Abstract
We present the case of a mixed martial arts (MMA) cage fighter who presented to the emergency department with a right sided common carotid artery pseudoaneurysm as a result of a neck trauma at an MMA event. We discuss the management of blunt force neck trauma, differential diagnosis, imaging findings and review the literature on blunt cerebrovascular injury following blunt force injury to the neck.
Keywords: Blunt cerebrovascular trauma, Carotid arterial injury, thyroid horn fracture, pseudoaneurysm, trauma
CASE REPORT
A 36 year old mixed martial arts (MMA) cage fighter presented to the emergency department with shortness of breath. He had received a blunt trauma to the neck from a kick in an MMA fight. Physical examination revealed that he was tachypnoeic with a respiratory rate of 22 breaths per minute and hypoxic with oxygen saturations of 89%. Asymmetrical swelling of the right neck soft tissues was present as was a large right upper chest haematoma. A right sided pharyngeal haematoma was present on evaluation of the oropharynx.
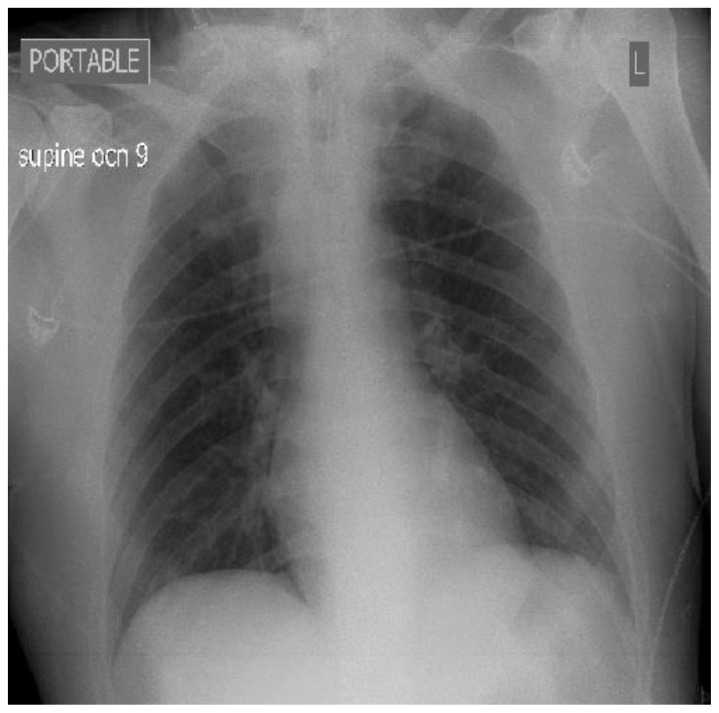
Chest radiograph demonstrated slight widening of the mediastinum (Figure 1).
Figure 1.
36 year old male imaged following right sided blunt neck trauma resulting in a right carotid artery pseudoaneurysm. Portable supine plain film chest x ray demonstrating slight widening of the mediastinum.
Findings: Slight mediastinal widening.
Technique: Portable Supine Plain film chest X-ray, Fujifilm FDR Go system, 80 kVp, 30
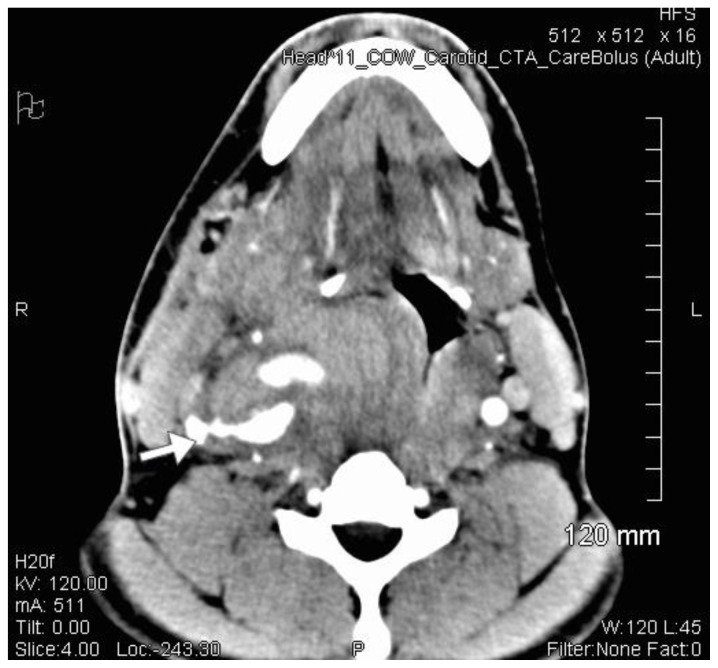
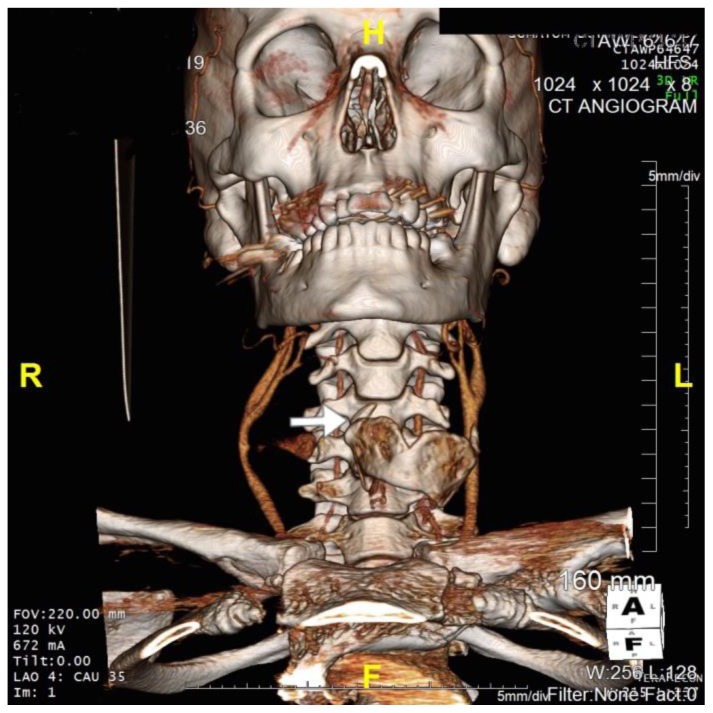
CT carotid angiogram demonstrated a right common carotid artery pseudoaneurysm (Figure 2). 3D CT imaging suggested blunt trauma to the neck had resulted in a thyroid cartilage fracture and a penetrating injury of the right carotid resulting in pseudoaneurysm formation (Figure 3).
Figure 2.
36 year old male imaged following blunt force trauma to right side of the neck. Axial CT Angiogram of the Carotid arteries demonstrating a right sided Carotid artery pseudoaneurysm.
Findings: Right sided carotid artery pseudoaneurysm.
Technique: Siemens SOMATOM 64 slice CT scanner, Axial CT Angiogram, 511mA, Contrast: 80mls Omnipaque 300 at 4.5mls/second, 120kV, 1mm slice thickness
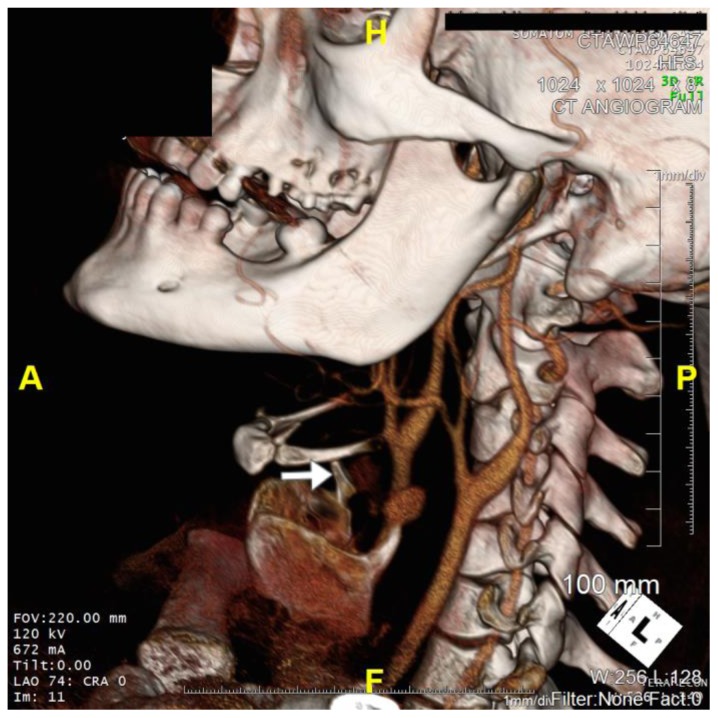
Figure 3.
36 year old male imaged following blunt force trauma to right side of the neck resulting in a right carotid artery pseudoaneurysm.
Findings: Fracture of the right superior horn of the thyroid cartilage (white arrow), with lateral bowing of the common carotid artery proximal to the carotid bifurcation suggesting extramural compression from pseudoaneurysm seen on CTA imaging.
Technique: Siemens SOMATOM 64 slice CT scanner, Coronal 3D CT reformat, 672mA, 120KV, 1mm slice thickness)
There was mild tracheal compression as a result of the mass effect from the adjacent pseudoaneurysm.
Findings were confirmed surgically where the right superior horn of the thyroid cartilage was removed and the pseudoaneurysm was repaired.
The surgery went without complication and the patient went on to make a full recovery both physically and neurologically.
DISCUSSION
Both blunt and penetrating neck trauma may result in carotid arterial injury, including vessel laceration, dissection, occlusion, fistula, or pseudoaneurysm formation. Traumatic internal carotid artery injury is associated with a mortality of up to 40%, with up to 45% of survivors demonstrating a residual neurological deficit [1].
Etiology
Blunt cerebrovascular injury (BCVI) describes a spectrum of carotid arterial intimal injuries. These may occur as the result of direct trauma or intimal stress from neck rotation.
BCVI occurs in approximately 1%(0.18–2.7%) of blunt trauma cases and may affect multiple vessels (18–23%) with a 70% association with cervical spine fractures[2,3,4]. In one series carotid pseudoaneurysm formation was associated with trauma, prior endarterectomy and carotid atherosclerosis in 35.7%, 35.7% and 28.6% of cases respectively[5].
Demographics
Carotid pseudoaneurysm has an incidence of 2.5–3 per 100,000 patient years, with a 1–3% incidence after significant blunt neck trauma. It has a very slight male preponderance (53–57% male: 43–47% female), risk factors for carotid pseudoaneurysm formation are trauma (blunt, penetrating), hypertension, atherosclerosis, Ehlers Danlos syndrome, Marfans syndrome, Osteogenesis Imperfecta, Fibromuscular dysplasia, Hyperhomocystinemia, large vessel cerebral vasculitis and instrumentation/surgery of the carotid arteries.
A review of 82 patients with carotid arterial trauma in 1995 demonstrated mortality and stroke rates of 21% and 41% respectively in patients with internal carotid artery injuries and 11% stroke rate in those with common carotid arterial injuries[6].
BCVI may cause dissection of the common carotid artery, which may evolve into a pseudoaneurysm in up to 30% of patients[7]. On review of the literature review from 1970 to date on Pubmed and Embase revealed a total of 42 cases of post traumatic carotid artery pseudoaneurysm formation, with the common carotid involved in 24% of patients, and the internal carotid involved in the remaining 76% of cases[8,9].
Clinical Findings
This case was unusual in that it was ‘mixed’ pathogenesis where the blunt trauma had in-fact resulted in a thyroid cartilage fracture which in turn lead to carotid artery pseudoaneurysm formation, which would be more in keeping with a penetrating injury pattern than a direct blunt force injury. Clinical findings in cases of carotid pseudoaneurysm can vary considerably depending on aetiology, with the 30 day risk of transient ischaemic attack/stroke/death reported at between 0–23% and the risk of cranial nerve injury reported between 2.2–44%, there is also a small risk of delayed stroke (after 30 days) reported between 6.2–8%[5].
Imaging Findings
Ultrasound findings
A pseudoaneurysm is formed when a breach in the blood vessel wall allows blood to leak through the wall and become contained in the adventitia. An aneurysm, however is an abnormal dilation of all 3 layers of the blood vessel wall. Pseudoaneurysms are generally at a higher risk of rupture as there is less support for the vessel wall and there is a direct communication of blood flow between the vessel wall and the aneurysm wall. A pseudoaneurysm results in turbulent flow within the wall of the abnormality resulting in non laminar ultrasound flow signal, most of the characteristic sonographic signs of this pathology are based on detection of this flow disturbance within the wall of the pseudoaneurysm. Several studies have confirmed the diagnostic validity of using Duplex ultrasound for the detection of arterial lesions due to trauma in the neck with sensitivity between 92–100%[10,11]. There can be characteristic forward and backward swirling blood flow. On colour Doppler a characteristic “ying yang sign”, caused by a communicating channel between the parent artery resulting in swirling pattern of flow is typical and on pulsed Doppler imaging there can be occasional “to and fro” pattern of flow[12].
CT findings
Non enhanced CT may demonstrate hypoattenuating smooth walled sac adjacent to an artery usually with communication, with occasional intermediate/high attenuation material (haemorrhage) adjacent to the pseudoaneurysm indicating pseudoaneurysm rupture. CT brain may also indicate evidence of cerebral ischaemia/infarction due to rupture of the pseudoaneurysm or associated vessel stenosis/thrombosis. CT angiography is the preferred diagnostic modality to make the radiological diagnosis of carotid pseudoaneurysm[13]. In particular, the “yin-yang” sign is a finding that may be seen on contrast enhanced CT scans and is often helpful in differentiating a partially thrombosed aneurysm from other pathology[14].
MRI findings
MRI imaging may demonstrate intramural thrombus formation, allowing assessment of the pseudoaneurysm sac size and detection of extramural haemosiderin deposition, indicative of active/previous haemorrhage/extravasation. Depending on the age of haemorrhage/blood products, the pattern of signal attenuation on T1and T2 weighted images is: hyper acute (bleeding less than 24 hours old): T1 isointense, T2 hyper intense, T2*GRE Hyperintense, Acute haemorrhage (bleeding 24 – 72 hours in age) T1 Hypointense becoming progressively hyperintense, T2 hypointense, Early subacute bleeding (from day 3 – 7 days post bleed) T1 hyper intense, T2 hypointense, Late subacute bleeding (from day 7–14) T1 hyperintense, T2 hyperintense, Chronic blood (>14 days in age) T1 iso or hypointense signal, T2 hypointense. Magnetic resonance angiography may be used to assess pseudoaneurysm anatomy in certain instances, for example, in patients with an allergy to iodinated contrast media or those with a contraindication to CT imaging. Vessel Flow voids on T2 weighted imaging may be used to predict vessel patency/degree of luminal occlusion/arterial blood flow[15].
Angiographic findings
Formal angiography can be useful in delineating vascular anatomy including feeding vessels, vascular outflow, vessel occlusion and size of pseudoaneurysm. It may demonstrate partial thrombosis of the pseudoaneurysm, arteriovenous fistula if present, active contrast blush indicative of active extravasation into the soft tissues surrounding the pseudoaneurysm. It may also be used as a treatment modality in the form of stent placement or endovascular closure[16].
Treatment & Prognosis
This patient underwent open primary repair of the traumatic pseudoaneurysm with resection and primary anastomosis. The fractured segment of the thyroid cartilage was removed at time of operation. The patient made a full clinical recovery without neurological sequelae. There are several surgical approaches to repair of carotid artery pseudoaneurysms which have been described since Sir Astley Cooper’s first description of the method of proximal artery ligation, including resection with primary anastomosis, clipping, resection with saphenous venous bypass, resection with prosthetic bypass, plication and patch angioplasty and Innominate to common carotid bypass[5,17]
Differential Diagnosis
Carotid artery pseudoaneurysm has many clinical mimics. An important part of the pre-imaging work up includes identifying a history of neck trauma, atherosclerosis or instrumentation of the neck vessels, however, it is important to exclude other diagnoses which may be pertinent including: Carotid artery aneurysm, Carotid artery dissection, carotid artery blowout syndrome/carotid artery rupture, neck haematoma, carotid body tumour/glomus tumour/chemodectoma, carotid-jugular AV fistula, branchial cleft cyst, lymphoma, reactive lymphadenopathy and neurogenic tumours.
BCVI may be delayed with non-specific signs/symptoms including:
Arterial haemorrhage
Cervical bruit in a patient < 50
Expanding cervical haematoma
Focal neurologic deficit
Neurologic examination incongruous with head CT scan findings
Stroke on secondary CT scan
The Modified Denver screening criteria was developed to identify patients who should be screened for BCVI, and the BIFFL scale describes the arterial injury on CT Angiogram or Digital Subtraction Angiography (DSA)[18,19,20]. The BIFFL scale classifies the spectrum of BCVI, ranging from grade 1 (mild intimal injury or irregular intima) to grade V (complete vessel transection)
Carotid arterial aneurysm
Carotid arterial aneurysm is an important differential diagnosis when considering carotid artery pseudoaneurysm as it can have a significant morbidity/mortality if missed or incorrectly diagnosed. Ultrasound features of carotid artery aneurysm include: irregular widening of the carotid artery with an adjacent mixed-echoic oval lesion indicative of the aneurysmal sac, with turbulent flow demonstrated in the aneurysmal artery, with or without thrombosis/partial thrombosis of the aneurysmal sac. CT brain/neck (unenhanced) may demonstrate evidence of stroke/cerebral ischaemia, saccular/calcified mass adjacent/contiguous with the carotid indicative of aneurysmal sac and is useful in the detection of haemorrhage. CT angiography is very useful in the diagnosis demonstrating aneurysmal dilatation of the carotid artery (either saccular or fusiform enlargement), with or without evidence of mural thrombus. 3D CT reformats allow better anatomical localisation and peri-operative planning for the surgical team. MRI can be useful in the diagnosis and management where CT is contraindicated or the patient has an allergy to iodinated contrast media. MRI demonstrates dilation of the vessel lumen with high signal within the artery. Low signal mural thrombus may be present. Coronal MIP images taken from TOF vascular imaging demonstrate the fusiform/saccular dilation of the vascular anatomy. Some centres use formal angiography as their gold standard for the diagnosis and management of carotid artery aneurysms, especially where endovascular approaches are favoured. Formal angiography/Digital Subtraction Angiography demonstrate the exact size, anatomy, location, orientation of the aneurysmal dilatation and allows visualisation of the feeding vessels and the distal vasculature precisely.
Carotid artery dissection
Carotid artery dissection is an important diagnosis for the radiologist to be cognizant of in cases of blunt cerebrovascular injury/blunt force injury to the neck. Ultrasonography may demonstrate absent internal carotid artery flow in high grade stenosis, biphasic flow in the carotid bulb, high resistance flow pattern in the ipsilateral ICA or evidence of collateral flow across the Circle of Willis on transcranial Doppler ultrasound. Non enhanced CT imaging: may demonstrate cerebral infarction/ischaemia suggestive of the vascular occlusion/narrowing proximal to the ischaemic event or hyper attenuating focus in the upper ICA indicative of extramural haematoma.
CT angiography may demonstrate an abnormal vessel contour, narrowed eccentric lumen surrounded by a crescent shaped mural thrombus, intimal flap or dissecting aneurysm/associated pseudoaneurysm.
Magnetic resonance imaging is useful, particularly T1 fat saturation/T2weighted imaging sequences, demonstrating high signal crescent sign in the wall of the artery, absent blood flow, evidence of cerebral ischaemia, abnormal vessel contour on MRA/maximum intensity projection (MIP). Formal carotid angiography/Digital Subtraction Angiography may demonstrate abnormalities of the vessel wall, string sign (thinning/narrowing of the luminal diameter), string and pearl sign (tapering of the lumen following a widened/normal luminal diameter) or pseudo aneurysm formation
Carotid artery blowout syndrome/rupture
Carotid artery blowout syndrome is a life threatening emergency and must be dealt with expediently to avoid permanent patient disability or death. Often times neck imaging will not be available and the diagnosis will be made clinically on the ward (without imaging), with evidence of a rapidly enlarging neck mass in association with localising neurological signs/stroke or decreased GCS. This must be treated as a surgical/endovascular emergency as the risk of permanent neurological sequelae increases with time to intervention. Ultrasound findings may show an extraluminal pattern of blood flow on Doppler imaging (indicative of active extravasation). CT brain/neck (non-enhanced): may demonstrate evidence of neck mass adjacent to carotid indicative of haemorrhage. There may be features of cerebral ischaemia or lobar infarction. CT angiography may demonstrate contrast extravasation into the soft tissues surrounding the carotid/tracking of contrast inferiorly into the mediastinum, with narrowing of the lumen of the involved carotid artery due to extramural compression by the enlarging haematoma.
MR imaging is not recommended in the acute setting due to the acquisition time, but, it may demonstrate T1 isointense/T2 iso/hyperintensity surrounding the carotid/carotid sheath region or obscure the image of the normal carotid anatomy. MR angiography may demonstrate active gadolinium extravasation outside of the limits of the vessel wall. Formal angiography/Digital Subtraction Angiography: may show rupture of the carotid artery and active extravasation of contrast which may be used for treatment/covered stent placement.
Neck haematoma
Neck haematoma may occur in cases of blunt cerebrovascular injury and are challenging as they can obscure/distort vascular anatomy. It is important to delineate where the bleeding is arising from, be it acute haemorrhage or quiescent haemorrhage and anatomically whether it is arising from a small branch of the external carotid artery, branch vessel arterial haemorrhage or venous haemorrhage. Ultrasound: dependent on the age of haematoma findings may differ; acute haematoma: liquid/hypoechoic mass in the neck adjacent to the carotid artery. CT brain/neck (unenhanced): evidence of neck mass adjacent to carotid indicative of haemorrhage with or without associated mass effect.
CT Angiography: may demonstrate active extravasation into the neck tissues
MRI: dependent on the age of haemorrhage, acute haematoma; surrounding the carotid/carotid sheath region.
Formal angiography/DSA: may locate active arterial bleed/source of haemorrhage, as a possible treatment option in terms of embolisation/coiling the source of the bleeding.
Carotid body tumour/glomus tumour/chemodectoma
Carotid body mass lesions may, rarely, be mistaken for a structural abnormality of the carotid artery, therefore the context in which the imaging request occurs is essential to the correct work up and diagnosis. The carotid body is a small highly specialised organ located at the bifurcation of the common carotid artery in the neck and plays an important role in acute adaptation to hypoxia or physiological changes in response to changes in altitude. Although slow-growing and benign, they can cause significant morbidity because of their proximity to major arteries and nerves in the head and neck and can result in extramural compression of the carotid arteries, hypoglossal or glossopharyngeal nerves in the neck[21].
Ultrasound imaging demonstrates a hypoechoic/weakly echogenic neck mass adjacent to the carotid vessels. Doppler imaging demonstrates increased vascularity within the lesion. CT brain/neck (unenhanced) may show a soft tissue density with splaying of the ICA and ECA. CT Angiography may demonstrate bright, early enhancement of lesion with splaying of the carotid artery. MRI is diagnostic with T1 Iso/hypointensity to muscle signal, “salt and pepper” appearance is pathognomonic, with intense enhancement following gadolinium administration. Formal angiography/Digital Subtraction Angiography: splaying/widening of the gap between the internal/external carotid vessels, known as the “lyre sign” (instrument held by statue of Apollo), with the ascending pharyngeal artery being main vascular supply to these lesions[22, 23].
Carotid-jugular AV fistula
Carotid-jugular arteriovenous fistulae are uncommon arteriovenous shunts following blunt force trauma to the neck. They are rarely detected within the acute phase following injury and they are especially prone to complications such as high output cardiac failure, atrial fibrillation and distal embolisation. The presence of a continuous thrill or bruit in the neck, high output cardiac failure or the Nicoladoni sign (slowing of the heart with compression of the arteriovenous fistula) should increase the clinical suspicion for the presence of an arteriovenous fistula[24,25,26]. Ultrasound may show Doppler signal with anterograde flow from carotid artery to internal jugular vein or branch vessel, high velocity turbulent flow within the fistula and an arterialised waveform within the Internal jugular vein, can provide haemodynamic information regarding fistula (e.g. velocity). CT brain/neck (unenhanced) may demonstrate dilation of the internal jugular vein due to expansion from high velocity flow from the adjacent carotid. CT angiography will demonstrate contrast flowing from the carotid artery in the arterial phase of the scan into the internal jugular vein (early appearance of contrast in the vein in the arterial phase), which is diagnostic.
MR: FLAIR sequence imaging may reveal venous congestion in the cerebral parenchyma, MR Angiography demonstrates arteriovenous shunting with early gadolinium enhancement in the internal jugular vein in the arterial phase. Formal angiography/DSA will provide the diagnosis of arteriovenous shunting with a dilated tortuous, arterialised vein, also providing the possibility for coil embolisation of the fistulous connection.
Branchial cleft cyst
Ultrasound imaging will demonstrate a sharply demarcated cystic lesion with posterior acoustic enhancement (70%), imperceptible cyst walls, with variable echogenicity (branchial cleft cysts are anechoic in 41% of cases). CT brain/neck (unenhanced) demonstrates a sharply circumscribed, well demarcated cystic structure, thin wall, fluid density inside cyst, “tail-sign” extension of the cyst wall between the ECA and ICA may be seen.
MR imaging demonstrates: T1: Variable signal, T2: hyperintense (high fluid content), T1 + contrast: no enhancement of lesion.
Lymphoma
Ultrasonography demonstrates a well defined, hypo echoic/hyperechoic mass, with occasional central necrotic region which appears ill defined/echo lucent, hilar and peripheral doppler signal indicative of hilar and peripheral vascularity. Nodal vascularity can be used as a conjugate measure of response to systemic treatment.
CT brain/neck: demonstrates a solid/cystic mass lesion; the mainstay imaging modality, used for staging disease (using the Lugano classification), provides descriptive information regarding tumour bulk, lymph node diameter, FDG PET-CT (fusion imaging) used to assess response to treatment.
MRI: used in the assessment of CNS lymphoma for determination of extent/anatomy of disease distribution. Head and neck lymphomas demonstrate low signal attenuation on T1 imaging/low to high intensity on T2 weighted imaging/low enhancement following gadolinium administration.
Reactive lymphadenopathy
Ultrasound demonstrates a hypoechoic, echogenic hilus, predominant hilar vascularity, short axis–to–long axis ratio [S/L] < 0.5 (except submandibular and parotid groups), when the ratio is > 0.5 there is a high probability of sinister aetiology. Maximum limit for axial diameter of normal and reactive nodes is 9 mm for subdigastric and submandibular nodes and 8 mm for all other neck nodal groups. Loss of fatty hilum, focal necrosis, cystic change with necrosis are independent predictors of non reactive aetiology.
CT brain/neck (unenhanced): demonstrated by ill defined mass in one or more of the lymph node areas of the neck, either a discrete mass 10–15mm in diameter, or, multiple nodes 6–15mm in diameter. Occasional necrotic/suppurative nodal disease dependent on aetiology. May have associated neck fascial/retropharyngeal space abscess, particularly, if associated with inoculation/infection following penetrating injury.
MRI: T1 weighted imaging demonstrates intermediate nodal intensity, high T2* signal intensity in reactive/inflamed nodes, strong post contrast enhancement is seen and occasional rim enhancement is specific for suppurative lymphadenitis.
Neurogenic tumours
Ultrasound: well-defined, ovoid or round hypoechoic mass, in association with neural tissue/nerves, occasional loculation/cystic changes, rare vascularity. CT brain/neck: mass lesion, well circumscribed, adjacent to/continguous with nerve tissue with low level contrast enhancement (reflects the predominance of Antoni B components), cystic components most specific for Antoni A type, occasional low density material within the centre of the mass (20–30 HU) vs. higher density at the periphery (60–70 HU).
MRI: Homogenous, isointense signal to muscle on T1 imaging, T2 weighted imaging demonstrates peripheral high intensity and central relatively low intensity, occasional “target sign” on T1 imaging post gadolinium demonstrating central high intensity by focal enhancement and peripheral low intensity.
We performed a literature review over the past 45 years revealed three cases of hyoid bone induced carotid arterial injury. No cases of carotid injury as a result of thyroid cartilage fracture were identified, hence to the authors knowledge this is a unique case in the radiology literature, particularly given the aetiology of mixed martial arts blunt force trauma[27].
MMA is increasingly popular at amateur and professional levels. Sadly it has resulted in significant morbidity and mortality. Frequently the ‘target’ of these fights includes the head and neck area. It is likely we shall all see more of this and it is important to be cognizant that the blunt forces upon which it is based can lead to ‘mixed’ blunt and penetrating injuries.
Conclusion
Carotid arterial injury most commonly occurs as a result of penetrating neck trauma. Other potential causes include iatrogenic injury, blunt trauma, atherosclerosis and vasculitis. In young patients with a recent history of blunt neck trauma presenting with asymmetrical neck swelling, carotid arterial injury in association with injury to the structures of the hypopharynx should be considered as they can cause unusual injury patterns.
TEACHING POINT
In blunt trauma to the neck in young patients presenting with asymmetrical neck swelling, carotid artery injury in association with injury to the structures of the hypopharynx should be considered as they can cause unusual injury patterns.
Table 1.
Summary table for carotid pseudoaneurysm.
| Definition | A pseudoaneurysm is formed when a breach in the blood vessel wall allows blood to leak through the wall and become contained in the adventitia. An aneurysm, however is an abnormal dilation of all 3 layers of the blood vessel wall. Pseudoaneurysms are generally at a higher risk of rupture as there is less support for the vessel wall and there is a direct communication of blood flow between the vessel wall and the aneurysm wall. |
| Etiology |
|
| Incidence | 2.5–3/100,000, 1–3% following significant blunt neck trauma |
| Gender ratio | 53–57% male: 43–47% female (slight male preponderance) |
| Risk factors |
|
| Treatment |
|
| Prognosis |
|
| Imaging findings |
|
Table 2.
Differential diagnosis table for carotid pseudoaneurysm.
| Diagnosis | Clinical features | Ultrasound findings | CT findings | MRI findings | Angiography/DSA findings |
|---|---|---|---|---|---|
| Carotid Pseudoaneurysm | Blunt force/penetrating injury, neck haematoma/swelling, oropharyngeal swelling, stroke/TIA/cranial nerve deficits | Colour Doppler Ultrasound: “to and fro” waveform, variable echogenicity, extraluminal pattern of blood flow | CT Neck: high attenuation material adjacent to the pseudo aneurysm CTA Carotid: Contrast material enhanced pseudoaneurysm sac, bowing of the normal anatomy, abnormality of the normal vascular lumen of the carotid artery |
MRI/MRA Carotid: may demonstrate intramural thrombus formation, allows assessment of the pseudoaneurysm sac size and detection of extramural haemosiderin deposition, indicative of previous haemorrhage/ extravasation. Please see Imaging findings section of the text for details on dating haemorrhage | Formal Angiography/DSA: Saccular pseudoaneurysm, assessment of diameter, feeding vessels/distal vessels, narrowing of the ICA/ECA, active extravasation |
| Carotid Artery Dissection | History of neck trauma or sudden shearing force to neck, post endarterectomy, TIA/stroke/carotid occlusion | Ultrasound: may demonstrate absent internal carotid artery flow in high grade stenosis, biphasic flow in the carotid bulb, high resistance flow pattern in the ipsilateral ICA or evidence of collateral flow across the Circle of Willis on transcranial Doppler ultrasound | CT brain/neck(unenhanced): may demonstrate cerebral infarction/ischaemia or hyper attenuating focus in the upper ICA indicative of extramural haematoma CTA: may demonstrate abnormal vessel contour, narrowed eccentric lumen surrounded by present shaped mural thrombus, intimal flap, dissecting aneurysm |
MRI: (T1 fat saturation/T2weighted imaging) High signal crescent sign in the wall of the artery, absent blood flow, cerebral ischaemia, abnormal vessel contour on MRA/ maximum intensity projection (MIP) | Formal angiography/DSA: abnormalities of the vessel wall, string sign, string and pearl sign, pseudo aneurysm formation |
| Carotid Artery Aneurysm | Post blunt force injury to neck/mycotic aneurysm/post instrumentation, pulsatile swelling/mass, embolic phenomena/TIA/Stroke. Rupture - rapidly expanding neck mass | Ultrasound: irregular widening of the carotid artery with an adjacent mixed-echoic oval lesion indicative of the aneurysmal sac, with turbulent flow demonstrated in the aneurysmal artery, with or without thrombosis/partial thrombosis of the aneurysmal sac | CT brain/neck (unenhanced): evidence of stroke/cerebral ischaemia, saccular/calcified mass adjacent/contiguous with the carotid indicative of aneurysmal sac, useful in the detection of haemorrhage CTA: aneurysmal dilatation of the carotid artery (either secular or fusiform enlargement), with or without evidence of mural thrombus. 3D CT reformats allow better anatomical localisation and peri-operative planning |
MRI: Dilation of the vessel lumen with hyperattenuation of the artery. Low signal mural thrombus may be present. Coronal MIP images taken from TOF vascular imaging demonstrate the fusiform/saccular dilation of the vascular anatomy | Formal angiography/DSA: demonstrate the exact size, anatomy, location, orientation of the aneurysmal dilatation. Allows visualisation of the feeding vessels and the distal vasculature precisely |
| Carotid blowout syndrome/Carotid artery rupture | Post instrumentation/ trauma in patient with underlying mural defect in the carotid artery, presents with rapid exsanguination and swelling of the neck, TIA/Stroke/Death if not treated immediately | Ultrasound: Extraluminal pattern of blood flow on Doppler imaging | CT brain/neck (unenhanced): evidence of neck mass adjacent to carotid indicative of haemorrhage, may be features of cerebral ischaemia/infarction CTA: contrast extravasation into the soft tissues surrounding the carotid/tracking of contrast inferiorly into the mediastinum |
MRI: not recommended in the acute setting, evidence of blood products around the carotid dated as per the imaging findings section of the text | Formal angiography/DSA: rupture of the carotid artery and active extravasation of contrast |
| Neck haematoma | May result from any of the above aetiologies, post trauma to the carotid or a branch vessel, may be asymptomatic if small. If large may cause compressive effects on the carotid artery resulting in stroke, or the Internal jugular vein resulting in venous /occlusion/infarction or airway compromise | Ultrasound: dependent on the age of haematoma; acute haematoma: liquid/hypoechoic mass in the neck adjacent to the carotid artery | CT brain/neck (unenhanced): evidence of neck mass adjacent to carotid indicative of haemorrhage with or without associated mass effect CTA: may demonstrate active extravasation into the neck tissues |
MRI: dependent on the age of haemorrhage, may be used to assess and date age of blood products as outlined in the Imaging findings section of the text | Formal angiography/DSA: may locate active arterial bleed/source of haemorrhage |
| Carotid Body tumour/Glomus tumour/Chemodectoma | Present as a mass in the neck resulting in unilateral paralysis of the tongue, tinnitus, globus sensation, in many instances are incidentally discovered on imaging for other clinical indications | Ultrasound: hypoechoic/weakly echogenic neck mass adjacent to the carotid vessels. Doppler imaging demonstrates increased vascularity within the lesion | CT brain/neck (unenhanced): soft tissue density, bright, early enhancement, splaying of the ICA and ECA CTA: bright, early enhancement of lesion with splaying of the carotid artery |
MRI: T1: Iso/hypointense to muscle signal, “salt and pepper” appearance, intense enhancement following gadolinium enhancement | Formal angiography/DSA: splaying of the carotid vessels, lyre sign, ascending pharyngeal artery is main vascular supply |
| Branchial cleft cyst | Congenital defect of branchial cleft development in utero, present with transilluminable lateral neck mass, may present with abscess or infection | Ultrasound: sharply demarcated, posterior acoustic enhancement (70%), imperceptible cyst walls, variable echogenicity (anechoic 41%) | CT brain/neck (unenhanced): sharply circumscribed, well demarcated cystic structure, thin wall, fluid density inside cyst, “tail-sign” extension of the cyst wall between the ECA and ICA | MRI: T1: Variable signal, T2: hyperintense, T1 + contrast: no enhancement | not applicable |
| Carotid Jugular AV fistula | Post trauma to the neck, particularly where there is trauma to the carotid, post carotid artery aneurysm or carotid blowout syndrome. May present with tinnitus, headache, vertigo, cranial nerve deficits or venous haemorrhage | Ultrasound: Doppler signal with anterograde flow from carotid artery to internal jugular vein or branch vessel, high velocity turbulent flow within the fistula and an arterialised waveform within the Internal jugular vein, can provide haemodynamic information regarding fistula (e.g. velocity) | CT brain/neck (unenhanced): dilation of the internal jugular vein CTA: will demonstrate contrast flowing from the carotid artery in the arterial phase of the scan into the internal jugular vein (early appearance of contrast in the vein in the arterial phase) |
MRI: FLAIR MR imaging may reveal venous congestion in the cerebral parenchyma, MRA demonstrates arteriovenous shunting with early gadolinium enhancement in the internal jugular vein in the arterial phase | Formal angiography/D SA: arteriovenous shunting with a dilated tortuous, arterialised vein |
| Lymphoma | Presents with unilateral enlarged neck mass along classical cervical lymph node groups, may present as multifocal lymphadenopathy, B symptoms; drenching night sweats, weight loss, cachexia. May also involve paratracheal nodal groups | Ultrasound: well defined, hypo echoic/hyperechoic mass, with occasional central necrotic region which appears ill defined/echo lucent, hilar and peripheral doppler signal indicative of hilar and peripheral vascularity. Nodal vascularity can be used as a conjugate measure of response to systemic treatment. | CT brain/neck: demonstrates a solid/cystic mass lesion; the mainstay imaging modality, used for staging disease (using the Lugano classification), provides descriptive information regarding tumour bulk, lymph node diameter, FDG PET CT (fusion imaging) used to assess response to treatment. | MRI: used in the assessment of CNS lymphoma for determination of extent/anatomy of disease distribution. Head and neck lymphomas demonstrate low signal attenuation on T1 imaging/low to high intensity on T2 weighted imaging/low enhancement following gadolinium administration |
not applicable |
| Neurogenic tumours | May present with compressive symptoms in the neck, e.g. radiculopathy, localised pain, or may invade into bony structures resulting in architectural compromise. May be incidentally discovered on imaging performed for another indication. | Ultrasound: well-defined, ovoid or round hypoechoic mass, in association with neural tissue/nerves, occasional loculation/cystic changes, rare vascularity | CT brain/neck: mass lesion, well circumscribed, adjacent to/contiguous with nerve tissue with low level contrast enhancement (reflects the predominance of Antoni B components), cystic components most specific for Antoni A type, occasional low-density material within the centre of the mass (20–30 HU) vs higher density at the periphery (60–70 HU) | MRI: Homogenous, isointense signal to muscle on T1 imaging, T2 weighted imaging demonstrated peripheral high intensity and central relatively low intensity, occasional “target sign” on T1 imaging post gadolinium demonstrating central high intensity by focal enhancement and peripheral low intensity | not applicable |
| Reactive lymphadenopathy | May present with prodromal viral or bacterial illness, may be unofficial or multifocal, may be suppurative in the case of tuberculosis or pyogenic bacteria strains. May present with compressive symptoms associated with mass effect. May coalesce to form an organised collection require percutaneous drainage. | Ultrasound: hypoechoic, echogenic hilus, predominant hilar vascularity, short axis–to–long axis ratio [S/L] < 0.5 (except submandibular and parotid groups), when the ratio is > 0.5 there is high probability of sinister aetiology. Max limit for axial diameter of normal and reactive nodes is 9 mm for subdigastric and submandibular nodes and 8 mm for all other neck nodal groups. Loss of fatty hilum, focal necrosis, cystic change with necrosis are independent predictors of non-reactive aetiology. | CT brain/neck (unenhanced): demonstrated by ill-defined mass in one or more of the lymph node areas of the neck, either a discrete mass 10–15mm in diameter, or, multiple nodes 6–15mm in diameter. Occasional necrotic/suppurative nodal disease dependent on aetiology. May have associated neck fascial/ retropharyngeal space abscess, particularly, if associated with inoculation/infection following penetrating injury |
MRI: T1 weighted imaging demonstrates intermediate nodal intensity, high T2 signal intensity in reactive/inflamed nodes, strong post contrast enhancement is seen, and occasional rim enhancement is specific for suppurative lymphadenitis | not applicable |
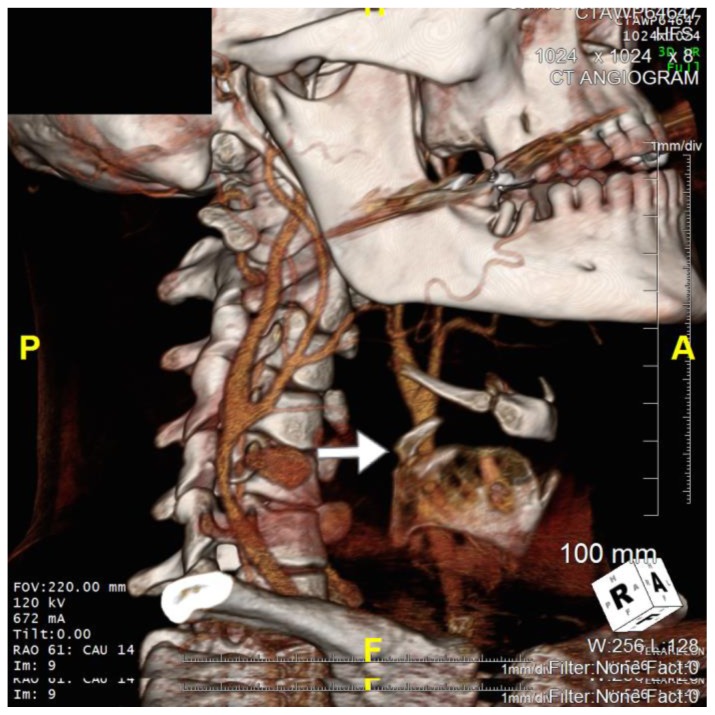
Figure 4.
36 year old male imaged following blunt force trauma to right side of the neck resulting in a right carotid artery pseudoaneurysm.
Findings: Fracture of the right superior horn of the thyroid cartilage (white arrow), with lateral bowing of the common carotid artery proximal to the carotid bifurcation suggesting extramural compression from the pseudoaneurysm seen on CTA imaging
Technique: Siemens SOMATOM 64 slice CT scanner, Right Anterooblique (RAO) view, Axial 3D CT reformat, 672mA, 120kV, 1mm slice thickness, Tilt 0 degrees, RAO 61 degrees, CAU 14 degrees)
Figure 5.
36 year old male imaged following blunt force trauma to right side of the neck resulting in a right carotid artery pseudoaneurysm.
Findings: Fracture of the right superior horn of the thyroid cartilage (white arrow), with lateral bowing of the common carotid artery proximal to the carotid bifurcation suggesting extramural compression from the pseudoaneurysm seen on CTA imaging
Technique: Siemens SOMATOM 64 slice CT scanner, Axial Left Anterooblique (LAO) projection 3D CT reformat, 672mA, 120kV, 1mm slice thickness, Tilt 0 degrees, LAO 74 degrees, Cranial 0 degrees)
ACKNOWLEDGEMENTS
Special thanks to Professor Leo P. Lawler
ABBREVIATIONS
- BCVI
Blunt Cerebrovascular Injury
- CNS
Central Nervous System
- CT
Computed Tomography
- CTA
Computed Tomography Angiography
- DSA
Digital Subtraction Angiography
- ECA
External Carotid Artery
- FLAIR
Fluid Attenuated Inversion Recovery
- HU
Hounsfield Units
- ICA
Internal Carotid Artery
- IJV
Internal Jugular vein
- MMA
Mixed Martial Arts
- MRI
Magnetic Resonance Imaging
- PET
Positron Emission Tomography
- TOF
Time of Flight
REFERENCES
- 1.Welling R, Kakkasseril J, Peschiera J. Management of blunt injury to the internal carotid artery. J Trauma. 1987;27:1221–6. doi: 10.1097/00005373-198711000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Schneidereit NP, Simons R, Nicolaou S, et al. Utility of screening for blunt vascular neck injuries with computed tomographic angiography. J Trauma. 2006;60(1):209–15. doi: 10.1097/01.ta.0000195651.60080.2c. 5. [DOI] [PubMed] [Google Scholar]
- 3.Sliker CW. Blunt cerebrovascular injuries: imaging with multidetector CT angiography. Radiographics. 2008;28(6):1689–708. doi: 10.1148/rg.286085521. [DOI] [PubMed] [Google Scholar]
- 4.Fox C, Gillespie D, Weber M, et al. Delayed evaluation of combat related penetrating neck trauma. J Vasc Surg. 2006;44:86–92. doi: 10.1016/j.jvs.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 5.Garg Karan, et al. Presentation and management of carotid artery aneurysms and pseudo aneurysms. J Vasc Surg. 2012;55(6):1618–1622. doi: 10.1016/j.jvs.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 6.Cogbill TH, Moore EE, Meissner M, et al. The spectrum of blunt injury to the carotid artery: a multicenter perspective. J Trauma. 1994;37:473–9. doi: 10.1097/00005373-199409000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252–8. doi: 10.1097/BSD.0b013e3180cab162. [DOI] [PubMed] [Google Scholar]
- 8.Ramadam F, Rutledge R, Oller D, Howell P, Baker C, Keagy B. Carotid Artery Trauma: A review of contemporary trauma centre experiences. J Vasc Surg. 1995 Jan;21(1):46–55. doi: 10.1016/s0741-5214(95)70243-1. discussion 55–67. [DOI] [PubMed] [Google Scholar]
- 9.Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Elliott JP, et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg. 1999;178(6):517–22. doi: 10.1016/s0002-9610(99)00245-7. (Level III evidence) [DOI] [PubMed] [Google Scholar]
- 10.Montalvo BM, et al. Color Doppler songraphy in penetrating injuries of the neck. AJNR Am J Neuroradiol. 1996;17:943–951. [PMC free article] [PubMed] [Google Scholar]
- 11.Ginzberg E, Montalvo B, LeBlang S, Nunez D, Martin L. The use of diplex ultrasonography in penetrating neck trauma. Arch Surg. 1996;131:691–693. doi: 10.1001/archsurg.1996.01430190013002. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoud MZ, et al. “ To-and-fro” waveform in the diagnosis of arterial pseudoaneurysms. World J Radiol. 2015;7(5):89–99. doi: 10.4329/wjr.v7.i5.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson SE, et al. Imaging features of pseudo aneurysms of the hand in Children and Adults. AJR Am J Roentgenol. 2003;180(3):659–64. doi: 10.2214/ajr.180.3.1800659. [DOI] [PubMed] [Google Scholar]
- 14.Lupattelli T. The Yin Yang Sign. Radiology. 2006;238:1070–1071. doi: 10.1148/radiol.2383031884. [DOI] [PubMed] [Google Scholar]
- 15.Phan T, Huston J, Bernstein MA, Riederer SJ, Brown RD. Contrast-enhanced magnetic resonance angiography of the cervical vessels: experience with 422patients. Stroke. 2001;32:2282–6. doi: 10.1161/hs1001.096046. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, et al. Treatment of traumatic internal carotid artery pseudoaneurysms with the Willis covered stent: a prospective study. J Trauma. 2011 Apr;70(4):816–22. doi: 10.1097/TA.0b013e3181f892af. [DOI] [PubMed] [Google Scholar]
- 17.Painter TA, Hertzer NR, Beven EG, O’Hara PJ. Exracranial carotid aneurysms: a report of six cases and review of the literature. J Vasc Surg. 1985;2:312–318. [PubMed] [Google Scholar]
- 18.Bromberg WJ, Collier BC, Diebel LN, Dwyer KM, Holevar MR, Jacobs DG, et al. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma. 2010;68(2):471–7. doi: 10.1097/TA.0b013e3181cb43da. (Evidence based guideline) [DOI] [PubMed] [Google Scholar]
- 19.Cothren CC, Moore EE, Biffl WL, Ciesla DJ, Ray CE, Johnson JL, et al. Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg. 2004;139(5):540–5. doi: 10.1001/archsurg.139.5.540. discussion 545–6. [DOI] [PubMed] [Google Scholar]
- 20.Foreman PM, et al. Reliability assessment of the Biffl Scale for blunt traumatic cerebrovascular injury as detected on computer tomography angiography. J Neurosurg. 2017 Jul;127(1):32–35. doi: 10.3171/2016.7.JNS16849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baysal B, Myers EM. Etiopathogenesis and clinical presentation of carotid body tumors. Microscopy Research and Technique. 2002;59(3):256–261. doi: 10.1002/jemt.10200. [DOI] [PubMed] [Google Scholar]
- 22.Netterville JL, Reilly KM, Robertson D, Reiber ME, Armstrong WB, Childs P. Carotid body tumors: a review of 30 patients with 46 tumors. Laryngoscope. 1995;105(2):115–126. doi: 10.1288/00005537-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Olsen KD. Tumors and surgery of the parapharyngeal space. Laryngoscope. 1994;104(5) supplement 63:1–28. doi: 10.1288/00005537-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Nandapalan V, O’Sullivan DG, Siodlak M, Charters P. Acute airway obstruction due to ruptured aneurysmal arteriovenous fistula: common carotid artery to internal jugular vein. J Laryngol Otol. 1995;109:562–4. doi: 10.1017/s0022215100130725. [DOI] [PubMed] [Google Scholar]
- 25.Droll KP, Lossing AG. Carotid-jugular arteriovenous fistula: case report of an iatrogenic complication following internal jugular vein catheterization. J Clin Anesth. 2004;16:127–9. doi: 10.1016/j.jclinane.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Ezemba N, Ekpe EE, Ezike HS, et al. Traumatic common carotid-jugular fistula: report of 2 cases. Tex Heart Inst J. 2006;33:81–3. [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider CG, Kortmann H. Pseudoaneurysm of the common carotid artery due to ongoing trauma from the hyoid bone. J Vasc Surg. 2007;45(1):186–187. doi: 10.1016/j.jvs.2006.08.075. [DOI] [PubMed] [Google Scholar]