Abstract
Despite the wealth of information available for the reverse transcriptase (RT)-associated ribonuclease H (RNaseH) domain of lentiviruses, gammaretroviruses and long terminal repeat containing retrotransposons, exploiting this information in the form of an RNaseH inhibitor with high specificity and low cellular toxicity has been disappointing. However, it is now becoming increasingly evident that the two-subunit HIV-1 RT is a highly versatile enzyme, undergoing major structural alterations in order to interact with, position and ultimately hydrolyze the RNA component of an RNA/DNA hybrid. Thus, in addition to targeting the RNaseH active site, identifying small molecules that bind elsewhere and disrupt catalysis allosterically by impairing conformational flexibility is gaining increased attention. This review summarizes current progress towards development of both active site and allosteric RNaseH inhibitors.
The ribonuclease H (RNaseH) domain of retroviral reverse transcriptase (RT), in addition to nonspecifically hydrolyzing the RNA strand of the RNA/DNA replication intermediate, catalyzes highly specific hydrolytic events that are critical to synthesis of integration-competent double-stranded proviral DNA from the RNA genome of the infecting particle [1]. Prominent among these is precise removal of the RNA primers that initiate (-) and (+) strand DNA synthesis (a host-coded tRNA and the polypurine tract, respectively), since these events ultimately define 5´ and 3´ long terminal repeat sequences essential for efficient integration of viral DNA. With respect to (+) strand synthesis, generating the polypurine tract 3´ terminus also mandates a mechanism whereby this sequence is accurately recognized when embedded within the replication intermediate. The observation over two decades ago that mutating active site residues of the RNaseH domain of HIV-1 RT eliminates activity [2] and results in loss of virus infectivity [3] demonstrates the necessity for this function and that the retrovirus-associated activity cannot be complemented by a host enzyme. Together, these observations define the C-terminal RT-associated RNaseH domain as an additional and important target in the development of future combination antiretroviral regimens.
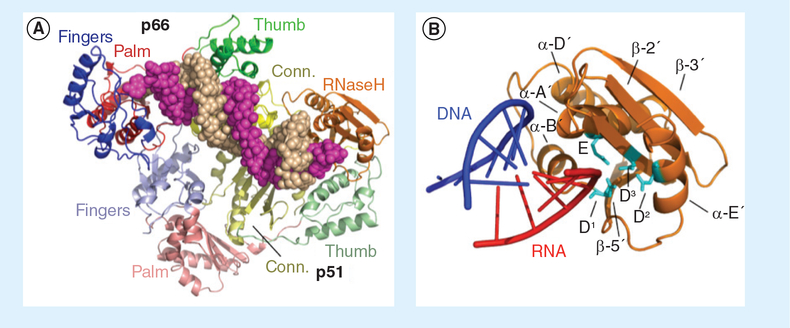
For the nucleoside- and non-nucleoside-derived DNA polymerase inhibitors (NRTIs and NNRTIs, respectively), there is a wealth of data to guide structure-based drug design since the complex of HIV-1 RT containing the NNRTI nevirapine was solved in 1992 by Kohlstaedt and Steitz [4]. In contrast, high-resolution structures of HIV-1 RT containing an inhibitor bound to the RNaseH active site have only recently become available following the initial report in 2009 by Himmel et al. [5]. Although the ease with which the current generation of RNaseH active site inhibitors can be displaced from their binding site in the presence of the nucleic acid substrate represents a major obstacle, the recently-reported structure of HIV-1 RT containing an RNA/DNA hybrid and an NNRTI (Figure 1A) [6] provides a plausible model where the hybrid has ready access to the RNaseH active site (Figure 1B). This model indicates that significant structural alterations within and between the p66 and p51 subunits of the parental p66/p51 heterodimer (Figure 1) are a prerequisite to correctly accommodating the duplex, thus, it may be possible to identify non-active site inhibitors that occupy a site within, or close to, the RNaseH domain and restrict conformational flexibility. Indeed, although a high-resolution co-crystal structure is unavailable, recent data suggest that vinylogous ureas and thienopyrimidinones might fulfill this requirement. The goal of this article is to extend previous reviews by providing an updated account of progress towards developing HIV-1 RNaseH inhibitors that interact outside the RNaseH active site. The reader is also encouraged to read recent reviews by Tramontano and Di Santo [7], and Ilina et al. [8].
Figure 1. p66/p51 HIV-1 reverse transcriptase containing an RNA/DNA hybrid.
(A) Fingers, palm, thumb and connection subdomains are color coded blue, red, green and yellow, respectively, with the darker and lighter colors representing the p66 and p51 subunits, respectively. The p66 C-terminal RNaseH domain is depicted in gold. RNA and DNA strands of the hybrid are depicted as magenta and sand-colored spheres, respectively. (B) Close-up of the p66 RNaseH domain containing portions of the RNA/DNA hybrid described by Lapkouski et al. [6]. Structural elements have been outlined, and catalytic residues (cyan) are: D1: Asp498; D2: Asp549; D3: Asp443; E: Glu478. RNA and DNA strands of the RNA/DNA hybrid are depicted in red and blue, respectively. RNaseH: Ribonuclease H.
Metal-chelating active site inhibitors
N-hydroxyimides
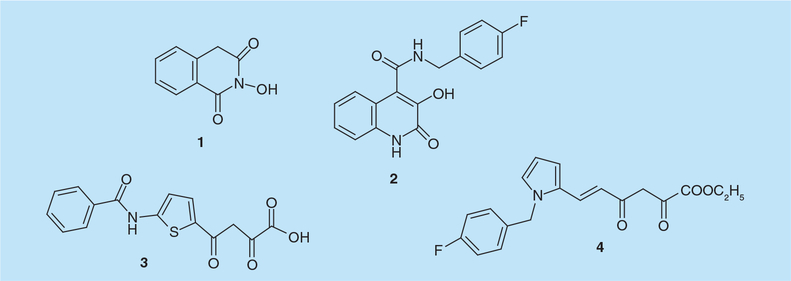
N-hydroxyimides were initially reported to inhibit the endonuclease-dependent influenza RNA-dependent RNA polymerase. 2-hydroxy-4H-isoquinoline-1,3-dione (compound 1, Figure 2) was shown to compete with the RNA substrate in a metal-dependent mode (IC50 = 15 μM) suggesting a direct interaction between its oxygen atoms and the active-site metal ions [9]. This observation provided a pharmacophore model comprising three oxygen atoms able to mimic the presumed interactions of the substrate, nucleophile and leaving group oxygens in the transition state with the metal ions present in the active site of influenza virus endonuclease [9]. Since HIV-1 RNaseH activityalso involves a two metal ion mechanism, N-hydroxyimides were subsequently tested for their effect on HIV-1 RNaseH and the prototype, compound 1, was inhibitory, with an IC50 value of 0.6–1 μM [10]. Loss of activity when the hydroxyl group was replaced with a methoxy or an amino function demonstrates a requirement for the oxygen ligands. Compound 1 was inactive against Escherichia coli RNase HI, and inhibited RNA-dependent DNA polymerase activity of HIV-1 RT only at significantly higher concentrations. Preliminary structure–activity relationship (SAR) data suggested that substitutions on the phenyl moiety increased both potency and selectivity [10] while crystallographic studies indicate that the flexible ‘His-loop’ near the RNaseH C-terminus was stabilized in the presence of 1 [11].
Figure 2.
N-hydroxyimide and diketo acid-derived HIV-1 ribonuclease H inhibitors.
Compound 1 was subsequently used to synthesize derivatives with a variety of substitutions in position 7 of the isoquinoline ring in an effort to obtain dual HIV-1 RNaseH and integrase (IN) inhibitors [12]. Although the most potent RNaseH inhibitors were equally active on IN, all compounds of this series exhibited high cytotoxicity in cell culture. A second series, obtained by synthesizing analogs substituted at position 4 by alkyl and arylalkyl chains and generating more amphiphilic molecules [13], showed little improvement and, while some compounds inhibited HIV-1 IN in the low micromolar range, none inhibited viral replication due to their cytotoxicity [13]. Physicochemical studies on N-hydroxyimides showed results consistent with a 1:1 stoichiometry of the Mg2+ complexes and that metal binding was strictly dependent on the enolization ability of the compounds [12]. Further 3H- and 13C-NMR, IR and ESR studies on 1 and analogs bearing electron-withdrawing or electron-donating moieties in position 4 of the isoquinoline ring confirmed this model and their facile enolization [14]. More recently, substituted 3-hydroxyquinolin-2(1H)-ones were reported to selectively inhibit HIV-1 RNaseH [15]. N-(4-fluorobenzyl)-3-hydroxy-2-oxo-1,2-dihydroquinoline-4-carboxamide (compound 2, Figure 2) inhibited HIV-1 RNaseH with an IC50 value of 19 μM, but was cytotoxic (CC50 = 29 μM). UV spectrophotometry, 1HNMR and 13C NMR spectrometry, together with studies on reduction of the intrinsic fluorescence of the isolated RNaseH domain, confirmed the ability of 2 to chelate the Mg2+ ions in the RNaseH catalytic site [15]. Further SAR studies showed that halogen substitution at position 6 of 2 did not significantly alter RNaseH inhibition, while the introduction of a halogen in position 8 significantly reduced its inhibitory potency [15].
Diketo acids
The two-metal-ion active site binding pharmacophore model elaborated for N-hydroxyimides also explained the mode of action by which 2,4-diketo acids (DKAs) interacted with the influenza endonuclease active site [9]. DKAs were previously reported to inhibit influenza virus endonuclease [16] and HIV-1 IN [17] and since these two enzymes, together with RNaseH, are members of the polynucleotidyl phosphotransferase enzyme family, they were also tested on HIV-1 RNaseH by Merck laboratories [18]. 4-[5-(benzoylamino)thien-2-yl]-2,4-dioxobutanoic acid (BTDBA, compound 3, Figure 2) was shown to: inhibit HIV-1 RNaseH with an IC50 value of 3.2 μM, independent of the order of addition; inhibit a hybrid protein comprising the E. coli RNaseH scaffold and the HIV-1 RNaseH domain (p15-EC) with an IC50 value of 4.7 μM; lack inactivity on the E. coli RNaseH. Isothermal titration calorimetry showed a BTDBA dissociation constant of 8.9 μM, supporting the hypothesis that BTDBA might interact with metal ions in the RNaseH active site independently of the presence or absence of the nucleic acid substrate [18]. BTDBA has been subsequently modeled into the HIV-1 RNaseH domain assuming the basic interaction of the DKA group with the metal ions of the active site [11]. According to this model, its benzoylamide moiety may extend towards Trp266, Leu422 and Trp426 of the p51 subunit. Notably, BTDBA has been shown to inhibit HIV-1 RT-catalyzed strand transfer activity synergistically with either an NRTI, an NNRTI and phosphonoformic acid (PFA, Foscarnet). Consistent with the requirement of both DNA polymerase and RNaseH activities for DNA strand transfer, these results suggest that future cell-penetrant regimens combining RT-associated DNA polymerase and RNaseH inhibitors may have therapeutic benefit [19].
Starting from a parallel screen for HIV-1 IN inhibitors [20,21] the DKA derivative 6-[1-(4-fluorophenyl)methyl-1H-pyrrol-2-yl)]-2,4-dioxo-5-hexenoic acid ethyl ester (RDS 1643, compound 4, Figure 2) was shown to inhibit the HIV-1 RNaseH activity with an IC50 value of 13 μM [22]. RDS 1643 did not inhibit RDDP, AMV and E. coli RNaseH activities, while it inhibited HIV-1 IN at higher concentrations (IC50 = 90 μM). Further biochemical characterization indicated that RDS 1643 activity was substrate-independent, and spectrophotometric analysis indicated it interacted with the active site divalent metal ions [22]. Notably, RDS 1643 inhibited replication of both wt and NNRTI-resistant viruses with EC50 values of 7–14 μM and a selective index of 5.
Hydroxytropolones
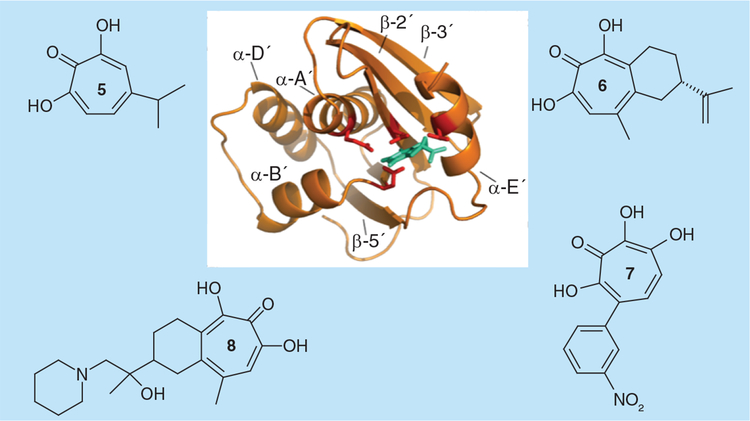
2-hydroxy-2,4,6-cycloheptatrien-1-one (tropolone) derivatives with a 7-OH substitution, such as 2,7-dihydroxy-4–1(methylethyl)-2,4,6-cycloheptatrien-1-one(β-thujaplicinol, compound 5, Figure 3) and 1,2,3,4-tetrahydro-5–7-dihydroxy-9-methyl-2-(1-methylethenyl)-6H-benzocyclohepten-6-one, or manicol, (compound 6, Figure 3) were first identified as potent and selective HIV-1 RNaseH inhibitors via high-throughput screening of a National Cancer Institute library of pure natural products [23]. β-thujaplicinol derives from the heartwood of several Cupressaceae plants (e.g., Thuja plicata, Thuja occidentalis and Chamaecyparis obtusa), inhibiting HIV-1, HIV-2, E. coli and human RNaseH activities with IC50 values of 0.2, 0.77, 50 and 5.7 μM, respectively. Manicol more potently inhibited HIV-2 and human RNases H (IC50 = 1.7 and 3.5 μM, respectively) than the HIV-1 and E. coli enzymes (IC50 = 60 and 40 μM, respectively). Importantly, the related tropolone analog 2-hydroxy-4-(methylethyl)-2,4,6-cycloheptatrien-1-one (β-thujaplicin) that lacks the 7-OH group of the heptatriene ring was inactive, suggesting that the 2,7-dihydroxy tropo-lone function is important for metal chelation at the RNaseH active site [23]. β-thujaplicinol and manicol were also shown to inhibit the HIV-1 IN-catalyzed strand transfer reaction in the micromolar range (IC50 = 21 and 71 μM, respectively) by chelating one or two Mg2+ in the active site [24]. Pre-steady state biochemical studies further demonstrated that β-thujaplicinol is unable to inhibit RNaseH cleavage if added after the RT-substrate complex is formed, suggesting that the RNA/DNA substrate may block access to the RNaseH active site, and confirmed that it does not inhibit HIV-1 RDDP activity [25]. It was also proposed that, in addition to coordinating one of the active site divalent cations, the central tropolone ring of β-thujaplicinol could form π-stacking interactions with the side chain of Tyr501, suggesting that its binding site could lie between the RNaseH active site and Tyr501 of the RNaseH primer grip [25]. Subsequent studies provided biochemical evidence that β-thujaplicinol is a slow-binding RNaseH inhibitor and suggested that the presence of a bound RNA:DNA substrate is necessary to achieve its maximal binding to RT [5]. A 2.80 Å resolution co-crystal of HIV-1 RT containing β-thujaplicinol confirmed that it indeed binds at the RNaseH active site: all three oxygen atoms of the β-thujaplicinol tropolone ring coordinate the two divalent cations and the inhibitor forms ionic or hydrogen bond interactions with the side chains of residues Glu478, Asp498, His539 and Asp549 [5]. Overall, it has been proposed that β-thujaplicinol forms a stable inhibitory complex with both RT and RNA:DNA substrate that directly prevents the attacking water and the scissile phosphate from being properly positioned for catalysis.
Figure 3.
Natural product (5 & 6) and synthetic (7 & 8) active site α-hydroxytropolone-derived HIV-1 ribonuclease H inhibitors.
Hydroxytropolone derivatives have been synthesized by different research teams. A series of monosubstituted, disubstitued, 3,7-dihydroxytropolones, previously shown to inhibit inositol monophosphatase by binding the two Mg2+ ions of the catalytic site [26], was shown to inhibit both HIV-1 RNaseH and RDDP functions, as well as both HIV-1 IN 3´-processing and strand transfer activities, in the low-micromolar range [27]. The most potent derivative, the 4-monosubstituted 3,7-dihydroxytropolone, SP47 (compound 7, Figure 3), inhibited HIV-1 RT and IN catalytic activities with IC50 values in the 0.7–2.3 μM range. SAR studies showed that C-7-modified and O-methylated tropolones were inactive and confirmed the hypothesis that active derivatives inhibit HIV-1 RNaseH activity by binding divalent metal ions in the active site. Importantly, HIV-1 RDDP inhibition by 3,7-dihydroxytropolones was also reported to be very sensitive to Mg2+ concentration and it was proposed that metal in the DNA polymerase catalytic site is required for binding, and that these compounds compete for Mg2+ chelation. 3,7-dihydroxytropolones, however, were found to be cytotoxic [27].
A series of manicol derivatives substituted at its alkene moiety were synthesized and demonstrated to inhibit HIV-1 RNaseH activity in the submicromolar range, with the most potent derivative, 5,7-dihydroxy-2-(1-hydroxy-1-methyl-2-piperidin-1-yl-ethyl)-9-methyl-1,2,3,4-tetrahydrobenzocyclohepten-6-one (compound 8, Figure 3), exhibiting an IC50 value of 0.8 μM, an EC50 value of 10 μM and minimal cytotoxicity at 50 μM [28]. A 2.70 Å crystal structure resolution of HIV-1 RT containing an NNRTI and manicol showed that the carbonyl oxygen and both hydroxyls of the tropolone ring coordinate the divalent cations in a manner similar to that previously observed in the RT-β-thujaplicinol structure. However, unlike β-thujaplicinol, manicol was shown to form extensive contacts with the imidazole ring of His539, including contacts with interatomic distances of under 3 Å. Modeling studies further indicated that a number of additional interactions with His539 could be achieved by introducing other groups into manicol. Notably, two manicol analogs inhibited the RNA-dependent DNA polymerase activity, suggesting they may be capable of occupying a second site on HIV-1 RT [28].
Hydroxypyrimidines
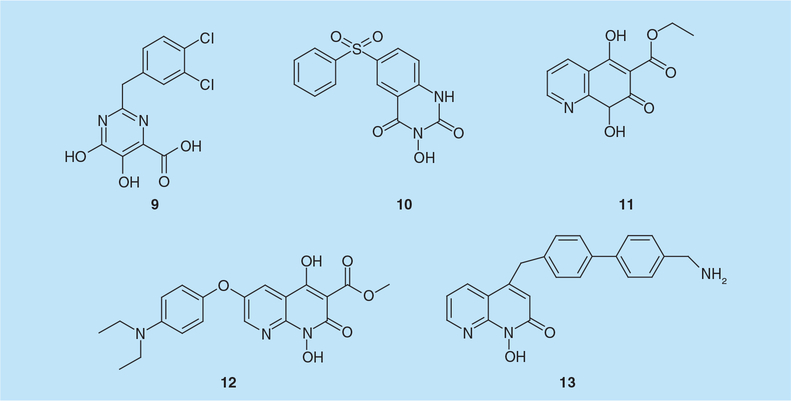
Combined electronic analysis of N-hydroxyimides, DKAs and hydroxytropolones in the context of dual metal chelation indicated that a doubly-anionic species may be necessary for effective coordination of both Mg2+ ions by RNaseH active site inhibitors. However, given their intrinsic problems of low stability under aqueous conditions, pyrimidinol carboxylic acids, previously reported as stable HIV IN [29] and HCV polymerase inhibitors [30], were selected as a scaffold for synthesis of new RNaseH inhibitors [31]. Among this series, the most interesting compound was the di-substituted analog 2-(3,4-dichlorobenzyl)-5,6-dihydroxypyrimidine-4-carboxylic acid (9; Figure 4) that selectively inhibited RNaseH activity with an IC50 value of 1.2 μM [31]. Preincubating enzyme with inhibitor/Mg2+ prior to adding the RNA/DNA substrate significantly quenched the initial burst of catalysis. However, RNaseH activity more closely resembled the inhibitor-free conditions when Mg2+ was absent during preincubation. In conjunction with a similar order-of-addition analysis of β-thujaplicinol, the work of Kirschberg et al. supported a metal-dependent mechanism of action for hydroxypyrimidines [31]. Crystal analysis also showed that the divalent cation activating the water molecule for nucleophilic attack is coordinated by the two phenolic oxygen atoms of 9. In addition, binding between the C2 aromatic substituent and the imidazole ring of His539 was observed, consistent with the improved potency observed with the methylene linker [31,32]. None of the reported pyrimidinol carboxylic acids, however, displayed antiviral activity in vivo [31], which could be ascribed to low cell permeability or high protein binding. In a second report, the N-hydroxyquinazolinedione analog 3-hydroxy-6-(phenylsulfonyl)quinazo-line-2,4(1H,3H)-dione (10; Figure 4) was found to inhibit HIV-1 RNaseH activity with an IC50 value of 0.2 μM and be inactive on human H1 RNaseH [32]. The 3-hydroxy-H-quinazoline-2,4-dione scaffold of 10 was also co-crystallized with a truncated HIV-1 RNaseH domain (p15-Ec) and demonstrated to coordinate the divalent ions the same manner as 9. However, in contrast to compound 9, the phenyl substituent of 10 interacted with the imidazole group of the His539 side chain with a parallel displacement (with two π-ring systems lying parallel to each other when 10 is bound to RNaseH) [32].
Figure 4.
Hydroxypyrimidine and naphthyridinone-derived HIV-1 ribonuclease H active site inhibitors.
Naphthyridinones
The naphthyridine pharmacophore was originally described as an effective HIV-1 IN inhibitor by coordinating divalent ions at the active site [33], and subsequently shown to inhibit HIV-1 RNaseH by Merck Research Laboratories [34]. The naphthyridine derivative ethyl 1,4-dihydroxy-2-oxo-1,2,3,4-tetrahydroquinoline-3-carboxylate (MK1, 11; Figure 4) inhibited HIV-1 RNaseH in vitro with an IC50 value of 0.11 μM and HIV-1 replication with an EC50 value of 2.8 μM, displaying minimal cytotoxicity at 50 μM [34]. A 2.8 Å resolution crystal structure revealed direct binding of MK1 to the RNaseH active site in a single orientation chelating the two cations and making additional interactions with Gly444, Ser499, Ala538, His539, Val552 and Ser553 [34]. Interestingly, crystal studies also showed that another derivative, methyl 6-(4-(diethylamino)phenoxy)-1,4-dihydroxy-2-oxo-1,2,3,4-tetrahydro-1,8-naphthyridine-3-carboxylate (MK3, 12; Figure 4) (IC50 and EC50 = 0.22 and 9.0 μM, respectively), bound to the enzyme distant from RNaseH site, interacting with Leu100, Val108, Tyr181, Tyr183, Asp186, Leu187, Lys223, Phe227, Leu228, Trp229 and Leu234, which are adjacent to the NNRTI pocket previously identified as the target of hydrazones [34,35].
Additional SAR studies on 1-hydroxy-1,8-naphthyridin-2(1H)-one derivatives lead to 4-((4´-(aminomethyl)-[1,1´-biphenyl]-4-yl) methyl)-1-hydroxy-1,8-naphthyridin-2(1H)-one (13; Figure 4) that inhibited HIV-1 RNaseH activity with an IC50 value of 45 nM, and the RT-associated RDDP and IN activities with IC50 values of 13 and 24 μM, respectively [36]. In single-cycle infectivity assay, 13 inhibited replication of wt and IN inhibitor-resistant HIV-1 with an EC50 value of 0.19 μM and showed a selective index of 17, while it was inactive in multicycle viral replication assays due to high toxicity [36].
Nitrofuran-2-carboxylic acid carbamoylmethyl ester derivatives
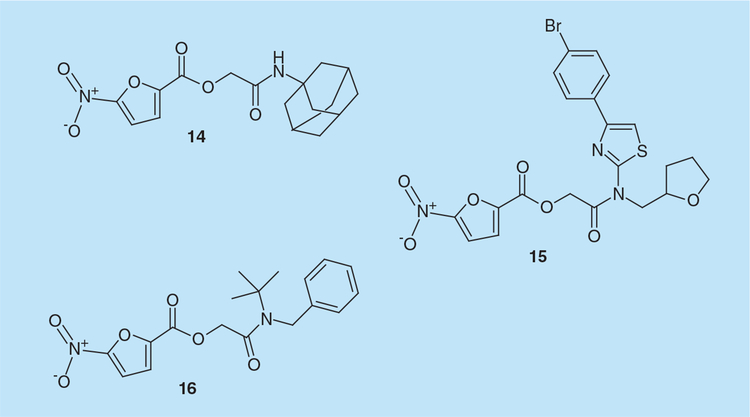
Screening of 20,000 low-molecular-weight compounds representing key structural features of three million chemicals identified derivatives of 5-nitro-furan-2-carboxylic acid carbamoylmethyl ester (NAMCE) as RNaseH inhibitors [37]. 5-nitro-furan-2-carboxylic acid adamantan-1-carbamoylmethylester (14; Figure 5) and 5-nitro-furan-2-carboxylic acid [[4-(4-bromo-phenyl)-thiazol-2-yl]-(tetrahydrofuran-2-ylmethyl)-carbamoyl]-methyl ester (15; Figure 5) inhibited HIV-1 RNaseH activity with IC50 values of 30 and 27 μM, respectively, did not inhibit E. coli RNaseH, and were poorly active on humanRNaseH1. Cell-culture studies showed that 15 inhibited HIV-1 replication with an EC50 value of 25 μM [37]. Docking studies indicated that the nitrofuran group of 14 chelates the two Mg2+ions and orients toward His539, possibly to form a hydrogen bond, while its carbonyl oxygen atom in the amide group may interact with the side chain of Ser553 and its adamantan group can make hydrophobic contacts with the Lys550 side chain. For 15, in addition to the above interactions, a nitrogen atom in the thiazole group was proposed to interact with Asp549 by hydrogen bonding [37].
Figure 5.
Nitrofuran-2-carboxylic acid carbamoylmethylester-derived HIV-1 ribonuclease H inhibitors.
Three series of NAMCE analogs were subsequently synthesized by the following strategies:
Conversion of the hydrophobic substitute bound to the amide group to other substitutes;
Conversion of the nitrofuran ring to several other chemical structures;
Conversion of the ester linkage to an amide bond [38].
While most analogs designed according to the first strategy exhibited a similar potency as the lead compounds 14 and 15, 2-([4-acetoxybenzyl][tert-butyl]amino)-2-oxoethyl 5-nitrofuran-2-carboxylate (16; Figure 5) inhibited HIV-1 RNaseH with an IC50 value of 0.9 μM. Analogs designed according to the second and third strategies were mainly inactive. SAR studies showed that:
The connection of the nitrofuran group and the hydrophobic moiety is essential for activity and that its conversion into an amide bond results in loss of activity;
The hydrophobic moiety connecting to carbonyl carbon could be substituted without loss of activity;
A nitrofuran core is indispensable potency [38].
Molecular modeling studies suggested that NAMCE derivatives occupy the RNaseH active site with their oxygen atoms coordinating the divalent metal ions [38].
Thiocarbamates & triazoles
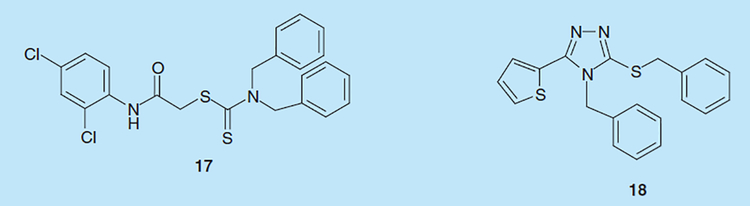
High-throughputscreening efforts at Wyeth Research identified thiocarbamates and triazoles as inhibitors HIV-1 RNaseH [39]. 2-((2,4-dichlorophenyl)amino)-2-oxoethyl dibenzyl carbamodithioate (17; Figure 6) inhibited HIV-1 RNaseH activity with an IC50 of 5 μM and virus replication with an EC50 of 1.3 μM, and a selectivity index >100 [39]. Among triazole derivatives,4-benzyl-3-(benzylthio)-5-(thiophen-2-yl)-4H-1,2,4-triazole (18; Figure 6) inhibited HIV-1 RNaseH activity with an IC50 of <2 μM, viral replication with an EC50 of 4 μM and a selectivity index >130 [39]. Time of addition studies confirmed that these compounds acted at the level of reverse transcription.
Figure 6.
Thiocarbamate and triazole-derived HIV-1 ribonuclease H active site inhibitors.
Allosteric RNaseH inhibitors
Hydrazones
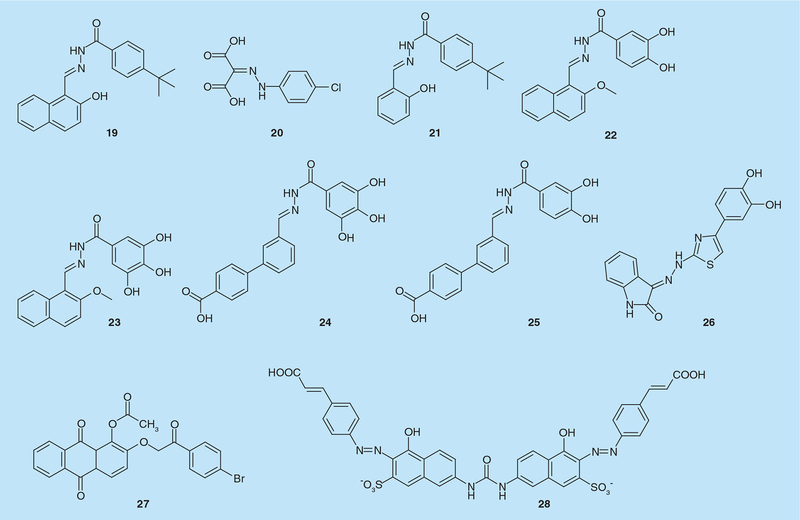
The first hydrazone derivative with anti-RT activity was N-(4-tert-butylbenzoyl)-2-hydroxy-1-naphthaldehyde hydrazone (BBNH, 19; Figure 7) [40]. BBNH was demonstrated to inhibit both enzymatic activities of HIV-1 RT in vitro with IC50 values in the low micromolar range (IC50 = 0.8–3.4 μM), depending on the template:primer used in the assay [40]. BBNH was also active on mutant RTs resistant to NNRTIs and inhibited HIV-1 replication in infected cells. Considering its chelation properties, it was hypothesized that Mg2+ coordination was the main mechanism of action for RNaseH inhibition, while allosteric inhibition of RNA-dependent DNA polymerase activity was proposed. According to this hypothesis, BBNH exerted its multitarget inhibition by binding to spatially separate sites in the DNA polymerase and RNaseH domains. The interaction between hydrazones and the RNaseH active site was predicted by molecular modeling, involving π–π stacking between the naphthyl ring and the Tyr501 of the p66 subunit [41].Site-directed mutagenesis showed that BBNH was sixfold less potent on a Tyr501Trp mutant and completely inactive on Tyr501Arg RT [41]. Further derivatization yielded 4-chlorophenylhydrazoneof mesoxalic acid (CPHM, 20; Figure 7) that likewise inhibited DNA strand transfer reaction by specifically targeting RNaseH (IC50 = 2.2 μM) [42]. CPHM has two carboxylic groups, partially supporting a chelation-related mechanism of action. Interestingly, while CPHM inhibited E. coli RNaseH activity, no inhibition of the RNaseH activity of murine or avian RTs was detected.
Figure 7.
Hydrazones, anthraquinones and naphthalene sulfonic derivatives as allosteric inhibitors of HIV-1 ribonuclease H activity.
Substituting the BBNH naphthyl ring with a phenyl ring produced (4-t-butylbenzoyl)-2-hydroxy-1-salicylyl hydrazone (BBSH, compound 21, Figure 7), where the π–π stacking interaction with Tyr501 was lost: consequently, the compound failed to inactivate RNaseH function, but retained its ability to inhibit DNA polymerase activity (IC50 = 4.7 μM) [43]. Moreover, BBNH and BBSH binding were shown to affect the interaction of the p66 and p51 HIV-1 RT subunits, decreasing the Gibbs free energy of dimer destabilization by 3.7–3.8 kcal mol-1 [43]. Therefore, N-acylhydrazones were proposed to bind to RT at a site overlapping, but distinct from, the NNRTI binding site, and this binding was suggested to affect dimer stability. This hypothesis was confirmed when derivative (E)-3,4-dihydroxy-N-((2-hydroxynaphthalen-1-yl)methylene)benzohydrazide(DHBNH, 22; Figure 7) was co-crystallized with HIV-1 RT and shown to bind to a pocket >50 Å from the RNaseH active site and distinct from the NNRTI-binding site, interacting with active site residues Asp186, Tyr188 and DNA polymerase primer grip residue Trp229 [35]. DHBNH inhibited HIV-1 RNaseH activity noncompetitively with an IC50value of 0.5 μM [35]. Notably, hydra-zone derivatives with bulky substitutions at the para position of DHBNH benzoyl ring more effectively inhibited DNA polymerase activity while retaining equivalent RNaseH inhibition ability. This finding was correlated to the possible access of the bulky substitution to the NNRTI-binding pocket [35]. However, a second binding site close to the RNaseH active site was not excluded, since RNaseH inhibition was fully retained even in presence of saturated concentration of nevirapine that should prevent DHBNH binding.
Further molecular docking studies on a series of hydrazone analogs proposed a new binding-mode in which the compound trihydroxybenzoyl naphthylhydrazone (THBNH, 23; Figure 7) should fill a large pocket formed between RT and the RNA/DNA hybrid substrate, establishing hydrogen bonds with Asn418 and the RNA:DNA substrate and hydrophobic interactions with p66 residues Tyr405, Trp406 and Lys424, as well as p51 residues Pro420, Pro421 and Val423 [44]. Since these residues are close and partially involved in positioning of the RNA primer, hydrazone binding to such a pocket could affect RNaseH primer grip efficacy in RNaseH functioning. Moreover, when the DHBNH naphthyl ring system was replaced with a flexible and extended biphenyl system possessing a carboxylate moiety in the distal phenyl ring, retaining a 3,4,5-trihydoxybenzoyl group in the acylidrazide portion, the analog (E)3´-((2-(3,4,5-trihydroxybenzoyl) hydrazono)methyl)-[1,1´-biphenyl]-4-carboxylic acid (BHMP07, compound 24, Figure 7) inhibited both DNA polymerase and RNaseH activity with IC50values of 0.3 and 0.2 μM, respectively [45]. Notably, deleting one of the three hydroxyl groups (BHMP03, 25; Figure 7) led to selective inhibition of RNaseH function (IC50 = 0.4 μM). NMR studies showed the interaction of BHMP07 with the RNaseH domain was Mg2+ independent, involving residues in the substrate handle region (Asp499, Ala502, Ile505, Ile506 and Ile526) [45]. RNaseH activity of mutant Ala502Phe and Ala502Gly RTs was substantially resistant to BHMP07, BHMP03 and DHBNH [45]. NMR analysis on the interaction between BHMP07 and full-length RT confirmed binding to a site located between the RNaseH active site and the region encompassing helices B and D [13] of the RNaseH domain [46]. DHBNH was also successfully used in virtual screening to identify new scaffolds able to allosterically inhibit both DNA polymerase and RNaseH functions [47,48]. The hydrazonoindolin-2-one derivative identified (E)-3-(2-(4-(3,4-dihydroxyphenyl)-thiazol-2-yl)hydrazono)indolin-2-one (compound 26, Figure 6) was shown to inhibit both RNaseH and RDDP activities with IC50 values of 2.0 and 1.4 μM, respectively. Notably, 26 retained significant potency against NNRTI-resistant RT mutants and was not cytotoxic, even though it failed to inhibit virus replication [47,48].
Anthraquinones
Screening of plant natural metabolites amide identified a new series of emodin [49] and alizarin anthraquinone derivatives [50,51] that inhibited both HIV-1 RT and HCV RNA polymerase [15]. Interestingly, compound 1-acetoxy-9,10-dioxo-9,10-dihydroanthracen-2-yl-4-bromobenzoate (KNA-53, 27; FIGURE 7) inhibited both RNaseH and RNA-dependent DNA polymerase activity of wt and Lys103Asn RTs (IC50 = 22 and 9 μM, respectively) but only the RNaseH function of Tyr181Cys RT (IC50 = 22 μM). Mode of action and molecular dynamic simulation studies suggested a putative anthraquinone binding site in the pocket originally reported for hydrazone derivatives [35,50]. Consequently, it has been hypothesized that anthraquinone binding to RT may influence the manner through which the RNA:DNA hybrid is positioned in the nucleic acid binding cleft, possibly modifying its trajectory toward the RNaseH catalytic site [50].
Naphthalene sulfonic derivatives
Naphthalene sulfonic acid derivatives were first reported to preferentially inhibit the RT-associated DNA polymerase activity (i.e., IC50 = 1–4 μM vs 15–50 μM for RNaseH activity) [52]. Further derivatization yielded (4-hydroxy-7-[[[[5-hydroxy-6-[(4 cinnamylphenyl)azo]-7-sulfo-2-naphthalenyl]amino]-carbonyl]amino]-3-[(4-cinnamylphenyl)]azo (KM-1, 28; Figure 7) that inhibited both RNA-dependent DNA polymerase and RNaseH activities with IC50 values of 24–100 nM [53]. KM-1 was subsequently shown to interfere with nucleic acid binding, suggesting it functioned by distorting RT conformation, thereby mis-aligning the RNA:DNA hybrid at the active site and weakening its binding affinity [54].
Vinylogous ureas & thienopyrimidinones
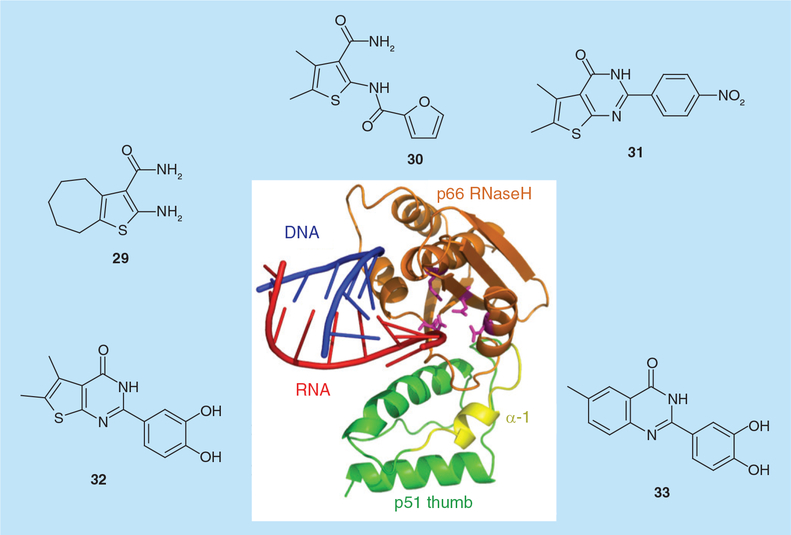
High-throughput screening of natural and synthetic compounds at the National Cancer Institute identified the vinylogous ureas 2-amino-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxamide (29; Figure 8; IC50 = 2.0 μM) and N-[3-(aminocarbonyl)-4,5-dimethyl-2-thienyl]-2-furancarboxamide (30; Figure 8; IC50 = 3.2 μM) as modestly potent inhibitors of the HIV-1 and HIV-2 RNases H [55]. In the absence of a crystal structure, protein footprinting implicated p51 residue Cys280 in inhibitor binding, suggesting these compounds occupied a site between the p51 thumb subdomain and the p66 RNaseH domain. Since: first, p51 thumb residues Cys280–Thr290 and p66 RNaseH residues Pro537–Glu546 constitut ~33% of the buried surface at the p51–p66 dimer interface [56,57], and second, x-ray crystal-lography [6] indicates that binding of the RNA/DNA hybrid is accompanied by movement of the p51 thumb and p66 RNaseH domain as a single unit (Figure 8), 29 and 30 most likely inhibit the subdomain/subunit flexibility that is an integral component of catalysis. Additional structure–activity studies identified the cyclized thienopyrimidinone, 5,6-dimethyl-2-(4-nitrophenyl)thieno[2,3-d]pyrimidin-4(3H)-one (31; Figure 8) with sub-micromolar activity in vitro (IC50 = 0.85 μM).
Figure 8. Allosteric inhibition of HIV-1 ribonuclease H activity by vinylogous ureas and thienopyrimidinone and benzopyrimidinone derivatives.
The central cartoon depicts the p66 C-terminal RNaseH domain (gold, with active site carboxylates in magenta) supported by the p51 thumb subdomain (green). α-helix I, proposed as the binding site for compounds 29–33 by a combination of protein footprinting and site-directed mutagenesis, is depicted in yellow. RNaseH: Ribonuclease H.
To more precisely determine the binding site for this class of RNaseH inhibitor, horizontal alanine scanning between p51 thumb residues Lys275 and Thr286 was combined with a limited vertical scan of Cys280. These studies revealed that enzymes harboring Val276Ala and Arg284Ala were approximately eightfold more sensitive to 31 (IC50 = 0.13 and 0.14 μM, respectively), while p51 mutants Cys280Ala and Thr286Ala were highly resistant (IC50 > 50 μM and 28.2 μM, respectively) [58]. Cys280 vertical scanning mutants were also highly resistant, implicating p51 α-helix I as the site of inhibitor binding. At the same time, the observation that a single Cys280 mutation conferred drug resistance raised the possibility that the equivalent mutation might be easily selected in culture. In order to address this possibility, a panel of drug-resistant and -sensitive recombinant enzymes was created to screen additional thienopyrimidinones with a goal of identifying compounds that were active against drug-resistant variants. This strategy identified 2-(3,4-dihydroxyphenyl)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-one (32; Figure 8), a catechol derivative with equal activity against wild type RT (IC50 = 0.26 μM) and the Cys280Ala variant (IC50 = 0.32 μM) [58]. An interesting feature of thienopyrimidinone-derived RNaseH inhibitors is their ability to destabilize HIV-1 RT in both the absence and presence of the nucleic acid substrate, evidenced by a drop in the melting temperature of the enzyme by as much as 5°C [59]. This is in contrast to active site RNaseH inhibitors, which increase the melting temperature by 2–3°C. Last, our recent studies have indicated that while 3,4-dihydroxyphenyl is critical for activity, the thiophene ring can be replaced with an isosteric benzene ring without loss of activity (33; Figure 8; IC50 = 0.41 μM) [Masaoka T et al., Unpublished Data].
Although there have been toxicity concerns with catechol-containing drugs, based on the ability of the catechol-derived semiquinone radical to participate in production of a super-oxide anion radical, the use of apomorphine and entacapone to treat Parkinson’s disease, as well as the benzazipine derivative fenaldopam to treat hypertension [60] illustrates it is possible to o vercome such toxicity.
Future perspective
Although progress of RNaseH inhibitors towards clinical trials has been challenging, the notion of targeting this critical RT-associated activity should not be dismissed. While the ease that active site inhibitors can be displaced by the nucleic acid substrate is disappointing for further development of this structural class, vinylogous ureas and thienopyrimidinones provide encouraging evidence that allosteric inhibition of RNaseH activity could be achieved. Indeed, continuing in this direction would have the obvious advantage of avoiding inhibition of related host enzymes that belong to the polynucleotidyl phosphotransferase superfamily, prominent among which is human RNaseH, an enzyme that is essential for removing ribonucleotides that have been mis-incorporated into DNA [61]. The recent crystal structure of HIV-1 RT containing an NNRTI and an RNA/DNA hybrid has clearly shown the necessity for enzyme flexibility in accommodating and processing the RNA/DNA replication intermediate [6]. Thus, targeting small-molecule binding pockets in and around the RNaseH domain with a goal of altering subdomain and subunit motion should be given further consideration. Finally, recent studies by Tavis et al. [62] have shown that drug-discovery strategies aimed at other viruses of clinical significance, such as hepatitis B virus can be guided by, and gain from, our experience with HIV-1 RNaseH.
Executive summary.
Reverse transcriptase-associated ribonuclease H & its role in HIV-1 replication
Ribonuclease H activity nonspecifically degrades RNA of the HIV RNA/DNA replication intermediate.
RNaseH activity is also required for accurate removal of the (−) and (+) strand RNA primers.
RNaseH activity is indispensible for HIV replication.
Active site RNaseH inhibitors
The pharmacophore of most RNaseH active site inhibitors suggests they function by chelating the divalent metal required for catalysis.
Order-of-addition experiments suggest RNaseH active site inhibitors are ineffective against the enzyme–substrate complex.
Cellular enzymes of the polynucleotidylphosphotransferase family present a major obstacle when developing active site RNaseH inhibitors.
Allosteric RNaseH inhibitors
Non-nucleoside reverse transcriptase inhibitors in clinical use illustrate that regions outside the DNA polymerase and RNaseH active sites can be targeted.
Small molecules binding to the RNaseH domain could alter trajectory of the nucleic acid substrate as it enters the active site.
RNaseH domain residues contribute significantly to the buried surface at the reverse transcriptase dimer interface, suggesting this as a target for allosteric inhibition.
Acknowledgments
Financial & competing interests disclosure
A Corona and E Tramontano were supported by RAS grant LR 7/2007 CRP-24915 and MIUR PRIN 2012, G Tocco by Ministero dell ‘Universita, dell’ Istruzione e della Ricerca MIUR – PRIN 2008 and Fondazione Banco di Sardegna, and T Masaoka and SFJ Le Grice by the Intramural Research Program of the National Cancer Institute, NIH, Department of Health and Human Services, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Key Terms
- Ribonuclease H:
An activity of the retroviral reverse transcriptase responsible for degrading RNA of the RNA/DNA hybrid produced by reverse transcription.
- Reverse transcriptase:
The retroviral enzyme responsible for converting the ssRNA genome into dsDNA.
- Nucleoside reverse transcriptase inhibitor:
Incorporated into the growing DNA chain at the DNA polymerase active site but cannot be extended due to a 3´deoxyribose modification.
- Non-nucleoside reverse transcriptase inhibitor:
Binds to HIV-1 reverse transcriptase outside the DNA polymerase active site to cause a conformational change that is incompatible with catalysis.
References
- 1.Le Grice SF. Human immunodeficiency virus reverse transcriptase: 25 years of research, drug discovery, and promise. J. Biol. Chem 287(49), 40850–40857 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schatz O, Cromme FV, Gruninger-Leitch F et al. Point mutations in conserved amino acid residues within the C-terminal domain of HIV-1 reverse transcriptase specifically repress RNaseH function. FEBS Lett. 257(2), 311–314 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Schatz O, Cromme F, Naas T et al. Inactivation of the RNaseH domain of HIV-1 reverse transcriptase blocks viral infectivity In: Oncogenesis and AIDS. Papas TS (Ed.). Portfolio Publishing Company, TX, USA, 293–303 (1990). [Google Scholar]
- 4.Kohlstaedt LA, Wang J, Friedman JM et al. Crystal structure at 3.5 Å resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 256(5065), 1783–1790 (1992). [DOI] [PubMed] [Google Scholar]
- 5.Himmel DM, Maegley KA, Pauly TA et al. Structure of HIV-1 reverse transcriptase with the inhibitor beta-thujaplicinol bound at the RNaseH active site. Structure 17(12), 1625–1635 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapkouski M, Tian L, Miller JT et al. Complexes of HIV-1 RT, NNRTI and RNA/DNA hybrid reveal a structure compatible with RNA degradation. Nat. Struct. Mol. Biol 20(2), 230–236 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tramontano E, Di Santo R. HIV-1 RT-associated RNaseH function inhibitors: recent advances in drug development. Curr. Med. Chem 17(26), 2837–2853 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Ilina T, Labarge K, Sarafianos SG et al. Inhibitors of HIV-1 reverse transcriptase-associated ribonuclease H activity. Biology (Basel) 1(3), 521–541 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkes KE, Ermert P, Fassler J et al. Use of a pharmacophore model to discover a new class of influenza endonuclease inhibitors. J. Med. Chem 46(7), 1153–1164 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Klumpp K, Hang JQ, Rajendran S et al. Two-metal ion mechanism of RNA cleavage by HIV RNaseH and mechanism-based design of selective HIV RNaseH inhibitors. Nuc. Acids Res 31(23), 6852–6859 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klumpp K, Mirzadegan T. Recent progress in the design of small molecule inhibitors of HIV RNaseH. Curr. Pharm. Des 12(15), 1909–1922 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Billamboz M, Bailly F, Barreca ML et al. Design, synthesis, and biological evaluation of a series of 2-hydroxyisoquinoline-1,3(2H,4H)-diones as dual inhibitors of human immunodeficiency virus type 1 integrase and the reverse transcriptase RNaseH domain. J. Med. Chem 51(24), 7717–7730 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Billamboz M, Bailly F, Lion C et al. 2-hydroxyisoquinoline-1,3(2H,4H)-diones as inhibitors of HIV-1 integrase and reverse transcriptase RNaseH domain: influence of the alkylation of position 4. Eur. J. Med. Chem 46(2), 535–546 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Billamboz M, Bailly F, Lion C et al. Magnesium chelating 2-hydroxyisoquinoline-1,3(2H,4H)-diones, as inhibitors of HIV-1 integrase and/or the HIV-1 reverse transcriptase ribonuclease H domain: discovery of a novel selective inhibitor of the ribonuclease H function. J. Med. Chem 54(6), 1812–1824 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Suchaud V, Bailly F, Lion C et al. Development of a series of 3-hydroxyquinolin-2(1H)-ones as selective inhibitors of HIV-1 reverse transcriptase associated RNaseH activity. Bioorg. Med. Chem. Lett 22(12), 3988–3992 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Tomassini J, Selnick H, Davies ME et al. Inhibition of cap (m7GpppXm)-dependent endonuclease of influenza virus by 4-substituted 2,4-dioxobutanoic acid compounds. Antimicrob. Agents Chemother 38(12), 2827–2837 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazuda DJ, Felock P, Witmer M et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 287(5453), 646–650 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Shaw-Reid CA, Munshi V, Graham P et al. Inhibition of HIV-1 ribonuclease H by a novel diketo acid, 4-[5-(benzoylamino)thien-2-yl]-2,4-dioxobutanoic acid. J. Biol. Chem 278(5), 2777–2780 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Shaw-Reid CA, Feuston B, Munshi V et al. Dissecting the effects of DNA polymerase and ribonuclease H inhibitor combinations on HIV-1 reverse-transcriptase activities. Biochemistry 44(5), 1595–1606 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Costi R, Di Santo R, Artico M et al. 6-aryl-2,4-dioxo-5-hexenoic acids, novel integrase inhibitors active against HIV-1 multiplication in cell-based assays. Bioorg. Med. Chem. Lett 14(7), 1745–1749 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Costi R, Santo RD, Artico M et al. 2,6-bis(3,4,5-trihydroxybenzylydene) derivatives of cyclohexanone: novel potent HIV-1 integrase inhibitors that prevent HIV-1 multiplication in cell-based assays. Bioorg. Med. Chem 12(1), 199–215 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Tramontano E, Esposito F, Badas R et al. 6-[1-(4-fluorophenyl)methyl-1H-pyrrol-2-yl)]-2,4-dioxo-5-hexenoic acid ethyl ester a novel diketo acid derivative which selectively inhibits the HIV-1 viral replication in cell culture and the ribonuclease H activity in vitro. Antiviral Res. 65(2), 117–124 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Budihas SR, Gorshkova I, Gaidamakov S et al. Selective inhibition of HIV-1 reverse transcriptase-associated ribonuclease H activity by hydroxylated tropolones. Nucleic Acids Res. 33(4), 1249–1256 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenova EA, Johnson AA, Marchand C et al. Preferential inhibition of the magnesium-dependent strand transfer reaction of HIV-1 integrase by alpha-hydroxytropolones. Mol. Pharmacol 69(4), 1454–1460 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Beilhartz GL, Wendeler M, Baichoo N et al. HIV-1 reverse transcriptase can simultaneously engage its DNA/RNA substrate at both DNA polymerase and RNaseH active sites: implications for RNaseH inhibition. J. Mol. Biol 388(3), 462–474 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piettre SR, Andre C, Chanal MC et al. Monoaryl- and bisaryl-dihydroxytropolones as potent inhibitors of inositol monophosphatase. J. Med. Chem 40(26), 4208–4221 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Didierjean J, Isel C, Querre F et al. Inhibition of human immunodeficiency virus type 1 reverse transcriptase, RNaseH, and integrase activities by hydroxytropolones. Antimicrob. Agents Chemother 49(12), 4884–4894 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung S, Himmel DM, Jiang JK et al. Synthesis, activity, and structural analysis of novel alpha-hydroxytropolone inhibitors of human immunodeficiency virus reverse transcriptase-associated ribonuclease H. J. Med. Chem 54(13), 4462–4473 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summa V, Petrocchi A, Matassa VG et al. 4,5-dihydroxypyrimidine carboxamides and N-alkyl-5-hydroxypyrimidinone carboxamides are potent, selective HIV integrase inhibitors with good pharmacokinetic profiles in preclinical species. J. Med. Chem 49(23), 6646–6649 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Koch U, Attenni B, Malancona S et al. 2-(2-thienyl)-5,6-dihydroxy-4-carboxypyrimidines as inhibitors of the hepatitis C virus NS5B polymerase: discovery, SAR, modeling, and mutagenesis. J. Med. Chem 49(5), 1693–1705 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Kirschberg TA, Balakrishnan M, Squires NH et al. RNaseH active site inhibitors of human immunodeficiency virus type 1 reverse transcriptase: design, biochemical activity, and structural information. J. Med. Chem 52(19), 5781–5784 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Lansdon EB, Liu Q, Leavitt SA et al. Structural and binding analysis of pyrimidinol carboxylic acid and N-hydroxy quinazolinedione HIV-1 RNaseH inhibitors. Antimicrob. Agents Chemother 55(6), 2905–2915 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hazuda DJ, Anthony NJ, Gomez RP et al. A naphthyridine carboxamide provides evidence for discordant resistance between mechanistically identical inhibitors of HIV-1 integrase. Proc. Natl Acad. Sci. USA 101(31), 11233–11238 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su HP, Yan Y, Prasad GS et al. Structural basis for the inhibition of RNaseH activity of HIV-1 reverse transcriptase by RNaseH active site-directed inhibitors. J. Virol 84(15), 7625–7633 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Himmel DM, Sarafianos SG, Dharmasena S et al. HIV-1 reverse transcriptase structure with RNaseH inhibitor dihydroxy benzoyl naphthyl hydrazone bound at a novel site. ACS Chem. Biol 1(11), 702–712 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams PD, Staas DD, Venkatraman S et al. Potent and selective HIV-1 ribonuclease H inhibitors based on a 1-hydroxy-1,8-naphthyridin-2(1H)-one scaffold. Bioorg. Med. Chem. Lett 20(22), 6754–6757 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Fuji H, Urano E, Futahashi Y et al. Derivatives of 5-nitro-furan-2-carboxylic acid carbamoylmethyl ester inhibit RNaseH activity associated with HIV-1 reverse transcriptase. J. Med. Chem 52(5), 1380–1387 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Yanagita H, Urano E, Matsumoto K et al. Structural and biochemical study on the inhibitory activity of derivatives of 5-nitrofuran-2-carboxylic acid for RNaseH function of HIV-1 reverse transcriptase. Bioorg. Med. Chem 19(2), 816–825 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Di Grandi M, Olson M, Prashad AS et al. Small molecule inhibitors of HIV RT ribonuclease H. Bioorg. Med. Chem. Lett 20(1), 398–402 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Borkow G, Fletcher RS, Barnard J et al. Inhibition of the ribonuclease H and DNA polymerase activities of HIV-1 reverse transcriptase by N-(4-tert-butylbenzoyl)-2-hydroxy-1-naphthaldehyde hydrazone. Biochemistry 36(11), 3179–3185 (1997). [DOI] [PubMed] [Google Scholar]
- 41.Arion D, Sluis-Cremer N, Min KL et al. Mutational analysis of Tyr-501 of HIV-1 reverse transcriptase. Effects on ribonuclease H activity and inhibition of this activity by N-acylhydrazones. J. Biol. Chem 277(2), 1370–1374 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Davis WR, Tomsho J, Nikam S et al. Inhibition of HIV-1 reverse transcriptasecatalyzed DNA strand transfer reactions by 4-chlorophenylhydrazone of mesoxalic acid. Biochemistry 39(46), 14279–14291 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Sluis-Cremer N, Arion D, Parniak MA. Destabilization of the HIV-1 reverse transcriptase dimer upon interaction with N-acyl hydrazone inhibitors. Mol. Pharmacol 62(2), 398–405 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Felts AK, Labarge K, Bauman JD et al. Identification of alternative binding sites for inhibitors of HIV-1 ribonuclease H through comparative analysis of virtual enrichment studies. J. Chem. Inf. Model 51(8), 1986–1998 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong Q, Menon L, Ilina T et al. Interaction of HIV-1 reverse transcriptase ribonuclease H with an acylhydrazone inhibitor. Chem. Biol. Drug Des 77(1), 39–47 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christen MT, Menon L, Myshakina NS et al. Structural basis of the allosteric inhibitor interaction on the HIV-1 reverse transcriptase RNaseH domain. Chem. Biol. Drug Des 80(5), 706–716 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Distinto S, Esposito F, Kirchmair J et al. Identification of HIV-1 reverse transcriptase dual inhibitors by a combined shape-, 2D-fingerprint- and pharmacophore-based virtual screening approach. Eur. J. Med. Chem 50, 216–229 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Distinto S, Maccioni E, Meleddu R et al. Molecular aspects of the RT/drug interactions. Perspective of dual inhibitors. Curr. Pharm. Des 19(10), 1850–1859 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Tatyana K, Francesca E, Luca Z et al. Inhibition of HIV-1 ribonuclease H activity by novel frangula-emodine derivatives. Med. Chem 5(5), 398–410 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Esposito F, Kharlamova T, Distinto S et al. Alizarine derivatives as new dual inhibitors of the HIV-1 reverse transcriptase-associated DNA polymerase and RNaseH activities effective also on the RNaseH activity of non-nucleoside resistant reverse transcriptases. FEBS J 278(9), 1444–1457 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tramontano E, Kharlamova T, Zinzula L et al. Effects of new quinizarin derivatives on both HCV NS5B RNA polymerase and HIV-1 reverse transcriptase associated ribonuclease H activities. J. Chemother 23(5), 273–276 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Mohan P, Loya S, Avidan O et al. Synthesis of naphthalenesulfonic acid small molecules as selective inhibitors of the DNA polymerase and ribonuclease H activities of HIV-1 reverse transcriptase. J. Med. Chem 37(16), 2513–2519 (1994). [DOI] [PubMed] [Google Scholar]
- 53.Skillman AG, Maurer KW, Roe DC et al. A novel mechanism for inhibition of HIV-1 reverse transcriptase. Bioorg. Chem 30(6), 443–458 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Wang LZ, Kenyon GL, Johnson KA. Novel mechanism of inhibition of HIV-1 reverse transcriptase by a new non-nucleoside analog, KM-1. J. Biol. Chem 279(37), 38424–38432 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Wendeler M, Lee HF, Bermingham A et al. Vinylogous ureas as a novel class of inhibitors of reverse transcriptase-associated ribonuclease H activity. ACS Chem. Biol 3(10), 635–644 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srivastava S, Sluis-Cremer N, Tachedjian G. Dimerization of human immunodeficiency virus type 1 reverse transcriptase as an antiviral target. Curr. Pharm. Des 12(15), 1879–1894 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Tachedjian G, Aronson HE, de los Santos M et al. Role of residues in the tryptophan repeat motif for HIV-1 reverse transcriptase dimerization. J. Mol. Biol 326(2), 381–396 (2003). [DOI] [PubMed] [Google Scholar]
- 58.Chung S, Miller JT, Johnson BC et al. Mutagenesis of human immunodeficiency virus reverse transcriptase p51 subunit defines residues contributing to vinylogous urea inhibition of ribonuclease H activity. J. Biol. Chem 287(6), 4066–4075 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masaoka T, Chung S, Caboni P et al. Exploiting drug-resistant enzymes as tools to identify thienopyrimidine inhibitors of human immunodeficiency virus reverse transcriptase-associated ribonuclease H. J. Med. Chem 56, 5436–5445 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang DP, Ji HF, Tang GY et al. How many drugs are catecholics. Molecules 12(4), 878–884 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nick McElhinny SA, Kumar D, Clark AB et al. Genome instability due to ribonucleotide incorporation into DNA. Nat. Chem. Biol 6(10), 774–781 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tavis JE, Cheng X, Hu Y et al. The hepatitis B virus ribonuclease H is sensitive to inhibitors of the human immunodeficiency virus ribonuclease H and integrase enzymes. PLoS Pathog. 9(1), e1003125(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]