Abstract
Background
Validation of elimination of trachoma as a public health problem is based on clinical indicators, using the WHO simplified grading system. Chlamydia trachomatis (Ct) infection and anti-Ct antibody responses (anti-Pgp3) have both been evaluated as alternative indicators in settings with varying levels of trachoma. There is a need to evaluate the feasibility of using tests for Ct infection and anti-Pgp3 antibodies at scale in a trachoma-endemic country and to establish the added value of the data generated for understanding transmission dynamics in the peri-elimination setting.
Methodology/Principal findings
Dried blood spots for serological testing and ocular swabs for Ct infection testing (taken from children aged 1–9 years) were integrated into the pre-validation trachoma surveys conducted in the Northern and Upper West regions of Ghana in 2015 and 2016. Ct infection was detected using the GeneXpert PCR platform and the presence of anti-Pgp3 antibodies was detected using both the ELISA assay and multiplex bead array (MBA). The overall mean cluster-summarised TF prevalence (the clinical indicator) was 0.8% (95% CI: 0.6–1.0) and Ct infection prevalence was 0.04% (95%CI: 0.00–0.12). Anti-Pgp3 seroprevalence using the ELISA was 5.5% (95% CI: 4.8–6.3) compared to 4.3% (95%CI: 3.7–4.9) using the MBA. There was strong evidence from both assays that seropositivity increased with age (p<0.001), although the seroconversion rate was estimated to be very low (between 1.2 to 1.3 yearly events per 100 children).
Conclusions/Significance
Infection and serological data provide useful information to aid in understanding Ct transmission dynamics. Elimination of trachoma as a public health problem does not equate to the absence of ocular Ct infection nor cessation in acquisition of anti-Ct antibodies.
Author summary
Trachoma is a disease caused by Chlamydia trachomatis (Ct). Validation of elimination of trachoma as a public health problem is based on clinical indicators. Antibody and infection data may provide a better understanding of transmission dynamics in elimination settings. Dried blood spots (DBSs) for antibody testing and ocular swabs for Ct infection testing were integrated into the pre-validation trachoma surveys conducted in the Northern and Upper West regions of Ghana in 2015 and 2016. Ct infection was detected using the GeneXpert PCR platform and the presence of anti-Ct antibodies were detected using both the ELISA and multiplex bead array (MBA). Very little infection was identified (0.04%). The conclusions from the ELISA and MBA testing were similar, with evidence of an association between increasing seroprevalence and age in 1-9-year olds. Infection and serological data provide useful insights into transmission dynamics. Even if an EU meets trachoma elimination targets, this may not reflect complete interruption of transmission of ocular Ct infection.
Introduction
Trachoma is a disease caused by Chlamydia trachomatis (Ct). Repeated ocular infections [1] result in inflammation leading to conjunctival scarring, trichiasis (in-turned eyelashes which touch the eye) and ultimately corneal opacity (CO). The intervention strategy for trachoma is the World Health Organization (WHO)-endorsed SAFE strategy (S: Surgery for trachomatous trichiasis (TT); A: Antibiotics to clear Ct infection; F: Facial cleanliness and E: Environmental improvement to reduce transmission of Ct) [2,3]. Successful implementation of this strategy has resulted in a reduction in trachoma prevalence across many endemic countries [4–7]. WHO has set a goal of global elimination of trachoma as a public health problem by 2020 [8].
For trachoma elimination to be validated, countries must provide evidence that three criteria have been met. First, each previously-endemic evaluation unit (EU; populations of 100,000–250,000 people) must have reached and sustained, for at least two years, a prevalence of trachomatous inflammation—follicular (TF) in 1–9-year-olds of less than 5%. Second, each previously endemic EU must have reached a prevalence of TT previously unknown to the health system in ≥15-year-olds of less than 0.2%. Third, there must be an appropriately-resourced system to identify and manage incident trichiasis cases [9].
The WHO elimination thresholds for trachoma are based on clinical diagnostic indicators [9], using the simplified grading system [10]. However, TF has been shown to correlate poorly with Ct infection in low prevalence settings [11–14]. A follicular inflammatory response is known to persist for many weeks after infection has been cleared [15,16]. The presence of follicles deep to the upper tarsal conjunctiva is not a sign unique to Ct infection; a number of non-chlamydial pathogens including Haemophilus influenzae may elicit a similar response [14,17]. As such, the positive predictive value of a clinical diagnosis of TF for Ct infection can be reduced in low prevalence settings [18] where other aetiologies may account for a high proportion of TF. In the context of trachoma elimination, a lack of specificity of TF as an indicator will make it increasingly difficult to ensure that EUs are correctly categorised as endemic or not and that valuable resources are not wasted by unnecessarily prolonging interventions [19]. There are also concerns over the inter-grader agreement for diagnosis of TF, which becomes increasingly difficult to demonstrate [20] as trachoma prevalence decreases. As a result, there is a considerable interest in exploring whether and how alternative indicators could provide more objective evidence of elimination of trachoma as a public health problem, or be used as tools for post-validation surveillance [21].
Tests for anti-Ct antibody and Ct infection have been evaluated as alternative markers in settings with varying levels of trachoma [22–26]. In general, there has been very little or no Ct infection identified in areas where TF prevalence is below the elimination threshold [25–28]. Nucleic acid amplification tests (NAATs) including polymerase chain reaction (PCR) are highly specific and sensitive for ocular Ct infection [29,30]. The Cepheid GeneXpert platform is an automated, cartridge-based NAAT platform used widely across Africa for detection of Mycobacterium tuberculosis [31] that can detect Ct infection using different primers [29]. While a good test for Ct infection may have advantages over a proxy indicator, such as a sign of eyelid inflammation, collecting and analysing conjunctival swabs can be time-consuming, require specialist resources and personnel, and be potentially cost-prohibitive for national eye care or neglected tropical disease programmes [30]. The presence of anti-Ct antibodies, measured by multiplex bead array (MBA) [32,33], enzyme-linked immunosorbent assay (ELISA) [32,34,35] or lateral flow assay [32,36,37], may reflect cumulative exposure to Ct and when evaluated against age, represent transmission intensity over time [23,25,38,39]. Studies to date have predominantly focused on the detection of antibodies to Pgp3 [23,25,38], a conserved Ct plasmid protein found in both urogenital and ocular serovars [40]. The prevalence of anti-Pgp3 antibodies correlates fairly well with the prevalence of TF [22,23,25,26,38,39]. In post-elimination settings, the prevalence of Pgp3 seropositivity in children has shown either no increase with age or only minimal increases with increasing age [22,25,26,38].
The feasibility of generating district-level data for Ct infection and anti-Ct antibodies and how to interpret them for programmatic decision-making is still to be determined. A better understanding of the age-prevalence profiles in the post-elimination setting is also needed [9]. In 2015–2016, Ghana conducted a set of population-based surveillance surveys that demonstrated that all EUs previously endemic for trachoma had maintained the elimination threshold of <5% TF in the absence of large scale antibiotic treatment [41]. We integrated ocular swabs and DBSs into the surveys, providing an opportunity to evaluate the feasibility of using tests for Ct infection and anti-Pgp3 antibodies at scale in a trachoma-endemic country. We also compared antibody data collected by ELISA in Ghana to MBA data run at the Centers for Disease Control and Prevention (CDC), USA. These data were also used to evaluate whether measures of infection or Pgp3 antibody response have added value for understanding transmission dynamics in the peri-elimination setting.
Methods
Ethics statement
The study was approved by the Ghana Health Service (GHS) Ethics Review Committee (Reference GHS-ERC: 03/07/15) and the London School of Hygiene & Tropical Medicine Research Ethics Committee (Reference 10285). CDC involvement was determined not to constitute engagement in human subjects research, as CDC staff had no interaction with study participants.
Written informed consent was sought from caregivers of all children who participated in this study. Children who were able to provided verbal assent. Individuals with active trachoma were given 1% tetracycline eye ointment.
Study area
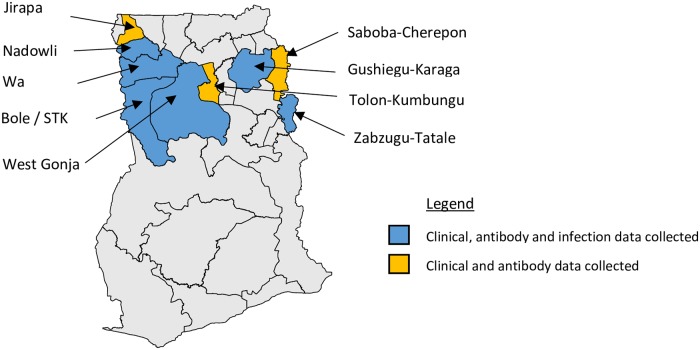
The study was conducted in the Northern and Upper West regions of Ghana, Fig 1. Surveys were conducted between November 2015 and April 2016.
Fig 1. Map of the evaluation units, indicating data types generated in each unit.
Adapted from an open source map retrieved from www.mapmaker.com.
Baseline assessments of trachoma prevalence were conducted in all 18 EUs between 1999 and 2003 [41]. A total of four EUs had a TF prevalence (in some cases combined TF/TI prevalence) of above 10% in children aged 1–5 years, five had a prevalence of 5–9.9% and nine had a prevalence of less than 5%. Based on WHO recommendations [42], GHS implemented the SAFE strategy, delivering EU-wide mass drug administration (MDA) of azithromycin in the EUs with TF prevalence >10% and antibiotic distribution targeted at community level in the EUs with TF prevalence <10%).
In 2008, impact surveys were conducted and all 18 EUs were declared to have reached or maintained the TF elimination threshold [43]. In 2011, GHS implemented a surveillance strategy that involved annual community and school screening for detection of TF and TT [41]. Eight communities identified during impact surveys or surveillance to have TF ≥5% were given three years of azithromycin MDA.
Survey design
A series of two-stage cluster-sampled population-based surveys were conducted in all 18 EUs as part of the Ghana pre-validation surveillance process [41]. A sub-set of nine EUs had additional indicators collected and evaluated, the results of which are the focus of this paper. Clinical, antibody and infection data were collected from six EUs (Table 1 and Fig 1). An additional three EUs were sampled for clinical and antibody data only (Table 1 and Fig 1). The EUs selected were chosen to represent a range of baseline TF prevalence and provide geographical spread. Infection data were not collected from the additional three EUs because of financial and time constraints related to the analysis.
Table 1. Number of samples analysed by evaluation unit.
| Sample | Evaluation Unit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bole/ Sawla-Tuna-Kalpa | West Gonja | Zabzugu-Tatale | Tolon Kumbungu | Gushiegu Karaga | Saboba Cherepon | Wa | Nadowli | Jirapa | Total | |
| DBS and TF | 1,307 | 1,148 | 1,345 | 1,361 | 1,367 | 1,202 | 1,357 | 1,363 | 1,280 | 11,730 |
| Ocular swabs | 1,312 | 925 | 1,349 | ND | 1,372 | ND | 1,364 | 1,366 | ND | 7,688 |
DBS: dried blood spot; ND: Not done; TF: trachomatous inflammation—follicular
The primary sampling unit was a community (village), selected with probability proportional to population size, and the secondary sampling unit was the household, selected using compact segment sampling. All children aged between one and nine years residing in the selected households were eligible for inclusion. The sample size calculations and sampling criteria were based on TF parameters and have been detailed elsewhere [41].
Survey data were collected electronically using a secure Open Data Kit-based Android smartphone application (LINKS, Task Force for Global Health, Atlanta, GA, USA; https://linkssystem.org) [44]. Data were uploaded to a cloud-located server with password-protected access only available to identified study investigators.
Clinical assessment
All graders were certified using Global Trachoma Mapping Project (GTMP) methodologies, described elsewhere [20]. Due to the expected low prevalence of trachoma in Ghana, the graders were trained and certified by examining children in Sokoto, Nigeria, where a number of districts still have TF prevalence estimates above the elimination threshold [7]. Each grader had to achieve a minimum kappa score of 0.7 for TF in an inter-grader agreement test with a grader trainer who had been certified by the GTMP.
Children aged 1–9 years were assessed for all five signs of trachoma (TF, trachomatous inflammation–intense (TI), trachomatous scarring (TS), TT and CO) as per the WHO simplified grading criteria [10].
Sample collection
Ocular swabs for infection testing were collected by passing a dry sterile polyester-tipped swab horizontally along the upper tarsal conjunctiva of the left eye, at least three times, rotating the shaft 120° with each pass. Control procedures were put into place to avoid field contamination, in particular washing hands at each new household, changing gloves between each examinee, and ensuring the end of the swab, once it had touched the conjunctiva, was placed directly into and broken off within a tube, which was sealed without further swab contact. Negative controls were taken after every 50 swabs by passing a clean swab in the air within five centimetres of a child’s eyes. Collected swabs were kept cool in the field and then refrigerated at 4°C for up to one week before being shipped on ice packs to Noguchi Memorial Institute for Medical Research (NMIMR) in Accra, where they were stored at -20°C until the time for analysis. Specimens were limited to one freeze/thaw cycle to reduce potential DNA degradation [29].
Dried blood spots (DBSs) were collected for serological testing. After cleaning with an alcohol-soaked swab, the participant’s finger was pricked using a sterile single-use lancet and the blood collected directly onto filter paper (Trop-Bio, Townsville, Australia). The filter paper had six projections, each calibrated to collect 10 μL of blood. The filter papers were air-dried in the shade then individually packed in sealable plastic bags and stored in a larger (gallon-size) sealable plastic bag with desiccant. DBSs were refrigerated at 4°C for up to one week before being shipped at ambient temperature to Accra and stored at -20°C until analysis.
Nucleic acid and antibody testing
PCR and ELISA were done at NMIMR in Accra, Ghana. MBA analysis was performed at the CDC in Atlanta, USA.
Ocular swabs were analysed for the presence of Ct DNA using the GeneXpert IV machine (Cepheid, Sunnyvale, USA) and GeneXpert CT/NG Assay (Cepheid, Sunnyvale, USA). Swabs were eluted using sterile diethylpirocarbonate (DEPC) water and pooled into groups of five samples as per a published pooling strategy [45]. The individual samples that made up a positive pool were tested separately to identify the positive sample(s). Results were reported as Ct-positive, negative or indeterminate (invalid, error or no result). The GeneXpert can produce an invalid result if there is failure of the sample adequacy control, which requires human DNA in the sample, or specimen processing control, indicating that amplification was inhibited [30]. Indeterminate pools were re-tested using a new aliquot of the specimen and a new cartridge. Control swabs collected in the field were analysed individually. Two Ct positive and two Ct negative processing controls were run at the beginning of each week.
DBSs were tested for antibodies to the Ct antigen Pgp3 using the semi-quantitative ELISA assay, described elsewhere [32,34]. Briefly, Immulon 2HB 96-well plates (ThermoFisher Scientific, Waltham, MA) were sensitized with 50 μL of Pgp3 antigen (500 ng/mL concentration) overnight at 4°C. DBSs and serum samples were diluted 1:50 in PBS containing 0.3% Tween-20 and 5% milk powder (PBST-milk) and stored overnight at 4°C. The next day, wells were washed with PBST (0.3% Tween-20 in PBS) and then blocked with PBST for one hour. Sample (50 μL) was added to wells and incubated for two hours at room temperature. Wells were then washed with PBST and incubated with 50 μL anti-human IgG conjugated to horseradish peroxidase (HRP) (1:10,000 dilution) (Southern Biotech, Birmingham, AL) to detect bound antibody. After four washes with PBST, 50 μL of 3, 3′, 5, 5′-tetramethylbenzidine (TMB) developing reagent (KPL, Gaithersburg, MD) was added to the wells and the reaction was stopped with 50 μL 1N H2SO4 after the predetermined interval. The optical density (OD) at 450 nm was read using an ELx808 Absorbance Microplate Reader (Biotek, Winooski, USA). OD values were corrected for background absorbance by subtracting the average OD of the two wells containing PBST-milk. The blanked OD values for all samples and controls were then normalised against the 200 U standard included on the same plate [34].
For the MBA, samples were tested in single-wells with Pgp3-coupled beads, as previously described [46,47]. Briefly, one DBS extension was diluted 1:320 in PBS containing 0.5% casein, 0.3% Tween 20, 0.5% polyvinyl alcohol, 0.8% polyvinylpyrrolidone, 0.02% sodium azide and 3 μg/mL E. coli extract (Buffer B). Coupled beads (2500 per antigen) were incubated with 40 μL of diluted sample per well in a 96-well filter plate (Millipore, Billerica, MA) for 1.5 hours. Wells were washed three times with PBS containing 0.05% Tween 20 (PBST2) and incubated with 50 ng biotinylated mouse anti-human IgG (Southern Biotech, Birmingham, AL) and 20 ng biotinylated mouse anti-human IgG4 (Southern BioTech) for 45 minutes to detect bound antibody. Wells were washed three times with PBST2 and incubated with 250 ng phycoerythrin-labelled streptavidin (Invitrogen, South San Francisco, CA) for 30 minutes to detect bound secondary antibody. After three washes with PBST2, wells were incubated for 30 minutes with 0.5% BSA, 0.05% Tween 20, 0.02% sodium azide in PBS to remove any non-specific binding. After one wash, wells were suspended in 125 μL of PBS and read on a Bio-Plex 200 instrument (Bio-Rad, Hercules, CA) equipped with Bio-Plex manager 6.0 software (Bio-Rad). The median fluorescence intensity (MFI) with the background from the blank well (Buffer B alone) subtracted out (MFI-bg) was recorded for each antigen for each sample. All samples were analysed masked to demographic and examination findings.
Statistical analysis
Only individuals with complete serological, infection and clinical data (or serological and clinical data, in EUs where ocular swabs were not collected) were included in the analysis. The dataset was presumed to be self-weighted but the analysis was adjusted (using STATA’s svy command) for the cluster sampling methodology.
Individuals were classified as seropositive or seronegative based on normalised OD values on the ELISA platform, and on the MFI-bg, after a log (x + 1) transformation, for the MBA. This transformation took into account that the MFI-bg included values of zero. The seropositive cut-off was defined using a finite mixture model based on maximum likelihood methods [34], with the threshold for seropositivity set as the mean of the Gaussian distribution of the seronegative population, plus four standard deviations [38].
To examine force of infection (FoI), the rate at which susceptible individuals acquire infection, the seroconversion rate (SCR), the rate at which seronegative individuals become seropositive, was estimated using a simple reversible catalytic model (RCM) fitted to seroprevalence in yearly age groups, using maximum likelihood estimates. Evidence for a change in SCR over time was explored by comparing two models using the profile likelihood method; the first model assumed constant transmission over time and the second assumed a potential change in the FoI at a specified time point [48].
Statistical analysis was conducted using R 3.4.0 [49] and STATA 12.0 [50]. The data were adjusted for age and gender based on the Ghana 2010 census [51]. The adjusted cluster-summarised mean prevalence was calculated for all the data and at the level of the EU. Bootstrap estimation was used to determine confidence intervals around prevalence estimates, based on 10,000 iterations and taking the 2.5th and 97.5th centiles.
For the serology data, chi-square tests were used to determine univariate associations. The non-parametric test for trend was used to determine an increase in seropositivity with age. Positive univariate associations of seropositivity at the individual level (age, EU, baseline TF endemicity) and gender (included a priori) were included in multivariate logistic regression models. The likelihood ratio test was used to determine the model of best fit. Regression was used for analysis of associations between continuous variables at the level of the EU. The geometric mean antibody titre was calculated using a log (x+1) transformation to take into account zero values.
A measure of cluster-level heterogeneity was determined by calculating the intra-cluster correlation coefficient (ICC) and design effect (DE). The ICC reveals how strongly observations in the same cluster resemble each other. The DE is the ratio of the variance in the collection of observations amassed using cluster sampling to the variance assuming the same sample size had been generated using simple random sampling [52]. The ICC and DE for this dataset were determined using STATA.
Results
Demographic information
Overall 96.0% of children resident in the selected households were examined, 3.6% were absent at the time of the survey and 0.4% refused to participate. A total of 11,730 DBSs were collected across nine districts, analysed by ELISA and matched to demographic and clinical data, Table 1. A total of 10,902 DBSs were also analysed by MBA. The number of samples taken by age group are detailed in Table 2.
Table 2. Number of samples analysed by age.
| Sample | Age (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total | |
| DBS and TF | 833 | 1,361 | 1,652 | 1,638 | 1,650 | 1,448 | 1,148 | 983 | 1,017 | 11,730 |
| Ocular swabs | 556 | 894 | 1,057 | 1,092 | 1,073 | 957 | 756 | 640 | 663 | 7,688 |
DBS: dried blood spot; TF: trachomatous inflammation—follicular
A total of 7,688 ocular swabs were taken across six districts, analysed and matched to demographic and clinical data. Overall, 50.2% of individuals surveyed were male and the median age was 5 years old.
Clinical data
Across all EUs, 1.0% of individuals (n = 112; 95%CI: 0.8–1.2) had TF in one or both eyes; of those, 67.9% (n = 76) had bilateral TF. There was no evidence of an association between TF and age (z = -0.48; p = 0.63). No TT was identified in children. The median age of individuals with TF was 4.5 years and 53.6% were female.
The overall cluster-summarised mean TF prevalence was 0.8% (95%CI:0.6–1.0), with an EU-level range of 0.5–1.1%, Table 3.
Table 3. Cluster-summarised mean prevalence of trachomatous inflammation—follicular (TF), Chlamydia trachomatis (Ct) infection and Pgp3 seropositivity by ELISA and MBA in children aged 1–9 years across selected evaluation units in Northern and Upper West regions of Ghana.
| Indicator | Evaluation unit | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Northern Region | Upper West Region | ||||||||||
| Bole/ Sawla-Tuna-Kalpa (STK) | West Gonja | Zabzugu-Tatale | Tolon Kumbungu | Gushiegu Karaga | Saboba Cherepon | Wa | Nadowli | Jirapa | Total | ||
| Baseline TF prevalence | 5–9.9% | ≥10% | 5–9.9% | ≥10% | <5% | <5% | ≥10% | <5% | 5–9.9% | NA | |
| TF | % | 0.5 | 1.1 | 1.0 | 0.8 | 0.7 | 0.8 | 1.1 | 0.8 | 0.5 | 0.8 |
| 95%CI (n) | 0.2–1.0 (9) | 0.4–1.2 (14) | 0.5–1.5 (18) | 0.4–1.3 (15) | 0.3–1.1 (12) | 0.3–1.3 (9) | 0.5–1.9 (13) | 0.3–1.5 (12) | 0.1–1.0 (10) | 0.6–1.0 (112) | |
| DE | 1.61 | 1.27 | 2.88 | 0.80 | 0.86 | 0.86 | 1.11 | 1.98 | 1.68 | 1.81 | |
| Ct infection | % | 0.07 | 0.0 | 0.2 | ND | 0.0 | ND | 0.0 | 0.0 | ND | 0.04 |
| 95%CI (n) | 0.00–0.12 (1) | 0.0–0.0 (0) | 0.0–0.6 (3) | ND | 0.0–0.0 (0) | ND | 0.0–0.0 (0) | 0.0–0.0 (0) | ND | 0.0–0.1 (4) | |
| DE | 1.01 | 1 (NA) | 3.01 | ND | 1 (NA) | ND | 1 (NA) | 1 (NA) | ND | 2.49 | |
| Seropositive by ELISA (anti-Pgp3 antibodies) | % | 8.2 | 3.9 | 7.2 | 2.5 | 4.0 | 6.1 | 7.4 | 5.0 | 5.9 | 5.5 |
| 95%CI (n) | 5.7–11.1 (100) | 2.4–5.4 (47) | 4.3–10.6 (90) | 1.5–3.6 (37) | 2.3–6.1 (56) | 4.3–8.6 (74) | 5.0–10.1 (105) | 3.8–6.2 (71) | 4.0–8.3 (82) | 4.8–6.3 (662) | |
| DE | 1.97 | 2.19 | 4.25 | 1.32 | 2.21 | 1.76 | 2.98 | 0.92 | 1.74 | 2.49 | |
| Seropositive by MBA (anti-Pgp3 antibodies) | % | 8.4 | 3.9 | 5.5 | 2.0 | 3.0 | 3.2 | 6.4 | 2.8 | 3.7 | 4.3 |
| 95%CI (n) | 6.3–10.6 (90) | 2.3–5.7 (39) | 3.3–8.3 (66) | 1.1–3.1 (25) | 1.8–4.5 (39) | 2.1–4.6 (37) | 4.1–8.9 (61) | 1.9–3.8 (39) | 2.3–5.3 (48) | 3.7–4.9 (444) | |
| DE | 2.23 | 1.17 | 3.75 | 1.50 | 1.77 | 0.72 | 2.20 | 1.37 | 1.68 | 2.30 | |
Cluster-summarised mean prevalence reported, except for baseline TF prevalence
ND: Not done NA: Not applicable DE: Design effect
Infection data
Four infections were identified, giving a cluster-summarised mean prevalence of 0.04% (95%CI:0.00–0.12), Table 3. The four samples were from two different clusters, one from a community in Bole and the other three from separate households of a single community in Zabzugu-Tatale. Both of these EUs had baseline TF prevalences of 5–9.9% in children aged 1–5 years. The median age of those infected was 6.5 years.
A total of 83 samples (1.1%) had indeterminate PCR results. All control swabs were negative for Ct DNA.
Serological data
The seropositive cut-off (four standard deviations from the mean of the seronegative population) for the ELISA was 1.091 OD450nm and for the MBA was 5.188 for the log of the MFI-bg.
The overall cluster-summarised mean seroprevalence was 5.5% (95%CI: 4.8–6.3) by ELISA and 4.3% (95%CI: 3.7–4.9) by MBA. Pgp3 seropositivity by ELISA differed by EU (F stat = 3.61; p = 0.001), with the highest seroprevalence in Bole/Sawla-Tuna-Kalpa (8.2% 95%CI: 5.7–11.1) and lowest in Tolon-Kumbungu (2.5%; 95%CI: 1.5–3.6) (Table 3). This pattern in EU seropositivity was also reflected by the MBA results.
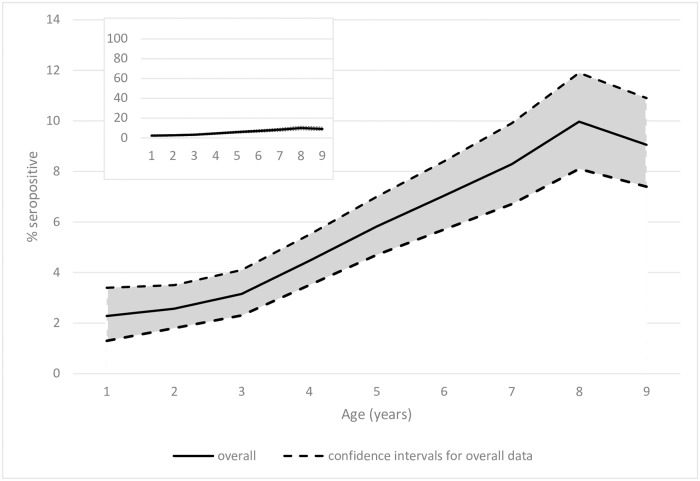
Seropositivity increased with age; this association held when analysing results from either platform (p<0.001) (Fig 2).
Fig 2. Seropositivity by age for the ELISA platform: Overall data 95%CI envelopes; inset graph shows the overall seropositivity by age using a 100% y-axis scale.
The model of best fit for the RCM was a singular SCR for the time period studied. Overall, there was a low seroconversion rate of 1.3 yearly events per 100 children (95%CI: 1.1–1.6), using the ELISA data. A similarly low SCR was reported using MBA data, with 1.2 yearly events per 100 children (95% CI: 0.9–1.6).
Pgp3 seropositivity was also associated with baseline EU-level TF prevalence (p<0.001) after controlling for potential confounders. The highest proportions of seropositive children were in those EUs that had a baseline TF prevalence of 5–9.9% in children aged 1–9 years. In the multi-variate model, there was an interaction observed between age and baseline TF prevalence: EUs with lower baseline TF prevalence estimates had comparatively greater odds of having older children who were seropositive by ELISA as opposed to younger children (p<0.001) (Table 4). Analysis of the seropositivity data generated by the MBA resulted in the same conclusions.
Table 4. Odds ratios for anti-Pgp3 seropositivity by ELISA, by evaluation unit-level baseline trachomatous inflammation—follicular (TF) prevalence and age of the individual, accounting for gender and EU.
| TF prevalence at baseline | Age group (comparator age: 1–3 years) | |
|---|---|---|
| 4–6 years | 7–9 years | |
| <5% | 3.4 | 4.4 |
| 5–9.9% | 1.7 | 3.1 |
| >10% | 2.3 | 3.5 |
The overall geometric mean of normalised ODs was 0.49 for the ELISA and 13.58 MFI-bg for the MBA platform. The geometric mean antibody titre increased with age (p<0.001). The strongest antibody responses (top 10% antibody titres of all seropositive individuals) were detected in children from the EUs with the highest seroprevalence estimates (for ELISA data: R2 = 0.68, p = 0.006).
Heterogeneity of data
An analysis of heterogeneity of data for the three indicators suggests some variability within clusters (Table 3). The overall DE for clinical data was quite low at 1.81 (EU-level range 0.80–2.88) and an ICC of 0.01 (cluster-level TF prevalence range 0–7.1%). The DE was higher for serologic data, 2.49 for the ELISA (2.30 for MBA data) with a corresponding ICC of 0.03 (cluster-level seroprevalence range 0–32.1%). There was variation in DE across EUs, ranging from 0.92 to 4.25 for the ELISA data and 0.72 to 3.75 for the MBA data, with the highest values in Zabzugu-Tatale. In the two clusters that were found to have infection, there was also high seropositivity (>15% seroprevalence using the ELISA and MBA). The high heterogeneity of the serology data (in Zabzugu-Tatale) was largely driven by one cluster that had infection (5.4%) and the highest proportion of seropositive individuals (32.1%). After removing that cluster from the DE calculations, the DE for (ELISA) serology dropped from 4.25 to 1.97 (3.75 to 0.90 for the MBA data). In EUs where infection was detected the DE was 3.01 (Zabzugu-Tatale) and 1.01 (Bole/Sawla-Tuna-Kalpa).
Discussion
Ghana has met the active trachoma criterion for elimination of trachoma as a public health problem, a measure based on TF parameters [41,53]. The collection of alternative indicators in pre-validation surveillance surveys allowed us to generate a more complete understanding of transmission dynamics in this setting. As evidenced in this study, elimination of trachoma as a public health problem does not equate to the absence of ocular Ct infection nor cessation in acquisition of anti-Ct antibodies. There are a number of potential explanations for this finding.
Infection was detected at very low levels (0.04%) and from limited sites, similar to findings from other studies in analogous settings which found no or very low prevalence of Ct [18,23,26,27]. These infection cases could be false positives, given the specificity of the assay [29,30,45], or a result of cross-contamination in the swab collection or analysis process. Equally, we cannot rule out the possibility that these were urogenital strains, acquired, for example, by transfer to children’s eyes, as a result of poor parental hand hygiene. However, the triangulation of serological, clinical and infection data in the communities suggests these are true Ct cases, whether ocular or urogenital strains. A cross-sectional study cannot tell us whether infection in these communities is transient or it is persistent and a potential risk for recrudescence. It is necessary to follow-up these select communities to determine if there is evidence of continued ocular Ct transmission over time and provide an opportunity to evaluate a model for post-validation surveillance.
Pooling of ocular swab samples was used in this study, a process known to be particularly cost-efficient in settings with low infection prevalence [45,54–56]. However, there are some concerns pooling can reduce the sensitivity of the test. Evidence suggests the impact would be minimal and likely to affect those individuals with lower ocular bacterial load [57], who are likely to be less important as drivers of transmission [58,59]. A slight loss in sensitivity would be tolerable where programmatic decision-making relied on EU-level classification; the specificity of the test, however, is critical. The GeneXpert machine is a relatively simple, mobile platform which is closed and self-contained, minimising potential cross-contamination [30], and was successfully used in Ghana. However, even with a simple platform to use and with pooling of samples for analysis, a key limitation of routinely including tests for infection in programmatic surveys will be the time and cost required to analyse the samples using NAATs.
Overall seroprevalence in children aged 1–9 years was similar here to estimates reported for other elimination or post-intervention settings [25,38]. The seroprevalence data by age allows estimation of transmission intensity over time. In Ghana there was evidence of a statistically significant increase in seropositivity with age (p<0.001). Studies in post-MDA settings in Tanzania and The Gambia found a similar increase in seropositivity with age [25,38]. Other studies in settings where elimination thresholds have been sustained over a number of years have found no association of seropositivity with age [22,26], which may be a reflection of interruption of transmission but also potentially a lack of power to be able to detect low rates of seroconversion. The data from Ghana suggested a history of low level ongoing seroconversion (1.2 to 1.3 events per 100 children per year) across the Northern and Upper West regions. Ghana stopped district-level antibiotic MDA at least eight years before the time of this study, and the single SCR reported likely reflects this, as any significant change in SCR would probably have occurred before most children enrolled in this study were born. Therefore, the SCR likely reflects a stable FoI in a post-elimination setting. These findings reinforce the idea that very low levels of on-going Ct transmission are not inconsistent with trachoma elimination as a public health problem. As some estimates suggest that more than 150 infections are needed over a lifetime to develop TT [1], the level of transmission we estimated in Ghana is highly unlikely to be of public health concern.
It is difficult to directly compare the seropositivity rates across studies because there is currently no agreed standard methodology for defining the threshold used to determine seropositivity [34]. We used an internally calibrated approach, which has the advantage that it does not rely on external positive or negative controls from different populations. This methodology could artificially define a seronegative and seropositive group [60] and inflate the number of “low intensity” positives. We used a conservative cut-off threshold of four standard deviations from the mean of the seronegative population to increase specificity and reduce likelihood of this outcome. Another difficulty in interpreting the serological data is that antibodies to Pgp3 do not distinguish between urogenital and ocular infection, and Ct exposure could have occurred at birth through ocular or respiratory infection from a mother with genital Ct [61]. This is an important consideration, however, although there is a paucity of data on the prevalence of sexually transmitted infections in Ghana, where it is documented it is reported to be relatively low [62–64] and urogenital Ct infection is not believed to be high in the Northern and Upper West regions of the country. If vertical transmission was a major driver of antibody acquisition, then it could be expected that the gradient of the age seroprevalence curve would be flat or even negative with increasing age [38].
It is noted that the use of an RCM is a simplification of real-world transmission dynamics, and using data from a single cross-sectional study to determine two linked RCM parameters is problematic. However, the SCR estimated here is similar to that generated in other studies in comparable environments [38]. Longitudinal data would help to generate better estimates of SCR [65].
In this study, we compared data obtained from the Pgp3 ELISA run in-country to MBA data generated at CDC. While the overall prevalences differed, the qualitative conclusions drawn from the data did not change and in particular the SCR estimates from the two methodologies did not differ significantly. While quantitative data collected on different platforms are difficult to compare, the SCR data presented here suggest a robustness of anti-Pgp3 antibody data. ELISA is relatively inexpensive and the protocol has been shown to be implemented effectively in trachoma-endemic countries. A key advantage of the MBA platform is that it allows for integrated serosurveillance of multiple pathogens [66], however it is unlikely to be widely available in the near future in trachoma-endemic countries, many of which are reluctant to export biological samples for analysis. A rapid test such as a lateral flow assay [36] might be the best format for application to trachoma elimination.
Infection and in particular serological data provide useful insights into transmission dynamics. Even if an EU meets trachoma elimination targets, this may not reflect complete interruption of transmission of Ct infection.
Supporting information
(DOCX)
Acknowledgments
Thanks to the surveys teams, including all ophthalmic nurses, data recorders, drivers and all other district health personnel. We are grateful to the survey communities and participants for their involvement in this study. A special thanks to the team of laboratory staff from the Bacteriology Department at NMIMR, headed by Professor Dorothy Yeboah-Manu, for the analysis of the samples. We are also grateful to Kristen Renneker for her help in developing the study database and training the data recorders and Enan Adamani for providing support during the survey activities. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work received financial support from the Coalition for Operational Research on Neglected Tropical Diseases (COR-NTD), which is funded at The Task Force for Global Health primarily by the Bill & Melinda Gates Foundation, by the United Kingdom Department for International Development, and by the United States Agency for International Development through its Neglected Tropical Diseases Program. Sightsavers and CDC provided some additional funds for the procurement of supplies and consumables. TFGH were not involved in the study design, data collection and analysis, decision to publish nor preparation of the manuscript.
References
- 1.Gambhir M, Basanez MG, Burton MJ, Solomon AW, Bailey RL, et al. (2009) The development of an age-structured model for trachoma transmission dynamics, pathogenesis and control. PLoS Negl Trop Dis 3: e462 10.1371/journal.pntd.0000462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis V, Turner V, WHO Programme for the Prevention of Blindness (1995) Achieving community support for trachoma control: a guide for district health work (WHO/PBL/93.36). Geneva: World Health Organisation.
- 3.Taylor HR, Burton MJ, Haddad D, West S, Wright H. (2014) Trachoma. Lancet. 384: 2142–52. 10.1016/S0140-6736(13)62182-0 [DOI] [PubMed] [Google Scholar]
- 4.Hammou J, El Ajaroumi H, Hasbi H, Nakhlaoui A, Hmadna A, et al. (2017) In Morocco, the elimination of trachoma as a public health problem becomes a reality. Lancet Glob Health 5: e250–e251. 10.1016/S2214-109X(17)30023-2 [DOI] [PubMed] [Google Scholar]
- 5.Ngondi J, Onsarigo A, Matthews F, Reacher M, Brayne C, et al. (2006) Effect of 3 years of SAFE (surgery, antibiotics, facial cleanliness, and environmental change) strategy for trachoma control in southern Sudan: a cross-sectional study. Lancet 368: 589–595. 10.1016/S0140-6736(06)69202-7 [DOI] [PubMed] [Google Scholar]
- 6.Ngondi J, Gebre T, Shargie EB, Adamu L, Ejigsemahu Y, et al. (2009) Evaluation of three years of the SAFE strategy (Surgery, Antibiotics, Facial cleanliness and Environmental improvement) for trachoma control in five districts of Ethiopia hyperendemic for trachoma. Trans R Soc Trop Med Hyg 103: 1001–1010. 10.1016/j.trstmh.2008.11.023 [DOI] [PubMed] [Google Scholar]
- 7.Mpyet C M N, Adamu MD, Ladan M, Willis R, Umar MM, et al. (2018) Impact survey results after SAFE strategy implementation in fifteen Local Government Areas of Kebbi, Sokoto and Zamfara States, Nigeria. Ophthalmic Epidemiology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO (1997) Future approaches to trachoma control: Report of a global scientific meeting, Geneva, 17–20 June 1996. Geneva: World Health Organization.
- 9.WHO (2016) Validation of elimination of trachoma as a public health problem. Geneva: WHO.
- 10.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR (1987) A simple system for the assessment of trachoma and its complications. Bull World Health Organ 65: 477–483. [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon AW, Harding-Esch E, Alexander ND, Aguirre A, Holland MJ, et al. (2008) Two doses of azithromycin to eliminate trachoma in a Tanzanian community. N Engl J Med 358: 1870–1871. 10.1056/NEJMc0706263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton MJ, Holland MJ, Makalo P, Aryee EA, Sillah A, et al. (2010) Profound and sustained reduction in Chlamydia trachomatis in The Gambia: a five-year longitudinal study of trachoma endemic communities. PLoS Negl Trop Dis 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keenan JD, Lakew T, Alemayehu W, Melese M, Porco TC, et al. (2010) Clinical activity and polymerase chain reaction evidence of chlamydial infection after repeated mass antibiotic treatments for trachoma. Am J Trop Med Hyg 82: 482–487. 10.4269/ajtmh.2010.09-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burr SE, Hart JD, Edwards T, Baldeh I, Bojang E, et al. (2013) Association between ocular bacterial carriage and follicular trachoma following mass azithromycin distribution in The Gambia. PLoS Negl Trop Dis 7: e2347 10.1371/journal.pntd.0002347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey RL, Hampton TJ, Hayes LJ, Ward ME, Whittle HC, et al. (1994) Polymerase chain reaction for the detection of ocular chlamydial infection in trachoma-endemic communities. J Infect Dis 170: 709–712. [DOI] [PubMed] [Google Scholar]
- 16.Keenan JD, Lakew T, Alemayehu W, Melese M, House JI, et al. (2011) Slow resolution of clinically active trachoma following successful mass antibiotic treatments. Arch Ophthalmol 129: 512–513. 10.1001/archophthalmol.2011.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton MJ, Hu VH, Massae P, Burr SE, Chevallier C, et al. (2011) What is causing active trachoma? The role of nonchlamydial bacterial pathogens in a low prevalence setting. Invest Ophthalmol Vis Sci 52: 6012–6017. 10.1167/iovs.11-7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baral K, Osaki S, Shreshta B, Panta CR, Boulter A, et al. (1999) Reliability of clinical diagnosis in identifying infectious trachoma in a low-prevalence area of Nepal. Bull World Health Organ 77: 461–466. [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon AW, Engels D, Bailey RL, Blake IM, Brooker S, et al. (2012) A diagnostics platform for the integrated mapping, monitoring, and surveillance of neglected tropical diseases: rationale and target product profiles. PLoS Negl Trop Dis 6: e1746 10.1371/journal.pntd.0001746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon AW, Pavluck AL, Courtright P, Aboe A, Adamu L, et al. (2015) The Global Trachoma Mapping Project: Methodology of a 34-Country Population-Based Study. Ophthalmic Epidemiol 22: 214–225. 10.3109/09286586.2015.1037401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Strategic and Technical Advisory Group on Neglected Tropical Diseases (2015) Technical consultation on trachoma surveillance, September 11–14, 2014. Task Force for Global Health, Decatur, USA (WHO/HTM/NTD/2015.02). Geneva: World Health Organization.
- 22.Zambrano AI, Sharma S, Crowley K, Dize L, Munoz BE, et al. (2016) The World Health Organization Recommendations for Trachoma Surveillance, Experience in Nepal and Added Benefit of Testing for Antibodies to Chlamydia trachomatis Pgp3 Protein: NESTS Study. PLoS Negl Trop Dis 10: e0005003 10.1371/journal.pntd.0005003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin DL, Bid R, Sandi F, Goodhew EB, Massae PA, et al. (2015) Serology for trachoma surveillance after cessation of mass drug administration. PLoS Negl Trop Dis 9: e0003555 10.1371/journal.pntd.0003555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cama A, Müller A, Taoaba R, Butcher RMR, Itibita I, et al. (2017) Prevalence of signs of trachoma, ocular Chlamydia trachomatis infection and antibodies to Pgp3 in residents of Kiritimati Island, Kiribati. PLOS Neglected Tropical Diseases 11: e0005863 10.1371/journal.pntd.0005863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West SK, Munoz B, Weaver J, Mrango Z, Dize L, et al. (2016) Can We Use Antibodies to Chlamydia trachomatis as a Surveillance Tool for National Trachoma Control Programs? Results from a District Survey. PLoS Negl Trop Dis 10: e0004352 10.1371/journal.pntd.0004352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West SK, Zambrano AI, Sharma S, Mishra SK, Munoz BE, et al. (2017) Surveillance Surveys for Reemergent Trachoma in Formerly Endemic Districts in Nepal From 2 to 10 Years After Mass Drug Administration Cessation. JAMA Ophthalmol 135: 1141–1146. 10.1001/jamaophthalmol.2017.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding-Esch EM, Sillah A, Edwards T, Burr SE, Hart JD, et al. (2013) Mass treatment with azithromycin for trachoma: when is one round enough? Results from the PRET Trial in the Gambia. PLoS Negl Trop Dis 7: e2115 10.1371/journal.pntd.0002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macleod CK, Butcher R, Mudaliar U, Natutusau K, Pavluck AL, et al. (2016) Low Prevalence of Ocular Chlamydia trachomatis Infection and Active Trachoma in the Western Division of Fiji. PLoS Negl Trop Dis 10: e0004798 10.1371/journal.pntd.0004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dize L, West S, Williams JA, Van Der Pol B, Quinn TC, et al. (2013) Comparison of the Abbott m2000 RealTime CT assay and the Cepheid GeneXpert CT/NG assay to the Roche Amplicor CT assay for detection of Chlamydia trachomatis in ocular samples from Tanzania. J Clin Microbiol 51: 1611–1613. 10.1128/JCM.00519-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenson A, Dize L, Mkocha H, Munoz B, Lee J, et al. (2013) Field evaluation of the Cepheid GeneXpert Chlamydia trachomatis assay for detection of infection in a trachoma endemic community in Tanzania. PLoS Negl Trop Dis 7: e2265 10.1371/journal.pntd.0002265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons LM, Somoskovi A, Gutierrez C, Lee E, Paramasivan CN, et al. (2011) Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 24: 314–350. 10.1128/CMR.00059-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gwyn S, Cooley G, Goodhew B, Kohlhoff S, Banniettis N, et al. (2017) Comparison of Platforms for Testing Antibody Responses against the Chlamydia trachomatis Antigen Pgp3. Am J Trop Med Hyg 97: 1662–1668. 10.4269/ajtmh.17-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammie PJ, Moss DM, Brook Goodhew E, Hamlin K, Krolewiecki A, et al. (2012) Development of a new platform for neglected tropical disease surveillance. Int J Parasitol 42: 797–800. 10.1016/j.ijpara.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 34.Migchelsen SJ, Martin DL, Southisombath K, Turyaguma P, Heggen A, et al. (2017) Defining Seropositivity Thresholds for Use in Trachoma Elimination Studies. PLoS Negl Trop Dis 11: e0005230 10.1371/journal.pntd.0005230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wills GS, Horner PJ, Reynolds R, Johnson AM, Muir DA, et al. (2009) Pgp3 antibody enzyme-linked immunosorbent assay, a sensitive and specific assay for seroepidemiological analysis of Chlamydia trachomatis infection. Clin Vaccine Immunol 16: 835–843. 10.1128/CVI.00021-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gwyn S, Mitchell A, Dean D, Mkocha H, Handali S, et al. (2016) Lateral flow-based antibody testing for Chlamydia trachomatis. J Immunol Methods 435: 27–31. 10.1016/j.jim.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 37.Sun MJ, Zambrano AI, Dize L, Munoz B, Gwyn S, et al. (2017) Evaluation of a field test for antibodies against Chlamydia trachomatis during trachoma surveillance in Nepal. Diagn Microbiol Infect Dis 88: 3–6. 10.1016/j.diagmicrobio.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Migchelsen SJ, Sepulveda N, Martin DL, Cooley G, Gwyn S, et al. (2017) Serology reflects a decline in the prevalence of trachoma in two regions of The Gambia. Sci Rep 7: 15040 10.1038/s41598-017-15056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin DL, Wiegand R, Goodhew B, Lammie P, Black CM, et al. (2015) Serological Measures of Trachoma Transmission Intensity. Sci Rep 5: 18532 10.1038/srep18532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen D, Lei L, Lu C, Galaleldeen A, Hart PJ, et al. (2010) Characterization of Pgp3, a Chlamydia trachomatis plasmid-encoded immunodominant antigen. J Bacteriol 192: 6017–6024. 10.1128/JB.00847-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debrah O, Mensah EO, Senyonjo L, de Souza DK, Hervie TE, et al. (2017) Elimination of trachoma as a public health problem in Ghana: Providing evidence through a pre-validation survey. PLoS Negl Trop Dis 11: e0006099 10.1371/journal.pntd.0006099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solomon AW, Zondervan M; Kuper, H; Buchan JC; Mabey DCW; Foster, A (2006) Trachoma control: a guide for programme managers. Geneva: WHO.
- 43.Yayemain D, King JD, Debrah O, Emerson PM, Aboe A, et al. (2009) Achieving trachoma control in Ghana after implementing the SAFE strategy. Trans R Soc Trop Med Hyg 103: 993–1000. 10.1016/j.trstmh.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 44.Pavluck A, Chu B, Mann Flueckiger R, Ottesen E (2014) Electronic data capture tools for global health programs: evolution of LINKS, an Android-, web-based system. PLoS Negl Trop Dis 8: e2654 10.1371/journal.pntd.0002654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dize L, West SK, Mkocha H, Quinn TC, Gaydos CA (2015) Evaluation of pooled ocular and vaginal swabs by the Cepheid GeneXpert CT/NG assay for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae compared to the GenProbe Aptima Combo 2 Assay. Diagn Microbiol Infect Dis 81: 102–104. 10.1016/j.diagmicrobio.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodhew EB, Priest JW, Moss DM, Zhong G, Munoz B, et al. (2012) CT694 and Pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis 6: e1873 10.1371/journal.pntd.0001873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moss DM, Priest JW, Boyd A, Weinkopff T, Kucerova Z, et al. (2011) Multiplex Bead Assay for Serum Samples from Children in Haiti Enrolled in a Drug Study for the Treatment of Lymphatic Filariasis. The American Journal of Tropical Medicine and Hygiene 85: 229–237. 10.4269/ajtmh.2011.11-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SL, et al. (2005) Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci U S A 102: 5108–5113. 10.1073/pnas.0408725102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R Core Team (2014) R: A Language and Environment for Statistical Computing. In: R Foundation for Statistical Computing. [Google Scholar]
- 50.Statacorp. (2011) Stata Statistical Software: Release 12. College Station TX: StataCorp LP.
- 51.Ghana Statistical Service (2013) 2010 Population and Housing Census. Accra, Ghana.
- 52.Campbell MK, Grimshaw JM, Elbourne DR (2004) Intracluster correlation coefficients in cluster randomized trials: empirical insights into how should they be reported. BMC Medical Research Methodology 4: 9–9. 10.1186/1471-2288-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO (2018) Ghana eliminates trachoma, freeing millions from suffering and blindness. WHO AFRO.
- 54.Diamant J, Benis R, Schachter J, Moncada J, Pang F, et al. (2001) Pooling of Chlamydia laboratory tests to determine the prevalence of ocular Chlamydia trachomatis infection. Ophthalmic Epidemiol 8: 109–117. [DOI] [PubMed] [Google Scholar]
- 55.Dize L, West S, Quinn TC, Gaydos CA (2013) Pooling ocular swab specimens from Tanzania for testing by Roche Amplicor and Aptima Combo 2 assays for the detection of Chlamydia trachomatis: accuracy and cost-savings. Diagn Microbiol Infect Dis 77: 289–291. 10.1016/j.diagmicrobio.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ray KJ, Zhou Z, Cevallos V, Chin S, Enanoria W, et al. (2014) Estimating community prevalence of ocular Chlamydia trachomatis infection using pooled polymerase chain reaction testing. Ophthalmic Epidemiol 21: 86–91. 10.3109/09286586.2014.884600 [DOI] [PubMed] [Google Scholar]
- 57.Morre SA, van Dijk R, Meijer CJ, van den Brule AJ, Kjaer SK, et al. (2001) Pooling cervical swabs for detection of Chlamydia trachomatis by PCR: sensitivity, dilution, inhibition, and cost-saving aspects. J Clin Microbiol 39: 2375–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Last A, Burr S, Alexander N, Harding-Esch E, Roberts CH, et al. (2017) Spatial clustering of high load ocular Chlamydia trachomatis infection in trachoma: a cross-sectional population-based study. Pathog Dis 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solomon AW, Holland MJ, Burton MJ, West SK, Alexander ND, et al. (2003) Strategies for control of trachoma: observational study with quantitative PCR. Lancet 362: 198–204. 10.1016/S0140-6736(03)13909-8 [DOI] [PubMed] [Google Scholar]
- 60.West SK, Munoz B, Kaur H, Dize L, Mkocha H, et al. (2018) Longitudinal change in the serology of antibodies to Chlamydia trachomatis Pgp3 in children residing in a trachoma area. Sci Rep 8: 3520 10.1038/s41598-018-21127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hobson D, Rees E (1977) Maternal genital chlamydial infection as a cause of neonatal conjunctivitis. Postgrad Med J 53: 595–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pepin J, Deslandes S, Khonde N, Kintin DF, Diakite S, et al. (2004) Low prevalence of cervical infections in women with vaginal discharge in west Africa: implications for syndromic management. Sex Transm Infect 80: 230–235. 10.1136/sti.2003.007534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Opoku BK, Sarkodie YA (2010) Prevalence of Genital Chlamydia and Gonococcal Infections in at Risk Women in the Kumasi Metropolis, Ghana. Ghana Medical Journal 44: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubbink JH, Verweij SP, Struthers HE, Ouburg S, McIntyre JA, et al. (2018) Genital Chlamydia trachomatis and Neisseria gonorrhoeae infections among women in sub-Saharan Africa: A structured review. International Journal of STD & AIDS 29: 806–824. [DOI] [PubMed] [Google Scholar]
- 65.Sepulveda N, Stresman G, White MT, Drakeley CJ (2015) Current Mathematical Models for Analyzing Anti-Malarial Antibody Data with an Eye to Malaria Elimination and Eradication. J Immunol Res 2015: 738030 10.1155/2015/738030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Priest JW, Jenks MH, Moss DM, Mao B, Buth S, et al. (2016) Integration of Multiplex Bead Assays for Parasitic Diseases into a National, Population-Based Serosurvey of Women 15–39 Years of Age in Cambodia. PLoS Negl Trop Dis 10: e0004699 10.1371/journal.pntd.0004699 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.