Abstract
Genital human papillomavirus (HPV) is the world’s most commonly diagnosed sexually transmitted infection, and high-risk HPV types are strongly linked to cervical dysplasia and carcinoma. Puerto Ricans are among the US citizens with higher HPV prevalence and lower screening rates and access to treatment. This bleak statistic was as a motivation to detect biomarkers for early diagnosis of HPV in this population. We collected both urine and cervical swabs from 43 patients attending San Juan Clinics. Cervical swabs were used for genomic DNA extractions and HPV genotyping with the HPV SPF10-LiPA25 kit, and gas chromatography-mass spectrometry (GC-MS) was employed on the urine-derived products for metabolomics analyses. We aimed at discriminating between patients with different HPV categories: HPV negative (HPV-), HPV positive with simultaneous low and high-risk infections (HPV+B) and HPV positive exclusively high-risk (HPV+H). We found that the metabolome of HPV+B is closer to HPV- than to HPV+H supporting evidence that suggests HPV co-infections may be antagonistic due to viral interference leading to a lower propensity for cervical cancer development. In contrast, metabolites of patients with HPV+H were significantly different from those that were HPV-. We identified three urinary metabolites 5-Oxoprolinate, Erythronic acid and N-Acetylaspartic acid that discriminate HPV+H cases from negative controls. These metabolites are known to be involved in a variety of biochemical processes related to energy and metabolism and may likely be biomarkers for HPV high-risk cervical infection. However, further validation should follow using a larger patient cohort and diverse populations to confirm our finding.
Introduction
The association between mucosotropic Human Papilloma Virus (HPV) infections and cervical cancer is unequivocal, with the virus being considered the etiologic agent for cervical carcinoma [1, 2]. To date, despite that 180 different HPV types have been sequenced, only about 50 strains infect the epithelium of the genital tract where they can cause cancer or persist asymptomatically [3, 4]. The high-risk HPV types (oncogenic) includes genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, and 58 that are associated with cervical, vulvar, vaginal, and anal cancer progression, while low-risk types such as 6, 11, 40, 42, 43, 44, 53, 54 and 74 are associated with warts and low-grade anal lesions [5]. Puerto Rico is the region with the lowest cervical cancer screening rates in the US, which has also substantial economic and access barriers to preventive strategies and treatments [6], and a higher prevalence of HPV than compared to those in the US (34% vs 27%) [7, 8].
Screening practices include HPV testing in conjunction with cervical cytology (Pap smear) during a pelvic examination especially for women above the age of 30 years [9]. This test has shown to reduce the incidence of mortality from cervical cancer [10]. However, the test has many barriers that include embarrassment in the screening method that can also contribute to low screening rates in certain cultures such as in Puerto Rico, where screening is significantly low [6]. Therefore, there is a definite need for alternative and supplementary HPV-related infections early detection tests that some authors have discussed before [11]. As an alternative, we aimed to determine if the analysis of urinary metabolites, could be a reliable approach for the screening of patients with cervical HPV infections since the urine is widely used for identification of metabolic biomarkers in cancer [12, 13]. Moreover, as the collection of urine is non-invasive, the biomaterial is very abundant and has a relatively stable composition of proteins—often used to detect prostate and bladder cancer or even relate to inflammatory bowel disease in children [13–15]. Indeed, methods such as HPV DNA urine testing has been used to identify abnormal cells in adolescent girls who do not wish to have a vaginal examination [16, 17]. In fact, it was reported that self-collected urine can be used for HPV DNA detection matching perfectly with the HPV DNA types identified in the corresponding cervical scrapes [18, 19]. This supports our hypothesis that the metabolic changes observed in urine samples could be directly related to the type of cervical HPV infection. Thus, we aimed to determine if changes in urinary metabolites, could be an alternative and reliable approach for the screening of patients with cervical HPV infections.
Urine biofluid samples, to our knowledge, have not been used in conjunction with metabolomics to discriminate patients with cervical HPV infections. However, a recent study suggested that methylation of both host and viral genes in urine has been feasible for cervical cancer screening [20]. Such recent evidence has suggested that biomarkers for cervical cancer may be washed such as exfoliated cells and debris in the urine, a kind of liquid biopsy–that could facilitate the diagnostics of non-urothelial malignant cells such as cervical cancer [21].
In the last decade, there has been an increased trend in the use of “omics” approaches to study cancer biology [22–25]. Among these approaches, metabolomics has been shown to potentially identify relevant biomarkers for cancer detection or for the development of new drug targets. Mass spectrometry-based metabolomics techniques are being used to uncover metabolites in different cancer types [23, 24, 26] due to their reliability and reproducibility [27].
Taken together these evidence supports our study, aimed at testing if urine could be used as a non-invasive method for the detection of cervical HPV infections by evaluating the association between cervical HPV types and urinary metabolites.
Materials and methods
Patient recruitment and sampling
Women undergoing gynecology evaluation at the University of Puerto Rico and San Juan City clinics (San Juan Metropolitan area), who did not meet the exclusion criteria, were recruited to the study. The exclusion criteria were: 1) antibiotics taken in the prior 2 months; 2) history of regular urinary incontinence; 3) treatment for or suspicion of prior toxic shock syndrome; 4) candidiasis; 5) active urinary tract infections; 6) active STDs; and 7) vaginal irritation at the time of screening. The study was approved by the Ethics Committees of the UPR-Medical Sciences Campus IRB (Protocol ref. 1050114/June 2014), San Juan City Hospital and the Inter American University of Puerto Rico IRB (Protocol ref. 1182327–2014) as part of a larger cervical microbiome study. All subjects were informed (both verbally and in writing) of the sampling procedure, risks and benefits of the study, gave written informed consent and signed HIPAA forms, in accordance with the Declaration of Helsinki.
Urine biofluid was self-collected at the time of gynecology evaluation, from 43 healthy reproductive-age women (21–50 years old), with the ability to provide informed consent. Metadata categories we collected from the interview/visit included age, BMI and smoking (S1 Table). All samples were stored at -80°C and processed for further metabolite extraction. Additionally, conventional cervical cytological test (Pap smear) was obtained for cytology diagnostics. Cervical swabs were obtained from the patients using sterile Catch-All Specimen Collection Swabs (Epicentre Biotechnologies, Madison WI), and placed in MoBio bead tubes with buffer (MoBio PowerSoil™ kit, MoBio, Carlsbad CA) for genomic DNA extractions using the MoBio PowerSoil™ kit, following the manufacturer’s instructions. As these patients were recruited as part of a cervical microbiome study, the cervical genomic DNA extractions used both for HPV typing and microbiota analyses, were done using the standard MoBio soil kit as suggested by the Manual of Procedures of the Human Microbiome Project protocol [28].
Human Papilloma Virus genotyping
For HPV genotyping we used a short-polymerase chain reaction-fragment assay (Labo Bio-medical Products (LBP), Rijswijk, The Netherlands, licensed Innogenetics technology) using the cervical genomic DNA. The assay uses SPF10 primers to amplify a 65-bp fragment of the L1 open reading frame of HPV genotypes, followed by a Reverse-Hybridization step. In the first step, the 65-bp PCR fragment assay amplifies HPV genotypes: 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, and 74. In the second step, the amplified fragments underwent a line probe assay by reverse-hybridization, to determine the specific HPV type compared to the kit-provided controls [29].
Metabolomics procedure and analysis
Metabolite extraction and derivatization
Two hundred μL of liquefied urine samples were mixed with 800 μL of the methanol-water mixture (8:1 v/v), vortexed for 1 min and centrifuged at 13000 rpm X 10 min at 4°C. After centrifugation, supernatants were collected, placed in glass vials, and evaporated to dryness using a SpeedVac (Savant AS160, Farmingdale, NY). The metabolite samples were first derivatized by methoxyamination by adding 50 μL of 20 mg/mL solution of methoxyamine hydrochloride (Sigma-Aldrich) in pyridine (Sigma-Aldrich) and incubated at 37°C for two hours. Trimethylsilylation was subsequently performed by adding 50 μL of N-methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA+1% TMCS, Sigma-Aldrich), incubating for 1 h at 65°C and centrifuged at 13000 rpm X 10 min at RT. Supernatants were transferred to analytical vials and stored at -20°C or diluted in hexane (1:50) prior to the GC-MS analysis.
Metabolites separation by GC and detection by MS
Metabolites were fractionated by gas chromatography-mass spectrometry (GC-MS) (GCMS-QP2010, Shimadzu Scientific). The chromatography conditions were as follows: RXI-5MS (0.25 mm inner diameter, 0.25 μm D.F., 30 m) (Restek), split injection (ratio = 15), the injection volume of 1 μL. The inlet temperature was 280 °C; the ion source temperature was 200 °C; interface temperature was 150 °C. The oven temperature was set at 100 °C for 1 min, and then programmed from 100 °C to 290 °C at 8 °C/min, and held at 290 °C for 16 min. Helium was used as the carrier gas at a constant linear velocity of 39 cm/sec. The sample aliquot was injected in split mode (ratio = 15). Mass spectra were obtained for each metabolite on a Shimadzu GCMS-QP2010 mass spectrometer after electron impact ionization (EI, 70 eV, ion source temperature 200°C) in full scan mode between 35 and 700 amu. Mass spectral library searches of the major chromatographic peaks were conducted using the GCMS Labsolution data analysis software (Shimadzu) equipped with NIST14/2014/EPA/NIH database.
Bioinformatics analysis
Genotyping results were grouped into three main categories: HPV negative (HPV-), HPV positive with simultaneous low and high-risk infections (HPV+B), and HPV positive exclusively with high-risk genotypes (HPV+H). Studies had found multiple HPV genotypes associated with neoplasias [30], including low-risk types [31]. Recently HPV research has also grouped patients with both high-risk and low-risk infections in studies relating HPV diversity and prevalence in Hispanic populations [32, 33].
For metabolomics analysis, peak intensities for each metabolite were collected, composed as the data matrix and processed using Metaboanalyst 4.0 [34, 35] unless otherwise specified. Data integrity check was performed according to default settings on the Metaboanalyst interface. Thus, obtained datasets were evaluated by Principal Component Analysis to identify samples-outliers being outside the Hotelling T2 95% confidence ellipse [36]. Next, identified outliers (two in HPV+B and one HPV+H group) and samples that did not produce a total ion chromatogram (Table 1) were removed from further analysis. The resultant data were normalized by log-transformation and Pareto scaling to improve the pattern recognition for untargeted metabolomics data. Changes between groups were analyzed via the Holm-Sidak test for multiple comparisons with GraphPad Prism version 7.0c (GraphPad Software, San Diego, CA). The α level for significance was set at 0.05. The supervised Partial Least-Squares Discriminant Analysis (PLS-DA) model was used to identify the metabolic differences between groups. To evaluate the model performance, class labels were permuted 2,000 times to identify whether differences between groups were significant. For permutation test statistics we selected separation distance, which was defined as the ratio of the between-group sum of squares and the within-group sum of squares (B/W ratio). Group’s performance was evaluated by using the leave-one-out cross-validation (LOOCV) method. Evaluation of Variable Importance in Projection (VIP) scores, estimated the importance of each variable in the projection used within the PLS-DA model. Variables with a VIP score ≥ 1.0 were considered important in a given model. The diagnostic ability of these variables as potential biomarkers for the detection of HPV infections in urine, was assessed by measuring the area under the curve values (AUC), using the receiver operating characteristic curves (ROC) method. Variables with a AUC ≥ 0.9 were considered important for diagnostic purposes [37].
Table 1. Sample IDs and groupings by HPV-risk, genotypes and cytology.
| # | Sample ID |
Genotyping Result |
Cytology | HPV Risk |
HPV Status |
Group |
|---|---|---|---|---|---|---|
| 1 | 35 | Negative | LGSIL | Negative | Negative | HPV- |
| 2 | 50 | Negative | ASCUS | Negative | Negative | HPV- |
| 3 | 53** | Negative | HGSIL | Negative | Negative | EXC |
| 4 | 58 | Negative | LGSIL | Negative | Negative | HPV- |
| 5 | 61 | Negative | HGSIL | Negative | Negative | HPV- |
| 6 | 69 | Negative | HGSIL | Negative | Negative | HPV- |
| 7 | 70 | Negative | HGSIL | Negative | Negative | HPV- |
| 8 | 75 | Negative | HGSIL | Negative | Negative | HPV- |
| 9 | 79 | Negative | NSIL | Negative | Negative | HPV- |
| 10 | 16 | 16,66,6 | LGSIL | Both | Positive | HPV+B |
| 11 | 18* | 16,6,53 | HGSIL | Both | Positive | EXC |
| 12 | 21 | 31,33,42,44,74 | HGSIL | Both | Positive | HPV+B |
| 13 | 22 | 16,31,39,45,66,68,44,53,74 | LGSIL | Both | Positive | HPV+B |
| 14 | 25* | 16,33,66,6 | HGSIL | Both | Positive | EXC |
| 15 | 26 | 16,51,56,6,34,44,53,74 | LGSIL | Both | Positive | HPV+B |
| 16 | 27 | 39,74 | LGSIL | Both | Positive | HPV+B |
| 17 | 28 | 54,56 | HGSIL | Both | Positive | HPV+B |
| 18 | 30 | 16,39,52,53,56 | HGSIL | Both | Positive | HPV+B |
| 19 | 31** | 31,52,6,74 | LGSIL | Both | Positive | EXC |
| 20 | 34 | 16,56,74 | HGSIL | Both | Positive | HPV+B |
| 21 | 36 | 18,44,74 | HGSIL | Both | Positive | HPV+B |
| 22 | 47 | 31,33,44 | HGSIL | Both | Positive | HPV+B |
| 23 | 48 | 33,42 | ASCUS | Both | Positive | HPV+B |
| 24 | 49 | 33,42 | HGSIL | Both | Positive | HPV+B |
| 25 | 51 | 51,53 | ASCUS | Both | Positive | HPV+B |
| 26 | 63 | 51,53,66 | HGSIL | Both | Positive | HPV+B |
| 27 | 65 | 54,45,51 | NA | Both | Positive | HPV+B |
| 28 | 66 | 31,6 | HGSIL | Both | Positive | HPV+B |
| 29 | 17 | 16,66 | HGSIL | H-risk | Positive | HPV+H |
| 30 | 19 | 35 | HGSIL | H-risk | Positive | HPV+H |
| 31 | 20 | 66 | LGSIL | H-risk | Positive | HPV+H |
| 32 | 32 | 52 | LGSIL | H-risk | Positive | HPV+H |
| 33 | 33 | 16 | HGSIL | H-risk | Positive | HPV+H |
| 34 | 37 | 31 | LGSIL | H-risk | Positive | HPV+H |
| 35 | 43 | 51 | LGSIL | H-risk | Positive | HPV+H |
| 36 | 44* | 56 | LGSIL | H-risk | Positive | EXC |
| 37 | 56 | 18,35 | ASCUS | H-risk | Positive | HPV+H |
| 38 | 60 | 51,52,66 | LGSIL | H-risk | Positive | HPV+H |
| 39 | 62 | 16 | LGSIL | H-risk | Positive | HPV+H |
| 40 | 68 | 68 | HGSIL | H-risk | Positive | HPV+H |
| 41 | 72** | 45 | HGSIL | H-risk | Positive | EXC |
| 42 | 74** | 31 | NA | H-risk | Positive | EXC |
| 43 | 78** | 52 | ASCUS | H-risk | Positive | EXC |
*—outlier removed from metabolomics analysis;
**—sample did not produce the total ion chromatogram; EXC—samples excluded from the metabolomics analysis.
Cytology categories: NA-undetermined; LGSIL Low-grade squamous intraepithelial lesion, HGSIL high-grade squamous intraepithelial lesion, ASCUS Atypical squamous cells of undetermined significance, and NSIL Negative for squamous intraepithelial lesion.
Results and discussion
Cervical HPV genotyping of the 43 patients revealed a total of 34 HPV positive patients and 9 negative to HPV infections (HPV-). Of the 34 HPV positives, 15 were exclusively high-risk types (HPV+H) and 19 had simultaneously high-risk and low-risk infections, ranging from 2 to 9 simultaneous HPV genotypes (HPV+B) (Table 1). We did not find any patients positive only for low-risk HPV types. These 43 patients were mostly non-smokers, and although socio-demographic and cytology metadata information was available, due to the modest sample size these categories did not pass the PLS-DA model validation.
The distribution of multiple HPV infections is common but with different HPV co-infection prevalence rates in different countries. Thus, it was documented that out of the 2,478 samples from the Costa Rican HPV Vaccine Trial, 43.2% had multiple HPV type infections [38]. In a study of 5,000 samples from the Centralized Cervical Cancer Screening Program of British Columbia, 33% were positive for more than one HPV type [39]. In Brazil, a study recruiting 2,113 women for a 1-yearand a 4-year period, showed an HPV co-infection prevalence of 12.3% and 22.3% correspondingly [40]. Relatively similar to the co-infection prevalence in Costa Rica, our study found 44.2% of Puerto Rican woman positive for multiple HPV type infections (Table 1). Other studies performed in Venezuela, revealed Amerindian populations with a 75% HPV prevalence, and with 23 different HPV types, a study that discriminated between exclusively low-risk HPV, exclusively high-risk and co-infections by both HPV types [32], as well as another study that made grouping between low-risk and high-risk types [33].
A total of twenty-four metabolites were identified in samples from all groups according to their electron impact mass spectra by comparison to the NIST14 spectral mass library (Table 2).
Table 2. Human urine metabolome found in HPV+B vs HPV- and HPV+H vs HPV- groups.
| # | Metabolite | RT | Fragment ions | VIP | HPV+B Adj P |
HPV+H Adj P |
|---|---|---|---|---|---|---|
| 1 | Lactic acid | 9.67 | 189, 233, 261 | 0.47 | 1.000 | 0.988 |
| 2 | Acetic Acid | 9.90 | 189, 219, 247 | 1.11 | 1.000 | 0.599 |
| 3 | Glycine | 10.69 | 147, 218, 246 | 0.47 | 1.000 | 0.993 |
| 4 | 2-Hydroxybutyric acid | 11.24 | 189, 247, 275 | 0.55 | 1.000 | 0.988 |
| 5 | Proline | 11.55 | 184, 258, 328 | 0.22 | 0.987 | 0.993 |
| 6 | Methylmalonic acid | 11.83 | 147, 189, 289, | 0.61 | 1.000 | 0.988 |
| 7 | Urea | 12.09 | 147, 231, 273 | 0.20 | 1.000 | 0.993 |
| 8 | 5-Oxoprolinate | 13.18 | 75, 158, 186 | 1.82 | 0.303 | 0.009* |
| 9 | Threonine | 13.27 | 130, 246, 290 | 0.61 | 1.000 | 0.983 |
| 10 | Succinic acid | 13.47 | 147, 189, 289, | 0.57 | 1.000 | 0.955 |
| 11 | Glycerol | 15.07 | 171, 189, 347 | 0.35 | 1.000 | 0.993 |
| 12 | 5-Oxoproline | 15.97 | 147, 272, 300 | 1.23 | 1.000 | 0.205 |
| 13 | Glutaric acid | 16.13 | 73, 147, 303 | 1.20 | 1.000 | 0.365 |
| 14 | N-Acetylaspartic acid | 16.65 | 73, 147, 346 | 1.65 | 0.575 | 0.024* |
| 15 | 2-Butenoic acid | 17.11 | 189, 247, 275 | 0.45 | 1.000 | 0.988 |
| 16 | Hippuric acid | 17.64 | 77, 105, 236 | 0.73 | 1.000 | 0.983 |
| 17 | 4-Hydroxybutyric acid | 17.72 | 73, 147, 275 | 1.62 | 1.000 | 0.035* |
| 18 | 4-Hydroxyphenylacetic acid | 17.83 | 75, 205, 324 | 0.68 | 1.000 | 0.955 |
| 19 | Erythronic acid | 18.28 | 147, 289, 331 | 1.69 | 1.000 | 0.016* |
| 20 | 2-Hydroxyglutaric acid | 19.04 | 147, 207, 433 | 0.32 | 1.000 | 0.993 |
| 21 | Glyceric acid | 19.95 | 73, 231, 391 | 0.04 | 1.000 | 0.993 |
| 22 | Aconitic acid | 20.8 | 73, 147, 459 | 1.46 | 1.000 | 0.120 |
| 23 | Citric acid | 23.14 | 357, 459, 591 | 1.27 | 0.909 | 0.599 |
| 24 | Uric acid | 25.47 | 73, 567 | 1.11 | 1.000 | 0.685 |
RT—Retention time; VIP—Variable importance in projection scores identified via PLS-DA analysis for the PC1; Adj P–Adjusted P value determined using the Holm-Sidak method in comparison to HPV- group, α = 0.05.
*—indicate metabolite that matches selected criterion.
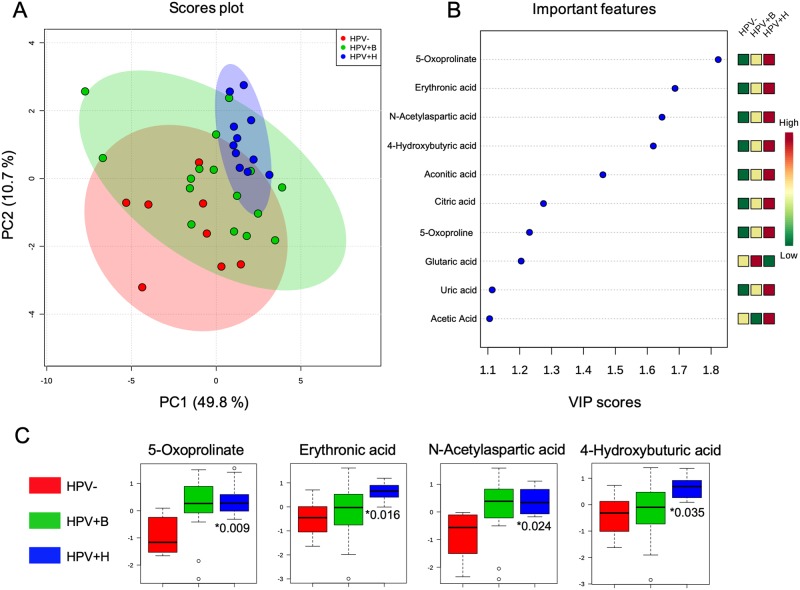
To identify potential biomarkers discriminating between patients with HPV infections and those that had no infections, we performed PLS-DA analysis using the normalized metabolite intensities as variables. The PLS-DA was used to analyze three groups of patients. PLS-DA scores plot of HPV- and HPV+H displayed satisfactory separation at the 95% level with minor overlap between source ellipses, while the separation between HPV- and HPV+B was less significant suggesting similarity in the metabolites abundances between HPV- and HPV+B. The PLS-DA showed that 60.5% of the total explained variance in the data was represented by the first two principal components (PC1–49.8% and PC2–10.7%). The permutation test showed significant separation distance between groups (p < 5e-04) (Fig 1A).
Fig 1. Metabolites discriminating HPV-, HPV+B and HPV+H patients.
(A) Partial-least discriminant analysis (PLS-DA) score plot. (B) Variable importance in projection (VIP) plot with cut-off ≥ 1.0. (C) Box-and-whisker plots depict the most significant metabolites, with the top two: 5- Oxoprolinate and N-Acetylaspartic acid changes in HPV+B and HPV+H groups relative to HPV-. *Adjusted P value. Y-axis shows the normalized relative abundance. RT—Retention time; VIP—Variable importance in projection scores identified via PLS-DA analysis for the PC1; Adj P–Adjusted P value determined using the Holm-Sidak method in comparison to HPV- group, α = 0.05. *—indicate metabolite that matches selected criterion.
This suggests that in our study, the metabolome of HPV+B group is closer to HPV- than to HPV+H. Thus, it is very likely that the pattern of HPV genotype co-infections in HPV+B group does exhibit a rather antagonistic effect of the different HPV risk types, resulting in a similar metabolomic profile as in HPV-. The effects of multiple interactions between co-infecting HPV types on carcinogenesis are not well studied and data reported to date are controversial. Some reports suggested that multiple HPV infections could trigger synergistic effects enhancing the development of carcinogenesis [41, 42] while others reported their antagonistic interactions, that likely may reduce the risk of cervical cancer [43–45]. In addition, despite the popular opinion that multiple HPV infections are associated with the higher risk of cervical cancer compared to those with single HPV infection, several studies conducted in different counties identified that multiple HPV infections are not necessarily correlated with the severity of cervical abnormalities [5, 46, 47]. Therefore, there is a need to perform more studies evaluating the incidence of multiple type HPV infections in different populations, and identification of any interaction between HPV types in the incidence of cervical cancer.
We next identified ten significant discriminatory metabolites (VIP score ≥1) responsible for the separation of groups that may have a clinical value in the diagnosis of HPV infection. Thus, we found higher abundance of 5-Oxoprolinate (VIP, 1.8), Erythronic acid (VIP, 1.7), N-Acetylaspartic acid (VIP, 1.6), 4-Hydroxybuturic acid (VIP, 1.6), Aconitic acid (VIP, 1.5), Citric acid (VIP,1.3), 5-Oxoproline (VIP, 1.2), Glutaric acid (VIP, 1.2), Uric acid (VIP, 1.1) and Acetic acid (VIP, 1.1) (Table 2, Fig 1B). These metabolites are known to be involved in multiple biochemical processes, such as energy metabolism, pentose phosphate pathway, γ-glutamyl cycle and futile 5-Oxoproline cycle.
To determine whether these metabolic changes were significant, we performed the Holm-Sidak corrections for multiple comparisons (α = 0.05) using the following group combinations HPV- with HPV+B, HPV- with HPV+H and HPV+B with HPV+H. We found that 5-Oxoprolinate, Erythronic acid, N-Acetylaspartic acid, and 4-Hydroxybuturic acid were significantly elevated in HPV+H group compare to HPV- (Table 2, Fig 1C). As expected, we have not identified significant changes in HPV+B vs HPV-, since the metabolome of HPV+B group is closer to HPV- than to HPV+H. However, multiple test comparisons have not identified changes between HPV+B vs HPV+H. The biological significance of this is to be determined, and requires a using a larger patient cohort.
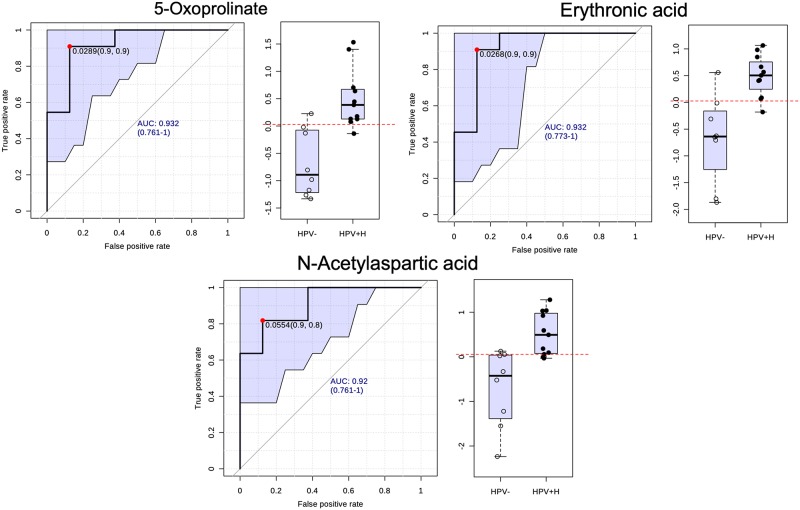
To identify the diagnostic potential of the four most significant metabolites 5-Oxoprolinate, Erythronic acid, N-Acetylaspartic acid and 4-Hydroxybuturic acid as prognostic biomarkers for high-risk HPV infection, we conducted an additional ROC analysis (Fig 2). The analysis showed the greatest AUC values in HPV+H vs HPV- including 5-Oxoprolinate (AUC, 0.92), Erythronic acid (AUC, 0.92) and N-Acetylaspartic acid (AUC, 0.91). Taken together, 5-Oxoprolinate, Erythronic acid and N-acetylaspartic acid could serve as prognostic biomarkers to discriminate high-risk HPV infections from non-infected controls.
Fig 2. Urine biomarkers predicted by ROC curve analysis curve with 95% confidence interval (shadowed).
Box-and-whisker plots show the distribution of abundance values of 5-Oxoprolinate, Erythronic acid and N-Acetylaspartic acid in HPV- vs HPV+H samples with the optimal cut-off as a horizontal dotted red line.
5-Oxoprolinate (the conjugate base of 5-Oxoproline) is an intermediate in the isomerization of glutathione to 5-Oxoproline via the action of γ-glutamyl cyclotransferase in the γ-glutamyl cycle. Elevation of 5-Oxoproline levels in blood and urine has been increasingly recognized as a cause of 5-Oxoprolinuria [48] that usually occurs in chronically ill, malnourished women with impaired renal function and chronic acetaminophen ingestion [49]. In addition, HPV infections in women are usually associated with a low level of glutathione [50], decreased expression of antioxidant enzymes and elevation of ROS levels in host cells [51]. Reduction in glutathione content augments an ATP-depleting futile 5-Oxoproline cycle via elimination of the feedback inhibition of γ-Glutamyl Cysteine Synthetase causing increase of γ-Glutamyl Cysteine that is subsequently metabolized to 5-Oxoproline which could cycle back into glutamic acid via action of 5-Oxoprolinase and at the cost of two ATP molecules without production of Glutathione [49, 52, 53].
Erythronic acid is a normal organic acid present in biofluid samples of healthy children and adults. However, its elevation in urine evidences a deficiency of the activity of Transaldolase—a key enzyme in the pentose phosphate pathway [54]. This can lead to various clinical manifestations including liver dysfunction hepatosplenomegaly, hepatic fibrosis in the pathophysiology of diabetes [55, 56].
N-Acetylaspartic acid is synthesized from Aspartate and Acetyl-CoA and via hydrolysis of N-Acetyl-aspartylglutamate. According to the literature, elevation of N-Acetylaspartic acid could be regulated by the Ras homolog gene family, member C guanosine triphosphatase, which is essential in transforming growth factor beta 1- induced epithelial-mesenchymal transition in cervical cancer cells [34]. To date, this metabolite is only known as a specific urinary marker for Canavan disease [57] which is caused by Aspartoacylase deficiency with abnormal accumulation of N-acetylaspartic acid in the brain and body fluids [58].
To date, HPV testing is increasingly used for screening in conjunction with the conventional cervical cytological test (Pap smear) or liquid-based cervical cytological test [9, 59] followed with the HPV DNA test [11]. Cervical cytology classification for squamous cell abnormalities includes Atypical Squamous Cells of Undetermined Significance (ASCUS), Negative for squamous intraepithelial lesion (NSIL), Low-Grade, and High-Grade Squamous Intraepithelial Lesion (LGSIL and HGSIL). Given that, these types of cervical screenings are invasive and time-consuming, the development of new diagnostic methods using biofluid samples including urine could provide a feasible alternative to HPV testing of cervical samples [60]. Thus, our study provides a new possibility for the detection of HPV+H infections in urine, via analysis of the content of 5-Oxoprolinate, Erythronic acid and N-Acetylaspartic acid using just 200 μL of the urine sample. The applicability of these metabolites as predictive urinary markers requires further investigation using a larger patient cohort, which is the limitation of the current study. In addition, assessment of Erythronic acid and N-Acetylaspartic acid abundance in urine are already routinely performed, via DNA mutation screenings for Transaldolase and Aspartoacylase correspondently. The existing use of these laboratory tests confirms its suitability for the diagnosis of HPV+H infections. Current diagnosis of 5-Oxoprolinuria is expensive and performed only in specialized laboratories via GC-MS. Nevertheless, this study suggests that mutational analysis of the 5-Oxoprolinase gene may be advisable for routine diagnostic purposes [61] which could also apply for the identification of HPV+H infections in clinical laboratories.
Conclusions
This study provided preliminary evidence for the successful detection of urine metabolites related to cervical high-risk HPV infections. Using the urine samples of the Puerto Rican woman followed by GC-MS analysis we have shown, that patients with high-risk HPV infections have the significantly higher abundance of 5-Oxoprolinate, Erythronic acid, and N-Acetylaspartic acid. Besides characterizing cervical HPV, we were able to relate high-risk HPV infections with urinary metabolites and defined 5-Oxoprolinate, Erythronic acid, and N-Acetylaspartic acid as possible prognostic biomarkers for high-risk HPV infections. We also found that patients with simultaneous high-risk and low-risk infections had a similar urinary metabolome with patients without infections supporting early evidence that suggests HPV co-infections may be antagonist due to viral interference leading to lower propensity in cervical cancer development. However, further validation should follow using a larger patient’s cohort to confirm our finding. In addition, it is advisable to perform more MS-based studies to evaluate differentially abundant metabolites and peptides in urine that may correlate not only with HPV genotypes but with cervical intraepithelial neoplasia stages and clinical status.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103475-15 and by the National Institute On Minority Health And Health Disparities Award Numbers 2U54MD007587 and U54 MD007600. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.van der Noordaa J. [Cancer and virus]. Ned Tijdschr Geneeskd. 1980;124(41):1726–1729. . [PubMed] [Google Scholar]
- 2.Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. [DOI] [PubMed] [Google Scholar]
- 3.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30 Suppl 5:F55–70. 10.1016/j.vaccine.2012.06.083 . [DOI] [PubMed] [Google Scholar]
- 4.Bernard HU, Burk RD, Chen Z, van Doorslaer K, zur Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401(1):70–79. 10.1016/j.virol.2010.02.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. The New England journal of medicine. 2003;348(6):518–527. 10.1056/NEJMoa021641 . [DOI] [PubMed] [Google Scholar]
- 6.Kunos CA, Massett HA, Galassi A, Walker JL, Good MJ, Báez Díaz L, et al. Leveraging National Cancer Institute Programmatic Collaboration for Uterine Cervix Cancer Patient Accrual in Puerto Rico. Frontiers in Oncology. 2018;8(102). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. The Journal of the American Medical Association. 2007;297(8):813–819. 10.1001/jama.297.8.813 [DOI] [PubMed] [Google Scholar]
- 8.Ortiz AP, Soto-Salgado M, Calo WA, Tortolero-Luna G, Perez CM, Romero CJ, et al. Incidence and mortality rates of selected infection-related cancers in Puerto Rico and in the United States. Infect Agent Cancer. 2010;5:10 10.1186/1750-9378-5-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qaseem A, Humphrey LL, Harris R, Starkey M, Denberg TD, Clinical Guidelines Committee of the American College of P. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161(1):67–72. [DOI] [PubMed] [Google Scholar]
- 10.Mahlck CG, Jonsson H, Lenner P. Pap smear screening and changes in cervical cancer mortality in Sweden. Int J Gynaecol Obstet. 1994;44(3):267–272. . [DOI] [PubMed] [Google Scholar]
- 11.Cox JT, Lorincz AT, Schiffman MH, Sherman ME, Cullen A, Kurman RJ. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 1995;172(3):946–954. . [DOI] [PubMed] [Google Scholar]
- 12.Arasaradnam RP, Wicaksono A, O’Brien H, Kocher HM, Covington JA, Crnogorac-Jurcevic T. Noninvasive Diagnosis of Pancreatic Cancer Through Detection of Volatile Organic Compounds in Urine. Gastroenterology. 2018;154(3):485–487 e481. 10.1053/j.gastro.2017.09.054 . [DOI] [PubMed] [Google Scholar]
- 13.Loras A, Trassierra M, Sanjuan-Herraez D, Martinez-Bisbal MC, Castell JV, Quintas G, et al. Bladder cancer recurrence surveillance by urine metabolomics analysis. Sci Rep. 2018;8(1):9172 10.1038/s41598-018-27538-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin FP, Su MM, Xie GX, Guiraud SP, Kussmann M, Godin JP, et al. Urinary metabolic insights into host-gut microbial interactions in healthy and IBD children. World J Gastroenterol. 2017;23(20):3643–3654. 10.3748/wjg.v23.i20.3643 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Rambla C, Puchades-Carrasco L, Garcia-Flores M, Rubio-Briones J, Lopez-Guerrero JA, Pineda-Lucena A. Non-invasive urinary metabolomic profiling discriminates prostate cancer from benign prostatic hyperplasia. Metabolomics. 2017;13(5):52 10.1007/s11306-017-1194-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vorsters A, Van den Bergh J, Micalessi I, Biesmans S, Bogers J, Hens A, et al. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur J Clin Microbiol Infect Dis. 2014;33(11):2005–2014. 10.1007/s10096-014-2147-2 . [DOI] [PubMed] [Google Scholar]
- 17.Enerly E, Olofsson C, Nygard M. Monitoring human papillomavirus prevalence in urine samples: a review. Clin Epidemiol. 2013;5:67–79. 10.2147/CLEP.S39799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prusty BK, Kumar A, Arora R, Batra S, Das BC. Human papillomavirus (HPV) DNA detection in self-collected urine. Int J Gynaecol Obstet. 2005;90(3):223–227. 10.1016/j.ijgo.2005.06.004 . [DOI] [PubMed] [Google Scholar]
- 19.Brinkman JA, Jones WE, Gaffga AM, Sanders JA, Chaturvedi AK, Slavinsky J Iii, et al. Detection of Human Papillomavirus DNA in Urine Specimens from Human Immunodeficiency Virus-Positive Women. Journal of Clinical Microbiology. 2002;40(9):3155–3161. 10.1128/JCM.40.9.3155-3161.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rurup R, van Trommel N, van Splunter A, Segerink L, Kenter G, Heideman D, et al. Detection of DNA methylation markers in urine of cervical cancer patients: a feasibility study. Int J Gynecol Cancer. 2016;26(3):170. [Google Scholar]
- 21.Flezar MS. Urine and bladder washing cytology for detection of urothelial carcinoma: standard test with new possibilities. Radiol Oncol. 2010;44(4):207–214. 10.2478/v10019-010-0042-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes VM, Kennedy AD, Panagakos F, Devizio W, Trivedi HM, Jonsson T, et al. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS One. 2014;9(8):e105181 10.1371/journal.pone.0105181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakanaka A, Kuboniwa M, Hashino E, Bamba T, Fukusaki E, Amano A. Distinct signatures of dental plaque metabolic byproducts dictated by periodontal inflammatory status. Sci Rep. 2017;7:42818 10.1038/srep42818 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei J, Xie G, Zhou Z, Shi P, Qiu Y, Zheng X, et al. Salivary metabolite signatures of oral cancer and leukoplakia. Int J Cancer. 2011;129(9):2207–2217. 10.1002/ijc.25881 . [DOI] [PubMed] [Google Scholar]
- 25.Vucic EA, Thu KL, Robison K, Rybaczyk LA, Chari R, Alvarez CE, et al. Translating cancer ‘omics’ to improved outcomes. Genome Res. 2012;22(2):188–195. 10.1101/gr.124354.111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kind T, Tolstikov V, Fiehn O, Weiss RH. A comprehensive urinary metabolomic approach for identifying kidney cancerr. Anal Biochem. 2007;363(2):185–195. 10.1016/j.ab.2007.01.028 . [DOI] [PubMed] [Google Scholar]
- 27.Strathmann FG, Hoofnagle AN. Current and future applications of mass spectrometry to the clinical laboratory. Am J Clin Pathol. 2011;136(4):609–616. 10.1309/AJCPW0TA8OBBNGCK . [DOI] [PubMed] [Google Scholar]
- 28.McInnes P, Cutting M. Manual of Procedures–Human Microbiome Project: Core Microbiome Sampling, Protocol A, HMP Protocol # 07–001. https://wwwncbinlmnihgov/projects/gap/cgi-bin/GetPdfcgi?id=phd0031902. 2010:pp 114.
- 29.Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37(8):2508–2517. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuschieri KS, Cubie HA, Whitley MW, Seagar AL, Arends MJ, Moore C, et al. Multiple high risk HPV infections are common in cervical neoplasia and young women in a cervical screening population. Journal of clinical pathology. 2004;57(1):68–72. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornall AM, Roberts JM, Garland SM, Hillman RJ, Grulich AE, Tabrizi SN. Anal and perianal squamous carcinomas and high-grade intraepithelial lesions exclusively associated with "low-risk" HPV genotypes 6 and 11. Int J Cancer. 2013;133(9):2253–2258. 10.1002/ijc.28228 . [DOI] [PubMed] [Google Scholar]
- 32.Vargas-Robles D, Magris M, Morales N, de Koning MNC, Rodriguez I, Nieves T, et al. High Rate of Infection by Only Oncogenic Human Papillomavirus in Amerindians. mSphere. 2018;3(3). 10.1128/mSphere.00176-18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godoy-Vitorino F, Romaguera JE, Zhao C, Vargas-Robles D, Ortiz-Morales G, Vázquez-Sánchez F, et al. Cervicovaginal fungi and bacteria associated with cervical intraepithelial neoplasia and high-risk Human Papillomavirus infections in a Hispanic population. Frontiers in microbiology. 2018;9(2533). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He X, Qian Y, Cai H, Yang S, Cai J, Wang Z. RhoC is essential in TGF-β1 induced epithelial-mesenchymal transition in cervical cancer cells. Oncology Letters. 2015;10(2):985–989. 10.3892/ol.2015.3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Research. 2009;37(Web Server issue):W652–W660. 10.1093/nar/gkp356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia J, Wishart DS. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat Protoc. 2011;6(6):743–760. 10.1038/nprot.2011.319 . [DOI] [PubMed] [Google Scholar]
- 37.Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics. 2013;9(2):280–299. 10.1007/s11306-012-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaturvedi AK, Katki HA, Hildesheim A, Rodríguez AC, Quint W, Schiffman M, et al. Human Papillomavirus Infection with Multiple Types: Pattern of Coinfection and Risk of Cervical Disease. The Journal of Infectious Diseases. 2011;203(7):910–920. 10.1093/infdis/jiq139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore RA, Ogilvie G, Fornika D, Moravan V, Brisson M, Amirabbasi-Beik M, et al. Prevalence and type distribution of human papillomavirus in 5,000 British Columbia women—implications for vaccination. Cancer Causes & Control. 2009;20(8):1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trottier H, Mahmud S, Costa MC, Sobrinho JP, Duarte-Franco E, Rohan TE, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(7):1274–1280. 10.1158/1055-9965.EPI-06-0129 . [DOI] [PubMed] [Google Scholar]
- 41.Orlando PA, Gatenby RA, Giuliano AR, Brown JS. Evolutionary ecology of human papillomavirus: trade-offs, coexistence, and origins of high-risk and low-risk types. J Infect Dis. 2012;205(2):272–279. 10.1093/infdis/jir717 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickson EL, Vogel RI, Geller MA, Downs LS Jr. Cervical cytology and multiple type HPV infection: a study of 8182 women ages 31–65. Gynecol Oncol. 2014;133(3):405–408. 10.1016/j.ygyno.2014.03.552 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silins I, Wang Z, Åvall- Lundqvist E, Frankendal B, Vikmanis U, Sapp M, et al. Serological evidence for protection by human papillomavirus (HPV) type 6 infection against HPV type 16 cervical carcinogenesis. Journal of General Virology. 1999;80(11):2931–2936. [DOI] [PubMed] [Google Scholar]
- 44.Senapati R, Nayak B, Kar SK, Dwibedi B. HPV genotypes co-infections associated with cervical carcinoma: Special focus on phylogenetically related and non-vaccine targeted genotypes. PLoS ONE. 2017;12(11):e0187844 10.1371/journal.pone.0187844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sundström K, Ploner A, Arnheim-Dahlström L, Eloranta S, Palmgren J, Adami H-O, et al. Interactions Between High- and Low-Risk HPV Types Reduce the Risk of Squamous Cervical Cancer. JNCI Journal of the National Cancer Institute. 2015;107(10):djv185 10.1093/jnci/djv185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kay P, Soeters R, Nevin J, Denny L, Dehaeck CM, Williamson AL. High prevalence of HPV 16 in South African women with cancer of the cervix and cervical intraepithelial neoplasia. Journal of medical virology. 2003;71(2):265–273. 10.1002/jmv.10479 . [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Wang P, Ren Y, Du J, Jiang J, Jia X, et al. Prevalence of High-Risk Human Papillomavirus (HR-HPV) Genotypes and Multiple Infections in Cervical Abnormalities from Northern Xinjiang, China. PLOS ONE. 2016;11(8):e0160698 10.1371/journal.pone.0160698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agrawal A, Kishlyansky M, Biso S, Patnaik S, Punjabi C. Common, yet elusive: a case of severe anion gap acidosis. Oxford Medical Case Reports. 2017;2017(9):omx054 10.1093/omcr/omx054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hundemer GL, Fenves AZ. Acquired 5-oxoproline acidemia successfully treated with N-acetylcysteine. Proceedings (Baylor University Medical Center). 2017;30(2):169–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwasniewska A, Tukendorf A, Semczuk M. [Frequency of HPV infection and level of glutathione in serum of women with cervix dysplasia]. Medycyna doswiadczalna i mikrobiologia. 1995;47(3–4):213–218. . [PubMed] [Google Scholar]
- 51.Williams VM, Filippova M, Filippov V, Payne KJ, Duerksen-Hughes P. Human Papillomavirus Type 16 E6* Induces Oxidative Stress and DNA Damage. Journal of Virology. 2014;88(12):6751–6761. 10.1128/JVI.03355-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emmett M. Acetaminophen toxicity and 5-oxoproline (pyroglutamic acid): a tale of two cycles, one an ATP-depleting futile cycle and the other a useful cycle. Clinical journal of the American Society of Nephrology: CJASN. 2014;9(1):191–200. 10.2215/CJN.07730713 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar A, Bachhawat AK. A futile cycle, formed between two ATP-dependant gamma-glutamyl cycle enzymes, gamma-glutamyl cysteine synthetase and 5-oxoprolinase: the cause of cellular ATP depletion in nephrotic cystinosis? Journal of biosciences. 2010;35(1):21–25. . [DOI] [PubMed] [Google Scholar]
- 54.Engelke UFH, Zijlstra FSM, Mochel F, Valayannopoulos V, Rabier D, Kluijtmans LAJ, et al. Mitochondrial involvement and erythronic acid as a novel biomarker in transaldolase deficiency. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease. 2010;1802(11):1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53(2):131–142. . [PubMed] [Google Scholar]
- 56.Banne E, Meiner V, Shaag A, Katz-Brull R, Gamliel A, Korman S, et al. Transaldolase Deficiency: A New Case Expands the Phenotypic Spectrum. JIMD reports. 2016;26:31–36. 10.1007/8904_2015_474 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Dirbashi OY, Rashed MS, Al-Qahtani K, Al-Mokhadab MA, Kurdi W, Al-Sayed MA. Quantification of N-acetylaspartic acid in urine by LC-MS/MS for the diagnosis of Canavan disease. Journal of inherited metabolic disease. 2007;30(4):612 10.1007/s10545-007-0635-6 . [DOI] [PubMed] [Google Scholar]
- 58.Bogner-Strauss JG. N-Acetylaspartate Metabolism Outside the Brain: Lipogenesis, Histone Acetylation, and Cancer. Frontiers in Endocrinology. 2017;8:240 10.3389/fendo.2017.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ronco G, For the New Technologies for Cervical Cancer Working G, Segnan N, For the New Technologies for Cervical Cancer Working G, Giorgi-Rossi P, For the New Technologies for Cervical Cancer Working G, et al. Human Papillomavirus Testing and Liquid-Based Cytology: Results at Recruitment From the New Technologies for Cervical Cancer Randomized Controlled Trial. JNCI: Journal of the National Cancer Institute. 2006;98(11):765–774. 10.1093/jnci/djj209 [DOI] [PubMed] [Google Scholar]
- 60.Kitchener HC, Owens GL. Urine testing for HPV. BMJ: British Medical Journal. 2014;349. [DOI] [PubMed] [Google Scholar]
- 61.Calpena E, Casado M, Martínez-Rubio D, Nascimento A, Colomer J, Gargallo E, et al. 5-Oxoprolinuria in Heterozygous Patients for 5-Oxoprolinase (OPLAH) Missense Changes. JIMD reports. 2013;7:123–128. 10.1007/8904_2012_166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.