Abstract
Aims:
von Willebrand factor (VWF) is an independent risk factor for adverse events in patients with non-valvular atrial fibrillation (NVAF). However, it is unclear if VWF level remains elevated and predictive of stroke during entire course of NVAF.
Methods and Results
In order to determine if VWF is a time-dependent blood variable, VWF antigen measured by latex immunoassay in 425 NVAF patients and 100 controls with normal sinus rhythm (NSR) was analyzed according to NVAF duration (<1 month: n = 76, 1–12 months: n = 98, and >12 months: n = 251). The mean VWF antigen level in NVAF patients with <1-month duration (167 ± 59%) was not different compared to those with 1–12 months (157 ± 50%, P = .24) and >12 months duration (156 ± 54%, P = .11) but higher compared to NSR controls (143 ± 48%, P = .003). Higher VWF level correlated with higher CHADS2 scores and with progressing intensity of blood stasis in the left atrium and thrombus formation in all three time periods of atrial fibrillation duration. Patients not treated with warfarin had VWF 30% higher in the first month compared to following months.
Conclusions:
von Willebrand Factor is steadily elevated throughout the course of dysrhythmia in NVAF patients treated with warfarin and in those with higher intensity of left atrium blood stasis.
Keywords: atrial fibrillation, duration of dysrhythmia, inflammation, von Willebrand Factor
1 |. INTRODUCTION
Atrial fibrillation carries an increased risk of first-lifetime stroke, recurrent stroke compared to the individuals with sinus rhythm, and greater stroke-related disability and mortality compared to strokes of other etiologies.1–4 Cardioembolic stroke from non-valvular atrial fibrillation (NVAF) begins with the development of left atrial appendage thrombus (LAAT).5–7 A number of clinical variables and echocardiographic measures of blood stasis in left atrium are linked with LAAT formation and stroke.8–12
von Willebrand factor (VWF), a large plasma glycoprotein that mediates platelet adhesion and aggregation, is a known marker of inflammation, endothelial perturbation, and thrombosis.13 Increasing VWF levels correlate with severity of left atrial blood stasis, left atrial appendage thrombosis, and increased stroke risk in NVAF patients.11,14 In fact, high plasma VWF level was found to be an independent risk factor for adverse events in NVAF.15 However, in order to reliably use VWF level measurement to refine stroke and bleeding risk stratification in NVAF, we need to establish stability of this biomarker throughout the clinical course of this dysrhythmia. CD40 ligand (CD40L), a sensitive indicator of inflammation associated platelet activation,16 was recently found to be significantly higher in NVAF but only during the first year of this dysrhythmia.17 Whether this time-dependent variation also exists for other inflammation and prothrombotic biomarkers including VWF in NVAF patients remains essentially unexplored.18–21
To determine if VWF is a time-dependent blood variable, plasma VWF antigen level was compared among NVAF patients with different duration of dysrhythmia (<1 month, 1–12 months, and >12 months) and in 100 controls with normal sinus rhythm (NSR).
2 |. METHODS
2. 1 |. Patient recruitment
Patient selection, recruitment, clinical and echocardiographic data collection, and assessment have previously been described.11 All patients with NVAF (cases) undergoing TEE (October 4, 2007-April 27, 2009) were approached for study participation. Exclusion criteria included (i) acute illness, stroke, myocardial infarction, or surgery within 30 days;(ii) valvular type of atrial fibrillation; (iii) prior unprovoked venous or arterial thrombosis; (iv) prior major bleeding unrelated to warfarin therapy; (v) liver disease; (vi) active malignancy; or (vii) hormonal stimulation (estrogen/progesterone therapy or pregnancy). Normal sinus rhythm controls were recruited from the Primary Care Internal Medicine clinic during their annual medical exam. These subjects had no known prior history of atrial fibrillation.
All patients gave written permission to use their clinical data and biological specimens for research purposes. This protocol was approved by the Institutional Review Boards of the Mayo Foundation (IRB 09–001493), and all research conduct was performed according to the ethical principles of the Declaration of Helsinki.
2. 2 |. Study definitions and event adjudication
Congestive heart failure (CHF) was defined as the presence of clinical symptoms and signs of heart failure within the last 3 months whether or not there was evidence of LV systolic dysfunction by echocardiography.9 Diabetes mellitus was confirmed based on the criteria recommended by the American Diabetes Association.22 Stroke, TIA, and systemic embolization were defined by criteria proposed by the American Heart Association.23 Inclusion required either electrocardiogram or Holter monitoring confirmation of atrial fibrillation. Atrial fibrillation was classified as “paroxysmal,” or “persistent,” or “permanent” in accordance with current guidelines.24 A CHADS2 score was assigned for both cases and controls.9
2. 3 |. Sample collection and plasma VWF assays
Twenty milliliters of citrated blood was collected by antecubital venipuncture using a 19 gauge thin-wall “butterfly” needle with a short plastic tube extension.
von Willebrand Factor: Ag in plasma was measured using HemosIL von Willebrand Factor Antigen latex immunoassay kits (Instrumentation Laboratory, Lexington, MA USA) with 2 ACL TOP coagulation system analyzers (Beckman Coulter, Brea, CA USA), following manufacturer’s instructions. Briefly, prediluted patient samples (1:1 plasma to diluent) and latex particle-enhanced immunoturbidity reagents were added to testing cuvettes. The lower limit of normal for VWF:Ag is 55 IU/dL.
2. 4 |. Statistical analysis
Categorical variables were presented as counts (%) and compared using Pearson’s chi-squared test. Continuous variables were presented as means ± standard deviation and median where appropriate and compared with the Student t-test or the non-parametric Kruskal- Wallis analysis of variance. In AF cases alone, demographic and clinical characteristics were compared by duration of AF: <1 month, 1–12 months, and >12 months. Pairwise correlation was calculated to assess the relationship between VWF levels and clinical variables including AF duration and CHADS2 score. Statistical testing used the two-tailed alpha level of 0.05 for significance. Data analyses were conducted using JMP (version 9.0.1, SAS Institute Inc, Cary, NC, USA).
3. |. RESULTS
3. 1 |. Patient population
Demographic and clinical characteristics of 452 NVAF cases and 100 NSR controls are presented in Table 1. The mean age of NVAF cases was similar compared to NSR controls. There were fewer females in NVAF cases compared with NSR controls. Prior stroke/TIA and warfarin therapy were more common among NVAF cases. The mean CHADS2 score and other demographic variables were similar between groups. TEE studies were performed to exclude intracardiac thrombi prior to electrophysiologic procedures (69%), cardioversion (17%), or for cardioembolic risk evaluation (14%).
TABLE 1.
Characteristics of VWF in NSR controls and NVAF cases
| Variable | Controls NSR (N = 100) |
Overall NVAF (N = 425) |
P | Duration of atrial fibrillation |
|||
|---|---|---|---|---|---|---|---|
| <1 mo (N = 76) |
1–12 mo (N = 98) |
>12 mo (N = 251) |
P | ||||
| Age, y (mean ± SD) | 63.4 ± 14 | 62.6 ± 13 | .5865 | 63.1 ± 15 | 61.8 ± 13 | 62.7 ± 12 | .76 |
| <65, n (%) | 51 (51%) | 242 (57%) | .2818 | 41 (54%) | 52 (53%) | 149 (59%) | .48 |
| 65–74 y, n (%) | 24 (24%) | 109 (26%) | .7333 | 16 (21%) | 35 (36%) | 58 (23%) | .032 |
| ≥75, n (%) | 25 (25%) | 74 (17%) | .0809 | 19 (19%) | 11 (11%) | 44 (18%) | .059 |
| Female, n (%) | 39 (39%) | 104 (25%) | .0033 | 11 (15%) | 19 (19%) | 74 (29%) | .012 |
| Congestive heart failure, n (%) | 15 (15%) | 100 (24%) | .0635 | 24 (32%) | 27 (28%) | 49 (20%) | .053 |
| Hypertension, n (%) | 50 (50%) | 253 (60%) | .0826 | 44 (58%) | 58 (59%) | 151 (60%) | .94 |
| Diabetes mellitus, n (%) | 21 (21%) | 58 (14%) | .0643 | 6 (8%) | 18 (18%) | 34 (14%) | .14 |
| Stroke/TIA prior, n (%) | 6 (6%) | 59 (14%) | .0313 | 7 (9%) | 10 (10%) | 42 (17%) | .12 |
| Antiplatelet therapy, n (%) | 51 (51%) | 203 (48%) | .5602 | 32 (42%) | 45 (46%) | 126 (50%) | .43 |
| Warfarin therapy, n (%) | 2 (2%) | 327 (77%) | <.0001 | 56 (74%) | 86 (88%) | 185 (74%) | .015 |
| Statin therapy, n (%) | 35 (35%) | 159 (37%) | .6530 | 22 (29%) | 36 (37%) | 101 (40%) | .20 |
| CHADS2, n (%) | |||||||
| mean ± SD | 1.23 ± 1.3 | 1.42 ± 1.4 | .2086 | 1.41 ± 1.2 | 1.36 ± 1.3 | 1.44 ± 1.4 | .90 |
| 0 | 36 (36%) | 129 (30%) | .3212 | 22 (29%) | 30 (31%) | 77 (31%) | .93 |
| 1 | 33 (33%) | 131 (31%) | 21 (28%) | 31 (32%) | 79 (32%) | ||
| ≥2 | 31 (31%) | 165 (39%) | 33 (43%) | 37 (38%) | 95 (38%) | ||
3. 2 |. VWF antigen levels by demographics and across duration of NVAF
The mean VWF antigen level in NVAF patients with <1-month duration (167 ± 59%) was not different compared to those with 1–12 months (157 ± 50%, P = .24) and >12 months duration (156 ± 54%, P = .11) but higher compared to NSR controls (143 ± 48%, P = .003). The relationship between VWF median antigen concentration, demographic, and clinical characteristics for cases stratified by NVAF duration is provided in Table 2. Globally, the median VWF antigen levels were higher in NVAF cases compared to controls[153% (121–185) vs 146% (104–177), P = .017]. In NVAF patients, VWF levels increased directly with aging however remained unchanged throughout the course of atrial fibrillation. A number of clinical variables were associated with significantly higher VWF levels including congestive heart failure, hypertension, and diabetes mellitus. The impact of these combined variables is evident in the CHADS2 assessment (Table 2, Figure 1). And yet, within these clinical characteristics and CHADS2 score values, VWF levels remained constant cross-time intervals.
TABLE 2.
VWF antigen levels by demographic characteristics in NSR controls and NVAF cases
| VWF antigen, % of normal [median (IQR)] |
||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Controls (N = 100) |
P | Overall cases (N = 425) |
P | Duration of atrial fibrillation |
|||
| <1 mo (N = 76) | 1–12 mo (N = 98) | >12 mo (N = 251) | P | |||||
| Overall VWF | 146 (104–177) | 153 (121–185) | .017 | 161 (130–193) | 152 (122–183) | 151 (119–185) | .29 | |
| Age, y | .089 | <.001 | ||||||
| <65 | 142 (99–172) | 140 (111–177) | .20 | 145 (111–176) | 140 (109–182) | 139 (111–178) | .57 | |
| ≥65 to <75 | 129 (96–182) | 162 (127–191) | .030 | 180 (116–220) | 163 (131–183) | 157 (124–191) | .59 | |
| Age, ≥75 y | .028 | <.001 | ||||||
| No | 139 (99–176) | 147 (113–180) | .023 | 160 (111–180) | 151 (118–183) | 144 (112–180) | .63 | |
| Yes | 164 (136–181) | 176 (146–216) | .027 | 182 (144–269) | 161 (149–197) | 184 (146–205) | .22 | |
| Gender | .67 | .080 | ||||||
| Female | 141 (99–177) | 161 (126–192) | .011 | 160 (137–255) | 169 (131–186) | 153 (123–191) | .55 | |
| Male | 147 (103–178) | 152 (116–183) | .14 | 161 (123–189) | 147 (120–182) | 150 (115–183) | .29 | |
| Congestive Heart failure | .69 | <.001 | ||||||
| No | 144 (100–177) | 145 (115–180) | .16 | 145 (111–176) | 145 (118–177) | 146 (113–182) | .90 | |
| Yes | 155 (121–177) | 175 (143–208) | .032 | 188 (162–254) | 174 (139–197) | 170 (139–200) | .12 | |
| Hypertension | .005 | <.001 | ||||||
| No | 129 (87–177) | 142 (110–175) | .038 | 141 (111–178) | 140 (104–167) | 142 (111–175) | .76 | |
| Yes | 150 (130–177) | 161 (128–192) | .24 | 163 (143–208) | 160 (132–187) | 157 (125–191) | .31 | |
| Diabetes mellitus | .10 | .019 | ||||||
| No | 146 (97–177) | 152 (118–182) | .011 | 162 (129–193) | 152 (119–177) | 148 (116–181) | .13 | |
| Yes | 144 (131–183) | 177 (130–196) | .28 | 143 (123–210) | 177 (129–191) | 177 (125–209) | .72 | |
| Stroke/TIA, prior | .15 | .058 | ||||||
| No | 144 (102–177) | 151 (119–184) | .016 | 161 (120–193) | 150 (121–180) | 147 (116–183) | .20 | |
| Yes | 170 (134–196) | 171 (134–190) | >.99 | 154 (138–215) | 177 (161–185) | 173 (128–193) | >.99 | |
| Antiplatelet therapy, n (%) | .74 | .60 | ||||||
| No | 143 (92–178) | 148 (118–184) | .077 | 147 (111–183) | 145 (114–190) | 148 (121–185) | .68 | |
| Yes | 146 (121–177) | 158 (126–185) | .064 | 165 (144–244) | 154 (134–178) | 153 (112–185) | .003 | |
| Warfarin therapy, n (%) | .054 | .016 | ||||||
| No | 144 (102–177) | 139 (108–177) | .52 | 176 (134–214) | 125 (101–164) | 135 (104–168) | .008 | |
| Yes | 208 (169–246) | 157 (126–186) | .22 | 157 (118–185) | 154 (126–183) | 158 (126–191) | .96 | |
| Statin therapy, n (%) | .107 | .10 | ||||||
| No | 137 (96–176) | 150 (115–184) | .021 | 161 (115–185) | 145 (110–181) | 150 (116–184) | .43 | |
| Yes | 151 (133–178) | 160 (131–186) | .29 | 163 (144–208) | 160 (136–184) | 155 (121–186) | .27 | |
| CHADS2 | .001 | <.001 | ||||||
| 0 | 102 (83–162) | 139 (107–168) | .037 | 128 (107–167) | 140 (102–166) | 142 (109–173) | .54 | |
| 1 | 149 (121–177) | 148 (119–184) | .64 | 160 (105–190) | 151 (122–189) | 139 (116–180) | .43 | |
| ≥2 | 155 (133–183) | 171 (139–198) | .19 | 180 (147–233) | 166 (133–185) | 173 (129–198) | .086 | |
FIGURE 1.
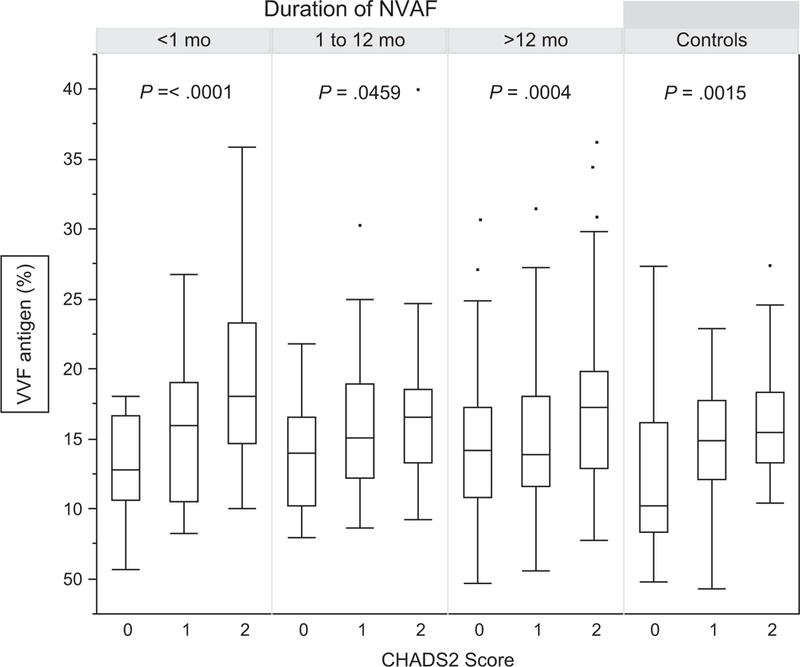
von Willebrand Factor (VWF) level by CHADS2 scores and duration of non-valvular atrial fibrillation (NVAF). Relationship between VWF antigen concentration and CHADS2 scores in patients with NVAF and control subjects with normal sinus rhythm analyzed by duration of NVAF. Measures are presented as mean ± standard deviation
For those cases not receiving warfarin, there was a striking impact of time on VWF (Table 2). VWF levels were nearly 30% higher in the first month in these subjects compared to following months (P = .008). NVAF patients receiving warfarin had VWF levels unchanged across time intervals (P = .96) although generally higher compared to those not on anticoagulation (P = .016). A similar phenomenon of higher VWF level within the first months of dysrhythmia was noted for aspirin taking NVAF cases (P = .003) but not treated with statins (P = .27).
Comparison of echocardiographic measures and VWF levels stratified by NVAF duration is provided in Table 3. The transition from paroxysmal to persistent to permanent NVAF is associated with a stepwise rise in VWF levels. Within NVAF types, however, there was consistency across time intervals. Higher VWF antigen was associated with a number of echocardiographic variables including left ventricular ejection fraction (P = .004), left atrial volume index (P = .018), aortic atheroma (P < .0001), left atrial appendage emptying velocity (P < .001), and spontaneous echo contrast (P < .001). The presence of left atrial appendage thrombus was also associated with higher VWF levels (P < .001). Within these echocardiographic variables, however, VWF levels again remained constant cross-time intervals. Significant drop of VWF levels after the first month of dysrhythmia was observed only in NVAF patients with left atrial appendage emptying velocity ≥25 to <50 cm/s2 (P = .007) and with mild spontaneous echocardiographic contrast (P = .027).
TABLE 3.
Echocardiographic measures and VWF antigen levels stratified by duration of atrial fibrillation
| VWF antigen, % of normal [median (IQR)] |
||||||
|---|---|---|---|---|---|---|
| Variable | Overall (N = 425) | P | Duration of atrial fibrillation |
|||
| <1 mo (N = 76) | 1–12 mo (N = 98) | >12 mo (N = 251) | P | |||
| Type of atrial fibrillation | ||||||
| Paroxysmal | 139 (109–177) | <.001 | 142 (96–176) | 140 (104–173) | 139 (112–179) | .91 |
| Persistent | 154 (126–182) | 152 (129–172) | 155 (125–177) | 159 (132–188) | .90 | |
| Permanent | 174 (132–194) | N/A | N/A | 174 (132–194) | ||
| LVEF ≤ 40% | ||||||
| No | 149 (120–184) | .004 | 161 (129–193) | 145 (120–177) | 149 (117–185) | .34 |
| Yes | 168 (133–192) | 166 (129–208) | 172 (149–208) | 169 (117–184) | .65 | |
| Left atrial volume index | ||||||
| <30 mL/m2 | 145 (103–176) | .018 | 180 (125–193) | 115 (97–173) | 139 (108–173) | .46 |
| ≥30 to <40 mL/m2 | 144 (118–175) | 161 (137–205) | 139 (118–163) | 143 (111–178) | .14 | |
| ≥40 to <60 mL/m2 | 161 (116–191) | 150 (101–250) | 156 (123–183) | 164 (119–191) | .74 | |
| ≥60 mL/m2 | 166 (138–185) | 163 (142–178) | 166 (152–191) | 160 (119–191) | .72 | |
| Aortic atheromatous disease | ||||||
| None | 139 (107–176) | <.001 | 147 (111–174) | 133 (100–177) | 139 (105–177) | .57 |
| Simple (<4 mm) | 159 (131–191) | 167 (142–208) | 159 (131–185) | 155 (126–192) | .65 | |
| Complex (>4 mm) | 175 (118–238) | 257 (97–308) | 132 (124–140) | 175 (118–234) | .23 | |
| Left atrial appendage emptying velocity | ||||||
| ≥75 cm/s2 | 149 (109–183) | <.001 | 166 (105–211) | 109 (92–183) | 158 (131–184) | .25 |
| ≥50 to <75 cm/s2 | 142 (109–175) | 135 (106–162) | 127 (116–147) | 151 (111–181) | .33 | |
| ≥25 to <50 cm/s2 | 152 (126–191) | 176 (145–212) | 158 (131–185) | 142 (115–191) | .007 | |
| <25 cm/s2 | 172 (139–199) | 169 (151–248) | 171 (134–199) | 173 (139–198) | .83 | |
| Spontaneous echo contrast | ||||||
| None | 142 (108–174) | <.001 | 142 (101–171) | 129 (102–160) | 146 (111–178) | .40 |
| Mild | 149 (125–189) | 171 (138–233) | 167 (133–194) | 142 (115–177) | .027 | |
| Moderate | 163 (128–192) | 164 (160–192) | 157 (126–176) | 175 (125–196) | .43 | |
| Severe | 173 (144–205) | 169 (140–260) | 178 (139–226) | 174 (144–188) | .65 | |
| Left atrial appendage thrombus | ||||||
| No | 152 (120–183) | <.001 | 161 (129–189) | 151 (121–179) | 150 (116–184) | .32 |
| Yes | 191 (154–240) | 256 (221–291) | 219 (189–287) | 178 (146–235) | .14 | |
4. |. DISCUSSION
The principal finding of the study is that VWF level is elevated in subjects with NVAF compared to normal sinus rhythm controls and correlates with clinical and echocardiographic features of high stroke risk irrespectively of arrhythmia duration. This indicates that previously reported15 predictive power of this biomarker is not time-dependent.
Inflammatory stimuli are known to drive endothelial cell release of VWF into the circulation.8 As such, elevated VWF levels may serve as a measure of endothelial cell perturbation and prothrombotic status. Whereas NVAF contains a notable inflammatory component,16–21 this finding fits neatly into this hypothesis. The degree of VWF elevation is directly associated with measures of left atrial blood stasis including spontaneous echo contrast, left atrial appendage emptying velocity, and ultimately left atrial appendage thrombus formation, again consistently throughout the time of dysrhythmia.
Elevated VWF persistently observed throughout the time of NVAF seems to be not affected by dysrhythmia burden. Although VWF is higher in permanent NVAF compared to persistent or paroxysmal type (Table 3), the levels are similar for each group in those who had dysrhythmia for <1 month, up to 12 months, or over 1 year.
The persistent endothelial response to NVAF however stands in marked contrast to the temporary platelet response. We have previously shown that the platelet activation marker—CD40L— is significantly elevated following initiation of NVAF.17 However, after 1 year of atrial fibrillation, the CD40L levels fall and do not differ from sinus rhythm controls. CD40L is rapidly expressed following platelet activation and induces endothelial cell stimulation leading to leukocyte recruitment and delivery of tissue factor.25–27 This temporal endothelial response to platelet CD40L is therefore quite different than the endothelial delivery of VWF which does not vary over time with this dysrhythmia. The mechanistic hypothesis may be chaotic or may indeed be quite simple. CD40L stands as a unique measure of platelet activation with subsequent endothelial activation. In contrast, VWF release from endothelial cells may result from a variety of stimuli including variations of flow characteristics and shear forces related to heart failure, hypertension with changes in wall tension, diabetes mellitus, direct injury, and aging.28,29 This is consistent with our observation that heart failure, hypertension, diabetes mellitus, and older age are associated with significantly higher level of VWF. Although both elevated VWF and CD40L promote a thrombotic response they may represent unique antithrombotic targets which may imply a more sophisticated strategy for selected patients over time. Whereas both biomarkers appear to be directly related to endothelial injury, it is perhaps some- what surprising that neither CD40L17 nor VWF in current study was impacted by statin therapy. Although there are data supporting pleiotropic effects of statins on endothelial function, vascular inflammation and thrombosis, statin-induced alterations to VWF level and activity are not fully elucidated.30 Recent meta-analysis showed generally lowering effect of statin therapy on VWF levels.30 However, only treatment with high doses of simvastatin and pravastatin used for more than 12 months but not with fluvastatin, atorvastatin, or rosuvastatin reduced VWF levels. None of the analyzed studies included NVAF patients. No impact of statin therapy on VWF level observed in our study might therefore reflect either specific drugs of the statin family used in our patients, their dosing, and duration of therapy; it might also represent an effect of specific clinical characteristics of NVAF patients including comorbidities and effect of other medications.
Persistency of VWF level may be also affected by anticoagulation therapy. NVAF patients receiving warfarin had consistently elevated VWF levels. In contrast, VWF levels declined significantly after the first month of atrial fibrillation for those patients not receiving warfarin. Warfarin therapy may be therefore somehow associated with endothelial cells stimulation. It is well known that endothelial cells participate intimately in the coagulation system through thrombomodulin, protein C activation, tissue factor metabolism and inhibition, and by fibrinolysis.13 It is therefore possible that inhibition of the vitamin K-dependent pathways alters endothelial responsiveness such that VWF production and release is augmented. Higher VWF level in NVAF patients treated with warfarin compared to those treated with aspirin was reported previously.31 Also, prior observation that patients treated with vitamin K antagonist have higher factor VIII level32 supports our finding of higher VWF in this treatment group because factor VIIII and VWF circulate in plasma as a tightly bound complex while free factor VIII is quickly degraded.13 In the end, these findings can- not be explained by selection bias such as coexistence of multiple comorbidities in warfarin-treated group compared to those not treated with anticoagulation or treated with aspirin as both patients with low- intermediate (0–1) and higher (≥2) CHADS2 scores had persistent VWF levels. Higher VWF level within the first period of NVAF followed by significant drop was observed in patients treated with aspirin. As aspirin has anti-inflammatory property, significant drop of VWF level after the first month might reflect this therapeutic effect. Previous reports that aspirin therapy has no effect on VWF level31 might not address time-dependent aspect of this phenomenon.
Progression of blood stasis within left atrium as indicated by decreasing left atrial appendage emptying velocity and intensification of spontaneous echocardiographic contrast was accompanied by progressive increase in VWF level, yet these levels remained stable throughout the duration of dysrhythmia. Only NVAF patients with mildly moderately decrease left atrium appendage emptying velocity and with mild echocardiographic contrast had significantly higher VWF levels within the first month of atrial fibrillation compared to those with longer duration of dysrhythmia. We are not able provide any explanation for this observation.
Several limitations of this study need to be mentioned. First, the study does not have longitudinal follow-up methodology. Ideally, a single cohort of patients with newly diagnosed atrial fibrillation should have serial testing for VWF changes over time without pharmacological or invasive interventions to change the natural course of dysrhythmia. However, this approach, although methodologically optimal, would be unethical. Second, we cannot exclude referral bias as only patients who had a TEE requested by their care provider were recruited. However, clinical characteristics of our patients do not differ from typical NVAF patients at our institution12 or participants of large multicenter studies.8–10 Third, duration of atrial fibrillation assessment may by imprecise in patients with asymptomatic dysrhythmia. Fourth, the blood type was not measured. Blood type “0″ is associated with lower than normal values of circulating VWF and impact outcome.33 Fifth, VWF level represents acute phase reactivity which raises concerns of reproducibility. A study of nearly 19 000 individuals however found that VWF reproducibility is similar to blood pressure and serum cholesterol.34 Lastly, we used CHADS2 score tool instead of currently indorsed CHA2DS2-VASc methodology.9 Our objective, however, was to discriminate NVAF patients with low, intermediate, and high stroke risk. As CHA2DS2-VASc system is mainly better identifying relatively low stroke risk patients,10 its use for our purpose would not be particularly advantageous.
5. |. CONCLUSIONS
VWF remains steadily elevated throughout the course of dysrhythmia in NVAF patients and its level correlates with CHADS2 scores and with higher intensity of left atrium blood stagnation irrespectively of duration of this dysrhythmia. Only those NVAF patients not treated with warfarin had VWF levels significantly higher in the first month compared to following months. Higher VWF level within the first months of dysrhythmia was also observed in aspirin taking cases but not in those receiving statins.
Acknowledgments
Funding information
Funded, in part, by Nr.17896 CR 20 grant from the Department of Internal Medicine, Mayo Clinic, and statistical support was provided by an internal grant from the Mayo Clinic Department of Cardiology
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
DISCLOSURES
The authors report no relationships that could be construed as a conflict of interest.
REFERENCES
- 1.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982;306:1018–1022. [DOI] [PubMed] [Google Scholar]
- 2.Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 3.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 4.Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke. Stroke 2005;36:1115–1119. [DOI] [PubMed] [Google Scholar]
- 5.Aberg H Atrial fibrillation. I. A study of atrial thrombosis and systemic embolism in a necropsy material. Acta Med Scand 1969;185:373–379. [PubMed] [Google Scholar]
- 6.Manning WJ, Silverman DI, Waksmonski CA, Oettgen P, Douglas PS. Prevalence of residual left atrial thrombi among patients with acute thromboembolism and newly recognized atrial fibrillation. Arch Intern Med 1995;155:2193–2198. [PubMed] [Google Scholar]
- 7.Klein AL, Grimm RA, Murray RD, et al. Assessment of cardioversion using transesophageal echocardiography investigators. Use of trans- esophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 2001;344:1411–1420. [DOI] [PubMed] [Google Scholar]
- 8.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the national registry of atrial fibrillation. JAMA 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 10.Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost 2012;107:1172–1179. [DOI] [PubMed] [Google Scholar]
- 11.Ammash N, Konik EA, McBane RD, et al. Left atrial blood stasis and von willebrand factor-adamts13 homeostasis in atrial fibrillation. Arterioscler Thromb Vasc Biol 2011;31:2760–2766. [DOI] [PubMed] [Google Scholar]
- 12.Melduni RM, Schaff HV, Lee HC, et al. Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality: a propensity score-matched analysis of 10 633 patients. Circulation 2017;135:366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggeri ZM, Savage B. Biological functions of von Willebrand factor. In: Ruggeri ZM, ed. Von Willebrand Factor and the Mechanisms of Platelet Function Berlin: Springer; 1998:79–109. [Google Scholar]
- 14.Conway DS, Pearce LA, Chin BS, Hart RG, Lip GY. Prognostic value of plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 994 patients with non-valvular atrial fibrillation. Circulation 2003;107:3141–3145. [DOI] [PubMed] [Google Scholar]
- 15.Roldán V, Marín F, Muiña B, et al. Plasma von Willebrand factor levels are an independent risk factor for adverse events including mortality and major bleeding in anticoagulated atrial fibrillation patients. J Am Coll Cardiol 2011;57:2496–2504. [DOI] [PubMed] [Google Scholar]
- 16.Choudhury A, Chung I, Panja N, Patel J, Lip GY. Soluble CD40 ligand, platelet surface CD40 ligand, and total platelet CD40 ligand in atrial fibrillation: relationship to soluble P-selectin, stroke risk factors, and risk factor intervention. Chest 2008;134:574–581. [DOI] [PubMed] [Google Scholar]
- 17.Cohoon KP, Mazur M, McBane RD, Wysokinski WE. Association of soluble CD40 ligand with duration of atrial fibrillation and left atrial blood stasis. JACC Clin Electrophysiol 2016;2:623–632. [DOI] [PubMed] [Google Scholar]
- 18.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein eleva- tion in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001;104:2886–2891. [DOI] [PubMed] [Google Scholar]
- 19.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol 2007;50:2021–2028. [DOI] [PubMed] [Google Scholar]
- 20.Kusayama T, Furusho H, Kashiwagi H, et al. Inflammation of left atrial epicardial adipose tissue is associated with paroxysmal atrial fibrillation. J Cardiol 2016;68:406–411. [DOI] [PubMed] [Google Scholar]
- 21.Lip GY, Patel JV, Hughes E, Hart RG. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke 2007;38:1229–1237. [DOI] [PubMed] [Google Scholar]
- 22.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167. [DOI] [PubMed] [Google Scholar]
- 23.Gillum RF, Fortmann SP, Prineas RJ, Kottke TE. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J 1984;108:150–158. [DOI] [PubMed] [Google Scholar]
- 24.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the american college of cardiology/american heart association task force on practice guidelines and the european society of cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the european heart rhythm association and the heart rhythm society. Europace 2006;8:651–745. [DOI] [PubMed] [Google Scholar]
- 25.Hassan GS, Merhi Y, Mourad W. CD40 ligand: a neo-inflammatory molecule in vascular diseases. Immunobiology 2012;217:521–532. [DOI] [PubMed] [Google Scholar]
- 26.Henn V, Slupsky JR, Gräfe M, et al. CD40 ligand on activated plate- lets triggers an inflammatory reaction of endothelial cells. Nature 1998;391:591. [DOI] [PubMed] [Google Scholar]
- 27.Bavendiek U, Libby P, Kilbride M, Reynolds R, Mackman N, Schonbeck U. Induction of tissue factor expression in human endothelial cells by cd40 ligand is mediated via activator protein 1, nuclear factor kappa b, and egr-1. J Biol Chem 2002;277:25032–25039. [DOI] [PubMed] [Google Scholar]
- 28.Freestone B, Gustafsson F, Chong AY, et al. Influence of atrial fibrillation on plasma von willebrand factor, soluble E-selectin, and N- terminal pro B-type natriuretic peptide levels in systolic heart failure. Chest 2008;133:1203–1208. [DOI] [PubMed] [Google Scholar]
- 29.Hou J, Liang Y, Gai X, et al. The impact of acute atrial fibrillation on the prothrombotic state in patients with essential hypertension. Clin Biochem 2010;43:1212–1215. [DOI] [PubMed] [Google Scholar]
- 30.Sahebkar A, Serban C, Ursoniu S, et al. Lipid and blood pressure meta- analysis collaboration (LBPMC) group. The impact of statin therapy on plasma levels of von Willebrand factor antigen. Systematic review and meta-analysis of randomised placebo-controlled trials. Thromb Haemost 2016;115:520–532. [DOI] [PubMed] [Google Scholar]
- 31.Lip GY, Lowe GD, Rumley A, Dunn FG. Increased markers of thrombo- genesis in chronic atrial fibrillation: effects of warfarin treatment. Br Heart J 1995;73:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passamonti SM, Bucciarelli P, Bader R, Martinelli I. Influence of anticoagulant therapy with vitamin K antagonists on plasma levels of coagulation factor VIII. Thromb Res 2010;126:243–245. 21. [DOI] [PubMed] [Google Scholar]
- 33.Blustin JM, McBane RD, Mazur M, et al. The association between thromboembolic complications and blood group in patients with atrial fibrillation. Mayo Clin Proc 2015;90:216–223. [DOI] [PubMed] [Google Scholar]
- 34.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387–1397. [DOI] [PubMed] [Google Scholar]


