regarding the macaques in the same cohort, basal ganglia infarction was present in the other 2 macaques with permanent MCA occlusion (animals RJJ3 and RCE3).54 However, only the macaque we report here had infarction restricted to the basal ganglia on the day of surgery (Figure 1), whereas the other 2 animals showed predominant infraction in the cortex. RCE3 was euthanized immediately after MRI on the surgery day (approved by IACUC), and RJJ3 was euthanized at 48 h after stroke due to poor neurologic condition (but no seizures were seen). Therefore, our current findings suggest that focal basal ganglia infarction may contribute to the initiation of seizures. However, a cause–effect relationship cannot be established solely on the timing of the 2 events in this report. As seen in a subpopulation of stroke patients25 and rat models,30 early seizures occur in a random fashion after stroke. However, our current macaque stroke case revealed the temporal evolution of the basal ganglia infarction before seizure manifestation, and this information might be helpful for investigating the pathophysiologic cascades of stroke-related seizures.
Basal ganglia infarction is usually caused by occlusion of the lenticulostriate arteries, which originate from the initial segment of the MCA and supply blood to most of the subcortical structures. By examining the MR angiography of all of our stroke macaques, we found that the main stem (M1) segment of the MCA was occluded on the day of surgery in all of the animals with basal ganglia infarction but not in those with infarction restricted to the cortex. Therefore, these perforating vessels could be occluded because of the unintended occlusion in the M1 segment during surgery (Figure 1). In addition, the animal with seizures lacked extensive cortical infarction, suggesting that this macaque had a strong collateral blood supply that protected her from cortical ischemic infarction. As a result, ischemic infarction was restricted to the basal ganglia of this macaque.
Prior studies in animal models and human patients have suggested the basal ganglia may function as a control circuit in seizures10,18 and therefore is a potential target for epilepsy treatment using deep brain stimulation.27 NHP models have been used in epileptic research.32,34,38 In addition, our present findings suggest that a macaque model with acute basal ganglia infarction may be useful to investigate the etiology of epileptic seizures and develop new treatment strategies.
In conclusion, the involvement of the basal ganglia in epileptic seizures of clinical patients suggests that these structures play an important role in various epileptic syndromes and might be targeted for neuromodulatory and pharmacologic treatment. Our current case report involving a rhesus macaque with stroke demonstrates the possible relationship between the temporal and spatial anatomic changes associated with a unilateral basal ganglia lesion after acute stroke insult and the accompanying clinical symptoms. The current findings may provide additional information regarding the involvement of the basal ganglia in seizures, given that prior clinical case reports did not provide information regarding the temporal and spatial evolution of the lesions.
Acknowledgments
We thank Sudeep Patel for C-arm operation and MRI data collection; Ruth Connelly, Wendy Williamson Coyne, and Juliet Brown for animal care during MRI, surgery, and after stroke; Dr Manuel Yepes for his valuable comments on this project; and Dr Anapatricia Garcia for necropsy. This project was supported in part by NCRR and currently by the Office of Research Infrastructure Programs (OD P51OD011132, P51RR000165 and OD P51OD011132) and by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000454 (XZ). Yerkes National Primate Research Center is a fully AAALAC-accredited facility.
References
- 1.Animal Welfare Act as Amended. 2013. 7 USC §2131–2159.
- 2.Apaydin H, Ozekmekci S, Yeni N. 1998. Posthemiplegic focal limb dystonia: a report of 2 cases. Clin Neurol Neurosurg 100:46–50. 10.1016/S0303-8467(97)00120-0. [DOI] [PubMed] [Google Scholar]
- 3.Bardutzky J, Shen Q, Henninger N, Bouley J, Duong TQ, Fisher M. 2005. Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke 36:2000–2005. 10.1161/01.STR.0000177486.85508.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman M, Slater LA, Leslie-Mazwi T, Simonsen CZ, Stuckey S, Chandra RV. 2017. Diffusion and perfusion MR Imaging in acute stroke: clinical utility and potential limitations for treatment selection. Top Magn Reson Imaging 26:77–82. 10.1097/RMR.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 5.Bekiesinska-Figatowska M, Mierzewska H, Jurkiewicz E. 2013. Basal ganglia lesions in children and adults. Eur J Radiol 82:837–849. 10.1016/j.ejrad.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Bentes C, Pimentel J, Ferro JM. 2001. Epileptic seizures following subcortical infarcts. Cerebrovasc Dis 12:331–334. 10.1159/000047730. [DOI] [PubMed] [Google Scholar]
- 7.Berges S, Moulin T, Berger E, Tatu L, Sablot D, Challier B, Rumbach L. 2000. Seizures and epilepsy following strokes: recurrence factors. Eur Neurol 43:3–8. 10.1159/000008120. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia KP, Marsden CD. 1994. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 117:859–876. 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt AA, Brucker JL, Almast J. 2018. Beyond stroke—uncommon causes of diffusion restriction in the basal ganglia. Emerg Radiol 25:87–92. [DOI] [PubMed] [Google Scholar]
- 10.Bouilleret V, Semah F, Biraben A, Taussig D, Chassoux F, Syrota A, Ribeiro MJ. 2005. Involvement of the basal ganglia in refractory epilepsy: an 18F-fluoro-L-DOPA PET study using 2 methods of analysis. J Nucl Med 46:540–547. [PubMed] [Google Scholar]
- 11.Burton K, Farrell K, Li D, Calne DB. 1984. Lesions of the putamen and dystonia: CT and magnetic resonance imaging. Neurology 34:962–965. 10.1212/WNL.34.7.962. [DOI] [PubMed] [Google Scholar]
- 12.Cook DJ, Teves L, Tymianski M. 2012. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature 483:213–217. 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- 13.Cook DJ, Tymianski M. 2012. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics 9:371–379. 10.1007/s13311-012-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Crespigny AJ, D'Arceuil HE, Maynard KI, He J, McAuliffe D, Norbash A, Sehgal PK, Hamberg L, Hunter G, Budzik RF, Putman CM, Gonzalez RG. 2005. Acute studies of a new primate model of reversible middle cerebral artery occlusion. J Stroke Cerebrovasc Dis 14:80–87. 10.1016/j.jstrokecerebrovasdis.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 15.De Renzi E, Faglioni P, Scarpa M, Crisi G. 1986. Limb apraxia in patients with damage confined to the left basal ganglia and thalamus. J Neurol Neurosurg Psychiatry 49:1030–1038. 10.1136/jnnp.49.9.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLong M, Wichmann T. 2009. Update on models of basal ganglia function and dysfunction. Parkinsonism Relat Disord 15 Suppl 3:S237–S240. 10.1016/S1353-8020(09)70822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dematteis M, Kahane P, Vercueil L, Depaulis A. 2003. MRI evidence for the involvement of basal ganglia in epileptic seizures: an hypothesis. Epileptic Disord 5:161–164. [PubMed] [Google Scholar]
- 18.Depaulis A, Moshe SL. 2002. The basal ganglia and the epilepsies: translating experimental concepts to new therapies. Epileptic Disord 4 Suppl 3:S7–S8. [PubMed] [Google Scholar]
- 19.Farid F, Mahadun P. 2009. Schizophrenia-like psychosis following left putamen infarct: a case report. J Med Case Reports 3:1–3. 10.4076/1752-1947-3-7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fross RD, Martin WR, Li D, Stoessl AJ, Adam MJ, Ruth TJ, Pate BD, Burton K, Calne DB. 1987. Lesions of the putamen: their relevance to dystonia. Neurology 37:1125–1129. 10.1212/WNL.37.7.1125. [DOI] [PubMed] [Google Scholar]
- 21.Fukuoka T, Osawa A, Ohe Y, Deguchi I, Maeshima S, Tanahashi N. 2012. Bilateral caudate nucleus infarction associated with a missing A1 segment. J Stroke Cerebrovasc Dis 21:908.e11–908.e12. 10.1016/j.jstrokecerebrovasdis.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Geary EK, Seidenberg M, Hermann B. 2009. Atrophy of basal ganglia nuclei and negative symptoms in temporal lobe epilepsy. J Neuropsychiatry Clin Neurosci 21:152–159. 10.1176/jnp.2009.21.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerdes JS, Keller SS, Schwindt W, Evers S, Mohammadi S, Deppe M. 2012. Progression of microstructural putamen alterations in a case of symptomatic recurrent seizures using diffusion tensor imaging. Seizure 21:478–481. 10.1016/j.seizure.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 25.Kim HJ, Park KD, Choi KG, Lee HW. 2016. Clinical predictors of seizure recurrence after the first postischemic stroke seizure. BMC Neurol 16:212 10.1186/s12883-016-0729-6.Erratum: Clinical predictors of seizure recurrence after the first postischemic stroke seizure. BMC Neurol 17: 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumral E, Evyapan D, Balkir K. 1999. Acute caudate vascular lesions. Stroke 30:100–108. 10.1161/01.STR.30.1.100. [DOI] [PubMed] [Google Scholar]
- 27.Laxpati NG, Kasoff WS, Gross RE. 2014. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics 11:508–526. 10.1007/s13311-014-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung T, Leung H, Soo YO, Mok VC, Wong KS. 2016. The prognosis of acute symptomatic seizures after ischaemic stroke. J Neurol Neurosurg Psychiatry 88:86–94. 10.1136/jnnp-2015-311849. [DOI] [PubMed] [Google Scholar]
- 29.Li CX, Patel S, Auerbach EJ, Zhang X. 2013. Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neurosci Lett 541:58–62. 10.1016/j.neulet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu XC, Dave JR, Chen Z, Cao Y, Liao Z, Tortella FC. 2013. Nefiracetam attenuates postischemic nonconvulsive seizures in rats and protects neuronal cell death induced by veratridine and glutamate. Life Sci 92:1055–1063. 10.1016/j.lfs.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Miyai I, Blau AD, Reding MJ, Volpe BT. 1997. Patients with stroke confined to basal ganglia have diminished response to rehabilitation efforts. Neurology 48:95–101. 10.1212/WNL.48.1.95. [DOI] [PubMed] [Google Scholar]
- 32.Mondragon S, Lamarche M. 1990. Suppression of motor seizures after specific thalamotomy in chronic epileptic monkeys. Epilepsy Res 5:137–145. 10.1016/0920-1211(90)90030-Y. [DOI] [PubMed] [Google Scholar]
- 33.Office of Laboratory Animal Welfare. [Internet]. 2002. Public health service policy on humane care and use of laboratory animals. [Cited 11 November 2018]. Available at: https://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf
- 34.Perez-Mendes P, Blanco MM, Calcagnotto ME, Cinini SM, Bachiega J, Papoti D, Covolan L, Tannus A, Mello LE. 2011. Modeling epileptogenesis and temporal lobe epilepsy in a nonhuman primate. Epilepsy Res 96:45–57. 10.1016/j.eplepsyres.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Pulsipher DT, Seidenberg M, Morton JJ, Geary E, Parrish J, Hermann B. 2007. MRI volume loss of subcortical structures in unilateral temporal lobe epilepsy. Epilepsy Behav 11:442–449. 10.1016/j.yebeh.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rektor I, Kuba R, Brazdil M, Chrastina J. 2012. Do the basal ganglia inhibit seizure activity in temporal lobe epilepsy? Epilepsy Behav 25:56–59. 10.1016/j.yebeh.2012.04.125. [DOI] [PubMed] [Google Scholar]
- 37.Rektor I, Tomcik J, Mikl M, Marecek R, Brazdil M, Rektorova I. 2013. Association between the basal ganglia and large-scale brain networks in epilepsy. Brain Topogr 26:355–362. 10.1007/s10548-012-0272-8. [DOI] [PubMed] [Google Scholar]
- 38.Ribak CE, Joubran C, Kesslak JP, Bakay RA. 1989. A selective decrease in the number of GABAergic somata occurs in preseizing monkeys with alumina gel granuloma. Epilepsy Res 4:126–138. 10.1016/0920-1211(89)90017-X. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Mercado R, Ford GD, Xu Z, Kraiselburd EN, Martinez MI, Eterovic VA, Colon E, Rodriguez IV, Portilla P, Ferchmin PA, Gierbolini L, Rodriguez-Carrasquillo M, Powell MD, Pulliam JV, McCraw CO, Gates A, Ford BD. 2012. Acute neuronal injury and blood genomic profiles in a nonhuman primate model for ischemic stroke. Comp Med 62:427–438. [PMC free article] [PubMed] [Google Scholar]
- 40.Senatorov VV, Satpute S, Perry K, Kaylie DM, Cole JW. 2013. Aphonia induced by simultaneous bilateral ischemic infarctions of the putamen nuclei: a case report and review of the literature. J Med Case Reports 7:1–5. 10.1186/1752-1947-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Q, Duong TQ. 2008. Quantitative prediction of ischemic stroke tissue fate. NMR Biomed 21:839–848. 10.1002/nbm.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverman IE, Restrepo L, Mathews GC. 2002. Poststroke seizures. Arch Neurol 59:195–201. 10.1001/archneur.59.2.195. [DOI] [PubMed] [Google Scholar]
- 43.Spetzler RF, Selman WR, Weinstein P, Townsend J, Mehdorn M, Telles D, Crumrine RC, Macko R. 1980. Chronic reversible cerebral ischemia: evaluation of a new baboon model. Neurosurgery 7:257–261. 10.1227/00006123-198009000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Stenerson MB, Collura CA, Rose CH, Lteif AN, Carey WA. 2011. Bilateral basal ganglia infarctions in a neonate born during maternal diabetic ketoacidosis. Pediatrics 128:e707–e710. [DOI] [PubMed] [Google Scholar]
- 45.Tong FC, Zhang X, Kempf DJ, Yepes MS, Connor-Stroud FR, Zola S, Howell L. 2015. An enhanced model of middle cerebral artery occlusion in nonhuman primates using an endovascular trapping technique. AJNR Am J Neuroradiol 36:2354–2359. 10.3174/ajnr.A4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vercueil L, Hirsch E. 2002. Seizures and the basal ganglia: a review of the clinical data. Epileptic Disord 4 Suppl 3:S47–S54. [PubMed] [Google Scholar]
- 47.Vuong J, Devergnas A. 2017. The role of the basal ganglia in the control of seizure. J Neural Transm (Vienna) 125:531–545. 10.1007/s00702-017-1768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Výtvarová E, Marecek R, Fousek J, Strycek O, Rektor I. 2017. Large-scale cortico–subcortical functional networks in focal epilepsies: the role of the basal ganglia. Neuroimage Clin 14:28–36. 10.1016/j.nicl.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner SJ, Begaz T. 2008. Basal ganglion stroke presenting as subtle behavioural change. Emerg Med J 25:459 10.1136/emj.2008.057968. [DOI] [PubMed] [Google Scholar]
- 50.Wang C, Chen Y, Zhang Y, Chen J, Ding X, Ming D, Du J. 2017. Quantitative EEG abnormalities in major depressive disorder with basal ganglia stroke with lesions in different hemispheres. J Affect Disord 215:172–178. 10.1016/j.jad.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 51.Wang S, Gu X, Paudyal R, Wei L, Dix TA, Yu SP, Zhang X. 2017. Longitudinal MRI evaluation of neuroprotective effects of pharmacologically induced hypothermia in experimental ischemic stroke. Magn Reson Imaging 40:24–30. 10.1016/j.mri.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Li Y, Paudyal R, Ford BD, Zhang X. 2015. Spatiotemporal assessment of the neuroprotective effects of neuregulin 1 on ischemic stroke lesions using MRI. J Neurol Sci 357:28–34. 10.1016/j.jns.2015.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wichmann T, DeLong MR. 1996. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol 6:751–758. 10.1016/S0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Tong F, Li CX, Yan Y, Kempf D, Nair G, Wang S, Muly EC, Zola S, Howell L. 2015. Temporal evolution of ischemic lesions in nonhuman primates: a diffusion and perfusion MRI study. PLoS One 10:1–15. 10.1371/journal.pone.0117290. Erratum: Temporal evolution of ischemic lesions in nonhuman primates: a diffusion and perfusion MRI study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Tong F, Li CX, Yan Y, Nair G, Nagaoka T, Tanaka Y, Zola S, Howell L. 2014. A fast multiparameter MRI approach for acute stroke assessment on a 3T clinical scanner: preliminary results in a nonhuman primate model with transient ischemic occlusion. Quant Imaging Med Surg 4:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Yan Y, Tong F, Li C, Jones B, Wang S, Meng Y, Muly EC, Kempf D, Howell L. 2018. Progressive assessment of ischemic injury to white matter using diffusion tensor imaging: a preliminary study of a macaque model of stroke. Open Neuroimaging J 12:30–41. 10.2174/1874440001812010030. [DOI] [PMC free article] [PubMed] [Google Scholar]