Abstract
Background:
There is well-documented systemic inflammatory response in xenograft recipients to the presence of a pig graft. Serum amyloid A (SAA) is an inflammatory marker that is elevated in various pathological states. The assay used to measure it is (i) simple, (ii) relatively inexpensive, and (iii) provides an answer within minutes.
Method:
The levels of SAA (n=11) and C-reactive protein (C-RP) (n=8) were measured retrospectively in the serum of baboons with pig kidney transplants, who received therapy with an IL-6R inhibitor and a TNF-α antagonist. Immunohistochemistry (IHC) was used to identify amyloid A and C-RP expression in the native livers and deposition in the pig kidney grafts.
Results:
One kidney graft underwent hyperacute rejection, 6 (55%) underwent acute antibody-mediated rejection, 3 baboons (27%) were euthanized for serious systemic infections, and one was euthanized for acute gastric dilatation. The SAA increased temporarily after kidney transplantation, and increased again by the day of euthanasia, indicating moderate (n=3) or significant (severe) (n=8) inflammation. In contrast, as the baboons were receiving tocilizumab, C-RP did not increase. There was greater expression of amyloid A in baboon livers (by IHC) than of C-RP (mean OD 53 vs 1, p<0.01), and greater deposition of amyloid A than C-RP in the pig kidney grafts (mean OD 24 vs 2, p<0.001). Plasma fibrinogen negatively correlated with the expression of amyloid A in the liver (r=−0.72, p<0.05). The results of the SAA assay correlated with amyloid A expression in the liver and deposition in the kidney grafts.
Conclusions:
SAA is a sensitive, but non-specific, marker for inflammation in baboons with pig kidney grafts, and is not affected by therapy that suppresses the response of C-RP. The SAA assay is a rapid, reliable, and relatively inexpensive method of following the inflammatory state of baboons with pig xenografts.
Keywords: Amyloid A; C-reactive protein; Inflammation; Kidney, pig; Serum amyloid A; Xenotransplantation
Introduction
In recent years, considerable progress has been made in the results of pig organ xenotransplantation in nonhuman primates, with survival of heterotopic heart and lifesupporting kidney transplants now extending for months or even years [1-4]. The indicators of impending graft failure are different from those seen after allotransplantation. Monitoring of the adaptive immune response, e.g., lymphocytes, T and B cells, is unhelpful, whereas indicators of coagulation dysregulation (e.g., reductions in platelet counts and plasma fibrinogen), and inflammation appear to be valuable [1, 5].
As there is a well-documented systemic inflammatory response in xenograft recipients (that has been termed systemic inflammation in xenograft recipients, SIXR) in response to the presence of a pig graft [5-7], we have searched for a reliable marker of this response. Initially, we suggested that C-reactive protein (C-RP) would be a reliable indicator that SIXR was occurring, but this was found to be unhelpful when the interleukin-6 receptor (IL-6R) was blocked by the specific IL-6R monoclonal antibody, tocilizumab [8]. Although the C-RP remained in the normal range, giving us a false picture of the state of inflammation in the recipient, other indicators, e.g., cytokines, chemokines, histones, suggested that a state of inflammation was continuing.
We also investigated extracellular histones and serum amyloid A (SAA) as potential markers of impending graft failure [8, 9]. SAA is an inflammatory marker that is elevated in various pathological states [10-13], and in recipients of kidney or heart allografts in the presence of rejection or infection [14-17]. SAA has the advantage that the assay used to measure it is (i) simple, (ii) relatively inexpensive, (iii) provides an answer within minutes, and (iv) can be carried out ‘at the bedside’ or, in the case of the experimental laboratory, at the side of the cage. Furthermore, it is not affected by the administration of tocilizumab or of soluble IL-6 blockade agents, such as siltuximab (unpublished data).
We therefore believe it has potential as an indicator that a xenograft may be at risk for injury, and thus would enable steps to be taken to prevent or reverse this. We have reviewed our most recent results of measuring SAA retrospectively in baboons with pig kidney grafts.
Materials and Methods
Animals
Baboons (n=11) (Papio species, Oklahoma University Health Sciences Center, Oklahoma City, OK) received a life-supporting kidney [1, 2]. All grafts were from genetically-engineered pigs (Revivicor, Blacksburg, VA). All were from GTKO pigs that expressed one or more human complement-regulatory proteins, and all except one expressed one or more human coagulation-regulatory proteins in the kidneys. Five expressed the human anti-apoptotic/anti-inflammatory protein, hemeoxygenase-1 (HO-1).
Anesthesia, intravascular catheter placement in baboons, and pig-to-baboon surgical procedures have been described previously [1, 2, 18, 19]. All animal care was in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Research Council (8th edition, revised 2011), and was conducted in an AAALAC-accredited facility. Protocols were approved by (Please insert from ethical approval document).
Immunosuppressive, anti-inflammatory, and adjunctive therapy
Two immunosuppressive regimens were used, with the only difference being in the maintenance immunosuppressive therapy administered (Table1). Five baboons received only FDA-approved agents (e.g., various combination of CTLA4-Ig, tacrolimus, rapamycin, mycophenolate mofetil), termed ‘conventional’ therapy, whereas the remaining 6 baboons received a regimen based on anti-CD40mAb (2C10R4; NIH NHP Reagent Resource, Boston, MA), which is not yet FDA-approved.
Table 1:
Maintenance immunosuppressive (IS) therapy, survival (in days), and cause for euthanasia in baboons with pig kidney grafts
| Baboon Number |
Maintenance IS therapy | Survival (days) | Cause for euthanasia |
|---|---|---|---|
| Long survival group | |||
| B10815 | Anti-CD40mAb-based | 90 | AHXR |
| B9313 | Anti-CD40mAb-based | 136 | Systemic Infection |
| B17315 | Anti-CD40mAb-based | 237 | Systemic Infection |
| B17615 | Anti-CD40mAb-based | 260 | Systemic Infection |
| Short survival group | |||
| B17415 | Anti-CD40mAb-based | 12 | AHXR |
| B3115 | Anti-CD40mAb-based | 4 | Acute gastric dilatation |
| B14214 | Conventional therapy* | 32 | AHXR |
| B5415 | Conventional therapy* | 15 | AHXR |
| B15815 | Conventional therapy* | 12 | AHXR |
| B9015 | Conventional therapy* | 13 | AHXR |
| B14115 | Conventional therapy* | 1 | Hyperacute rejection |
Conventional therapy consisted of only FDA-approved agents (various combinations of CTLA4-Ig, tacrolimus, rapamycin, mycophenolate mofetil).
AHXR = acute humoral xenograft rejection.
Anti-inflammatory therapy included the IL-6R inhibitor, tocilizumab (Actemra, Genentech, South San Francisco, CA ), administered throughout the post-transplant course, and the TNF-α antagonist, etanercept (Enbrel, Amgen Pfizer, Thousand Oaks, CA), administered for only the first two weeks.
Measurement of serum C-RP and SAA
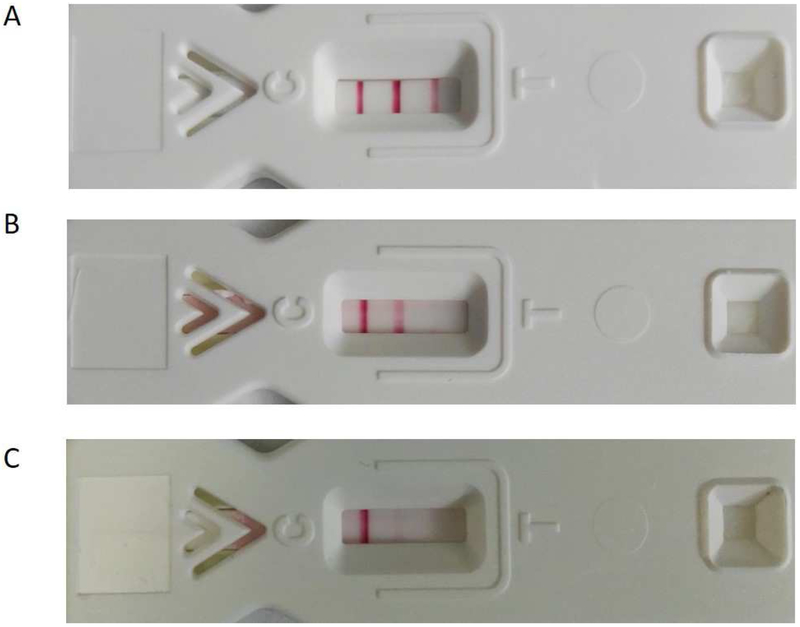
Serum samples were obtained from the baboons before and at intervals after pig kidney transplantation. C-RP (n=8) was measured by standard methods in two laboratories; samples were measured by the UPMC Central Laboratory in Pittsburgh (B9313, B17315, B17615, B17415), or by Antech Diagnostics (Atlanta, GA) (B10815, B5415, B15815, B9015). SAA (n=11) was measured with an OmniChekTM -SAA kit (Multispecies Rapid Test for Inflammation, Accuplex Diagnostics, Co Kildare, Ireland), as per the manufacturer’s instructions. The level of SAA was assessed by the number of red bands that appear and the intensity of the red color (Figure1).
Figure 1:
Representative results of assay for SAA showing (A) 3 lines (negative), indicating no active inflammation, (B) 2 lines, indicating mild-to-moderate inflammation, (C) 1 line, indicating significant inflammation.
Measurement of coagulation parameters
Blood samples were collected for platelet counts, and plasma fibrinogen and D-dimer were measured by the UPMC Central Laboratory in Pittsburgh or by Antech Diagnostics [5].
Immunohistochemistry (IHC) for C-RP and amyloid A
After tissues were obtained at euthanasia, samples were immediately fixed in formalin and embedded in paraffin, and sections with a thickness of 4μM were cut. Expression of C-RP and amyloid A in the baboon liver (n=8) and deposition of C-RP and amyloid A in the pig kidney (n=11) were measured by IHC. Negative controls were naïve pig liver and kidney tissues. Positive controls were baboon liver and kidney tissues from (i) a baboon that experienced an intense anaphylactic response to anti-thymocyte globulin (and thus was euthanized without undergoing pig kidney transplantation), and (ii) a baboon (that received tocilizumab) in which the pig kidney graft underwent hyperacute rejection [20].
The primary and secondary antibodies used were (i) for amyloid A, rat anti-amyloid A (Accuplex Diagnostics) (1:100), and goat anti-rat (Ab#97057, Abcam, Cambridge, MA) (1:200), and (ii) for C-RP, rabbit anti-C-RP (Cat#Ab32412, Abcam) (1:50), and goat antirabbit (Cat#Ab6721, Abcam) (1:1000). All slides were examined with an optical microscope (Olympus Optical, Tokyo, Japan).
Expression of amyloid A and C-RP was measured by two methods.
(i). Intensity and extent of staining:
The intensity of stain was graded by two independent observers as (i) negative (−) = score 0; (ii) weakly positive (+) = score 1; moderately positive (++) = score 2; strongly positive (+++) = score 3. The percentage of positively-stained cells was graded as (i) <5% = score 0; 5-25% = score 1; 25-50% = score 2; 50-75% = score 3; >75% = score 4. The formula for the total IHC score was determined by calculating the score of staining intensity x the score of positive-staining cells. A total score of 0 was negative (−); scores of 1-4 were considered weakly-positive (+); scores of 5-8 were moderately-positive (++); scores of 9-12 were highly-positive (+++).
(ii). Quantitative analysis of expression:
Measurement was made of the mean optical density using ImageJ software (National Institute of Mental Health, Bethesda, MA). Five visual fields of each sample were randomly selected, and 5 visual areas were randomly selected from each visual field. The mean optical density (MOD) was calculated.
Serum assays for TNF-α and IL-1 β cytokines in baboons
The serum levels of serum TNF-α and IL-1 β at specific time-points (before any therapy or procedure [Naive], on post-transplant day 1, and at euthanasia) were measured using cytometric bead array (CBA) with a human Inflammatory Cytokine Kit (#551811, BD Biosciences, San Jose, CA). LSR II flow cytometry (BD Biosciences) was used to collect data, and the data were analyzed using CBA analysis software (BD Bioscience).
Statistical analyses
Statistical analyses were performed using social sciences software GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). The quantity variables were expressed as mean +/− SD and were performed using a t test or nonparametric test. The numerical variables were expressed as n (%) or median (range), and were analyzed with Χ2 test or Fisher's exact test. Spearman’s rank correlation was used to analyze the correlation between MOD of amyloid A and coagulation parameters or cytokines. Values of p<0.05 were considered statistically significant.
Results
Clinical course
Based on the clinical course and graft histopathology, the baboons were divided into 2 groups – (i) short-term survivors (n=7) and long-term survivors (n=4). In the short-survival group, 1 pig kidney underwent hyperacute rejection, 5 underwent acute humoral xenograft rejection, and I was euthanized on day 4 for acute gastric dilatation (Table1). In the long-survival group, 3 baboons were euthanized for systemic infection, and 1 lost graft function at 90 days when immunosuppressive therapy was reduced too quickly (Table1). Mean survival in the short survival group was 13 days (range 1-32 days), and in the long-survival group was 181 days (90-260 days).
The baboons that received the anti-CD40mAb-based immunosuppressive regimen (n=6) included one with acute gastric dilatation (survival 4 days), one that underwent early antibody-mediated rejection with consumptive coagulopathy (as the graft was subsequently found not to express a human coagulation-regulatory protein), and 3 that required euthanasia for systemic infection (at 136, 237, and 260 days). Mean survival was 123 days. In those that received conventional immunosuppressive therapy, none survived longer than 32 days, and all underwent either hyperacute (n=1) or acute antibody-mediated (n=4) rejection. Mean survival was 15 days.
Serum C-RP and SAA levels in baboon recipients
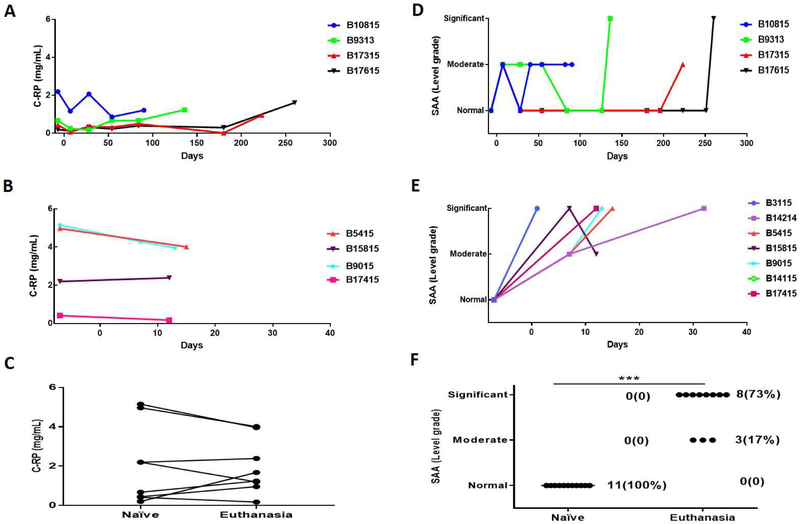
The baboons were divided into two groups based on their survival (a long-survival group: ≥90 days (n=4), and a short-survival group: <35 days (n=7) (Figure2A, E). SAA levels were measured in 11 baboons and CRP in 8 baboons.
Figure 2: Serum C-RP (left) and SAA (right) levels in baboons with pig kidney xenografts.
(A, B) The levels of C-RP in long-survival (A) and short-survival (B) baboons. (D, E) The levels of SAA in long-survival (D) and short-survival (E) baboons. (C, F) Comparisons of baboon serum C-RP (C) and SAA (F) pre-transplant (naive) and at euthanasia. Although there was no significant change in the level of C-RP between these two time-points (p=0.08), the level of SAA increased significantly (***p<0.001). (With regard to SAA, the results were categorized as normal [no inflammation], moderate, or significant [severe] inflammation).
Serum C-RP
As all of the baboons received tocilizumab, no significant increase in C-RP was observed after transplantation, though some increase occurred in the presence of systemic infection (B9313, B17315, B17615) (Figure2A,B). Overall, there was no significant difference in the level of C-RP between pre-transplantation (naive) and at the time of euthanasia (Figure2C).
SAA
Before xenotransplantation, the SAA assay indicated no inflammation. In both the long- and short-survival groups, the SAA showed an immediate moderate inflammatory response after the surgical procedure. In the long-survival baboons, there was no further increase (and usually a reduction) until the baboon developed either systemic infection (n=3) or antibody-mediated rejection (n=1) (Figure2D). In contrast, in the short-survival group, after the initial increase after the surgical procedure, the SAA continued to increase until the baboon was euthanized for antibody-mediated rejection or acute gastric dilatation (Figure2E). In all baboons in both groups, the SAA was increased at the time of euthanasia (moderate inflammation n=3, significant [severe] inflammation n=8) (Figure2F).
C-RP and amyloid A expression in baboon liver tissue
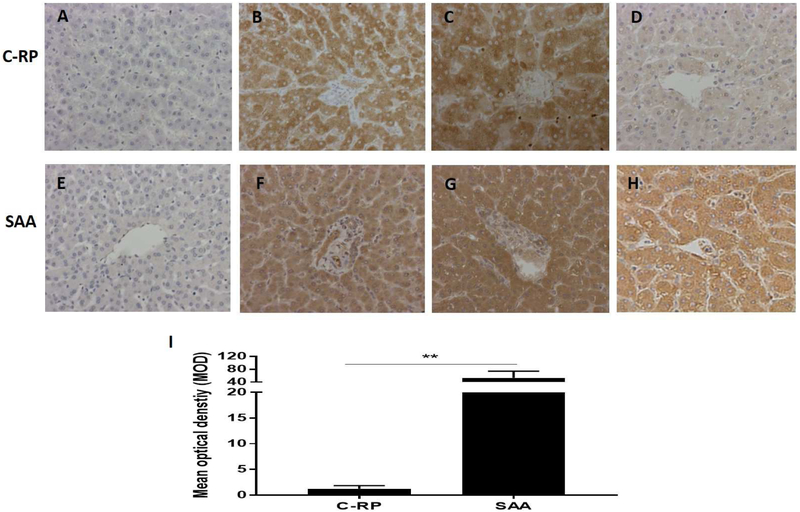
Neither C-RP nor amyloid A was detected in naive pig livers (negative controls) (Figures3A, E). In contrast, both C-RP and amyloid A were intensely expressed in the livers from the experiments considered positive controls (see Methods) (Figures3B, C, F, G). In all other baboons, expression of amyloid A was much greater than of C-RP (Figures3D, H), with the MOD of amyloid A being significantly higher than of C-RP (53 vs 1, p<0.01) (Figure3I)
Figure 3: Expression of C-RP (above) and amyloid A (below) in liver tissue detected by IHC (magnification x200).
(A, E) Expression of C-RP and amyloid A in a naive pig liver (negative control); (B, F) Expression of C-RP and amyloid A in the liver of a baboon that experienced anaphylactic shock (without transplantation) (positive control). (C, G) Expression of C-RP and amyloid A in the liver of a baboon with a pig kidney graft that underwent hyperacute rejection (while receiving the IL-6R inhibitor, tocilizumab, and the TNF-α antagonist, etanercept) (positive control). (D, H) Expression of C-RP and amyloid A in the liver of a representative baboon with a pig kidney graft that was undergoing antibody-mediated rejection (while receiving tocilizumab). There is considerable expression of amyloid A, but not C-RP, in the liver. (I) Comparison of C-RP and amyloid A expression in the livers of baboons with pig kidney grafts (while receiving tocilizumab). The difference in expression is significant (**p<0.01).
C-RP and amyloid A deposition in pig kidney tissue
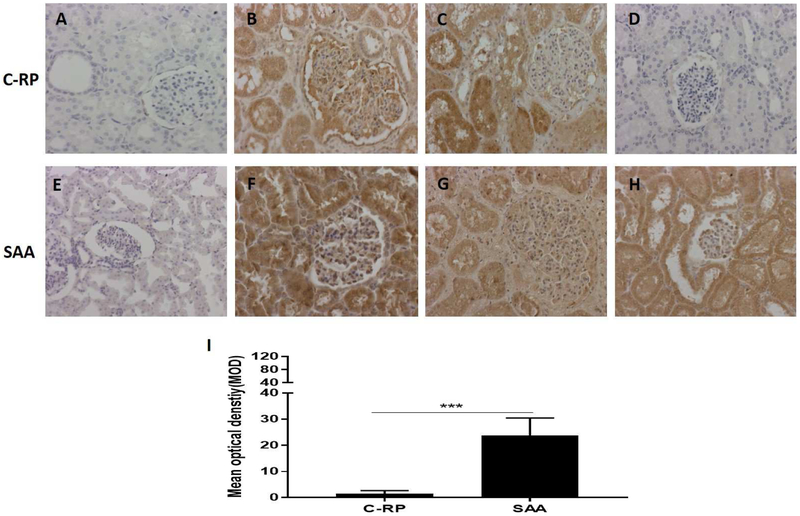
There was no deposition of C-RP or amyloid A in naive pig kidneys (negative controls) (Figures4A, E). In the kidneys considered to be positive controls, deposition of both C-RP and amyloid A was intense (Figures4B, C, F, G). In the pig kidneys in all other baboons, deposition of amyloid A, but not C-RP was considerable (Figures4D, H), with the MOD of amyloid A being significantly greater than of CRP (24 vs 2, p<0.001) (Figure4I).
Figure 4: Deposition of C-RP (above) and amyloid A (below) in kidney tissue detected by IHC (magnification x200).
(A, E) Expression of C-RP and amyloid A in a naive pig kidney (negative control); (B, F) Deposition of C-RP and amyloid A in the pig kidney in a baboon that experienced anaphylactic shock (without transplantation) (positive control). (C, G) Deposition of C-RP and amyloid A in a pig kidney xenograft that underwent hyperacute rejection after transplantation into a baboon (while receiving the IL-6R inhibitor, tocilizumab, and the TNF-α antagonist, etanercept) (positive control). (D, H) Deposition of C-RP and amyloid A in a representative pig kidney xenograft (in a baboon with rejection, while receiving tocilizumab). There is considerable deposition of amyloid A, but not C-RP, in the kidney. (I) Comparison of C-RP and amyloid A deposition in pig kidney xenografts in baboons (that were receiving tocilizumab). The difference in deposition is significant (***p<0.001).
Parameters of coagulation and cytokines
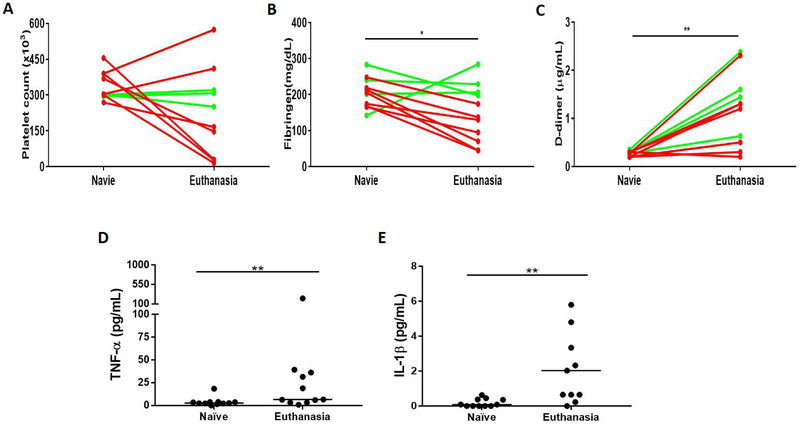
In the short-survival baboons, platelet counts and plasma fibrinogen fell rapidly and D-**dimer rose, whereas in the long-survival baboons these parameters remained largely stable until infection or rejection occurred [1, 2, 20]. Compared to naive baboons, the platelet counts significantly decreased in the short-survival baboons (p<0.05), but this did not occur in the long-survival baboons; overall, however, there was no correlation between the change in platelet count and SAA (Figure5A). Plasma fibrinogen levels decreased by the time of euthanasia (p<0.05) (Figure5B), and levels of D-dimer were significantly higher at euthanasia than pre-transplantation (p<0.01) (Figure5C).
Figure 5: Comparisons between SAA and selected coagulation parameters and cytokine levels in baboons with pig kidney grafts.
(A, B, C) Comparison of platelet count (left), plasma fibrinogen (middle), and D-dimer (right) and SAA at 2 time-points (pre-transplantation [Naïve] and at euthanasia). The plasma fibrinogen was significantly decreased (*p<0.05) and the D-dimer was significantly increased by the day of euthanasia (**p<0.01). (Red lines = short-survival baboons; green lines = long-survival baboons.) (D, E) Comparison of SAA and serum TNF-α (left) and serum IL-1 β (right) in baboons (receiving tocilizumab) at 2 time-points (pre-transplantation [Naïve] and at euthanasia). Both TNF-α and IL-1 β were significantly increased at euthanasia (**p<0.01).
All baboons received the IL-6R inhibitor, tocilizumab, and the TNF-α antagonist, etanercept. The serum level of IL-6 significantly increased after the administration of tocilizumab (Zhang G, et al. manuscript in progress). Both the levels of TNF-α and IL-1 β were significantly increased by the day of euthanasia (Figures5D, F).
Correlation between amyloid A expression in baboon livers and coagulation parameters or inflammatory cytokine levels
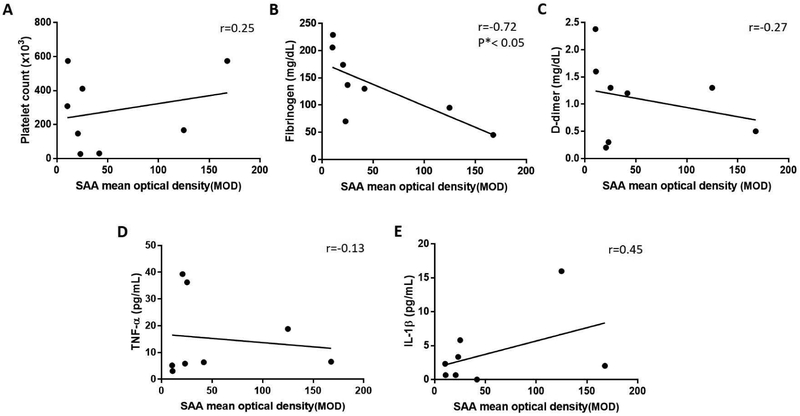
Our previous study indicated that, in baboons with pig organ grafts, systemic inflammation can activate coagulation [7]. Amyloid A is mostly produced by the liver, and so expression in the baboon liver provides an indication of the state of inflammation in the recipient. Therefore, we investigated correlations between amyloid A expression in the baboon liver with (i) coagulation parameters, and (ii) inflammatory cytokine levels. Although the plasma fibrinogen level negatively correlated with the expression of amyloid A in the liver (r=−0.72, p<0.05) (Figure6B), there was no correlation between platelet counts or D-dimer with amyloid A expression (Figures6A, C). We could not demonstrate a correlation between the expression of amyloid A in the liver and serum cytokine levels (TNF-α, IL-1 β) (Figures6D, E).
Figure 6: Correlation between amyloid A expression in the livers of baboons (with pig kidney xenografts) and selected coagulation parameters and serum cytokine levels.
(A, B, C) Correlation of the expression of amyloid A in the baboon liver (n=8) with platelet count (left), plasma fibrinogen (middle), and D-dimer (right). The expression of amyloid A in the baboon liver negatively correlated with the change in plasma fibrinogen (r=−0.72, *p<0.05). (D, E) Correlation of amyloid A expression in the baboon liver with TNF-α and IL-1 β. The expression of amyloid A in the baboon liver did not correlate significantly with the change in TNF-α (r=−0.13, p>0.05) or IL-1 β (r=0.45, p>0.05).
Comparison of the results of the SAA assay with (i) expression of amyloid A in the baboon liver and (ii) deposition of amyloid A on the pig kidney graft
The results of the SAA assay correlated with the expression of amyloid A in the baboon liver (Table2A) and with the deposition of amyloid A on the pig kidney grafts (Table2B).
Table 2:
Comparison of the results of the SAA assay with (A) amyloid A expression in the recipient baboon livers, and (B) amyloid A deposition in the pig kidney grafts
| A | |||
|---|---|---|---|
| Degree of inflammation | SAA | Amyloid A expression (IHC) |
P (SAA vs IHC) |
| Serum (n=8) n (%) |
Liver (n=8) n (%) |
||
| Significant | 6 (75) | 7 (88) | |
| Moderate | 2 (25) | 1 (12) | NS |
| Normal or negative | 0 (0) | 0 (0) | |
| B | |||
|---|---|---|---|
| Degree of inflammation | SAA | Amyloid A deposition (IHC) |
P (SAA vs IHC) |
| Serum (n=11) n (%) |
Kidney (n=11) n (%) |
||
| Significant | 8 (73) | 10 (91) | |
| Moderate | 3 (27) | 0 (0) | NS |
| Normal or negative | 0 (0) | 1 (9) | |
Discussion
It is well-known that inflammation leads to activation of the immune system, and is also associated with dysregulation of coagulation [21, 22]. After pig kidney xenotransplantation in baboons, inflammation may play an important role in exacerbating the immune response and coagulation dysregulation [7]. Therefore, prevention of, or the early identification and treatment of, inflammation may be important in prolonging survival of pig organ xenografts.
Measurement of serum C-RP is considered a sensitive marker of inflammation, and is monitored in inflammatory diseases [23]. In human organ allotransplantation, C-RP has been monitored to detect inflammation [24-26]. In pig organ xenotransplantation, our previous studies indicated that C-RP was a sensitive marker of acute inflammation [7], but, if the baboon recipients received the IL-6R inhibitor, tocilizumab, monitoring of C-RP no longer provided an indication of the state of the inflammatory response [5,8,27]. In vitro studies in our laboratory have confirmed this observation, and have also demonstrated that IL-6-neutralizing antibodies, e.g., sultixumab, also negate C-RP’s value as a marker of inflammation (Zhang G, et al, manuscript in preparation).
In the present study, in the presence of tocilizumab therapy, the level of C-RP did not significantly increase after pig kidney transplantation, unless terminal severe systemic infection developed, and thus was not a sensitive marker of the state of inflammation. Studies by others have indicated that SAA may be a more sensitive marker of inflammation than C-RP [14, 28], and so we monitored SAA in baboons undergoing pig kidney transplantation.
The level of SAA increased as a response to the surgical stress of kidney transplantation, but then decreased if the immunosuppressive therapy was fully effective (anti-CD40mAb-based regimen), but increased when the regimen was ineffective (conventional therapy) resulting in graft failure. Although a rise in SAA was not specific for rejection (as it occurred when a systemic infectious complication or acute gastric dilatation developed), it would at least inform us that an inflammatory response is developing, alerting us to the need for more intensive investigation (e.g., blood culture, graft biopsy) as to the cause, thus enabling us to take therapeutic steps to reverse the complication.
In our previous study, severe inflammation was associated with antibody-mediated rejection, whether this was early or late in the course of graft survival and whether it was related to inadequate immunosuppressive therapy or absence of expression of a human coagulation-regulatory protein in the graft. Whether the inflammatory response was a causative factor in the development of rejection or merely secondary to the response, remains uncertain, though Ezzelarab et al provided evidence that inflammation precedes the coagulation dysfunction seen in baboons with xenografts [7], suggesting that inflammation is a causative factor. In the present study, there was some correlation between SAA level and parameters known to indicate impending xenograft failure, e.g., a falling plasma fibrinogen.
Both SAA and CRP are acute phase proteins, produced largely in cells of hepatic origin, that can be induced by a variety of stimuli, including IL-6, TNF-α, and IL-1 β [29-31]. The present study indicates that, in the presence of tocilizumab therapy, the production of C-RP, but not SAA, is inhibited. This difference may be related to (i) C-RP being more specifically stimulated by IL-6 than SAA, or (ii) SAA being more sensitive to cytokine stimulation than C-RP [32], and therefore its production not being inhibited so much by tocilizumab. Although in the present study we did not document a significant correlation between SAA and IL-1 β, it is possible that IL-1 β or other cytokines stimulate SAA production more than they stimulate C-RP production. We have been unable to find any reports in the literature that clarify this point.
There are several methods for measuring SAA, e.g., radioimmunoassay (RIA), enzymelinked immunosorbent assay (ELISA), and microsphere capture enzyme immunoassay (MEIA). In the present study, we used a simple, rapid, and sensitive assay that can measure SAA in blood or serum, and does not require special equipment. The results of the assay appeared to correlate well with the findings of immunohistochemistry. The assay has recently been modified by the manufacturer to enable it to be more quantitative, with 4 (rather than 3) grades of inflammation now being differentiated. This modification should allow better differentiation between mild, moderate, and severe inflammation, and may possibly identify differences in the response between antibody-mediated rejection and other causes of inflammation, e.g., infection.
In conclusion, our study indicated that SAA is a simple, rapid, relatively inexpensive, and sensitive marker of inflammation in baboons with pig organ xenografts, even when they received anti-inflammatory therapy in the form of the IL-6R blockade agent, tocilizumab. In this respect, it proved a more valuable marker than C-RP. However, it was non-specific and did not differentiate between graft rejection and systemic infection. However, when carried out prospectively, it should prove useful in alerting us to the fact that an inflammatory response is developing or increasing, enabling further investigation of the cause and introduction of suitable therapy.
Supplementary Material
Highlight.
Inflammation, which can be measured by the serum amyloid A (SAA) assay and serum C-reactive protein (C-RP), increases the immune response to a xenograft.
However, measuring the C-RP does not reflect the state of inflammation when the recipient receives an IL-6 receptor inhibitor.
The SAA is a more reliable marker, and correlates with amyloid A expression in the recipient liver and with amyloid A deposition in the donor pig kidney.
The SAA assay is a simple, rapid, reliable and relatively inexpensive method to monitor inflammation.
Acknowledgements
Guoqiang Zhang is supported by the Ph.D. student foreign visiting program project from Nanchang University in China. Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959. Some of the baboons used in the study were from the Oklahoma University Health Sciences Center, Division of Animal Resources, which was supported by NIH P40 sponsored grant RR012317-09. We thank Dr. Keith Reimann for providing anti-CD40mAb from the NIH NHP Reagent Resource (contract HHSN2722001300031C), funded by AI126683 and OD010976.
Abbreviations
- C-RP
C-reactive protein
- IHC
immunohistochemistry
- IL-6R
interleukin-6 receptor
- MOD
mean optical density
- SAA
serum amyloid A
- SIXR
systemic inflammation in xenograft recipients
Footnotes
Disclosure of conflict of interest
No author has a conflict of interest.
Provenance and peer review
Not Commissioned, internally reviewed
Ethical approval
Protocols were approved by the University of Pittsburgh and University of Alabama at Birmingham Institutional Animal Care and Use Committees.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24(2). doi: 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant mode. Xenotransplantation. 2015;22:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 2016;7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Iwase H, Ekser B, Zhou H, et al. Further evidence for sustained systemic inflammation in xenograft recipients (SIXR). Xenotransplantation. 2015;22:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ezzelarab MB, Cooper D. Systemic inflammation in xenograft recipients (SIXR): A new paradigm in pig-to-primate xenotransplantation? Int J Surg. 2015;23(Pt B):301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ezzelarab MB, Ekser B, Azimzadeh A, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li T, Lee W, Hara H, et al. An investigation of extracellular histones in pig-to-baboon organ xenotransplantation. Transplantation. 2017;101:2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li T, Hara H, Ezzelarab MB, et al. Serum amyloid A as a marker of inflammation in xenotransplantation. European Journal of Inflammation.2018. 10.1177/2058739218780046 [DOI] [Google Scholar]
- [10].Lepedda AJ, Nieddu G, Zinellu E, et al. Proteomic analysis of plasma-purified VLDL, LDL, and HDL fractions from atherosclerotic patients undergoing carotid endarterectomy: identification of serum amyloid A as a potential marker. Oxid Med Cell Longev. 2013;2013:385214. doi: 10.1155/2013/385214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Qu J, L X, Liu Y, et al. Evaluation of procalcitonin, C-reactive protein, interleukin-6 & serum amyloid A as diagnostic biomarkers of bacterial infection in febrile patients. Indian J Med Res. 2015;141:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang XY, Zhang G, Jiang Y, et al. The prognostic value of serum C-reactive protein-bound serum amyloid A in early-stage lung cancer. Chin J Cancer. 2015;34:335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hwang YG, Balasubramani GK, Metes ID, et al. Differential response of serum amyloid A to different therapies in early rheumatoid arthritis and its potential value as a disease activity biomarker. Arthritis Res Ther. 2016; 18(1):108. doi: 10.1186/s13075-016-1009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Feussner G, Stech C, Dobmeyer J, et al. Serum amyloid A protein (SAA): a marker for liver allograft rejection in humans. Clin Investig. 1994;72:1007–1011. [DOI] [PubMed] [Google Scholar]
- [15].Hartmann A, Eide TC, Fauchald P, et al. Serum amyloid A protein is a clinically useful indicator of acute renal allograft rejection. Nephrol Dial Transplant. 1997;12:161–166. [DOI] [PubMed] [Google Scholar]
- [16].Muller TF, Vogl M, Neumann MC, et al. Noninvasive monitoring using serum amyloid A and serum neopterin in cardiac transplantation. Clin Chim Acta. 1998;276:63–74. [DOI] [PubMed] [Google Scholar]
- [17].Walker AH, Keevil BG, Yonan N. An investigation of the correlation between C-reactive protein, serum amyloid a concentration, and cardiac allograft rejection. Transplant Proc. 2002;34:1279–1280. [DOI] [PubMed] [Google Scholar]
- [18].Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yamamoto T, Hara H, Wang L, et al. Kidney xenotransplantation from genetically-engineered pigs in baboons: a comparison of two immunosuppressive regimens. Submitted. [DOI] [PubMed] [Google Scholar]
- [21].Levi M, Keller TT, van Gorp E, et al. Infection and inflammation and the coagulation system. Cardiovasc Res. 2003;60:26–39. [DOI] [PubMed] [Google Scholar]
- [22].Shrivastava S, Mcvey JH, Dorling A. The interface between coagulation and immunity. Am J Transplant. 2007;7:499–506. [DOI] [PubMed] [Google Scholar]
- [23].Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dahle DO, Mjoen G, Oqvist B, et al. Inflammation-associated graft loss in renal transplant recipients. Nephrol Dial Transplant. 2011;26:3756–3761. [DOI] [PubMed] [Google Scholar]
- [25].Minculescu L, Kornblit BT, Friis LS, et al. C-reactive protein levels at diagnosis of acute graft-versus-host disease predict steroid-refractory disease, treatment-related mortality, and overall survival after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:600–607. [DOI] [PubMed] [Google Scholar]
- [26].Oweira H, Lahdou I, Daniel V, et al. Early post-operative acute phase response in patients with early graft dysfunction is predictive of 6-month and 12-month mortality in liver transplant recipients. Hum Immunol. 2016;77:952–960. [DOI] [PubMed] [Google Scholar]
- [27].Min BH, Shin JS, Kim JM, et al. Delayed revascularization of islets after transplantation by IL-6 blockade in pig to non-human primate islet xenotransplantation model. Xenotransplantation. 2018;25(1). doi: 10.1111/xen.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest. 1996;26:427–435. [DOI] [PubMed] [Google Scholar]
- [29].Thorn CF, Lu ZY, Whitehead AS. Regulation of the human acute phase serum amyloid A genes by tumour necrosis factor-alpha, interleukin-6 and glucocorticoids in hepatic and epithelial cell lines. Scand J Immunol. 2004;59:152–158. [DOI] [PubMed] [Google Scholar]
- [30].Edbrooke MR, Foldi J, Cheshire JK, et al. Constitutive and NF-kappa B-like proteins in the regulation of the serum amyloid A gene by interleukin 1. Cytokine. 1991;3:380–388. [DOI] [PubMed] [Google Scholar]
- [31].Girgis RR, Ciarleglio A, Choo T, et al. A randomized, double-blind, placebo-controlled clinical trial of tocilizumab, an interleukin-6 receptor antibody, for residual symptoms in schizophrenia. Neuropsychopharmacology. 2018;43:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yamada T Serum amyloid A (SAA): a concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med. 1999;37:381–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.