Abstract
Aim:
To evaluate the clinical and financial impact of introducing electromagnetic navigation bronchoscopy (ENB) at a community center.
Methods:
This retrospective, single-arm, single-center study evaluated 90 consecutive patients who had undergone ENB in 2012. Radial probe endobronchial ultrasound was used to localize the lesion after initial ENB. ENB-aided diagnoses, follow-up procedures and treatments, and adverse events were collected through 2 years.
Results:
ENB was conducted for lung biopsy (86 patients), fiducial placement (five), and/or dye marking (two). ENB-aided diagnostic yield was 82.6% (71/86), including 36 malignant and 35 nonmalignant cases. NSCLC was stage I–II in 84.6%. There were four false negatives. Sensitivity and negative predictive value were 90.0 and 88.6%. Pneumothorax occurred in 6/90 (5/6 with chest tube) and minor bleeding in four. The downstream revenue of new ENB cases was US$363,654.
Conclusion:
ENB introduction provided high diagnostic yield, early-stage diagnosis, acceptable safety, and was financially justified.
KEYWORDS : cancer, electromagnetic navigation bronchoscopy, lung
Summary points.
Late-stage cancer comprises 57% of all lung cancer diagnoses with a 5-year survival rate of only 4%; thus, strategies to diagnose lung cancer at an earlier stage are critically important.
Low-dose computed tomography screening recommendations will also increase the need for minimally invasive options to diagnose nodules detected on computed tomography.
Electromagnetic navigation bronchoscopy (ENB) may aid in the localization and sampling of more peripheral lung lesions beyond the reach of conventional bronchoscopy and has a lower risk of iatrogenic pneumothorax than transthoracic biopsy.
ENB combined with radial endobronchial ultrasound has been shown to improve diagnostic yield for peripheral nodules in academic centers but there is less published experience in community settings, presenting a potential barrier to adoption.
Another consideration particularly important to community centers is justifying the acquisition cost of the system. Administrators require that the downstream clinical and economic benefits outweigh the initial expenditure.
The objectives of the current retrospective series were to evaluate diagnostic yield, complication rates, stage at diagnosis, the utility of ENB to localize lesions for treatment and the financial impact of ENB during the first year of use at a community center.
The study results suggest that ENB offers a financially justifiable, minimally invasive, high-yield diagnostic tool with significant clinical benefits to patients.
Lung cancer remains the deadliest cancer diagnosis among Americans, with an estimated 158,080 deaths in 2016 and a 5-year survival rate of only 4% for distant metastases [1]. Only 16% of lung cancers are detected at a localized stage when the prognosis is better [1]. With more diagnostic CT scans being performed and the addition of active lung cancer screening [2], many small peripheral nodules are being detected. Peripheral bronchoscopic biopsy with assistive modalities such as electromagnetic navigation bronchoscopy (ENB) allow navigation to these smaller peripheral lung lesions in a safe manner and provide earlier definitive diagnosis and possibly help avoid repeated follow-up CT scans.
There is little published literature of using ENB in a community setting. In addition, while the ENB ‘learning curve’ has been reported previously [3–5], there is limited information regarding outcomes during the first year of use with a nonexpert, nonacademic user. Pulmonary physicians have historically had poor success with small peripheral nodules [6], making entry into this diagnostic arena undesirable. Furthermore, the success of transthoracic needle aspiration (TTNA) made it unnecessary for a community physician to attempt to biopsy peripheral nodules given the historically low success rate of bronchoscopy. While the 3% reported pneumothorax rate for ENB [7] is lower than the 15–25% reported for computed tomography-guided TTNA [8], first-time community practitioners may doubt that the low risk would be reproducible.
New technology adoption at both academic and community practices also requires a financial commitment. From an administrative standpoint, the downstream clinical and economic benefit must outweigh the initial expenditure, expanding the availability of novel diagnostic tools to patients from a wider ‘draw area’. A prior study has examined the clinical and economic impact of introducing endobronchial ultrasound (EBUS) to a clinical practice [9]. To our knowledge, the economic justification of ENB for a community hospital had not yet been examined.
The current study retrospectively evaluated outcomes in 90 consecutive patients in the first year of ENB use at a community practice. The study objectives were to evaluate diagnostic yield and complication rates, the utility of ENB for fiducial marker placement and dye marking, the impact on lung cancer stage at diagnosis and the financial impact of ENB on the institution.
Methods
• Study design
This study was conducted in accordance with the amended Declaration of Helsinki and the Health Care Portability and Accountability Act of 1996. The protocol was approved by the Sterling Institutional Review Board (Atlanta, GA, USA; 4936–001; January 23, 2015); a consent waiver was granted for the retrospective collection of anonymous patient data.
ENB was introduced at Saint Thomas Health in January 2012. Records were retrospectively reviewed from 90 consecutive patients who had undergone ENB using the superDimension™ navigation system Version 6 (Medtronic, MN, USA) [7,10–12] between 1 January 2012 and 31 December 2012.
Patient demographics, lesion and procedural characteristics, diagnostic yield, pathology reports, imaging and adverse events were collected from 30 days preprocedure until March 2015. Patients were followed for confirmation of final diagnosis; any lost to follow-up were classified with an unconfirmed diagnosis. All follow-up procedures related to lung lesions during service were captured through 6 months postprocedure.
• Procedures
ENB was performed by the first author similar to prior reports [3,10–11] using general anesthesia with an endotracheal tube. Sampling instruments included cytology brush, needle-tipped cytology brush, transbronchial biopsy forceps, fine-needle aspiration and bronchoalveolar lavage.
Radial-probe EBUS (rEBUS) was introduced at the institution and used to localize the lesion after initial ENB navigation beginning with the third patient. A transition from the original version straight catheter to the directional Edge™ navigation catheter (Medtronic) was employed beginning with the 30th patient. An EBUS convex probe was utilized in the same setting for mediastinal staging purposes when appropriate. Rapid on-site evaluation (ROSE) of biopsy specimens was performed by cytologists in the endoscopy suite for all cases.
• Economic analyses
Economic analyses were conducted similarly to a published study [9]. All ENB direct reimbursement and downstream collections beginning on the day after the procedure were captured (including all facility and professional fees for all services and revenue related to lung lesions, for example, office visits, radiologic studies, consults to other specialties, pathology, hospitalizations, procedures and treatments related to ENB-aided diagnoses). Economic data were assessed for existing patients under care by Saint Thomas Health more than 30 days prior to the ENB procedure and patients new to Saint Thomas Health within 30 days of the ENB procedure and referred by physicians not affiliated with Saint Thomas Health for evaluation of lung nodules.
For patients who remained within Saint Thomas Health for treatment following the ENB-aided diagnosis, costs were directly calculated based on hospital records. For patients treated externally, downstream payments were estimated based on the total number of services using 2012 Centers for Medicare and Medicaid Services reimbursement rates for Nashville, including injectable drugs [13], oral drugs [14], physician payments [15], outpatient hospital rates [16] and inpatient hospital rates [17].
• Definitions & statistical analyses
Navigation success was calculated as the proportion of cases in which ENB was able to successfully navigate to the lung target. The initial diagnostic yield of the ENB procedure was defined as the proportion of patients in whom ENB aided in a definitive diagnosis (malignant, infection, inflammation, or benign) based on the final pathology results, out of all patients in whom a diagnostic biopsy was attempted. The final diagnostic yield (true positives plus true negatives), sensitivity, specificity, negative predictive value and positive predictive value were calculated based on all available follow-up procedures and tests through 2 years. A pathologic description of inflammation, infection, or benign alveolar changes was considered true negative if radiographic stability or resolution was documented at the end of 2-year follow-up.
Analyses were performed using SAS® Version 9.4 (SAS Inc., NC, USA). Data were summarized by descriptive statistics (for continuous variables) or frequencies and percentages (for categorical variables).
Results
• Patient, lesion & procedural characteristics
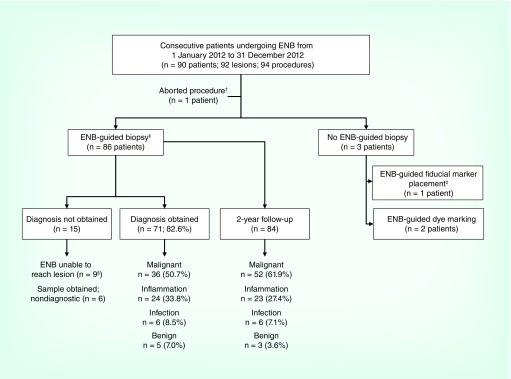
Ninety-four ENB procedures were conducted in 90 patients (92 lesions; Figure 1). Patient, lesion and procedural characteristics are shown in Table 1. The average lesion size was 22.7 ± 16.0 mm and 55.4% were <20 mm in diameter. The most common lesion location was the right upper lobe (34.8%). Approximately 64% of patients traveled more than 20 miles to obtain the ENB procedure. As mentioned above, rEBUS was used to confirm the lesion location after initial navigation with ENB in all but the first three patients.
Figure 1. . Purpose and outcomes of the electromagnetic navigation bronchoscopy procedures.
A total of 94 ENB procedures were conducted in 90 patients (92 lesions). ENB procedures were conducted for the purpose of obtaining a biopsy sample in 86 patients; of those, a diagnosis was obtained in 71/86 patients, for an initial diagnostic yield of 82.6%.
†In one patient the ENB procedure was aborted because the catheter was too short for the unusually tall patient.
‡A total of five patients underwent ENB-guided fiducial placement: four patients with both ENB-guided biopsy and fiducial placement and one with fiducial placement only.
§Includes two patients with ENB-guided EBUS (see text description).
EBUS: Endobronchial ultrasound; ENB: Electromagnetic navigation bronchoscopy.
Table 1. . Patient demographics and lesion characteristics.
| Patient demographic and lesion characteristic variables | n = 92 lesions in 90 patients |
|---|---|
| Age (years) | 65.6 ± 10.9 (90) (42–89) |
| Gender: | |
| – Female | 59/90 (65.6) |
| – Male | 31/90 (34.4) |
| Smoking history (current or former) | 68/90 (75.6) |
| Race: | |
| – African–American | 10/90 (11.1) |
| – Caucasian | 80/90 (88.9) |
| Approximate travel distance to health center: | |
| – ≤20 miles | 32/90 (35.6) |
| – 21–149 miles | 56/90 (62.2) |
| – ≥150 miles | 2/90 (2.2) |
| Lesions per patient | 1.02 ± 0.15 (90) |
| Size (mm): | 22.7 ± 16.0 (92) (4.0–100.0) |
| – <2 cm | 51/92 (55.4) |
| – ≥2 cm | 41/92 (44.6) |
| Location: | |
| – Right upper lobe | 32/92 (34.8) |
| – Right lower lobe | 22/92 (23.9) |
| – Left upper lobe | 22/92 (23.9) |
| – Left lower lobe | 10/92 (10.9) |
| – Right middle lobe | 6/92 (6.5) |
Data are presented as n (%) or mean ± standard deviation (n) (range).
• Diagnostic yield
ENB was conducted with the intent to biopsy a suspicious lesion in 86 patients (see Figure 1) and provided an initial diagnosis in 71/86 patients. The 15 patients without an initial ENB-aided diagnoses included 9 in whom ENB was unable to navigate to the lesion and 6 in whom ENB successfully navigated the lesion but a sample was obtained that was not sufficient to yield any diagnosis (Figure 1). In 2/9 with unsuccessful navigation, the lesions were more proximal than anticipated by evaluation of the computed tomography and were positioned just beyond subsegmental airways. These two lesions, which would not have been locatable with a traditional convex EBUS mediastinal survey, were located with ENB and marked with manual airway irritation to make a visible target for convex EBUS.
ENB successfully navigated to the target lesion in 77/86 patients, for a navigation success rate of 89.5%. Pathology results of ENB-aided biopsy samples yielded an initial diagnosis in 71/86 patients, for an initial diagnostic yield of 82.6%. These 71 ENB-aided diagnoses included 36 tissue samples read as malignant (50.7%), 24 read as inflammation (33.8%), six read as infection (8.5%) and five read as benign (7.0%), as shown in Table 2. Diagnosis of NSCLC was aided by ENB in 26 cases; of those, 84.6% were diagnosed at stage I–II. Initial diagnostic yield was 80.8% (21/26) using the straight catheter versus 83.3% (50/60) using the directional catheter, 37/47 (78.7%) in lesions <2 cm versus 34/39 (87.2%) in lesions ≥2 cm and 80.0% (24/30) in the first 30 patients versus 86.7% (26/30) in the last 30 patients.
Table 2. . Diagnosis outcomes.
| Patients (%) | |
|---|---|
| Initial diagnosis aided by ENB procedure | |
| Malignant tissue: | 36/71 (50.7) |
| – NSCLC: | 26/71 (36.6) |
| • Adenocarcinoma | 13/71 (18.3) |
| • Squamous carcinoma | 9/71 (12.7) |
| • Large cell/neuroendocrine tumor | 2/71 (2.8) |
| • Other NSCLC† | 2/71 (2.8) |
| – Small cell carcinoma | 2/71 (2.8) |
| – Sarcoma | 1/71 (1.4) |
| – Metastatic disease | 7/71 (9.9) |
| Nonmalignant tissue: | 35/71 (49.3) |
| – Inflammation | 24/71 (33.8) |
| – Infection | 6/71 (8.5) |
| – Benign‡ | 5/71 (7.0) |
| Final diagnosis after all follow-up§ | |
| Malignant | 52/84 (61.9) |
| Inflammation | 23/84 (27.4) |
| Infection | 6/84 (7.1) |
| Benign | 3/84 (3.6) |
†Other includes: NSCLC-not otherwise specified (n = 1) and sarcomatoid carcinoma (n = 1).
‡Includes four cases ultimately determined to be true negative (one read as atypical calls, two read as ‘no malignancy’, and one read as ‘benign alveolar tissue’) and one case ultimately determined to be false negative.
§Out of the 86 patients with ENB-aided biopsy attempted, follow-up was available in 84 patients.
ENB: Electromagnetic navigation bronchoscopy.
Follow-up was available in 84/86 patients with ENB-aided biopsies. One patient was lost to follow-up and one patient died before the diagnosis could be confirmed. Final diagnoses based on all available follow-up and procedures were 61.9% (52/84) malignant, 27.4% (23/84) inflammation, 7.1% (6/84) infection and 3.6% (3/84) benign (Table 2). There were four false negatives. The final diagnostic yield based on all follow-up procedures and tests through 2 years was 67/86 (77.9%; Table 3). The sensitivity, specificity, positive predictive value and negative predictive value of ENB to yield a definitive malignancy diagnosis based on 2-year follow-up were 90.0, 100, 100 and 88.6% (Table 3).
Table 3. . Electromagnetic navigation bronchoscopy aided diagnoses (n = 86 patients with biopsy attempted).
| Initial diagnostic yield: 71/86 (82.5%) | ||
| – Malignant | 36 | |
| – Nonmalignant (inflammation, infection, benign) | 35 | |
| No diagnosis obtained | 15 | |
| Final diagnostic yield†: 67/86 (77.9%) | ||
| Malignant | Nonmalignant | |
| – Malignant | True positive (A) (n = 36 patients) | False positive (B) (n = 0 patients) |
| – Nonmalignant | False negative (C) (n = 4 patients) | True negative (D) (n = 31 patients) |
| Sensitivity (A/[A+C]) | 90.0% | |
| Specificity (D/[B+D]) | 100% | |
| Positive predictive value (A/[A+B]) | 100% | |
| Negative predictive value (D/[C+D]) | 88.6% | |
†Calculated as ‘true negative’ plus ‘true positive’ out of all attempted electromagnetic navigation bronchoscopy-aided biopsies.
• Fiducial placement & dye marking
A total of five patients underwent ENB-guided fiducial placement, including four patients with both ENB-guided biopsy and fiducial placement and one patient with fiducial placement only. In one patient, biopsy and fiducial placement were conducted in the same ENB procedure. Fiducial markers were successfully deployed in all five patients. Of the four patients who received fiducial markers for stereotactic radiosurgery, all implanted fiducials remained in place at radiosurgery. The fifth patient received a fiducial marker on the same day as a wedge resection. Due to pneumothorax with placement, the marker had migrated and was not in the appropriate proximity of the lesion at the time of resection. Further resection allowed a benign diagnosis of granuloma to be established during the same surgery.
ENB-guided dye marking was conducted in two patients (nodule size 12 mm and 4 mm). In one patient, indigo carmine dye was used the day prior to surgery. Due to significant anthracosis, the black/gray dye could not be seen in the gray lung; however, the lesion was visualized as puckering of the pleura and successfully resected. In the second patient, dye marking was successfully followed by lobectomy on the same day. No spillage or diffuse staining was noted in either patient.
• Patient safety
Pneumothorax occurred in 6/90 patients (6.7%) of which 5/90 required a small-bore chest tube (5.6%; including four patients with pleural lesions and 1 with end-stage emphysema). Pneumothorax was secondary to fiducial wire placement in one of the four patients with pneumothorax (see above). The average lesion size for patients with pneumothorax was 14.8 mm. Four patients (4.4%) experienced minor bleeding.
• Downstream revenue generated by ENB
Of the 90 patients enrolled, 60 were existing patients and 30 were new to Saint Thomas Health at the time of ENB. Fifty-six patients (41 existing and 15 new) were treated within Saint Thomas Health while 24 (13 existing and 11 new) went elsewhere (some patients had treatment both within and external to Saint Thomas Health). The median direct collection per patient on the ENB day was US$2285. Treatments included surgery in 39.3% (35/89), radiation therapy in 20.5% (18/88) and chemotherapy in 21.3% (17/80). The total downstream collection following the ENB-aided diagnosis was US$1,097,782, including US$363,654 in collections from the 30 patients new to the center (Table 4).
Table 4. . Economic outcomes.
| Existing patients | New patients | All patients | |
|---|---|---|---|
| Follow-up treatment after ENB (number of patients): | |||
| – Surgery | 24/60 (40%) | 11/29 (37.9%) | 35/89 (39.3%) |
| – Radiation therapy | 14/59 (23.7%) | 4/29 (13.8%) | 18/88 (20.5%) |
| – Chemotherapy | 10/55 (18.2%) | 7/25 (28.0%) | 17/80 (21.3%) |
| Payments on ENB procedure day† | US$2325 | US$2186 | US$2285 |
| Mean downstream collections generated by ENB procedure (per patient) | US$15,296 | US$17,313 | US$15,910 |
| Total downstream collections generated by ENB procedure | US$734,218 | US$363,654 | US$1,097,782 |
Data reflect multiple procedures per patient; data not available for all patients.
†Median collections for all services on the ENB procedure day.
ENB: Electromagnetic navigation bronchoscopy.
Discussion
Many smaller, more peripheral lesions are beyond the reach of conventional bronchoscopy [6]. TTNA, while providing high diagnostic yield, has a high pneumothorax rate [8]. The current results demonstrate comparable diagnostics with improved safety compared with TTNA.
ENB provides an alternative, minimally invasive option to reach peripheral lung lesions, potentially aiding in an earlier diagnosis. However, prior results vary widely and there are few studies of ENB use in community practice. Wilson et al. reported on 279 peripheral lung lesions in an early community practice experience. Tissue samples were successfully obtained in 96% of lesions and 65% were diagnostic on the procedure day. With additional follow-up, 70% were diagnostic. Pneumothorax was observed in 3 patients [12]. In a more recent community thoracic surgery experience, diagnostic tissue was obtained in 86/101 cases (85%) with 6 pneumothoraces (5.8%) [18]. In a meta-analysis of 17 studies (1106 patients), the ENB diagnostic yield ranged from 60 to 94% [19]. In contrast, the AQuIRE registry of 266 cases using ENB combined with rEBUS reported a diagnostic yield of only 47.1% [20]. Of note, AQuIRE utilized low-frequency users with an average of only 14 ENB procedures per year. ENB was also employed in patients in whom traditional bronchoscopy and rEBUS were unsuccessful, likely contributing to the low yield. The AQuIRE registry also differed from the current study in the definition of diagnostic yield and the availability of follow-up information for all patients. Most published studies consider nonmalignant diagnoses to be true negative if there is no evidence of growth on serial computed tomography for at least 1 year [12,18,21–22]. In AQuIRE, inflammatory tissue or lymphocytes were considered nondiagnostic and follow-up information was not sufficient to calculate diagnostic yield with respect to the true negative rate. In the current study, 2-year follow-up data were available for 84/86 patients who underwent initial ENB-guided biopsy to allow a full analysis of sensitivity and negative predictive value.
In the current study, the initial and final diagnostic yields of 82.6 and 77.9%, respectively, are in the range of prior results. The learning curve of a user without fellowship training was likely aided by multiple factors, including use of rEBUS and ROSE, which were adopted based on published results [21,23–25]. Manufacturer training and on-site support was also used for early cases, per standard practice. Use of the 180° directional catheter for more difficult apical upper lobe and superior segment lesion also aided in the learning curve. General anesthesia use was not felt to impact yield, though this study does not directly compare to conscious sedation. Type of sedation did not have a significant impact on diagnostic yield in one prior report [22].
The goal of this study was to evaluate ENB for localizing peripheral lesions. In this study, 84/86 lesions were truly peripheral in nature. Two lesions, while more central than anticipated, remained parenchymal in nature and not in areas traditionally surveyed with convex EBUS. In these lesions, manual irritation marking with ENB was used to guide convex EBUS sampling, which would not have been possible with convex EBUS alone. This technique has been subsequently adopted by the user for similar lesions.
In the current study, 22/26 (84.6%) of patients with NSCLC were diagnosed at stage I–II. The overall (all procedures) rate of stage I–II lung cancer diagnosis at the study institution rose from 29.8% in 2010 to 44.7% in the first year following the integration of ENB [26]. While not all of these cases were diagnosed by ENB, it was a significant contributing factor. This trend toward earlier lung cancer diagnosis has been sustained in subsequent years and is felt to be directly related to the impact of ENB on the diagnosis and management algorithm. This result is consistent with a recent publication demonstrating that the proportion of stage I–II lung cancer diagnoses was 23% prior to the introduction of ENB compared with 40% after the introduction of ENB [27].
ENB technology was initially designed for lesions <2 cm. In this study, 55% of lesions met that criterion. Diagnostic yield was 79% in lesions <2 cm and 85% in lesions ≥2 cm. While larger lesions may seem more easily accessible with non-ENB bronchoscopic techniques, the inability to predict nondiagnostic results due to necrotic, highly vascular, large lesions in difficult locations still makes ENB the most reliable diagnostic approach. In large, heterogeneous lesions, ENB allows multiple, large-volume, multidirectional sampling in a single procedure with the same low risk of pneumothorax, along with ability to navigate to difficult-to-reach locations. Unlike computed tomography-guided TTNA, ENB also allows the potential for complementary EBUS-guided staging of the mediastinum and treatment planning with fiducial placement in a single anesthetic event. In addition to the lower risk profile, these should be primary factors in the decision-making algorithm of when to use ENB over computed tomography-guided TTNA, regardless of lesion size. First-time users may also find that limiting ENB usage to lesions <2 cm may reduce proficiency.
While ENB may aid in an earlier diagnosis and potentially lower downstream costs of treatment [28], the acquisition costs of the technology are higher than bronchoscopy or TTNA. One study has demonstrated that despite higher acquisition costs, ENB procedures were cost–effective compared with TTNA due to the reduced complication rate [29]. However, in the current healthcare environment, the system cost must be justified by evidence that the downstream clinical and economic benefit outweighs the initial expenditure. Pastis et al. demonstrated that the availability of new EBUS technology resulted in increased downstream revenue for the hospital system by drawing new patients who would have otherwise been treated elsewhere. In the current study, 30 patients were new to Saint Thomas Health at the time of the ENB procedure, generating US$363,654 in total payments. This downstream revenue justifies the initial expenditure, making the diagnostic option available to a wider population of patients. While it is feasible that the same revenue would have been generated by alternative diagnostic methods, this total payment represents the potential revenue generated by new patients coming to the practice to receive the ENB procedure.
While pneumothorax in this study is higher than the previously reported weighted mean of approximately 3%, it is within the range of reported rates [7,19] and represents a real-world example in a nonexpert, first-time user with relatively complex cases (four patients with pneumothorax had pleural lesions, one had end-stage emphysema and the average lesion size was 14.8 mm). Chest tube placement also occurred more frequently than anticipated in part due to the provider's clinical decision to place chest tubes in patients with unreliable radiographic follow-up due to a long travel distance. Subsequent years of ENB usage by this provider resulted in complication rates of 1–2% per year, similar to prior reports, partially attributable to avoidance of needle-tipped cytology brush and fine-needle aspiration in areas of pleura/fissure lesions and in cases of severe emphysema. Rapid and careful engagement of fluoroscopy with fiducial wire usage was also employed after the first observation of pneumothorax.
This study demonstrates the clinical and financial benefits of introducing ENB into a community practice. The hurdles of the learning curve, financial investment, complication rates and diagnostic yield were surmountable even for a first-time, nonacademic user. Earlier diagnosis aided by a minimally invasive, low-risk technology provided a clinically impactful outcome for patients and a strong financially rewarding service line for the institution. This technology should be considered in centers dedicated to growth in the lung cancer arena and is not meant for the occasional user. The learning curve is similar to that of other diagnostic tools such as EBUS and should not be considered prohibitive for nonexpert, nonfellowship-trained, community physicians. Given the Medicare adoption of lung cancer screening following the favorable 20% mortality benefit reported by the National Lung Screening Trial [30], programs will be required to develop algorithms for the diagnosis of incidental nodules found through screening. A multidisciplinary approach is necessary to ensure that the most clinically appropriate patients move on to a diagnostic work-up.
Limitations
This was a single-center, single-operator, retrospective, nonrandomized study. General anesthesia, ROSE and rEBUS availability at this user's institution may not be generalizable to other community centers.
Conclusion
This study suggests that ENB offers a minimally invasive, high-yield, diagnostic tool with significant clinical benefits to patients that is financially justifiable to the institution.
Future perspective
With the advent of carefully implemented screening programs, new diagnostic tools and personalized treatment modalities, the continuum of lung cancer care is evolving into a state analogous to breast and colon cancer management. Until a cure is found, innovations over the next 5–10 years will focus on streamlining care, reducing diagnoses and treatment times and extending survival.
Acknowledgements
The authors thank Lynn Peterson, Kimberly Owens and Elizabeth Pace of Saint Thomas Health and Jeffrey Potkul of Medtronic for their contributions to study design and data collection.
Footnotes
Author contributions
SK Garwood takes responsibility for the content of the manuscript and had full access to all data. SK Garwood, P ClenDening, S Pidgeon and K Hood made substantial contributions to conception and design of the study. SK Garwood, P ClenDening and LJ Wudel were responsible for data acquisition. Data were compiled by S Pidgeon and N Hevelone and analyzed by N Hevelone. SK Garwood and K Hood drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final version to be published.
Disclosure
Note of Prior Abstract Publication/Presentation: CHEST 2015 (poster).
Financial & competing interests disclosure
N Hevelone, KL Hood and S Pidgeon are full-time employees of Medtronic and assisted SK Garwood in data compilation (S Pidgeon), analysis (N Hevelone) and manuscript writing (KL Hood). The study was sponsored and funded by Medtronic (Minneapolis, MN), which provided input into the study design, data analysis and manuscript writing. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by KL Hood and was funded by Medtronic.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval and have followed the principles outlined in the Declaration of Helsinki. A consent waiver was granted by the institutional review board for the retrospective collection of anonymous patient data.
References
Papers of particular significance to the subject have been highlighted as: • of interest; •• of considerable interest
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chin J, Syrek Jensen T, Ashby L, Hermansen J, Hutter JD, Conway PH. Screening for lung cancer with low-dose CT – translating science into medicare coverage policy. N. Engl. J. Med. 2015;372(22):2083–2085. doi: 10.1056/NEJMp1502598. [DOI] [PubMed] [Google Scholar]
- 3.Lamprecht B, Porsch P, Wegleitner B, Strasser G, Kaiser B, Studnicka M. Electromagnetic navigation bronchoscopy (ENB): increasing diagnostic yield. Respir. Med. 2012;106(5):710–715. doi: 10.1016/j.rmed.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Leong S, Ju H, Marshall H, et al. Electromagnetic navigation bronchoscopy: a descriptive analysis. J. Thorac. Dis. 2012;4(2):173–185. doi: 10.3978/j.issn.2072-1439.2012.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makris D, Scherpereel A, Leroy S, et al. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur. Respir. J. 2007;29(6):1187–1192. doi: 10.1183/09031936.00165306. [DOI] [PubMed] [Google Scholar]
- 6.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl.):e142S–e165S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]; •• American College of Chest Physicians evidence-based clinical practice guidelines recommending electromagnetic navigation bronchoscopy (ENB) and complementary endobronchial ultrasound (radial-probe endobronchial ultrasound) in peripheral lesions difficult to reach with conventional bronchoscopy, provided equipment and expertise are available.
- 7.Gex G, Pralong JA, Combescure C, Seijo L, Rochat T, Soccal PM. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration. 2014;87(2):165–176. doi: 10.1159/000355710. [DOI] [PubMed] [Google Scholar]; • Meta-analysis of 15 trials evaluating ENB for the diagnosis of lung nodules.
- 8.Wiener RS, Wiener DC, Gould MK. Risks of transthoracic needle biopsy: how high? Clin. Pulm. Med. 2013;20(1):29–35. doi: 10.1097/CPM.0b013e31827a30c1. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Meta-analysis of 20 trials reporting complications following transthoracic needle lung biopsy.
- 9.Pastis NJ, Simkovich S, Silvestri GA. Understanding the economic impact of introducing a new procedure: calculating downstream revenue of endobronchial ultrasound with transbronchial needle aspiration as a model. Chest. 2012;141(2):506–512. doi: 10.1378/chest.11-0254. [DOI] [PubMed] [Google Scholar]; •• Study after which the current economic analysis was modeled. Evaluates downstream revenue generated by the introduction of endobronchial ultrasound-guided transbronchial needle aspiration into a medical practice.
- 10.Odronic SI, Gildea TR, Chute DJ. Electromagnetic navigation bronchoscopy-guided fine needle aspiration for the diagnosis of lung lesions. Diagn. Cytopathol. 2014;42(12):1045–1050. doi: 10.1002/dc.23164. [DOI] [PubMed] [Google Scholar]
- 11.Loo FL, Halligan AM, Port JL, Hoda RS. The emerging technique of electromagnetic navigation bronchoscopy-guided fine-needle aspiration of peripheral lung lesions: promising results in 50 lesions. Cancer Cytopathol. 2014;122(3):191–199. doi: 10.1002/cncy.21373. [DOI] [PubMed] [Google Scholar]
- 12.Wilson DS, Bartlett RJ. Improved diagnostic yield of bronchoscopy in a community practice: combination of electromagnetic navigation system and rapid on-site evaluation. J. Bronchology Interv. Pulmonol. 2007;14(4):227–232. [Google Scholar]
- 13.Centers for Medicare & Medicaid Services, January 2012 ASP Pricing Files. www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2012ASPFiles.html
- 14.RED BOOK™ Online. http://micromedex.com/products/product-suites/clinical-knowledge/redbook
- 15.Center for Medicare and Medicaid Services, Medicare Physician Fee Schedule. www.cms.gov/apps/physician-fee-schedule/overview.aspx
- 16.Centers for Medicare and Medicaid Services, Outpatient Prospective Payment System: CMS-1525-CN2, Addendum B. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices-Items/CMS-1525-CN2.html
- 17.Centers for Medicare and Medicaid Services, Inpatient Prospective Payment System (October 2011– September 2012). CMS-1518-CN4, Table 5. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Acute-Inpatient-Files-for-Download-Items/FY2012-FinalRule-CorrectionNotice-Files.html
- 18.Pearlstein DP, Quinn CC, Burtis CC, Ahn KW, Katch AJ. Electromagnetic navigation bronchoscopy performed by thoracic surgeons: one center's early success. Ann. Thorac. Surg. 2012;93(3):944–949. doi: 10.1016/j.athoracsur.2011.11.006. discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Chen S, Dong X, Lei P. Meta-analysis of the diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules. J. Thorac. Dis. 2015;7(5):799–809. doi: 10.3978/j.issn.2072-1439.2015.04.46. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Meta-analysis of 17 trials evaluating ENB for the diagnosis of lung nodules.
- 20.Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am. J. Respir. Crit. Care Med. 2016;193(1):68–77. doi: 10.1164/rccm.201507-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balbo PE, Bodini BD, Patrucco F, et al. Electromagnetic navigation bronchoscopy and rapid on site evaluation added to fluoroscopy-guided assisted bronchoscopy and rapid on site evaluation: improved yield in pulmonary nodules. Minerva Chir. 2013;68(6):579–585. [PubMed] [Google Scholar]
- 22.Bowling MR, Kohan MW, Walker P, Efird J, Ben Or S. The effect of general anesthesia versus intravenous sedation on diagnostic yield and success in electromagnetic navigation bronchoscopy. J. Bronchology Interv. Pulmonol. 2015;22(1):5–13. doi: 10.1097/LBR.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 23.Eberhardt R, Anantham D, Ernst A, Feller-Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am. J. Respir. Crit. Care Med. 2007;176(1):36–41. doi: 10.1164/rccm.200612-1866OC. [DOI] [PubMed] [Google Scholar]
- 24.Karnak D, Ciledag A, Ceyhan K, Atasoy C, Akyar S, Kayacan O. Rapid on-site evaluation and low registration error enhance the success of electromagnetic navigation bronchoscopy. Ann. Thorac. Med. 2013;8(1):28–32. doi: 10.4103/1817-1737.105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamprecht B, Porsch P, Pirich C, Studnicka M. Electromagnetic navigation bronchoscopy in combination with PET-CT and rapid on-site cytopathologic examination for diagnosis of peripheral lung lesions. Lung. 2009;187(1):55–59. doi: 10.1007/s00408-008-9120-8. [DOI] [PubMed] [Google Scholar]
- 26.American College of Surgeons and American Cancer Society. National Cancer Database. www.facs.org/search/cancer-programs?name=saint%20thomas%20health
- 27.Brown C, Ben-Or S, Walker P, Bowling M. The impact of electromagnetic navigational bronchoscopy on a multidisciplinary thoracic oncology program. J. Natl Compr. Canc. Netw. 2016;14(2):181–184. doi: 10.6004/jnccn.2016.0021. [DOI] [PubMed] [Google Scholar]; •• Evaluates the stage of lung cancer diagnosis in 286 patients evaluated prior to the introduction of ENB versus 290 patients evaluated after the introduction of ENB.
- 28.Pyenson B, Henschke C, Yip R, Dec E, Yankelevitz D. Offering Lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am. Health Drug Benefits. 2014;7(5):272–282. [PMC free article] [PubMed] [Google Scholar]
- 29.Deppen SA, Davis WT, Green EA, et al. Cost–effectiveness of initial diagnostic strategies for pulmonary nodules presenting to thoracic surgeons. Ann. Thorac. Surg. 2014;98(4):1214–1222. doi: 10.1016/j.athoracsur.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that low-dose computed tomography screening reduces lung cancer mortality in selected high-risk patients.