Abstract
The gastrointestinal microbiota (GM) plays a fundamental role in health and disease and contributes to the bidirectional signaling between the gastrointestinal system and brain. The direct line of communication between these organ systems is through the vagus nerve. Therefore, vagal nerve stimulation (VNS), a commonly used technique for multiple disorders, has potential to modulate the enteric microbiota, enabling investigation and possibly treatment of numerous neurologic disorders in which the microbiota has been linked with disease. Here we investigate the effect of VNS in a mouse model of amyotrophic lateral sclerosis (ALS). B6SJL-Tg(SOD1*G93A)dl1Gur (SOD1dl) and wildtype mice underwent ventral neck surgery to access the vagus nerve. During surgery, the experimental group received 1 h of VNS, whereas the sham group underwent 1 h of sham treatment. The third (control) group did not undergo any surgical manipulation. Fecal samples were collected before surgery and at 8 d after the initial collection. Microbial DNA was sequenced to determine the GM profiles at both time points. GM profiles did not differ between genotypes at either the initial or end point. In addition, VNS did not alter GM populations, according to the parameters chosen in this study, indicating that this short intraoperative treatment is safe and has no lasting effects on the GM. Future studies are warranted to determine whether different stimulation parameters or chronic use of VNS affect GM profiles.
Abbreviations: ALS, amyotrophic lateral sclerosis; GM, gastrointestinal microbiome; OTU, operational taxonomic unit; PERMANOVA, permutational multivariate ANOVA; SOD1, superoxide dismutase 1; SOD1dl, B6SJL-Tg(SOD1*G93A)dl1Gur; VNS, vagal nerve stimulation
The vagus nerve, the tenth and longest cranial nerve in the body, innervates numerous structures including the larynx, pharynx, heart, lungs, and gastrointestinal tract. The vagus nerve is composed of thousands of axons that work to provide a vast majority of the autonomic innervation in the body.39,70 This nerve plays a large role in interoceptive awareness and is often regarded as the body's ‘sixth sense.’12,61,73 Although the function of the vagus nerve is largely parasympathetic, it also provides somatic innervation, mainly to the muscles responsible for swallowing and upper airway function.6 The vagus nerve comprises A, B, and C fiber types, all of which are characterized by different conduction velocities and stimulation thresholds.14,39,55,60 This nerve consists of both afferent (80%) and efferent (20%) fibers that provide sensory and motor information to maintain homeostasis in nearly every organ system in mammals.12,39,43,54,65,70
Given the vagus nerve's wide-ranging anatomic targets and neuromodulatory effects, targeted manipulation of this nerve has a broad range of potential experimental and therapeutic applications. In fact, vagal nerve stimulation (VNS) has been used experimentally to establish the contribution of the vagus nerve to numerous behaviors, including immune function, mood, pain, and memory.70 Furthermore, there is immense interest in using implantable and noninvasive VNS devices to modulate essential functions within the body.39 VNS has been shown as an effective therapeutic strategy for diverse disorders, and various forms of VNS are currently FDA-approved for treating refractory epilepsy, depression, migraines, cluster headaches, and obesity.12,30,51,55,60,70-72 In addition, this technology is currently being explored in a multitude of other disorders, including arthritis, asthma, heart failure, gastroparesis, and inflammatory bowel disease, among many others.30,54,70 Despite the effectiveness of VNS as a treatment strategy, the richness and complexity of the information transmitted along the vagus nerve raises serious challenges that must be considered before widespread use of VNS.39 Because the vagus nerve innervates multiple organ systems, it is imperative to examine how using VNS to treat a disorder of one organ system might affect healthy function in another.
Numerous studies have been conducted on the safety of VNS in regard to cardiovascular and respiratory function.6,8,10,30 However, although studies have examined the effects of VNS on gastrointestinal function,45,46,50 no published study has investigated how VNS might influence gastrointestinal microbial populations.12 This dearth is surprising, given that the vagus nerve is the direct link between the CNS and gastrointestinal tract, serving as a complex bidirectional line of communication between these 2 organ systems.9,12,16,38 This brain–gut axis is essential for maintaining homeostasis and is greatly influenced by the gastrointestinal microbiota in both health and disease states, thus yielding its label as the ‘brain–gut–microbiota axis’.9,15,16,38,67
As part of this 3-component axis, the gastrointestinal microbiome (GM) consists of more than 1013 microorganisms, predominantly bacterial species.28,33 These commensal enteric bacteria are crucial for preventing invasion of pathogens and for maintaining gastrointestinal morphology, intestinal barrier function, normal digestion, mucosal immune function, and host metabolism.38 The bacteria in this population have a substantial capacity for secretory and metabolic activity that influence the signals sent and received by the gastrointestinal tract to and from the brain. Through this complex system, the brain controls motor, antiinflammatory, and secretory functions of the gastrointestinal tract, and the gastrointestinal viscera can return sensory messages to modulate nervous system function.9,12,13,16,38 Miscommunication between the 2 organ systems can elicit stress responses, influence mood and behavior, and has been linked to chronic diseases throughout the body, including obesity, inflammatory bowel disease, colorectal cancer, among many others.16,22,38,62,69 Therefore, alteration of the enteric microbiota has tremendous effects on both health and pathologic conditions.57
Moreover, the GM, through the vagus nerve, has been suggested as a contributor to the development of neurodevelopmental and neurodegenerative disorders, including Autism Spectrum Disorder, Alzheimer disease, and Parkinson disease.21,27,53,63 Amyotrophic lateral sclerosis (ALS) is another neurodegenerative disease with potential involvement of the GM. ALS is a fatal disease characterized by progressive loss of motor neurons. Typical clinical signs include limb paralysis, aspiration pneumonia due to swallowing impairment, and asphyxiation.32 Although the role of the gastrointestinal tract in ALS is largely unexplored, patients with ALS have exhibited delayed gastric emptying and extended colonic transit times,7,64 signifying abnormal gastrointestinal function. Perhaps most promising, a previous study demonstrated alterations in the GM and gastrointestinal morphology in a mouse model of ALS.69,75 Because many human patients with ALS have mutations in the superoxide dismutase 1 (SOD1) gene, the most common mouse models used to study ALS carry a mutated human SOD1 transgene.40 Recent studies using an SOD1 mouse model demonstrate that GM changes occur in young mice before the onset of disease and show that disease onset can be delayed by restoring the GM.69,75 These findings indicate that altered microbial populations and gastrointestinal pathology may contribute to the pathogenesis of ALS. Consequently, perhaps VNS could play a role in manipulating the gastrointestinal microbes that contribute to ALS and other neurologic diseases.
Whether VNS will have beneficial or detrimental effects on the microbial populations in the gastrointestinal tract is unclear currently. It is concerning that patients undergoing VNS for treatment of disease conditions may experience changes in their GM profiles, potentially contributing to other chronic diseases or negatively modulating the treatment effect. Alternatively, VNS might produce a favorable effect, positively influencing the treatment efficacy and proving useful as an entirely separate therapeutic strategy for disorders with a known gastrointestinal dysbiosis. Therefore, as the use of VNS becomes more prevalent for a multitude of disorders, it is essential to understand the potential off-target effects of this treatment on GM composition. Therefore, the primary objective of this study was to examine the effects of intraoperative VNS on GM profiles in healthy and neurologically diseased mice.
In this study, we used an SOD1dl mouse model of ALS,1,5 which has fewer copies of the mutated transgene, corresponding to a delayed onset of disease compared with the high transgene copy number SOD1 model used in the aforementioned studies.40,69,75 Primarily, we sought confirmation that commonly used experimental stimulation parameters for promoting swallowing and upper airway function did not alter the GM profiles of the mouse models used in our studies. Our secondary objective was to classify the GM composition of mice from our transgenic SOD1dl colony compared with age-matched WT controls to explore whether altered GM populations were present prior to disease onset, analogous to recent findings in the similar high-copy-number SOD1 model.69,75
Materials and Methods
Animals.
All experimental procedures performed in this study were reviewed and approved by the University of Missouri Animal Care and Use Committee (protocol no. 8980). The University of Missouri is USDA-licensed and AAALAC-accredited. The line of mice used in this study, B6SJL-Tg(SOD1*G93A)dl1Gur (SOD1dl), originally was purchased from Jackson Laboratories (Bar Harbor, ME), but a breeding colony has been maintained at the University of Missouri for 3 to 4 y. Mice undergo tail snips at weaning for genotyping purposes to discriminate transgenic from WT animals and confirm the copy number of the transgene. The animals for this study were moved from the barrier breeding room to a conventional room at least 1 mo (age, approximately 4 mo) prior to data collection and were housed in IVC (Tecniplast, West Chester, PA) with aspen chip bedding. Mice were group-housed by sex whenever possible. Mice had free access to food (Laboratory Rodent Diet 5001, Purina, St Louis, MO) and water. Room temperature was maintained between 20.0 °C and 26.0 °C, relative humidity was between 30% and 70%, and the photoperiod was a 12:12-h light:dark cycle. Standard enrichment (cotton squares) was provided to all cages.
All mice were of the same health status and were housed in the same room during the experiment; surgery was performed in a separate room in the laboratory, outside of the vivarium. At the time of the study, colony sentinels were tested quarterly and were considered free of the following agents: mouse hepatitis virus, minute virus of mice, mouse parvovirus, Sendai virus, Theiler murine encephalomyelitis virus, mouse rotavirus, Mycoplasma pulmonis, Pasteurella pneumotropica, Salmonella spp., mouse pneumonia virus, reovirus 3, lymphocytic choriomeningitis virus, Ectromelia, mouse adenovirus types 1 and 2, K virus, and polyoma virus. Fecal PCR analysis was used to detect pinworms in sentinel mice, whereas cage PCR assays (pooled swabs by room) were used to detect fur mites. According to the standard procedures for the room, mice were not tested for Helicobacter spp.
Experimental design.
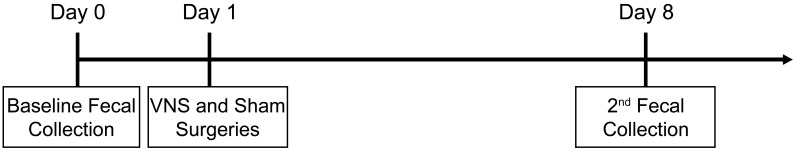
A total of 30 B6SJL-Tg(SOD1*G93A)dl1Gur (SOD1dl) mice and 30 age-matched WT controls were used for this study. Mice (age, 5 mo) were randomly selected from our SOD1dl colony. This time point is approximately 1 mo prior to disease onset, which typically is observed around 6 mo of age in our colony.17,56 Experimental procedures were performed over the course of 3 mo by using 7 cohorts of mice, depending on the availability of mice at the correct age. Mice were divided into 3 groups, each with 10 SOD1dl and 10 WT mice (equal sexes). The experimental group underwent surgery for 1 h of unilateral VNS. A second group underwent surgery for 1 h of sham stimulation. The control group did not undergo surgery and remained in the animal housing room throughout the study. As such, control animals were not housed with mice from other groups, but animals from the experimental and sham treatment groups were housed together randomly. Fecal samples were collected from individual mice 1 d prior to surgery (day 0) and 8 d later (that is, 1 wk after surgery). Fecal samples were stored at –80.0 °C until the end of the study, when microbial DNA was extracted and sequenced to characterize the GM at both time points. Figure 1 shows a timeline of experimental procedures.
Figure 1.
Timeline of fecal sample collections. All mice underwent baseline fecal collection at 5 mo of age (day 0). Mice in the experimental and sham groups underwent either vagal nerve stimulation (VNS) or sham stimulation surgeries on day 1. Mice in the control group remained in the vivarium, with no surgical manipulation. Feces were collected again from all mice at 8 d after the initial collection.
Surgical procedure.
Mice in the experimental and sham groups underwent surgery with a ventral neck approach to access the right cervical vagus nerve. Mice were anesthetized by using a ketamine (90 mg/kg SC; Henry Schein, Melville, NY)–xylazine (11.25 mg/kg SC; Akorn, Lake Forest, IL) cocktail. Half doses of ketamine were given subcutaneously as needed to maintain the surgical plane of anesthesia throughout the procedure. We chose injectable anesthesia because our lab has anecdotally experienced decreased efficacy of electrical stimulation in isoflurane-anesthetized mice. The eyes were lubricated to prevent drying, and the ventral neck was shaved and prepared aseptically for surgery. Mice were positioned in dorsal recumbency on a custom platform beneath a surgical microscope (model M125, Leica Microsystems, Buffalo Grove, IL). Core body temperature was maintained at 37 °C by using a homeothermic heating system (DC Temperature Controller; FHC, Bowdoin, ME), and reflexes were checked every 10 to 15 min. Supplemental oxygen (100%) was delivered through nose cone at a flow rate of 1 L/min during stimulation or sham treatment.
A midline neck incision was made from the suprasternal notch to the mandible. The salivary glands were gently retracted laterally, and the right vagus nerve was identified in its cervical location. After careful isolation from the carotid artery and jugular vein, the vagus nerve was placed on bipolar electrodes (FHC, Bowdoin, ME). Mice in the experimental group received VNS for 1 h while maintained at surgical depth of anesthesia. For mice in the sham group, the vagus nerve was placed on the electrodes for 1 h while they remained under surgical anesthesia.
For both groups, the vagus nerve was removed from the electrodes after 1 h of stimulation or sham treatment, and the neck incision was closed by using absorbable sutures (6-0 Monocryl, Ethicon, Somerville, NJ) and surgical glue (Tissumend II, Veterinary Products Laboratories, Phoenix, AZ). After suturing was complete, 0.3 mL of warm, sterile saline was administered subcutaneously; sustained-release buprenorphine (1 mg/kg SC; Zoopharm, Windsor, CO) and flunixin meglumine (2.2 mg/kg SC; Merck, Kenilworth, NJ) were given as separate injections for pain control. Mice were transferred to a clean, heated cage for recovery; mice were monitored at least every 10 to 15 min and were returned to their home cage once fully ambulatory. The home cages were placed half on, half off of a heated water blanket overnight and returned to the vivarium the following morning. All mice were monitored daily after surgery for any signs of pain, distress, or surgical complications. Control mice had no experimental manipulation beyond fecal collection at days 0 and 8.
VNS.
Mice in the experimental group received VNS for 1 h during surgery. The right vagus nerve was placed on the electrodes, with the anode positioned distally (Figure 2). Once positioned, the nerve was stimulated by using a constant current stimulator connected to a laptop equipped with LabChart software (ADInstruments, Colorado Springs, CO). Stimulation was verified by using a digital oscilloscope (Tektronix, Beaverton, OR) coupled to a current probe (Tektronix, Beaverton, OR) attached to the electrode leads. The nerve was stimulated according to the following parameters: biphasic 0.5-ms square-wave pulses (interstimulus interval, 0.1 ms) delivered at 20 Hz (stimulus intensity, 0.2 mA). We chose these parameters because of their effectiveness in peripheral nerve regeneration after injury2-4,34-37,68 and are commonly used parameters in our lab for various other projects to promote swallow and upper airway function in neurologic disorders.
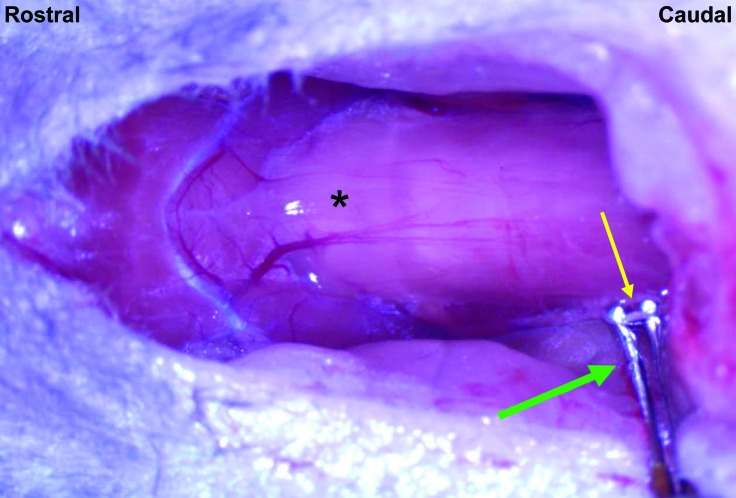
Figure 2.
Vagal nerve stimulation. The right vagus nerve (yellow arrow) was isolated in its cervical location and placed on bipolar electrodes (green arrow) for electrical stimulation delivered at 20 Hz with a 0.2-mA stimulus intensity. *, larynx.
Fecal collection.
Fecal collection took place early in the morning (0700 to 0800) after the lights were turned on in the housing facility. Mice temporarily were placed individually into empty autoclaved cages and allowed to defecate. Approximately 2 fecal pellets were collected aseptically from each mouse and placed into a sterile 2-mL tube containing a stainless steel bead (diameter, 0.5 cm; Penn Ball Bearing, Delran, NJ).24 Fecal samples were collected the day prior to surgery (day 0) and 1 wk after surgery (day 8). Fecal samples were stored at –80.0 °C prior to DNA extraction.
DNA extraction.
DNA was extracted from all samples within 3 consecutive days, by using previously described methods.24,26 Briefly, lysis buffer was placed in all tubes containing fecal samples. The samples were homogenized, followed by incubation at 70.0 °C for 20 min. After incubation, samples were centrifuged; the entire supernatant was transferred to a fresh tube, supplemented with 200 µL of 10 M ammonium acetate, and allowed to incubate on ice for 5 min. After centrifugation at 5000 × g for 5 min at room temperature, the supernatant was removed, mixed with an equivalent volume of isopropanol, and allowed to incubate on ice for 30 min. Precipitated nucleic acids were pelleted by centrifuging at 16,000 × g for 15 min at 4 °C, rinsed twice with 70% ethanol, resuspended in 10 mM Tris–1 mM EDTA, and then purified (DNeasy Blood and Tissue kit, Qiagen, Hilden, Germany) according to the manufacturer's instructions. DNA yields were determined through fluorometry (Qubit, Life Technologies, Carlsbad, CA) by using a reagent kit (Quant-iT BR dsDNA Kit, Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Preparation of 16S rRNA library and sequencing.
Extracted fecal DNA was sent to the University of Missouri DNA Core Facility for bacterial 16S rRNA amplification and sequencing according to a previously described protocol.24,26,42 Briefly, amplification of the V4 hypervariable region of the 16S rDNA gene with previously developed universal primers was used to create bacterial 16S rDNA amplicons. Amplicons were purified and evaluated by using an automated electrophoresis system (Fragment Analyzer, Advanced Analytical, Ankeny, IA). A fluorometer (Qubit, Life Technologies) was used for quantification purposes, and samples were sequenced by using a desktop sequencer (MiSeq, Illumina, San Diego, CA).
Informatics analysis.
Assembly, filtering, binning, and annotation of DNA sequences was performed at the University of Missouri Informatics Research Core Facility (Columbia, MO). Operational taxonomic units (OTU) were identified and given taxonomic assignments by using BLAST against the SILVA database of 16S rRNA sequences and taxonomy.24,26,42 Principal coordinate analysis of 1/4 root-transformed sequence data and α-diversity indices were performed at the University of Missouri Metagenomics Center by using open-access Past 3.18 software (https://folk.uio.no/ohammer/past/).
Statistics.
Differences in α-diversity and richness between genotypes on day 0 were evaluated by using t tests or Mann–Whitney rank–sum tests, depending on the results from Shapiro–Wilk normality tests. Differences in α-diversity and richness between treatment groups and genotypes on day 8 were tested by using 2-way ANOVA. Changes in α-diversity and richness among treatment groups and genotypes between days 0 and 8 were assessed by using 2-way ANOVA. All statistical analyses were performed by using SigmaPlot 12.5 (Systat Software, San Jose, CA). Differences between genotype GM compositions at day 0 were tested by using one-way permutational multivariate ANOVA (PERMANOVA) of ranked Bray–Curtis and unranked Jaccard distances by using PAST 3.18 software. Two-way PERMANOVA of ranked Bray–Curtis and unranked Jaccard distances were used to assess genotype and treatment effects on the GM profiles on day 8. Uncorrected P values less than 0.05 were considered significant.
Results
Surgical procedures.
Overall the SOD1dl and WT mice tolerated the surgical procedure well. Three mice (2 SOD1dl, 1 WT) in the experimental group received only 42 to 44 min of VNS treatment because they showed increased respiratory effort; in these mice, the treatment was stopped prematurely, and they recovered uneventfully. In addition, 2 WT mice in the experimental group stopped breathing during recovery. Although resuscitation with atipamezole (0.22 mg/kg; SQ) and chest compressions was attempted, the mice did not recover. Consequently, these mice were replaced with 2 additional age-matched WT mice to complete the study. Another 7 mice (4 SOD1dl, 3 WT; 2 VNS, 5 sham) developed bradypnea and mild respiratory distress after surgery. During recovery, these mice were given atipamezole (0.22 mg/kg SC) to reverse anesthesia, which restored respiration and facilitated recovery.
Fecal collection and DNA extraction.
Fecal samples were collected from all mice in the study at day 0, except for one SOD1dl mouse that did not defecate in a timely manner and therefore was replaced in the study. Fecal samples were obtained from all surviving mice (n = 60) on day 8. DNA was extracted from all samples; all but one day 0 sample (WT mouse) and 2 day 8 samples (1 WT and 1 SOD1dl) generated sufficient high-quality reads to be interpreted.
GM richness, diversity, and composition profiles.
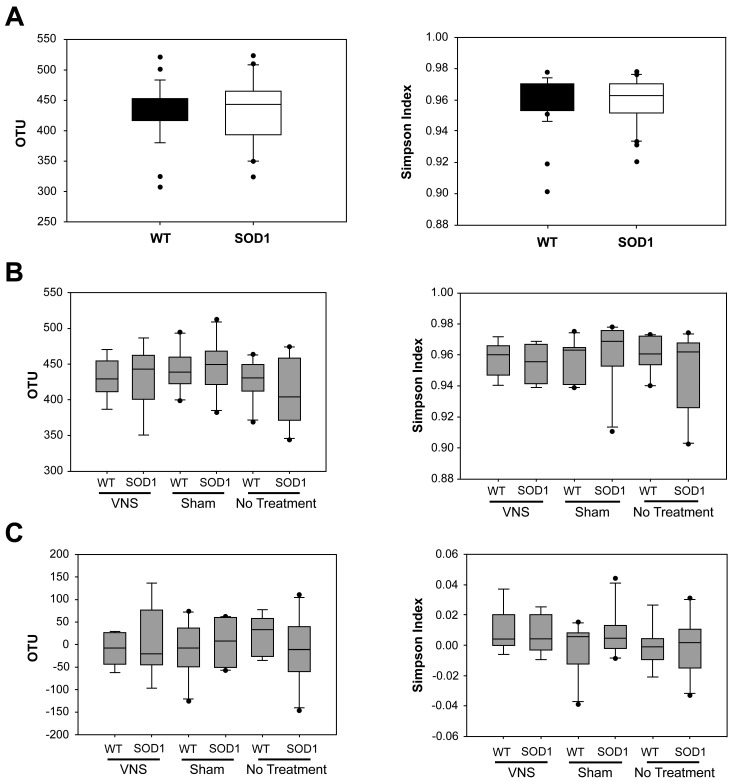
Prior to determining the GM composition, the number of OTUs (a measure of the richness of the sample) was quantified, and the relative abundance of those OTUs (that is, the diversity of the sample) was calculated for each fecal sample. Richness and diversity were compared between SOD1dl mice and age-matched WT controls at day 0 (before surgery) to determine whether differences between genotypes exist prior to disease onset in this model.69 According to Chao1 and Simpson indices, richness and diversity did not differ significantly between transgenic and WT mice at 5 mo of age (Figure 3 A). Similarly, richness and diversity were compared between the 3 treatment groups (VNS treated, sham, and control) and 2 genotypes at day 8, and no significant differences were found (Figure 3 B). Furthermore, changes in richness and diversity between days 0 and 8 was not significantly influenced by genotype or treatment group (Figure 3 C). Therefore, the overall number of OTUs and their relative abundance in each sample was not affected by genotype or treatment.
Figure 3.
Richness and α-diversity of fecal samples. Richness (left panels) was quantified according to the Chao1 index, whereas α-diversity (right panels) was characterized by using the Simpson index. (A) Neither richness or α-diversity differed significantly between genotypes at day 0. (B) Neither richness or α-diversity differed significantly between genotypes or treatment groups at day 8. (C) Neither richness nor α-diversity differed between day 0 and day 8 samples across genotypes or treatment groups. OTU, operational taxonomic unit; VNS, vagal nerve stimulation.
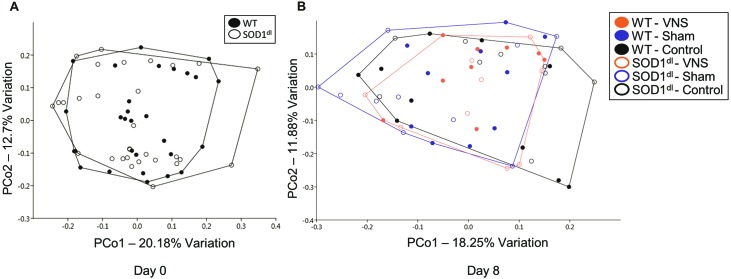
Next, we sought to determine whether the bacterial composition of the GM was unique to each genotype at day 0 and to each genotype and treatment group at day 8. In all mice, the most abundant bacteria included those in the Bacteroidales S24-7 group and the bacterial families of Rikenellaceae and Lachnospiraceae. Taking into account all detected OTUs, samples at each time point were compared by using principal coordinate analysis. Unexpectedly, there was no separation between genotypes at day 0 (Figure 4 A) or day 8 (Figure 4 B). Furthermore, there was no separation between treatment groups on day 8. At both time points, samples showed marked overlap, regardless of treatment group or genotype. Statistical analysis through PERMANOVA using Bray–Curtis and Jaccard indices confirmed no significant differences between the independent variables of this study. This finding suggests that GM profiles are not altered in presymptomatic SOD1dl mice compared with age-matched WT controls. In addition, the GM composition remained relatively stable over an 8-d time period, despite surgical procedures and electrical stimulation of the vagus nerve. We also confirmed that administration of antisedan after surgery to reverse anesthesia in 7 mice did not significantly influence the GM composition (data not shown).
Figure 4.
Principal coordinate analyses of the samples at (A) day 0 and (B) day 8. Operational taxonomic unit-level data were normalized through 1/4 root transformation. No distinct clustering or separation of samples between genotypes or treatment groups was evident at either time point. VNS, vagal nerve stimulation.
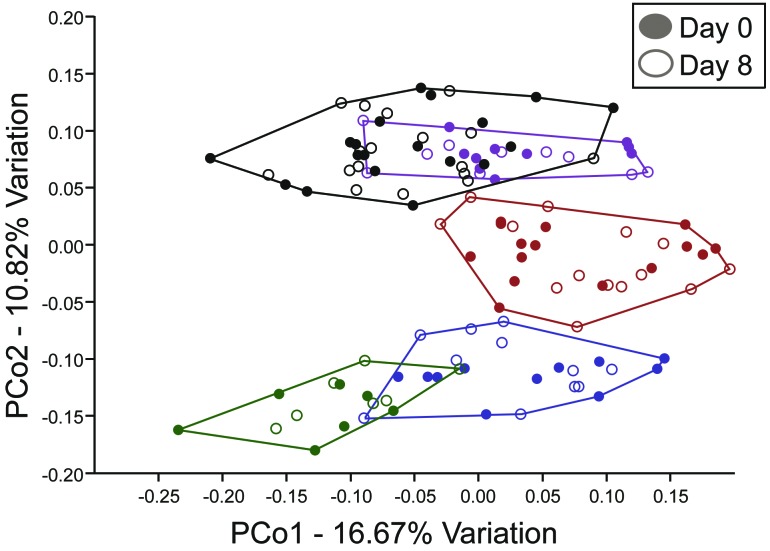
Interestingly but unsurprisingly, the GM composition varied depending on which breeding group from the SOD1dl colony produced the experimental animal, given that GM profiles are typically passed to offspring from the dam.38,41,52 The mice in the current study were born from 5 different breeder pairs within the SOD1dl colony. Breeder groups consisted of one affected SOD1dl male mated with a B6SJLF1/J female. Principal coordinate analysis showed distinct clustering of offspring produced from different breeding groups, regardless of genotype, treatment group, or time point (day 0 or 8; Figure 5). Results from 2-way PERMANOVA comparing mice from the different breeder groups and each time point of the study showed the GM composition differed significantly (P = 0.0001) between mice from the different breeding groups but not between time points. In addition, analyses were performed separately for the offspring from each breeder group to determine whether genotype or treatment effects differed significantly between mice from each breeding group; however, no significant results were found. These findings further support that neither genotype nor treatment affected the GM composition of the mice used in this study.
Figure 5.
Principal coordinate analyses of samples from both time points (days 0 and 8). Different colors represent the offspring from different breeding groups at both time points (n = 7 to 18 per group of offspring); filled symbols represent day 0 data; open symbols indicate day 8 data. GM profiles cluster among offspring born from the same breeder pairs. Samples cluster significantly between breeder groups (P < 0.0001, PERMANOVA). No separation according to genotype occurred when each breeder group was analyzed separately (data not shown).
Discussion
In summary, the results obtained from this study suggest that brief periods of intraoperative VNS, using the described stimulation parameters, do not have long-term effects on the GM composition in mice. In addition, GM profiles were similar between presymptomatic SOD1dl mice and their age-matched WT controls. Although GM profiles differed among offspring from different breeder pairs, the GM was consistent across both time points, regardless of genotype or treatment group. Given that this study is the first to investigate how VNS may modulate the GM, the lack of changes between treated and untreated mice does not automatically warrant dismissal of this experimental and potential therapeutic modality. Several explanations might account for the observed lack of GM modification due to treatment or genotype, and various study limitations must be discussed.
The first caveat of this study was that VNS was applied for a short duration (1 h) during a surgical procedure. Not only may surgery itself be a confounding variable, the short duration of VNS is minimally translatable to human patients, who receive implantable devices for VNS therapy.39,58,66 In the current study, the surgery variable was controlled by using a sham surgery group and a control group that did not receive either treatment or surgery. The surgical procedure and experimental protocol for this study were chosen to ensure that the VNS procedure does not alter the GM in the experimental mice undergoing the same procedures for different studies in our lab. It is reassuring to know that our experimental paradigm does not have lasting effects on the GM in our mouse models. However, for many human patients, VNS is often delivered through an implantable device enabling long-term therapy,39,58,66 which operates when patients are awake and freely living their lives. Chronic application of VNS likely would have a greater effect on GM populations than a brief exposure to VNS during surgery.12 Therefore, additional studies using implantable VNS devices that can deliver chronic VNS therapy outside of a surgical procedure are warranted.
A second limitation of this study was that only one set of stimulation parameters was used. Again, we selected these stimulation parameters because they are commonly used in our laboratory to promote normal swallowing49 and upper airway function. We are currently examining laryngeal nerve stimulation in the SOD1dl mouse model and intraoperative VNS stimulation in a mouse model of recurrent laryngeal nerve injury. The parameters used in our studies are based on numerous other published works indicating these parameters are useful in peripheral nerve regeneration.2-4,34-37,68 Although it is reassuring to confirm that the stimulation parameters used in our studies do not significantly alter the GM in our mouse models, how other stimulation parameters might affect the GM is unknown. Stimulation parameters consist of variables including the frequency, pulse width, waveform, amplitude, and continuity of the pulses delivered to the nerve.11 Furthermore, electrode design itself adds additional variables, which include the geometry, materials used, impedance, and the location of the electrode on the nerve.39 Finally, it is essential to consider the characteristics of the target neurons themselves. Given that the vagus nerve is composed of both afferent and efferent neurons, consisting of A, B, and C fiber types,14,39,55,60 different parameters and electrode designs might target some fiber types over others. Therefore, factors such as axonal diameter, conduction velocity, and threshold potential60 of the target axons directly influence the stimulation parameters that should be used for the intended function.55 Consequently, all variables must be optimized for the effective use of VNS in its proposed function, whether that is inhibiting seizures, promoting vocal function, or manipulating the GM.
Identifying the optimal VNS parameters for a given function is challenging. Even when VNS is applied in an approved manner, such as for refractory epilepsy, the optimal VNS parameters for each individual patient are unknown.39,55,71 The stimulation parameters used in the current study were designed to target degenerating motor neurons by sending signals to the cell bodies in the brainstem to trigger upregulation of neurotrophic factors and regeneration-associated genes to promote nerve regeneration.2,4,31,36,59 Therefore, it remains likely that the GM could be modulated by using different stimulus parameters and electrode orientations that specifically target the gastrointestinal tract. For instance, in a swine model of obesity, bilateral thoracic VNS was delivered as bipolar pulse trains consisting of 30-Hz, 500-μs pulses for 30 s every 5 min at a maximum intensity of 2 mA for 14 wk.66 In this swine model, there was a delay between the onset of simulation and the appearance of beneficial effects (that is, decreased weight gain, food consumption, and sweet cravings). Although VNS causes changes in the brain regions involved in the regulation of food intake, the underlying mechanisms of action for the observed beneficial effects of VNS in obesity are largely unknown.39,66 Given that the GM has been implicated in contributing to obesity,53 perhaps part of the equation of VNS efficacy in this obesity model is its GM modulation potential. In other words, VNS may be modulating the GM in experimental models where the gastrointestinal tract is targeted, thus contributing to the efficacy of treatment. However, this possibility has not been explored at this time.
Unexpectedly, we could not replicate the results of a recent study.69 This outcome likely was due to slight variations in experimental and statistical design, making comparisons between the 2 studies difficult. The previous study69 used the most common ALS mouse model, G93A-SOD1, which harbors high copy numbers of a transgene construct carrying a human SOD1 gene with a glycine-to-alanine transition at position 93.40,69 Fecal samples were collected from 2-mo-old mice to determine GM compositions prior to ALS symptom onset around 3 mo of age.69 The authors used qPCR analysis to show that transgenic mice had reduced levels of Butyrivibrio fibrisolvens, Escherichia coli, and Peptostreptococcus, which may have contributed to an observed shift in the GM profiles between affected and WT mice.69 In our present study, we used mice with the same transgenic construct, although it is present at lower copy numbers, corresponding to a delayed phenotypic onset of disease.1,18 We, too, explored GM profiles in transgenic and age-matched WT controls approximately 1 mo prior to disease onset in our colony (6 mo of age). However, we found no significant differences in GM compositions between genotypes in our study.
One explanation for why we were unable to replicate the previous results69 might be due to differences in the background strain. Our mice are maintained as a B6SJL hybrid strain. The background strain of mice in the aforementioned study was not specifically stated.40 However, background strain significantly influences disease phenotype in SOD1 mouse models of ALS.1,44 Age at onset of disease symptoms, such as tremor, loss of limb tone, and decreased grip strength, as well as duration of survival differ significantly by genetic background. Different background strains of mice harbor distinct GM profiles, which vary by institutional vendor24 and various husbandry and environmental factors.25,29 Therefore, background strain likely influences the potential differences observed in GM profiles between transgenic and age-matched WT mice.
In addition, it is worth noting that our present study had large sample sizes (30 SOD1dl and 30 WT mice) and equal distribution according to sex between genotypes, because ALS phenotype can vary markedly depending on sex.44 Due to our large sample size, mice used in this study comprised offspring from 5 different breeder pairs in our colony. For feasibility purposes, the study was performed by using 7 cohorts of mice. The mice in the previous study40 likely were offspring from a single litter, with the entire study conducted as a single run. Therefore, the question arises regarding whether genotype-dependent differences in GM profiles might have emerged between offspring from the same breeder pair. Therefore, we confirmed our negative results by investigating the GM profiles between offspring from each breeder pair. Again we found no significant differences between genotypes in this second analysis.
Diet and caloric intake are 2 additional factors that might alter the GM composition in the high-copy-number SOD1 strain used in the previous study69 but not the low-copy-number SOD1dl strain used in our study. These factors have been shown to greatly influence the GM.19,20,22,23,74 In our lab, we have demonstrated that the high-copy SOD1 strain develops dysphagia at weaning (approximately 3 to 4 wk of age).17 High-copy SOD1 mice have decreased swallowing,47 licking, and mastication rates48 that can be identified prior to the onset of limb paralysis. Therefore, the high-copy strain develops dysphagia during the developmental and maturation life stages. The low-copy SOD1dl strain develops similar symptoms of dysphagia; however, they do not develop swallowing impairment until fully mature (that is, near 6 mo of age), consistent with the onset of other ALS symptoms in this model. Therefore, potential variations in diet and total caloric intake between transgenic and WT mice during developmental stages might affect the GM more strongly in the high-copy SOD1 strain than in the low-copy model, which already has established and maintained a GM profile prior to disease onset.
In conclusion, this study assessed the GM modulatory potential of VNS in healthy and neurologically diseased mice. Although the GM profiles did not differ significantly between transgenic and WT controls or with VNS treatment, numerous possibilities remain regarding future explorations in this continuously expanding neuromodulatory technology. Some of our mice undergoing surgery experienced respiratory difficulty, but this side effect likely was due to the prolonged anesthesia produced through an injectable anesthetic regimen rather than to genotype or treatment factors. Therefore, short-term intraoperative VNS is a relatively safe procedure to perform and, under specific stimulation parameters, does not alter GM populations. Additional studies examining VNS with stimulation parameters that better target the gastrointestinal tract and using chronic VNS are warranted. Despite the neutral results from this study, VNS remains a promising experimental and therapeutic modality for manipulating gastrointestinal microbial communities.
Acknowledgments
We thank Kate Osman for the management of the SOD1dl mouse colony at the University of Missouri and for all of her assistance in the lab. In addition, we thank Giedre Turner for providing training to MH on fecal DNA extraction and for assisting with sample preparation for sequencing. Furthermore, MH acknowledges financial support from the NIH (T32 training grant 5T32OD011126-39) and Mu Phi Zeta for this project.
References
- 1.Acevedo-Arozena A, Kalmar B, Essa S, Ricketts T, Joyce P, Kent R, Rowe C, Parker A, Gray A, Hafezparast M, Thorpe JR, Greensmith L, Fisher EM. 2011. A comprehensive assessment of the SOD1G93A low-copy transgenic mouse, which models human amyotrophic lateral sclerosis. Dis Model Mech 4:686–700. 10.1242/dmm.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Majed AA, Brushart TM, Gordon T. 2000. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci 12:4381–4390. [PubMed] [Google Scholar]
- 3.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. 2000. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci 20:2602–2608. 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Majed AA, Tam SL, Gordon T. 2004. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol Neurobiol 24:379–402. 10.1023/B:CEMN.0000022770.66463.f7. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GM, Erwin KL, Byers N, Deitch JS, Augelli BJ, Blankenhorn EP, Heiman-Patterson TD. 2004. Effect of transgene copy number on survival in the G93A SOD1 transgenic mouse model of ALS. Brain Res Mol Brain Res 130:7–15. 10.1016/j.molbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Ardesch JJ, Sikken JR, Veltink PH, van der Aa HE, Hageman G, Buschman HP. 2010. Vagus nerve stimulation for epilepsy activates the vocal folds maximally at therapeutic levels. Epilepsy Res 89:227–231. 10.1016/j.eplepsyres.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Baltadzhieva R, Gurevich T, Korczyn AD. 2005. Autonomic impairment in amyotrophic lateral sclerosis. Curr Opin Neurol 18:487–493. 10.1097/01.wco.0000183114.76056.0e. [DOI] [PubMed] [Google Scholar]
- 8.Banzett RB, Guz A, Paydarfar D, Shea SA, Schachter SC, Lansing RW. 1999. Cardiorespiratory variables and sensation during stimulation of the left vagus in patients with epilepsy. Epilepsy Res 35:1–11. 10.1016/S0920-1211(98)00126-0. [DOI] [PubMed] [Google Scholar]
- 9.Bauer KC, Huus KE, Finlay BB. 2016. Microbes and the mind: emerging hallmarks of the gut microbiota-brain axis. Cell Microbiol 18:632–644. 10.1111/cmi.12585. [DOI] [PubMed] [Google Scholar]
- 10.Binks AP, Paydarfar D, Schachter SC, Guz A, Banzett RB. 2001. High-strength stimulation of the vagus nerve in awake humans: a lack of cardiorespiratory effects. Respir Physiol 127:125–133. 10.1016/S0034-5687(01)00252-3. [DOI] [PubMed] [Google Scholar]
- 11.Bollini CA, Cacheiro F. 2006. Peripheral nerve stimulation. Tech Reg Anesth Pain Manag 10:79–88. 10.1053/j.trap.2006.07.007. [DOI] [Google Scholar]
- 12.Bonaz B, Bazin T, Pellissier S. 2018. The vagus nerve at the interface of the microbiota–gut–brain axis. Front Neurosci 12:1–9. 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462. 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 14.Castoro MA, Yoo PB, Hincapie JG, Hamann JJ, Ruble SB, Wolf PD, Grill WM. 2011. Excitation properties of the right cervical vagus nerve in adult dogs. Exp Neurol 227:62–68. 10.1016/j.expneurol.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Cryan JF, Dinan TG. 2012. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712. 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 16.Cryan JF, O'Mahony SM. 2011. The microbiome–gut–brain axis: from bowel to behavior. Neurogastroenterol Motil 23:187–192. 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 17.Daghlas I MH, Kadosh M, Goding G, Mancini S, Dougherty D, Harris K, Robbins KA, Lever TE. 2015. Effect of gene copy number on dysphagia onset in SOD1-G93A transgenic mice. Amyotroph Lateral Scler Frontotemporal Degener 16 Sup 1:225 https://www.tandfonline.com/doi/abs/10.3109/21678421.2015.1098818 [Google Scholar]
- 18.Dal Canto MC, Gurney ME. 1997. A low expressor line of transgenic mice carrying a mutant human Cu–Zn superoxide dismutase (SOD1) gene develops pathological changes that most closely resemble those in human amyotrophic lateral sclerosis. Acta Neuropathol 93:537–550. 10.1007/s004010050650. [DOI] [PubMed] [Google Scholar]
- 19.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. 2013. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lartigue G, de La Serre CB, Raybould HE. 2011. Vagal afferent neurons in high-fat diet-induced obesity: intestinal microflora, gut inflammation, and cholecystokinin. Physiol Behav 105:100–105. 10.1016/j.physbeh.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doenyas C. 2018. Gut microbiota, inflammation, and probiotics on neural development in autism spectrum disorder. Neuroscience 374:271–286. 10.1016/j.neuroscience.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 22.DuPont AW, DuPont HL. 2011. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 8:523–531. 10.1038/nrgastro.2011.133. [DOI] [PubMed] [Google Scholar]
- 23.Eisenstein M. 2016. Microbiome: bacterial broadband. Nature 533:S104–S106. 10.1038/533S104a. [DOI] [PubMed] [Google Scholar]
- 24.Ericsson AC, Davis JW, Spollen W, Bivens N, Givan S, Hagan CE, McIntosh M, Franklin CL. 2015. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PLoS One 10:1–19. 10.1371/journal.pone.0116704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ericsson AC, Gagliardi J, Bouhan D, Spollen WG, Givan SA, Franklin CL. 2018. The influence of caging, bedding, and diet on the composition of the microbiota in different regions of the mouse gut. Sci Rep 8:4065 10.1038/s41598-018-21986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ericsson AC, Personett AR, Turner G, Dorfmeyer RA, Franklin CL. 2017. Variable colonization after reciprocal fecal microbiota transfer between mice with low and high richness microbiota. Front Microbiol 8:1–13. 10.3389/fmicb.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang X. 2015. Potential role of gut microbiota and tissue barriers in Parkinson's disease and amyotrophic lateral sclerosis. Int J Neurosci 126:771–776. [DOI] [PubMed] [Google Scholar]
- 28.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. 2010. Mood and gut feelings. Brain Behav Immun 24:9–16. 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 29.Franklin CL, Ericsson AC. 2017. Microbiota and reproducibility of rodent models. Lab Anim (NY) 46:114–122. 10.1038/laban.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garamendi-Ruiz I, Gomez-Esteban JC. 2017. Cardiovascular autonomic effects of vagus nerve stimulation. Clin Auton Res 1–12. 10.1007/s10286-017-0477-8. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31.Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VMK. 2007. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol 205:347–359. 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 32.Gil J, Funalot B, Verschueren A, Danel-Brunaud V, Camu W, Vandenberghe N, Desnuelle C, Guy N, Camdessanche JP, Cintas P, Carluer L, Pittion S, Nicolas G, Corcia P, Fleury MC, Maugras C, Besson G, Le Masson G, Couratier P. 2008. Causes of death amongst French patients with amyotrophic lateral sclerosis: a prospective study. Eur J Neurol 15:1245–1251. 10.1111/j.1468-1331.2008.02307.x. [DOI] [PubMed] [Google Scholar]
- 33.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. 2006. Metagenomic analysis of the human distal gut microbiome. Science 312:1355–1359. 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon T. 2016. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics 13:295–310. 10.1007/s13311-015-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon T, Amirjani N, Edwards DC, Chan KM. 2010. Brief postsurgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol 223:192–202. 10.1016/j.expneurol.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Gordon T, Brushart TM, Chan KM. 2008. Augmenting nerve regeneration with electrical stimulation. Neurol Res 30:1012–1022. 10.1179/174313208X362488. [DOI] [PubMed] [Google Scholar]
- 37.Gordon T, Udina E, Verge VM, de Chaves EI. 2009. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control 13:412–441. 10.1123/mcj.13.4.412. [DOI] [PubMed] [Google Scholar]
- 38.Grenham S, Clarke G, Cryan JF, Dinan TG. 2011. Brain–gut– microbe communication in health and disease. Front Physiol 2:1–15. 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guiraud D, Andreu D, Bonnet S, Carrault G, Couderc P, Hagege A, Henry C, Hernandez A, Karam N, Le Rolle V, Mabo P, Maciejasz P, Malbert CH, Marijon E, Maubert S, Picq C, Rossel O, Bonnet JL. 2016. Vagus nerve stimulation: state-of-the-art of stimulation and recording strategies to address autonomic function neuromodulation. J Neural Eng 13:041002 10.1088/1741-2560/13/4/041002. [DOI] [PubMed] [Google Scholar]
- 40.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T. 1994. Motor neuron degeneration in mice that express a human Cu–Zn superoxide dismutase mutation. Science 264:1772–1775. 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 41.Hart ML, Ericsson AC, Franklin CL. 2017. Differing complex microbiota alter disease severity of the IL10–/– mouse model of inflammatory bowel disease. Front Microbiol 8:1–15. 10.3389/fmicb.2017.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart ML, Meyer A, Johnson PJ, Ericsson AC. 2015. Comparative evaluation of DNA extraction methods from feces of multiple host species for downstream next-generation sequencing. PLoS One 10:1–16. 10.1371/journal.pone.0143334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatton KW, McLarney JT, Pittman T, Fahy BG. 2006. Vagal nerve stimulation: overview and implications for anesthesiologists. Anesth Analg 103:1241–1249. 10.1213/01.ane.0000244532.71743.c6. [DOI] [PubMed] [Google Scholar]
- 44.Heiman-Patterson TD, Deitch JS, Blankenhorn EP, Erwin KL, Perreault MJ, Alexander BK, Byers N, Toman I, Alexander GM. 2005. Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J Neurol Sci 236:1–7. 10.1016/j.jns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Królczyk G, Zurowski D, Dobrek L, Laskiewicz J, Thor PJ. 2001. The role of vagal efferents in regulation of gastric emptying and motility in rats. Folia Med Cracov 42:141–148. [PubMed] [Google Scholar]
- 46.Krolczyk G, Zurowski D, Sobocki J, Slowiaczek MP, Laskiewicz J, Matyja A, Zaraska K, Zaraska W, Thor PJ. 2001. Effects of continuous microchip (MC) vagal neuromodulation on gastrointestinal function in rats. J Physiol Pharmacol 52:705–715. [PubMed] [Google Scholar]
- 47.Lever TE, Braun SM, Brooks RT, Harris RA, Littrell LL, Neff RM, Hinkel CJ, Allen MJ, Ulsas MA. 2015. Adapting human videofluoroscopic swallow study methods to detect and characterize dysphagia in murine disease models. J Vis Exp 97:1–16. 10.3791/52319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lever TE, Gorsek A, Cox KT, O'Brien KF, Capra NF, Hough MS, Murashov AK. 2009. An animal model of oral dysphagia in amyotrophic lateral sclerosis. Dysphagia 24:180–195. 10.1007/s00455-008-9190-z. [DOI] [PubMed] [Google Scholar]
- 49.Lever TE, Simon E, Cox KT, Capra NF, O'Brien KF, Hough MS, Murashov AK. 2010. A mouse model of pharyngeal dysphagia in amyotrophic lateral sclerosis. Dysphagia 25:112–126. 10.1007/s00455-009-9232-1. [DOI] [PubMed] [Google Scholar]
- 50.Matyja A, Thor PJ, Sobocki J, Laskiewicz J, Kekus J, Tuz R, Koczanowski J, Zaraska W. 2004. Effects of vagal pacing on food intake and body mass in pigs. Folia Med Cracov 45:55–62. [PubMed] [Google Scholar]
- 51.McGregor A, Wheless J, Baumgartner J, Bettis D. 2005. Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. Epilepsia 46:91–96. 10.1111/j.0013-9580.2005.16404.x. [DOI] [PubMed] [Google Scholar]
- 52.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. 2015. The infant microbiome development: mom matters. Trends Mol Med 21:109–117. 10.1016/j.molmed.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naseer MI, Bibi F, Alqahtani MH, Chaudhary AG, Azhar EI, Kamal MA, Yasir M. 2014. Role of gut microbiota in obesity, type 2 diabetes, and Alzheimer's disease. CNS Neurol Disord Drug Targets 13:305–311. 10.2174/18715273113126660147. [DOI] [PubMed] [Google Scholar]
- 54.Paulon E, Nastou D, Jaboli F, Marin J, Liebler E, Epstein O. 2017. Proof of concept: short-term noninvasive cervical vagus nerve stimulation in patients with drug-refractory gastroparesis. Frontline Gastroenterol 8:325–330. 10.1136/flgastro-2017-100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelot NA, Grill WM. 2018. Effects of vagal neuromodulation on feeding behavior. Brain Res 1693:180–187. 10.1016/j.brainres.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robbins K, Allen M, Lever TE. 2015. Low copy number SOD1-G93A mice are better suited for dysphagia research compared to the high copy-number model. Amyotroph Lateral Scler Frontotemporal Degener 16 sup1:225–226. DOI: 10.3109/21678421.2015.1098818/0017 [Google Scholar]
- 57.Rodrigues Hoffmann A, Proctor LM, Surette MG, Suchodolski JS. 2015. The microbiome: the trillions of microorganisms that maintain health and cause disease in humans and companion animals. Vet Pathol 53:10–21. 10.1177/0300985815595517. [DOI] [PubMed] [Google Scholar]
- 58.Ryvlin P, Gilliam FG, Nguyen DK, Colicchio G, Iudice A, Tinuper P, Zamponi N, Aguglia U, Wagner L, Minotti L, Stefan H, Boon P, Sadler M, Benna P, Raman P, Perucca E. 2014. The long-term effect of vagus nerve stimulation on quality of life in patients with pharmacoresistant focal epilepsy: the PuLsE (Open Prospective Randomized Long-term Effectiveness) trial. Epilepsia 55:893–900. 10.1111/epi.12611. Erratum. Epilepsia 2014. 55:1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma N, Marzo SJ, Jones KJ, Foecking EM. 2010. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol 223:183–191. 10.1016/j.expneurol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 60.Simon B, Blake J. 2017. Mechanism of action of noninvasive cervical vagus nerve stimulation for the treatment of primary headaches. Am J Manag Care 23:S312–S316. [PubMed] [Google Scholar]
- 61.Strigo IA, Craig AD. 2016. Interoception, homeostatic emotions, and sympathovagal balance. Philos Trans R Soc Lond B Biol Sci 371:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun J, Chang EB. 2014. Exploring gut microbes in human health and disease: pushing the envelope. Genes Dis 1:132–139. 10.1016/j.gendis.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sorensen HT. 2015. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol 78:522–529. 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 64.Toepfer M, Folwaczny C, Klauser A, Riepl RL, Muller-Felber W, Pongratz D. 1999. Gastrointestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:15–19. 10.1080/146608299300079484. [DOI] [PubMed] [Google Scholar]
- 65.Ueda H, Suga M, Yagi T, Kusumoto-Yoshida I, Kashiwadani H, Kuwaki T, Miyawaki S. 2016. Vagal afferent activation induces salivation and swallowing-like events in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 311:R964–R970. 10.1152/ajpregu.00292.2016 [DOI] [PubMed] [Google Scholar]
- 66.Val-Laillet D, Biraben A, Randuineau G, Malbert CH. 2010. Chronic vagus nerve stimulation decreased weight gain, food consumption, and sweet craving in adult obese minipigs. Appetite 55:245–252. 10.1016/j.appet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 67.Wang HX, Wang YP. 2016. Gut–microbiota–brain axis. Chin Med J (Engl) 129:2373–2380. 10.4103/0366-6999.190667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong JN, Olson JL, Morhart MJ, Chan KM. 2015. Electrical stimulation enhances sensory recovery: a randomized controlled trial. Ann Neurol 77:996–1006. 10.1002/ana.24397. [DOI] [PubMed] [Google Scholar]
- 69.Wu S, Yi J, Zhang YG, Zhou J, Sun J. 2015. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan H, Silberstein SD. 2015. Vagus nerve and vagus nerve stimulation, a comprehensive review: part I. Headache 56:71–78. 10.1111/head.12647. [DOI] [PubMed] [Google Scholar]
- 71.Yuan H, Silberstein SD. 2015. Vagus nerve and vagus nerve stimulation, a comprehensive review: part II. Headache 56:259–266. 10.1111/head.12650. [DOI] [PubMed] [Google Scholar]
- 72.Yuan H, Silberstein SD. 2015. Vagus nerve and vagus nerve stimulation, a comprehensive review: part III. Headache 56:479–490. 10.1111/head.12649. [DOI] [PubMed] [Google Scholar]
- 73.Zagon A. 2001. Does the vagus nerve mediate the sixth sense? Trends Neurosci 24:671–673. 10.1016/S0166-2236(00)01929-9. [DOI] [PubMed] [Google Scholar]
- 74.Zhang C, Li S, Yang L, Huang P, Li W, Wang S, Zhao G, Zhang M, Pang X, Yan Z, Liu Y, Zhao L. 2013. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun 4:1–10. 10.1038/ncomms3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang YG, Wu S, Yi J, Xia Y, Jin D, Zhou J, Sun J. 2017. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin Ther 39:322–336. 10.1016/j.clinthera.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]