Abstract
Background:
A recent randomized trial of patients with primarily anterior circulation intracranial artery stenosis showed that intensive medical therapy was superior to intracranial stenting in preventing recurrent stroke. The rate of stroke recurrence or death in symptomatic intracranial vertebrobasilar stenosis with medical therapy alone may be especially high and rates compared to endovascular therapy need further study.
Methods:
We conducted a systematic review and meta-analysis of studies reporting the rates of stroke recurrence or death (the primary outcome) in symptomatic intracranial vertebrobasilar stenosis with medical or endovascular treatment over a minimum follow up period of 6 months. We included all studies in any language indexed in MEDLINE or EMBASE, supplemented by bibliography searches and by contacting authors. The secondary end points were stroke recurrence, basilar artery and vertebral artery stroke recurrence rates.
Results:
Twenty-three studies (591 medical treatment patients and 483 endovascular treatment patients) were included. The risk of combined stroke recurrence or death was 14.8 per 100 person-years (95%CI: 9.5–20.1) among medical group compared to 8.9 per 100 person-years (95%CI: 6.9–11.0) among endovascular group. The incidence rate ratio (IRR) was 1.3 (95%CI: 1.0–1.7). The stroke recurrence rate was 9.6 per 100 person-years (95%CI: 5.1–14.1) in the medical group compared to 7.2 per 100 person-years (95%CI: 5.5–9.0) in the endovascular group.
Conclusion:
Our results show that the risk of stroke recurrence or death or risk of stroke recurrence is comparable between medical and endovascular therapy. A small preventive effect of endovascular therapy may exist, particularly if the 30-day post-procedural risk could be reduced.
Keywords: stenosis, atherosclerosis, stroke, stent
INTRODUCTION
Posterior circulation stroke and TIA constitute a fifth of all cerebrovascular ischemic events.(1) Stroke recurrence after TIA or minor stroke is as high as 8–10%, and may be even higher after posterior circulation events.(2) Vertebrobasilar stenosis is associated with stroke recurrence risk that varies between 2.5%−5.5% per year (3) to 10–15% per year (4) and has been reported as high as 33% in the first month (1) with best medical therapy. Recently endovascular treatment with angioplasty and stenting has been used as an adjuvant treatment for patients with vertebrobasilar stenosis. The risk of stroke recurrence after endovascular therapy is reported ranging widely between 5.6% and 18%.(5,6) The differences in reported risk of stroke recurrence are due to differences in the follow up period, whether TIA was included as an outcome event, whether stroke in territories other than vertebrobasilar territories were included in stroke recurrence risk, and whether periprocedural events in endovascular treatment groups were included. This variability and lack of standardization in estimating the risk and benefit is clinically uninformative. In order to reduce this variability, we aimed to standardize the method of counting events and accounting for variability in follow up periods.
Defining the risk of stroke recurrence in the vertebrobasilar circulation is important because it might be higher than the risk of stroke in the anterior circulation.(7) The Warfarin-Aspirin Symptomatic Intracranial disease (WASID) study group reported risk of stroke recurrence of 23% in posterior circulation versus 8% in anterior circulation in the Aspirin group while it was 10% versus 2% in the Warfarin group.(7) Subjects with basilar stenosis were found to benefit from warfarin in a post-hoc analysis, which supports the argument of a different risk benefit estimate by stenosis location.(8) The Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial (9) did not report the risk for the vertebrobasilar stenosis separately. Although SAMMPRIS reported that aggressive medical therapy is superior to endovascular therapy with Wingspan™ stent overall, most patients in SAMMPRIS (65%) had anterior circulation stenosis which may have diluted any benefit in the vertebrobasilar stenosis subgroup.
We aimed to define the rates of stroke recurrence among patients with vertebrobasilar stenosis events only in medical and endovascular therapy.
METHODS
We conducted a systematic review and meta-analysis of cohort studies that examined vertebrobasilar circulation stenting. We followed the meta-analysis of observational studies (MOOSE) statement recommendations.(10) We searched MEDLINE (1948 to January 2013) and EMBASE (1980 to January 2013) electronic databases with no language restriction, supplemented with manual bibliography reviews for articles that fulfilled our inclusion criteria [Table 1].
Table 1 –
Search Strategy and Inclusion/Exclusion Criteria
|
Search Strategy: We combined four groups of search terms using Boolean operator and text words ($ denotes truncation).
|
Study Inclusion criteria:
|
Study Exclusion criteria:
|
Authors were contacted to obtain or clarify data in situations with studies reporting intracranial stenosis as a group without specifying data limited to the vertebrobasilar circulation, or with studies including both symptomatic and asymptomatic patients without reporting outcome in each group separately. We did not attempt to search the unpublished, non-peer reviewed literature.
Two reviewers (ARA and MHA) independently screened the titles and abstracts to exclude articles that conflicted with one of the inclusion criteria or fulfilled one of the exclusion criteria. Remaining articles were reviewed in full and retained only if they met all the inclusion criteria, and no single exclusion criteria had been identified. Any disagreements were resolved by consensus discussion between the two reviewers.
Studies characteristics, quality and outcome data were extracted by two reviewers for each language. Studies characteristics included age, sex, hypertension, diabetes mellitus, hyperlipidemia, smoking, TIA as qualifying events and whether they excluded severe stroke.
Quality assessment of each study was based on the study characteristics, methods of finding stenosis, stroke recurrence definition and follow up methods, excluding coexisting other etiologies and loss of follow up. Study quality was assessed in each of these categories.
The primary outcome was stroke recurrence or death. Stroke included both ischemic stroke and intracranial hemorrhage (ICH). Secondary outcomes were stroke recurrence (which include in the medical group stroke recurrence only and in the endovascular group, stroke recurrence and 30-day post-procedural death), stroke recurrence in basilar artery stenosis subgroup (including 30-day post-procedural stroke and death in endovascular group), and stroke recurrence in vertebral artery stenosis subgroup (including 30-day post-procedural stroke and death in endovascular group).
Statistical Methods
For the primary and secondary outcomes, we calculated the stroke recurrence rate and 95% confidence intervals (CI) as the weighted mean of stroke per 100 person-years. Nine of the 23 studies included anterior circulation stenosis and we used the median or mean follow up of the whole cohort in 8 of them. When a study reported zero events, a correction of 0.5 was introduced to allow for the estimation. Results are displayed using reported effect size estimations and Forest plots. Heterogeneity among the medical therapy group and the endovascular therapy group was assessed using the I2 statistics. Pooled estimate of the primary and secondary outcomes and 95% CI were calculated using random effects size models if heterogeneity was significant; otherwise we used a fixed effect size model. Studies that fulfilled our criteria were included in stroke recurrence rate analysis; however, only studies that reported death rate explicitly were included in the stroke or death rate analysis. We calculated incidence rate ratios (IRR) using Poisson regression comparing medical and endovascular group outcomes based upon the raw data from the included studies. Funnel plot analyses were used to assess for publication bias.
RESULTS
A total of 1740 studies were retrieved, 1716 from the database search and 24 from bibliographic searching. After screening, 23 studies met the inclusion criteria and were included in the stroke recurrence rate analysis; 17 studies explicitly reported death rate and were included in the calculation of stroke or death rate [eFigure-1]. Ten authors were contacted to obtain additional data on the vertebrobasilar circulation, of whom two provided additional data.
A funnel plot showed symmetric distribution in endovascular therapy but asymmetric in medical therapy (eFigure-2) as a result of one outlier study. Study quality is shown in eTable-1, with the proportion of high quality studies of 50% in the medical group and 35% in the endovascular group. All studies were retrospective except for two in the medical therapy(8,11) and three in the endovascular therapy(6,12,13) groups. Three studies in the medical(3,11,14) and one in the endovascular group did not report loss of follow up(15). Three studies did not define the methods of follow up used.(12,14,16) Five of the 8 studies in medical therapy(3,4,17–19) and 10 of 16 studies in endovascular therapy(5,6,12,17,20–25) included only vertebrobasilar circulation patients. In general, follow up and documenting of the stroke recurrence rate was complete by clinic visits or telephone interviews. The stroke recurrence definition was absent in all but two trials(4,17), and none of them included imaging criteria in the stroke recurrence definition. The loss to follow up rate was higher in the endovascular therapy group with median of 5% while in the medical therapy group the median was 0%.
A total of 1072 patients from the 23 published studies were included.(3–6,8,11–28) Of these, 592 patients were from the medical group and 480 patients from the endovascular group. However, only 403 were included in stroke and or death rate analysis in the endovascular therapy group as the remaining studies did not report the death rate explicitly. The medical and endovascular therapy group had comparable baseline characteristics [Table 2]. Age ranged from 57.0–71.5 for the medical group and 58.0–71.5 for the endovascular group. Hypertension was the most prevalent risk factor (>70%) with equal proportion from the medical and endovascular groups; except for one study.(28) One third of the patients in both groups had diabetes mellitus and hyperlipidemia was more commonly found in the endovascular group.
Table 2:
Study characteristics
| Author, year | Age | Male | BP | DM | Lipid | Smoking | TIA as qualifying events | Warfarin |
|---|---|---|---|---|---|---|---|---|
| Povedano, 2010(17) | 68 | 86.80% | 89% | 26% | 76% | 47% | 10% | NR |
| Mazighi, 2006*(11) | 63.3 | 71.60% | NR | NR | NR | NR | 48% | 52.94% |
| WASID, 2006(8) | 63.5 | 61.5% | 84% | 38% | 34% | 42% | 39% | 51% |
| Qureshi, 2003(4) | 64 | 54% | 81% | 41% | 34% | 42% | 29% | 47% |
| Thijs, 2000(14) | 66.7 | 59.60% | 73.1% | 26.9% | 38.5% | 42.30% | 55.80% | 53.84% |
| Woolfenden, 2000(18) | 71.5 | 67.90% | 78.6% | 32.1% | 35.6% | 21.40% | 67.90% | 90.9% |
| WASID, 1998(3) | 65.7 | 77.90% | 72.5% | 46% | 53% | 64.50% | 41% | 61.8% |
| Moufarrij, 1986(19,20) | 57 | 77% | 70% | 70% | NR | NR | 56.80% | NR |
| Li, 2012(20) | 59 | 83% | 86% | 43.30% | 13.30% | 53.30% | 40% | 10% |
| Jiang, 2010(6) | 58.5 | 92% | 78.4% | 29% | 80.5% | 66.2% | 72.6% | NAP |
| Povedano, 2010(17) | 63 | 84% | 84% | 24% | 60% | 40% | 44% | NR |
| Wittkugel, 2009(12) | 64.6 | 73.30% | NR | NR | NR | NR | NR | NAP |
| Miao Zhong-rong, 2008(21) | 59 | 87.50% | NR | NR | NR | NR | 75% | NAP |
| Fiorella, 2007(22) | 64.8 | 79.50% | NR | NR | NR | NR | NR | NAP |
| Freitas, 2007(15) | 59.3 | 75% | NR | NR | NR | NR | 33% | NAP |
| Wojak, 2006(26) | 62.5 | 76.70% | NR | NR | NR | NR | 46% | NAP |
| Hatano, 2005(16) | 71.5 | 84.20% | NR | NR | NR | NR | NR | NR |
| Kim, 2005(23) | 64 | 59% | 100% | 41% | 30% | 41% | NR | NAP |
| Weber, 2005(24) | 67 | 71% | NR | NR | NR | NR | 9.10% | NAP |
| Yu, 2005(5) | 69 | 83.30% | 83% | 50% | 72% | 33% | 44% | NR |
| Liu Jian-min, 2004(27) | 63 | 63.05% | 84.8% | 23.9% | 58.7% | NR | 89.13% | NAP |
| SSYLLVIA, 2004†(13) | 63.6 | 82% | 63.9% | 32.8% | 54.1% | 52.50% | 39.30% | NAP |
| Huang Qing-Hai, 2003(28) | 62.1 | 64.1% | 43.5% | 15.3% | NR | NR | 82.10% | NAP |
| Nahser, 2000(25) | 60 | 89% | 84% | 10.5% | 63.2% | 57% | 72% | NR |
excluded mRS>2;
excluded mRS>3;
NAP= not as protocol; NR = not reported; BP = blood pressure; DM – diabetes mellitus; TIA = transient ischemic attack; WASID = Warfarin Aspirin Symptomatic Intracranial Disease study;
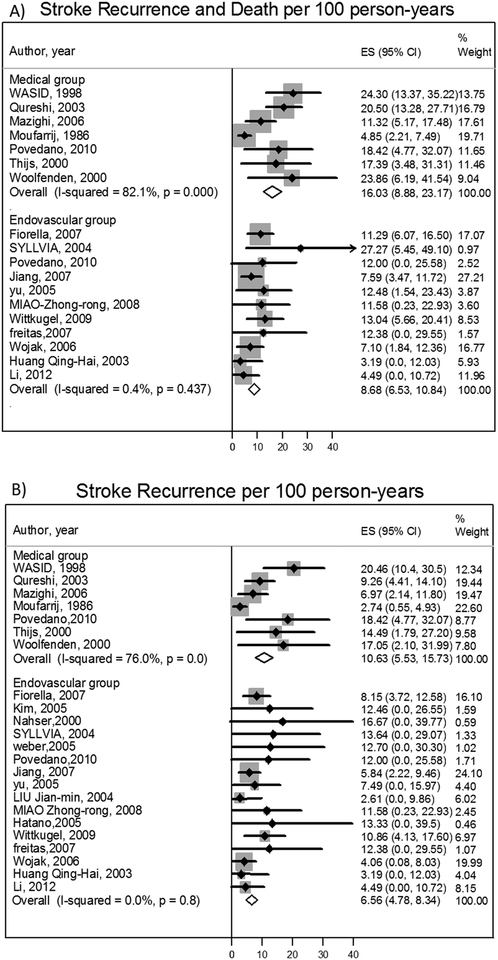
The stroke or death rate pooled estimate was 14.8 per 100 person-years (95%CI: 9.5, 20.1) in the medical therapy group and 8.9 per 100 person-years (95%CI: 6.9–11.0) in the endovascular group [IRR=1.3 (95%CI: 1.0–1.7)]. Sensitivity analysis, excluding the trials that included <20% asymptomatic patients and limiting to high quality studies only, showed consistent results eTable-2. There were 16 ICH events (8 in medical therapy group and 8 in endovascular therapy group). Stroke recurrence rate was 9.6 per 100 person-years (95%CI: 5.1–14.1) in the medical group and 7.2 per 100 person-years (95%CI: 5.5–9) in the endovascular group. Heterogeneity of effect was observed in the medically treated cohorts on the outcomes of stroke or death (I2=80.5%, p=0.0) and on stroke recurrence (I2=72.4%, p=0.0). This was not seen in the endovascular arm. The reason for this was the long follow up observed in one larger study accounting for a greater weighting in the calculation of event rates. The IRR for stroke recurrence was 1.1 (95%CI: 0.8–1.5). After excluding the study that causing the heterogeneity in the medical group, the stroke recurrence or death rate was 14.7 per 100 person-years (95% CI: 12.1, 17.3) and IRR was 1.6 (95% CI: 1.2, 2.0). Stroke recurrence was 10.1 per 100 person-years (95% CI: 7.3, 13.0) and IRR was 1.5 (95% CI: 1.0, 2.1).
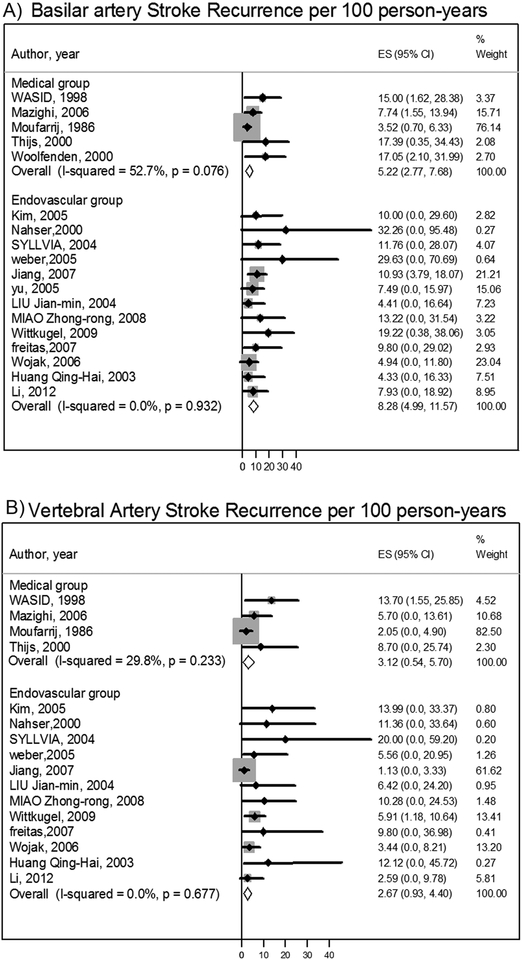
Basilar stroke recurrence rates were 9.2 per 100 person-years (95%CI: 5.0, 13.4) in the medical group and 8.9 per 100 person-years (95%CI: 5.7–12.1) in the endovascular group [IRR was 1.0 (95%CI: 0.7 −1.6)]. After removing the outlier study(19) causing the heterogeneity in the medical group, the basilar stroke recurrence rate was 10.7 per 100 person-years (95% CI: 7.4, 14.0). Vertebral artery stroke recurrence rates were 5.8 per 100 person-years (95%CI: 0.4, 11.2) in the medical therapy group and 4.9 per 100 person-years (95%CI: 2.7–7.0) in the endovascular group [IRR was 1.8 (95%CI: 1.0–3.4).] After removing the outlier study(19) that caused the heterogeneity in the medical group(19), the vertebral stroke recurrence rate was 8.8 per 100 person-years (95% CI: 3.1, 14.6). Both of these subgroup analyses excluded 4 studies,(8)(11)(14)(22) with large numbers of patients due to insufficient data, despite the fact that all authors had been contacted for more data. [Figures1–2]. The 30-day post-procedural stroke and death rate was 6.5% (95% CI: 4.2, 8.7).
Figure 1:
Stroke or death per 100 person-years in intracranial vertebrobasilar circulation stenosis and Stroke recurrence per 100 person-years in intracranial vertebrobasilar circulation stenosis.
Figure 2:
Stroke recurrence per 100 person-years in basilar artery stenosis and Stroke recurrence per 100 person-years in intracranial vertebral artery stenosis
DISCUSSION
Among patients with vertebrobasilar stenoses, endovascular therapy was associated with a non-significantly lower risk of stroke or death compared to medical therapy. A similar difference was found in the stroke recurrence rate, but both therapies were equivalent in the same territory stroke recurrence. Heterogeneity of effect was observed in the medical arm as a result of a single, outlier study that was conducted over 25 years ago. A sensitivity analysis, removing studies reporting on asymptomatic patients and low quality studies, showed a similar direction of effect without evidence of heterogeneity by study. Our stroke recurrence rate of 9.6 per 100 person-years was consistent with what was found in SAMMPRIS trial of stroke recurrence of 12% in medical group (combing anterior and posterior circulation).(9) However, our rate was less among endovascular group 7.2 per 100 person-years compared to the risk in SAMPRIS of 21%.
The stroke recurrence rate in the vertebrobasilar stenosis treated medically (9.6 per 100 person years) was similar to the reported estimate of anterior circulation risk in high quality studies. WASID found no significant difference in the risk of stroke recurrence between anterior 14% and posterior circulation stenosis 13%.(29) Importantly, we observed that mortality associated with intracranial vertebrobasilar stenosis is a major contributor to the overall primary outcome. The stroke or death rate was 14.8 per 100 person-years while the stroke recurrence was 9.6 per 100 person years in the medical group. WASID showed similar results as 22% (6/27) of events were vascular deaths not related to stroke.(29) This high mortality likely reflects that severity of atherosclerotic disease in this population and is expected to reduce the overall benefit from endovascular therapy.
Reduction of periprocedural events may be the key to improve benefit from endovascular therapy, particularly in basilar artery stenosis. The 30-day post procedural event rate constituted 51% of the numbers of total events in the endovascular therapy. The overall 30-day post-procedural stroke or death rate was 6.5%. Similar 30-day post-procedural risk was found between coronary stents and non-coronary stents (see eTable-2) and this risk was also similar to reported rates using the Wingspans® stent.(20) Our data were also consistent with a systematic review which showed a median rate of posterior circulation periprocedural complication risk of 8% (range 0%−50%) despite that a weighted mean showed 12% risk likely skewed by outlier studies.(30) Prior studies showed perforator stroke risk is higher in basilar stenting (8.3%) than in middle cerebral artery stenosis (2%).(31) Peri-procedural stroke is commonly due to occlusion of small perforating arteries and thought to be due to compression of plaque into these small arterial ostia arising from the parent trunk artery. Typically perforator stroke occurs within 24 hours of the procedure.(31)
Stent type did not alter the stroke recurrence rate (eTable-2). This is consistent with a prior study which found similar risk between the balloon mounted stent and self-expandable wingspan® stent in periprocedural complications and clinical outcomes.(32) The single study which used only wingspan® in vertebrobasilar stenosis has shown a similar risk of 6%.(20) SAMMPRIS reported a stroke recurrence of 21% in overall intracranial stenosis, which was driven by the intracranial hemorrhage rate and perforator stroke.(33) In contrast we found that the intracranial hemorrhage rate was similar between medical and endovascular groups (0.4 versus 0.8 per 100 person-years, respectively).
We found that >70 stenosis was not associated with higher risk of stroke recurrence (eTable-2). This finding is similar to what was reported in the Wingspan® registry.(34) In contrast, WASID 2006 found twice the risk of stroke recurrence at 19% with >70% stenosis compared to 10% risk with <70% stenosis.(29) We could not assess the risk between acute and subacute therapy because most studies did not provide adequate data about timing of the interventions.
Patients with basilar artery stenosis have a higher risk of stroke recurrence than those with vertebral artery stenosis in both medical and endovascular groups (Figure-2). Because both the natural history and interventional risk may vary by specific arterial anatomy, considering patients by arterial lesion location is an important consideration for future studies.
There are limitations to this meta-analysis. Patient selection bias, inherent to the observational studies will be translated to these pooled outcome estimates. Eight of 23 studies included telephone interview as a method of outcome assessment. The loss to follow up rate was higher in the endovascular therapy group with median of 7.6% while in medical therapy group the median was 0%. Only 7 of 23 studies have excluded other coexisting stroke etiologies. Most studies did not report the timing of the intervention (medical or endovascular) in relation to recent symptoms; this is relevant, because the risk of recurrence is time dependent. Statistically, the studies did not report individual patient data so we used the median follow up to estimate follow up years. There is potential for publication bias, particularly in the medical arm.
Our results show that the risk of stroke recurrence or death, or risk of stroke recurrence alone is comparable between medical and endovascular therapy. A small preventive effect of endovascular therapy may exist, particularly if the 30-day post-procedural risk could be reduced.
Supplementary Material
Contributor Information
Ahmad R Abuzinadah, Internal medicine department, King Abdulaziz University, Jeddah, KSA neuroahmad@gmail.com.
Mohammed H. Alanazy, Internal medicine department, King Saud University, Riyadh, KSA dranazy@gmail.com.
Mohammed A Almekhlafi, Internal medicine department, King Abdulaziz University, Jeddah, KSA. malmekhl@ucalgary.ca.
Yanjune Duan, University of Calgary, Calgary, Alberta, Canada yduan2004ca@yahoo.ca.
Haifeng Zhu, University of Calgary, Calgary, Alberta, Canada. hazhu@ucalgary.ca.
Michael Mazighi, Department of Neurology and Stroke Centre, Bichat University Hospital, INSERM U-698 and Paris-Diderot University, Paris, France. mikael.mazighi@bch.aphp.fr.
Helmi L. Lutsep, Oregon Health & Science University, Portland, Oregon, USA lutseph@ohsu.edu
Tyrone Donnon, Department of Community Health Sciences, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada tldonnon@ucalgary.ca.
Michael D Hill, Departments of Clinical Neurosciences, Medicine, Radiology and Community Health Sciences, Hotchkiss Brain Institute, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada. michael.hill@ucalgary.ca.
References
- (1).Gulli G, Khan S, Markus HS. Vertebrobasilar stenosis predicts high early recurrent stroke risk in posterior circulation stroke and TIA. Stroke 2009. August;40(8):2732–2737. [DOI] [PubMed] [Google Scholar]
- (2).Marquardt L, Kuker W, Chandratheva A, Geraghty O, Rothwell PM. Incidence and prognosis of > or = 50% symptomatic vertebral or basilar artery stenosis: prospective population-based study. Brain 2009. April;132(Pt 4):982–988. [DOI] [PubMed] [Google Scholar]
- (3).Prognosis of patients with symptomatic vertebral or basilar artery stenosis. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Stroke 1998. July;29(7):1389–1392. [DOI] [PubMed] [Google Scholar]
- (4).Qureshi AI, Ziai WC, Yahia AM, Mohammad Y, Sen S, Agarwal P, et al. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: a multicenter study. Neurosurgery 2003. May;52(5):1033–9; discussion 1039–40. [PubMed] [Google Scholar]
- (5).Yu W, Smith WS, Singh V, Ko NU, Cullen SP, Dowd CF, et al. Long-term outcome of endovascular stenting for symptomatic basilar artery stenosis. Neurology 2005. March 22;64(6):1055–1057. [DOI] [PubMed] [Google Scholar]
- (6).Jiang WJ, Du B, Hon SF, Jin M, Xu XT, Ma N, et al. Do patients with basilar or vertebral artery stenosis have a higher stroke incidence poststenting? J Neurointerv Surg 2010. March;2(1):50–54. [DOI] [PubMed] [Google Scholar]
- (7).Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology 1995. August;45(8):1488–1493. [DOI] [PubMed] [Google Scholar]
- (8).Kasner SE, Lynn MJ, Chimowitz MI, Frankel MR, Howlett-Smith H, Hertzberg VS, et al. Warfarin vs aspirin for symptomatic intracranial stenosis: subgroup analyses from WASID. Neurology 2006. October 10;67(7):1275–1278. [DOI] [PubMed] [Google Scholar]
- (9).Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011. September 15;365(11):993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000. April 19;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- (11).Mazighi M, Tanasescu R, Ducrocq X, Vicaut E, Bracard S, Houdart E, et al. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology 2006. April 25;66(8):1187–1191. [DOI] [PubMed] [Google Scholar]
- (12).Wittkugel O, Rosenkranz M, Burckhardt D, Niessen WD, Espersen T, Zeumer H, et al. Long-term outcome after endovascular treatment of high-risk patients with recurrently symptomatic intracranial stenoses of the posterior circulation. Rofo 2009. August;181(8):782–791. [DOI] [PubMed] [Google Scholar]
- (13).SSYLVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke 2004. June;35(6):1388–1392. [DOI] [PubMed] [Google Scholar]
- (14).Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology 2000. August 22;55(4):490–497. [DOI] [PubMed] [Google Scholar]
- (15).Freitas JM, Zenteno M, Aburto-Murrieta Y, Koppe G, Abath C, Nunes JA, et al. Intracranial arterial stenting for symptomatic stenoses: a Latin American experience. Surg Neurol 2007. October;68(4):378–386. [DOI] [PubMed] [Google Scholar]
- (16).Hatano T, Tsukahara T, Ogino E, Aoyama T, Nakakuki T, Murakami M. Stenting for vertebrobasilar artery stenosis. Acta Neurochir Suppl 2005;94:137–141. [DOI] [PubMed] [Google Scholar]
- (17).Povedano G, Zuberbuhler P, Lylyk P, Ameriso SF. Management strategies in posterior circulation intracranial atherosclerotic disease. J Endovasc Ther 2010. June;17(3):308–313. [DOI] [PubMed] [Google Scholar]
- (18).Woolfenden AR, Tong DC, Norbash AM, Ali AO, Marks MP, O’Brien MW, et al. Basilar artery stenosis: clinical and neuroradiographic features. J Stroke Cerebrovasc Dis 2000. Mar-Apr;9(2):57–63. [DOI] [PubMed] [Google Scholar]
- (19).Moufarrij NA, Little JR, Furlan AJ, Leatherman JR, Williams GW. Basilar and distal vertebral artery stenosis: long-term follow-up. Stroke 1986. Sep-Oct;17(5):938–942. [DOI] [PubMed] [Google Scholar]
- (20).Li J, Zhao ZW, Gao GD, Deng JP, Yu J, Gao L, et al. Wingspan stent for high-grade symptomatic vertebrobasilar artery atherosclerotic stenosis. Cardiovasc Intervent Radiol 2012. April;35(2):268–278. [DOI] [PubMed] [Google Scholar]
- (21).MIAO Z, WANG B, Li S, Zhu F, JI X, Li X, et al. Endovascular treatment of severe vertebrobasilar artery stenosis. Chin J Contemp Neurol Neurosurg 2008;8:525–529. [Google Scholar]
- (22).Fiorella D, Chow MM, Anderson M, Woo H, Rasmussen PA, Masaryk TJ. A 7-year experience with balloon-mounted coronary stents for the treatment of symptomatic vertebrobasilar intracranial atheromatous disease. Neurosurgery 2007. August;61(2):236–42; discussion 242–3. [DOI] [PubMed] [Google Scholar]
- (23).Kim DJ, Lee BH, Kim DI, Shim WH, Jeon P, Lee TH. Stent-assisted angioplasty of symptomatic intracranial vertebrobasilar artery stenosis: feasibility and follow-up results. AJNR Am J Neuroradiol 2005. Jun-Jul;26(6):1381–1388. [PMC free article] [PubMed] [Google Scholar]
- (24).Weber W, Mayer TE, Henkes H, Kis B, Hamann GF, Schulte-Altedorneburg G, et al. Stent-angioplasty of intracranial vertebral and basilar artery stenoses in symptomatic patients. Eur J Radiol 2005. August;55(2):231–236. [DOI] [PubMed] [Google Scholar]
- (25).Nahser HC, Henkes H, Weber W, Berg-Dammer E, Yousry TA, Kuhne D. Intracranial vertebrobasilar stenosis: angioplasty and follow-up. AJNR Am J Neuroradiol 2000. August;21(7):1293–1301. [PMC free article] [PubMed] [Google Scholar]
- (26).Wojak JC, Dunlap DC, Hargrave KR, DeAlvare LA, Culbertson HS, Connors JJ,3rd. Intracranial angioplasty and stenting: long-term results from a single center. AJNR Am J Neuroradiol 2006. October;27(9):1882–1892. [PMC free article] [PubMed] [Google Scholar]
- (27).Liu JM, Hong B, Huang QH, Xu Y, Zhao WY, Zhang L, et al. Safety and short-term results of stent-assisted angioplasty for the treatment of intracranial arterial stenosis. Zhonghua Wai Ke Za Zhi 2004. February 7;42(3):169–172. [PubMed] [Google Scholar]
- (28).Huang QH, Liu JM, Hong B, Xu Y, Zhang L, Zhang X, et al. Endovascular stent-assisted angioplasty for atherosclerotic intracranial stenosis, a clinical analysis of 39 cases. Zhonghua Yi Xue Za Zhi 2003. January 10;83(1):18–20. [PubMed] [Google Scholar]
- (29).Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006. January 31;113(4):555–563. [DOI] [PubMed] [Google Scholar]
- (30).Groschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke 2009. May;40(5):e340–7. [DOI] [PubMed] [Google Scholar]
- (31).Jiang WJ, Srivastava T, Gao F, Du B, Dong KH, Xu XT. Perforator stroke after elective stenting of symptomatic intracranial stenosis. Neurology 2006. June 27;66(12):1868–1872. [DOI] [PubMed] [Google Scholar]
- (32).Yue X, Yin Q, Xi G, Zhu W, Xu G, Zhang R, et al. Comparison of BMSs with SES for symptomatic intracranial disease of the middle cerebral artery stenosis. Cardiovasc Intervent Radiol 2011. February;34(1):54–60. [DOI] [PubMed] [Google Scholar]
- (33).Derdeyn CP, Fiorella D, Lynn MJ, Rumboldt Z, Cloft HJ, Gibson D, et al. Mechanisms of stroke after intracranial angioplasty and stenting in the SAMMPRIS trial. Neurosurgery 2013. May;72(5):777–95; discussion 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Nahab F, Lynn MJ, Kasner SE, Alexander MJ, Klucznik R, Zaidat OO, et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology 2009. June 9;72(23):2014–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.