Abstract
In the era of high-throughput molecular screening and personalized medicine, difficulty in determining whether cancer mutations are truly ‘actionable’ remains a gray zone in NSCLC. The most important prerequisite to perform such investigations is the tumor tissue retrieval via biopsy at diagnosis and after occurrence of resistance. Blood-based liquid biopsy as circulating tumor cells, circulating tumor DNA and exosomes can offer a fast and non-invasive method to elucidate the genetic heterogeneity of patients, the screening and patient stratification and give a dynamic surveillance for tumor progression and monitor treatments response. Here we prospectively discuss the three main approaches in the blood-biopsy field of lung cancer patients and its clinical applications in patient management. We also outline some of the analytical challenges that remain for liquid biopsy techniques in demonstrating that it could represent a true and actionable picture in lung cancer management for the implementation into clinical routine.
KEYWORDS : CTCs, ctDNA, early detection, exosomes, liquid biopsy, minimal residual disease, NSCLC, personalized medicine, resistance monitoring, therapy options, tumor burden, tumor evolution
Practice points.
Liquid biopsy qualifies different potential approaches for detection of body fluids carrying biomarkers in cancer patients. As well as a tissue biopsy, it is representative of the tumor tissue from which it is spread.
Blood-based liquid biopsy is a rapid blood-based and non-invasive way to obtain information on tumor-specific genetic alterations and tumor burden, and assess the dynamic tumor evolution during treatment of cancer patients.
Three main different sources of tumor DNA and RNA can be assessed in the circulation: cell-free circulating tumor DNA (ctDNA), circulating tumor cells (CTCs) and circulating extracellular vesicles (exosomes).
ctDNA represents a small fraction of total circulating free DNA and consists of small DNA fragments not associated with cells or cell fragments, originating from apoptotic and necrotic process and released into the bloodstream.
CTCs are intact, often viable cells shed into the bloodstream from primary and metastatic tumor sites and may constitute seeds for subsequent growth of tumor, self-seeding and additional tumors in distant organs.
Exosomes are membrane-encapsulated vesicles containing different types of nucleic acids and proteins from the cell they originate from, including cancer cells. Each subtype of lung cancer has its own DNA, RNA and/or protein signature in exosomes, dependent on its stage, metastatic power and drug sensitivity.
ctDNA, CTCs and exosomes have also currently been described in lung cancer as having potential application in early and advanced stages as prognostic, predictive markers or source of DNA for molecular profiling and tracking longitudinal resistance in patients.
Lung cancer can be considered as a genetic disease with a patient's specific mutational profile. This molecular information is currently obtained from tissue biopsies or surgical specimens that represent the gold standard source for diagnosis and in some cases are used to identify therapeutic options. Besides this, tumor staging and disease follow-up is currently assessed by clinical and radiological findings. However, it is not always possible to obtain enough biopsy material due to the invasive nature of the procedure and the poor performance status of many advanced lung cancer patients.
In addition, available tumor samples rarely offer a dynamic picture of disease progression and frequently show heterogeneity both at the histological and genetic level. All this evidence often makes a single, small biopsy less representative of the overall tumor.
The term ‘liquid biopsy’ is strictly referred to a blood test used for the isolation and characterization of circulating tumor cells (CTCs) based on their cytopathological features, by analogy to the definition of a ‘tissue’ biopsy. From a more general point of view, the term liquid biopsy indicates different potential approaches for detection of body fluids carrying biomarkers in cancer patients.
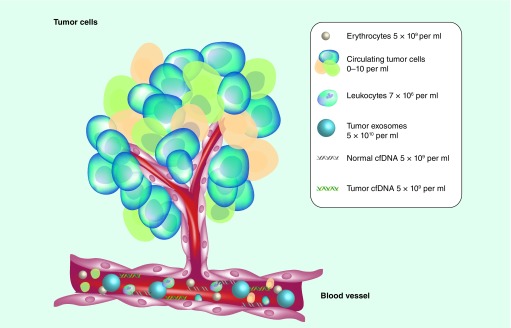
There are three main sources of tumor-derived DNA and RNA that can be assessed in the circulation in a non-invasive approach: ctDNA, [1,2] CTCs and circulating extracellular vesicles (exosomes) [3,4]. The term ctDNA refers to circulating tumor DNA fragments detectable in the circulation as naked nucleic acids, whereas CTCs represent whole, often viable, cells characterized by both physic-chemical properties and surface molecules which make them susceptible to isolation from blood. Exosomes are extracellular vesicles that contain RNA, DNA fragments, proteins and metabolites, and are released by several cell types (Figure 1).
Figure 1. . The primary tumors or metastasis are a composite of genetically heterogeneous cancer and stromal cells that can generate biomarkers detectable by liquid biopsy.
Tumors can shed circulating tumor cells into the bloodstream; apoptotic and necrotic process can instead originate cfDNA, released into the bloodstream. In addition, all cells from tumors can release exosomes, membrane-encapsulated vesicles containing different types of nucleic acids and proteins from the cells. All these elements (circulating tumor cells, cfDNA, exosomes) can be isolated from blood for molecular analysis.
cfDNA: Circulating-free DNA.
The evolution of scientific findings clarifies that as methods using CTC, ctDNA and exosomes evolve, they will likely have similar but also distinct clinical applications, reflecting their relative biologic and technologic strengths and limits.
Circulating tumor cells
CTCs represent a subset of tumor cells that leave the primary and metastatic tumor site, and are transported through the circulation to distant organs. Hence, CTCs emerge as fundamental prerequisites for subsequent metastasis development [5]. This hypothetical vision was first proposed in the early 1990s by Thomas Ashworth, who first described the ‘seed versus soil’ theory of tumor invasion and dissemination to explain the non-random formation of metastasis.
From a biological point of view, CTCs are typically defined as cells containing an intact viable nucleus, and that are cytokeratin positive, epithelial cell adhesion molecule (EpCAM) positive and not expressing CD45. Nevertheless, EpCAM and other markers are not always expressed on CTCs and are downregulated by processes such as epithelial–mesenchymal transition [6]. CTCs have been described in a wide range of epithelial cancers and the studies concerning their isolation and characterization improved the knowledge into the pathophysiology of the natural history of lung cancer [7–14]. Importantly, scientific evidences support the idea that, along with their role in promoting metastases, CTCs may be also found within the primary tumor and support its progression, through the so-called tumor self-seeding mechanism [15,16].
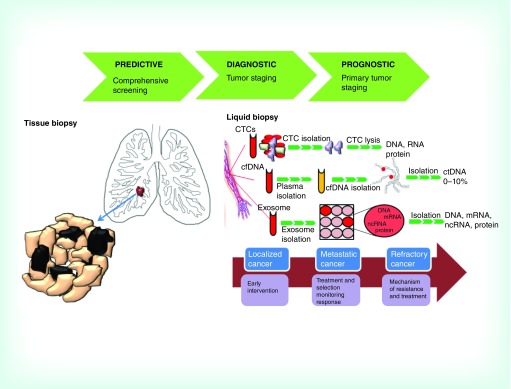
Besides the biological studies on CTCs, clinical studies and first trials using CTCs have been performed, also indicating promising advancements for patients’ care. Practical clinical applications of CTCs include the identification of prognostic, predictive and pharmacokinetic biomarkers. Moreover, such real-time longitudinal monitoring of CTC-derived genotypes may also mean CTCs can provide a novel non-invasive approach to identify drug sensitivity and resistance associated markers, thereby guiding therapeutic decisions (Figure 2).
Figure 2. . Main aspects of conventional tissue biopsy and liquid biopsy.
Tumor tissue represents the gold standard for molecular investigations but some problems exist regarding its obtainment and utility. In fact, apart from the invasiveness of the practice, tissue biopsy can give molecular information just at the diagnosis time and does not permit the detection of cancer heterogeneity and molecular cancer evolution. A valid non-invasive approach to assess the molecular profile of patients is through a liquid biopsy, where cancer biomarkers can be analyzed through a biofluid sample. CTCs, ctDNA and exosomes can offer a fast and non-invasive method to elucidate the genetic heterogeneity of patients and patients can give a dynamic surveillance for tumor progression and monitor treatments response.
cfDNA: Circulating-free DNA; CTC: Circulating tumor cell; ctDNA: Circulating tumor DNA; ncRNA: Non-coding RNA.
A correlation between the number of CTCs and prognosis in patients with metastatic disease has been currently extensively demonstrated in both patients with NSCLC and SCLC, in terms of progression-free survival and overall survival [17–20].
In a preliminary study by Dorsey et al., CTC counts also appear to reflect response to radiotherapy in patients with localized NSCLC and suggest a possible way for complementing standard radiographic imaging [21]. Enumeration of CTCs in early-stage cancer patients is more challenging than in metastatic disease, and some evidences exist suggesting that CTC counts can also help to predict prognosis in early-stage patients and post-surgery lung cancer to risk stratification [22,23]. Therefore, not only enumeration but also characterization of CTCs should be performed at different moments along the follow-up, thus contributing to the identification of different tumor cell subpopulations with different possible implications in patient prognosis [24]. Molecular and genetic characterization of CTCs can be additionally suggested as a form of non-invasive ‘liquid biopsy’. CTCs profiling could provide dynamic information about genotypic and phenotypic features of a tumor without the need of an invasive biopsy. In this context, a clear correlation has been observed between the molecular profile of the primary tumor with those of captured CTCs [25,26]. For instance, EGFR mutations have been successfully detected in CTCs from patients with advanced NSCLC using different molecular techniques with a variable but high concordance with results on primary tissues [27,28]. More interestingly, besides the detection of pre-existing mutations for diagnosis, the analysis of CTCs has been demonstrated to allow the monitoring of drug resistance over the course of treatment by revealing the emergence of drug resistance mutations.
A multi-institutional Stand-Up-To-Cancer collaboration reported an exploratory analysis on patients with EGFR-mutant tumors progressing on EGFR tyrosine kinase inhibitor (TKI) [2] therapy, comparing the T790M genotype from tumor biopsies with analysis of simultaneously collected CTCs and ctDNA. Despite not particularly exciting concordance of results, genetic assessment in liquid biopsy leads to the identification of a consistent fraction of patients in whom the concurrent tissue biopsy was negative or indeterminate. Moreover, in the recent work of Hata et al. it was observed that acquired resistance caused by the EGFR T790M gatekeeper mutation can occur either by selection of pre-existing mutated drug-resistant cancer cells or via genetic evolution of initially EGFRT790M-negative, drug-tolerant cells, with a high impact in the modulation of therapeutic opportunities to prevent or overcome resistance in the clinic [29]. The ability recently described to diagnose EML4–ALK gene rearrangement in CTCs shows that these cells could also have a clinical utility in ALK-positive NSCLCs to guide therapeutic management with ALK inhibitors. Moreover, ALK rearrangements found in CTCs reveal a strong correlation with a mesenchymal phenotype, in contrast to the heterogeneous epithelial–mesenchymal phenotypes in the patient's tumors, with migratory and invasive properties, and possibly a higher metastatic potential. These findings also underlay the role of CTCs as a unique compartment to identify tumor clones to clarify their role in the process of metastasis [30].
Besides all of these advantages, it is also important to consider that tumor cells in circulation have an heterogeneous nature and may exist as individual cells, some as clumps of tumor cells [31], some associated with blood cells such as platelets [32], and many if not most may be dead, non-viable cells [33–36]. Thus, although the potentialities of CTCs as prognostic and predictive cancer biomarkers, several issues concerning the technologies and methods of recognition and isolation from the more abundant blood cell populations have limited their broad translation into the clinical setting. It is only in recent years that technology has become available to reliably identify CTCs in peripheral blood.
To separate CTCs from normal cells, two main isolation approaches based on biological or physical properties are available in laboratory practice. The first one chooses molecular biomarkers that are exclusively expressed in tumor cells [37]. Currently, EpCAM expression is the most commonly used biomarker for distinction and subsequent purification of CTCs. Among these, the CellSearchTM System (Veridex LLC) method is the most used and US FDA approved for monitoring cancer patients [38].
Despite the major advantage of being semi-automated and highly reproducible, all the immunological-based methods have the limitation of the intrinsic variability of common epithelial specific markers used that are expressed at different levels and disease-related stages in CTCs and may be downregulated as a result of epithelial–mesenchymal transition as previously described [39]. These limitations can be partially addressed by using cocktails of antibodies or by negative filtration to remove blood cells from a sample and leave behind tumor cells, as for the CTC-iChip platform [40]. However, these new CTCs microfluidic devices have not been fully validated to date in terms of specificity, reproducibility and clinical relevance.
The simplest CTC selection method is probably size-based membrane filters, a method based on the assumption that CTCs are larger than blood cells [41]. The cytometric CTC isolation technique was first described by Vona and colleagues, and has since been used successfully to identify CTCs in NSCLC [42]. The main advantage of using the membrane filtration approach is that it does not require a reliance on antiepithelial antigen expression to capture cells, thus avoiding the lack of CTC detection because of epithelial–mesenchymal transition and tumor heterogeneity expression variability. However, recent studies have reported a considerable size overlap between CTCs and leucocytes, with a probable missing of a portion of CTCs [43].
In general, it is reasonable to comment that actually all CTCs separation strategies will miss some cancer cells, and a particular challenge will be in this context to answer the question of whether the cells collected are the ones that can seed new tumors.
Circulating-free DNA
Circulating cell-free DNA (cfDNA) was first described in 1948 by Mandel and Metais [44]. Despite this early finding, it has been clarified only 30 years later that cancer patients have extremely higher levels of circulating cfDNA as compared with healthy individuals as a consequence of the tumor growth and it has taken more than 20 years to corroborate evidence that cfDNA correlates with tumor stage and are frequently elevated in patients with metastatic cancers [45–49]. ctDNA represents only a small portion (0–10%) of total cfDNA and data regarding the origin, mechanism and rate of release into the blood are contradictory. The main hypothesis is that along the increment of tumor volume, an increased cell turnover occurs, enhancing apoptosis and cell necrosis processes, leads the accumulation of cellular debris within the tumoral mass, and DNA cross-over and releases into the circulation [50]. Thus, tumor DNA shedding essentially seems to be a passive phenomenon. It is a shared opinion that the apoptotic process inside primary tumor mass represents the most important source of the fraction of ctDNA in circulation. Such hypothesis is supported by the observation that the major part of circulating ctDNA discloses fragments that are multiples of 180–185 base pairs in length, typical of the apoptotic process [51].
The amount of ctDNA is influenced by tumor progression, turnover of tumor and tumor size, as well as clearance, degradation, and filtering by the blood and lymphatic circulation. Additionally, half-life could be different depending on mechanism of release [52].
The main advantage of ctDNA is that its extraction from blood is easier than CTCs isolation and requires no specific instrumental facilities. Analysis from bio-banked biofluids, such as frozen plasma, can be assessed. The most common published extraction methods for ctDNA are the commercially available spin column extraction kits [53]. Other reported methods of extraction include magnetic beads, phenol/chloroform extraction and alkaline salting [54–56]. The efficiency of ctDNA extraction remains technically challenging because tumor-derived ctDNA levels are often extremely low and can directly impact the outcome of mutation detection. In addition, methods to accurately determine the quantity and quality of total cfDNA are crucial to reliably compare and normalize the cfDNA for its use as a biomarker [57]. Moreover, it can be taken into account the instability of white blood cells after collection that breaks down post-blood draw, leading to an increase in total cfDNA with a consequent dilution of ctDNA fraction and more difficulties for mutation detection and decrease in sensitivity [58–62]. Current recommendations for assessing cfDNA include: plasma rather than serum samples, use of ethylenediaminetetraacetic acid or cfDNA collection tubes with processing within 4 h, double centrifugation and no more than three freeze–thaw cycles of plasma specimens [60].
Circulating ctDNA-based non-invasive methods show many potential clinical applications in oncology and can be used to detect and monitor specific and predictive biomarkers that are recommended for the proper treatment of cancer patients according to the molecular characterization of the specific cancer (Figure 2). Up to now, a lot of retrospective studies have indicated that detection of driver gene mutations in ctDNA of patients with NSCLC is feasible and reliable [63–66] and can represent a good predictor of clinical response with relevant implications for patient management. In particular for advanced NSCLCs, detection of EGFR L858R mutations in cfDNA can be performed using several platforms with a high sensitivity [67]. An additional recent study from Marchetti et al. provides the first strong correlation between the EGFR copy number mutation detection in the first days of treatment and clinical response to TKI treatments with relevant implications for NSCLCs management [68].
All these positive results close to many evidences of a match between ctDNA molecular profile and tumor site, led to the approval for the use of ctDNA analysis for EGFR mutation analysis for gefitinib in Europe in patients in whom a tumor sample was not evaluable, making it the first EGFR-TKI inhibitor for which ctDNA testing is included in the label [69–71]. cfDNA could represent a useful source for molecular profiling in patients with ALK-positive NSCLC who had tumors that had progressed during treatment with crizotinib. Two different groups of investigators recently reported that the variations in levels of ALK mutations in patients treated with different ALK inhibitors showed a good correlation with the course of disease and offer a valid alternative to serial tumor biopsy to study the evolution of resistance [72,73]. Ubiquitous and heterogeneous somatic mutations could be detected in ctDNA from early-stage NSCLCs by different techniques [74] and can be used to reveal clonal heterogeneity mechanisms and genetic processes of cancer evolution in individual patients.
Epigenetic alterations are a common phenomenon in NSCLC that can also be detected using several techniques in the ctDNA fragments [75,76], with a good agreement with methylation status in tumor itself [77]. The results of studies on methylated gene alterations in ctDNA of NSCLC patients indicated that hypermethylation of multiple genes played important roles in NSCLC pathogenesis and that the methylated genes in ctDNA might be potential candidate epigenetic biomarkers for NSCLC detection [78–82]. However, DNA methylation measurement shows lower specificity than genomic alterations detection, due to the methylation changes often detected in tumor surrounding normal tissues [83]. Here comes the question about the real clinical utility of these determinations in practical management of cancer patients.
Currently, we still lack the effective methods to predict which patients are disease free after surgery from those who have residual disease, depending largely on TNM staging system that stratifies patients by risk of recurrence and possible benefit from adjuvant therapy without addressing if residual tumor is present or not after surgical resection. However, evidences of future possible utility of monitoring ctDNA levels as personalized markers for the adjuvant therapy were provided [84]. Indeed, it has been reported that the amount of ctDNA in the blood of NSCLC patients may change depending on the evolution of the disease after surgery, therefore resected patients who experienced disease recurrence had detectable post-operative levels of ctDNA than patients who remained disease free with completely undetectable postoperative ctDNA levels.
Mutation testing of plasma also offers a minimally invasive option to characterize metastatic and/or resistant disease mechanisms when tissue or re-biopsy is unavailable and offers a feasible way for longitudinal and dynamic monitoring and tracking molecular resistance [85–87]. Clarification of the mechanisms of acquired resistance could help to determine an alternate therapy before clinical resistance happens. T790M can be detected in serial plasma samples from NSCLC patients receiving TKI before and after progression disease as a poor prognostic factor [88]. Moreover, ctDNA has also been applied to explore the novel mechanism of acquired resistance to third-generation EGFR-TKI, thus overcoming the major limitation of tumor re-biopsy. Thress et al. used next-generation sequencing to investigate the potential mechanism in ctDNA from lung cancer patients whose tumor had developed resistance to AZD9291, revealing the EGFR C797S as new mutation resistance in this pharmacological context [89].
Finally, ctDNA assessment can be used to reveal clonal heterogeneity mechanisms and genetic processes of cancer evolution in individual patients. It has been observed, for instance, that the only presence of the T790M mutation may be insufficient to confer EGFR-TKI resistance to tumor cells, suggesting that such mutation does not necessarily confer an EGFR-TKI resistance phenotype of tumor cells [90].
Exosomes
Exosomes are active extracellular membrane-encapsulated vesicles ranging in size from 40 to 140 nanometers in diameter, and contain different types of biological constituents released by all cell types [91–93]. Exosomes are highly heterogeneous [94] and likely reflect the phenotypic state of the cell that generates them. Similarly to cells, exosomes are composed of a lipid bilayer and, at any given point, can contain well known molecular constituents of a cell, including signal proteins and/or peptides, RNA, DNA and lipids [95,96]. The RNA includes miRNAs, mRNAs, and additional structural and non-coding RNAs [97,98]. The precise role of exosomes remains unknown. Early hypotheses suggested that exosomes may function both as cellular trashbags that expel excess and/or non-functional cellular components and extracellular mediators in cell-to-cell signaling by direct activation of surface-expressed ligands on the distant cells they may reach [99]. Owing to the fact that exosomes deliver information both to their close environment and distant organs, they are detectable in many biological fluids including plasma, serum and saliva, thus making them easily accessible for research [100].
Besides their physiological role, exosomes have been demonstrated to increase in quantity and heterogeneity when a pathological insult and condition occur. Indeed, blood of cancer patients is estimated to contain about 4000 trillion heterogeneous exosomes, compared with the 2000 trillion detected in normal individuals [101].
Exosomes were initially isolated from the peripheral blood circulation of cancer patients in 1979 [102] and they have been progressively reported as elevated in the systemic circulation of patients with breast, pancreatic and colon cancer. Experimental evidences support that exosomes play a bimodal role in cancer: they may either manipulate the local and systemic environment allowing cancer growth and dissemination or modulate the immune system to elicit or suppress the antitumor response [103–106], leading to tumor progression and metastases (Figure 2).
In the light of these data, the potential of tumor derived exosomes fraction isolated from the bloodstream of cancer patients as novel biomarkers has grown exponentially in the last few years. In this regard, many methodologies have been used to isolate and analyze exosomes but, to allow exosomes to enter the clinic, technical standardization is of primary importance. Biologic molecules into the exosomes are protected by a lipid bilayer membrane that confers high degree of stability and can be isolated and analyzed quite simply. Moreover, exosomes carry surface markers from the cells of origin, which can be used for enriched strategies, similar to CTCs [107]. Immunoaffinity bead-based capture methods, microfluidic chip methods and antibody-based exosome arrays using both label and label-free detection platforms have successfully been used to identify, separate, sort and enrich exosomes originating from different cell sources [108,109]. Following isolation, a variety of techniques have been employed to purify and detect the harbored biomarkers [110]. Nevertheless, the influence of disparate techniques on the results of downstream extracellular nucleic acid sequencing and profiling remains unclear, raising the need to provide a definition of standardization [111,112].
The first main potential in clinical oncology is based on the fact that exosomes reflect protein expression and DNA mutations of their originating diseased tissue. Moreover, being extremely abundant in plasma of patients, DNA and RNA packaged inside exosomes can easily be used to detect gene amplifications as well as mutations [113]. Whole genome sequencing revealed that exosomes in the serum of cancer patients contained the entire genomic double-stranded DNA, defined as exosomic DNA that is representative for the whole genomic DNA [113]. Additionally, driver mutations associated with tumors were identified in the exosomal DNA. Activated receptors of the EGFR family in NSCLC and cell adhesion molecules such as EpCAM in epithelial tumors can be detected as well [114,115]. A recent study reported by Nilsson et al. also demonstrated that exosomes released by cancer cells are vehicles capable of transferring tumor-derived EML4–ALK rearranged RNA into platelets that can easily be isolated from patients as a possible way for monitoring the patient response to crizotinib throughout the course of treatment [116].
Enriched and specific miRNAs in exosomes may also favor diagnosis and serve to monitor the progression of lung cancer. Exosome miRNA profile accurately reflects the tumor's profile. Overexpression of specific sets of miRNAs has been revealed comparing the miRNA expression profile of lung cancer samples with miRNA-derived circulating exosomes in NSCLCs and showed clinical associations and prognostic and predictive potential values with lung cancer stages [117].
The new proteomic technologies have significantly contributed to better clarify the protein profiling of exosomes. Analysis from cultured cells and body fluids demonstrates that tumor-derived exosomes express many proteins specifically related to the tumor cell proliferation, migration and invasion that can be purpose as prognostic and diagnostic markers [118].
Finally, in addition to the molecular information exosomes may provide for a better understanding of lung cancer pathology and progression, a further interesting application is represented by the possibility to ‘customize’ exosomes by reprogramming their production and manipulating their content to make them vehicles for cancer drugs, thus enhancing the development of novel diagnostic tools for NSCLC cure [119].
Conclusion
Liquid biopsies represent a new generation of biomarkers. While there remain significant obstacles to overcome in the methodologies used to capture, enumerate and molecularly analyze CTCs, cfDNA and exosomes, there is an abuandance of promising data to suppose that liquid biopsy are very promising and include early detection, assessment of molecular heterogeneity of the tumor, monitoring of tumor dynamics, identification of genetic determinants for targeted therapy, evaluation of early treatment response and monitoring of minimal residual disease to assessment of evolution of resistance in real-time. Future implementation of liquid biopsy approach will answer to a real question about the efficiency of these three different non-invasive sources of nucleic acid in presenting a true and actionable picture of patient's care arise in particular in the area of personalized medicine
Future perspective
The majority of patients treated with targeted therapies develop resistance, metastasis or recurrence. The most important prerequisite to perform molecular analysis is the acquisition of tumor tissue via biopsy at diagnosis and after the occurrence of resistance. To allow personalized medicine and overcoming the limitations of tissue sampling, a more accessible and minimally invasive way is needed.
Three major challenges are ongoing in current management of cancer patients treated with precision therapy.
• Biological material sampling & preservation
Tumor tissue unquestionably represents the gold standard for molecular investigations but some problems exist regarding its obtainment and utility. Biopsies increase the cost of patient care and are yet invasive procedures for patients despite often having no impact on disease outcome. Sampling also remains difficult and resulted in an inadequate amount of tissue for molecular analysis, especially for patients with advanced or metastatic NSCLC. Furthermore, the amount of cancer cells in each sample varies and is largely dependent on the tumor cellularity and size of resected tissue. This is further compounded by small tissue amounts from fine-needle aspirates or core needle biopsies, which often results in smaller amounts of material from molecular investigations if compared with surgically resected tissues. Classical storage of tissues also induces some molecular artifacts. The major part of tumor tissue is preserved in formalin-fixed paraffin-embedded (FFPE) blocks, which crosslink DNA and in some case result in FFPE samples inadequate for molecular analysis. In addition, formalin fixation can cause C>T transitions through deamination of cytosine, potentially leading to false positive results for genetic tests.
• Tumor heterogeneity
In addition to the critical aspects of tissue sampling, the most important limitation of tissue biopsy is probably the molecular heterogeneity of most advanced cancers. Cancers are highly heterogeneous; they show different areas of the same tumor displaying different genetic fingerprints. Similarly, heterogeneity can exist between different metastases of the same patient. Hence, with the biopsy from the primary tumor the loss of molecular information due to tumor heterogeneity is really possible.
• Longitudinal monitoring of cancer patients
The majority of patients treated with targeted therapies ultimately develop resistance, metastasis or recurrence. Longitudinal monitoring of patients with serial tissue sampling is not clinically practical with current invasive tissue biopsy techniques. A minimally invasive way to characterize and follow the molecular profile of the patient is needed, and especially capable of capturing molecular changes cancer cells undergo during the treatment.
• The potential of the blood-based liquid biopsy
Nucleic acid from ctDNA, exosomes and CTCs can provide the same genetic information of the primary tumor and, obviously, the access to the bloodstream has notable advantages. The first is the blood sampling is devoid of potential complications characterizing the biopsies. Liquid biopsy can also go beyond the problem of the cancer molecular heterogeneity because by blood sampling it is possible to collect circulating ctDNA from all patients’ tumors. In addition, it can be collected at any time during the course of the treatment to monitor genetic changes over time if compared with biopsy that only inform of the tumor genotype at the time point (Tables 1 & 2).
Table 1. . Overview and comparison of the main viable analysis on circulating tumor cells, circulating-free DNA and exosomes in lung cancer.
| Research field | Applications | CTCs | cfDNA | Exosomes |
|---|---|---|---|---|
| Mutations | Detection of point mutation, translocations, deletions, amplifications | Yes | Yes | Yes |
| Chromosomal abnormalities | FISH analysis | Yes | No | No |
| Epigenetic modifications | DNA methylation profiling | Yes | Yes | Yes |
| RNA transcription profiles | Determination of mRNA, ncRNA and RNA splice variant level | Yes | No | Yes |
| Protein profiles | Expression, phosphorylation and localization studies | Yes | No | Yes |
| Basic metastasis research | Cell morphology and clonality investigation, analysis in animal models | Yes | No | No |
cfDNA: Circulating-free DNA; CTC: Circulating tumor cell; ncRNA: Non-coding RNA.
Table 2. . The principal key characteristic specific or shared among conventional tissue biopsy, circulating tumor cells, circulating-free DNA and exosomes.
| Key characteristics | Tissue biopsy | CTCs | cfDNA | Exosomes |
|---|---|---|---|---|
| Invasiveness | Yes | No | No | No |
| Availability of the sample throughout the disease process | Re-biopsy needed | Yes | Yes | Yes |
| Equipment | Special instrumentation | Special instrumentation for cell identification | Simple blood collection | Simple |
| Isolation and processing | Complex and time consuming | Complex and time consuming | No isolation required, time saving | Easy-to-perform isolation, time consuming |
| Clinical utility | Early detection; targeted therapy; resistance | Early detection; targeted therapy | Early detection; targeted therapy resistance | Early detection targeted therapy drug delivery |
cfDNA: Circulating-free DNA; CTC: Circulating tumor cell.
• Overcoming the limits of liquid biopsy
The main important limit of the liquid biopsy could be the sensitivity that is limited by the fact that PCR-based methods are collectively capable of detecting rare sequence variants whose abundance is between 1000- and 10,000-fold lower than the most abundant background sequence. Hence, liquid biopsy could reasonably be negative in case of circulating cfDNA lower of such range. The potential of the liquid biopsy in patients’ cancer management is being clearly recognized and now often embedded in the planning of many clinical trials. The correct use of such methodology in a routinely clinical practice will need firstly a strong standardization of standardized workflow of analysis, including blood sampling, storage, processing and DNA/CTC extraction and quantification. Moreover, validation in large patients cohorts are demanded.
The values and limitations of CTCs and ctDNA tests might have different meaning during treatment and the usefulness of serial tests for lung cancer surveillance has to be still clarified. Data from large-scale, multi-institutional studies are needed to answer a real question about the efficiency of ‘liquid biopsy’ in presenting a true and actionable picture of lung patient's care in the area of personalized medicine.
Acknowledgements
The authors would like to thank A Rossi for the critical review of the manuscript, B Pasculli for the review of the ‘Exosomes section’ and A Guarnieri for the English revision.
Footnotes
Financial & competing interests disclosure
This work was supported by the AIRC/MGAF grant 12983 (to LA Muscarella). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ma M, Zhu H, Zhang C, Sun X, Gao X, Chen G. “Liquid biopsy”-ctDNA detection with great potential and challenges. Ann. Transl. Med. 2015;3(16):235. doi: 10.3978/j.issn.2305-5839.2015.09.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawaguchi T, Holland WS, Gumerlock PH. Methods for isolation and genetic analysis of circulating tumor DNA in patient plasma. Methods Mol. Med. 2003;85:257–262. doi: 10.1385/1-59259-380-1:257. [DOI] [PubMed] [Google Scholar]
- 3.Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat. Rev. Cancer. 2014;14(9):623–631. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]; • This report proposes a conceptual framework of circulating tumor cells assays and point out current challenges of circulating tumor cell research, which might structure this dynamic field of translational cancer research.
- 4.Kosaka N. Decoding the secret of cancer by means of extracellular vesicles. J. Clin. Med. 2016;5(2):22. doi: 10.3390/jcm5020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grover PK, Cummins AG, Price TJ, Roberts-Thomson IC, Hardingham JE. Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann. Oncol. 2014;25(8):1506–1516. doi: 10.1093/annonc/mdu018. [DOI] [PubMed] [Google Scholar]
- 7.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 2008;359(4):366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann. Oncol. 2009;20(7):1223–1229. doi: 10.1093/annonc/mdn786. [DOI] [PubMed] [Google Scholar]
- 9.Stott SL, Lee RJ, Nagrath S, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci. Transl. Med. 2010;2(25):25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 2012;7(2):306–315. doi: 10.1097/JTO.0b013e31823c5c16. [DOI] [PubMed] [Google Scholar]
- 11.Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjensvoll K, Nordgard O, Smaaland R. Circulating tumor cells in pancreatic cancer patients: methods of detection and clinical implications. Int. J. Cancer. 2014;134(1):1–8. doi: 10.1002/ijc.28134. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan JP, Nahed BV, Madden MW, et al. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov. 2014;4(11):1299–1309. doi: 10.1158/2159-8290.CD-14-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo X, Mitra D, Sullivan RJ, et al. Isolation and molecular characterization of circulating melanoma cells. Cell Rep. 2014;7(3):645–653. doi: 10.1016/j.celrep.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comen E, Norton L. Self-seeding in cancer. Recent Results Cancer Res. 2012;195:13–23. doi: 10.1007/978-3-642-28160-0_2. [DOI] [PubMed] [Google Scholar]
- 16.Comen E, Norton L, Massague J. Clinical implications of cancer self-seeding. Nat. Rev. Clin. Oncol. 2011;8(6):369–377. doi: 10.1038/nrclinonc.2011.64. [DOI] [PubMed] [Google Scholar]; •• The self-seeding model was proposed in this article to answer to many of the mysteries inherent to cancer metastasis. Reframing our understanding of metastasis within the self-seeding model offers new opportunities for prevention and cure of metastatic cancer.
- 17.Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011;29(12):1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 18.Normanno N, Rossi A, Morabito A, et al. Prognostic value of circulating tumor cells’ reduction in patients with extensive small-cell lung cancer. Lung Cancer. 2014;85(2):314–319. doi: 10.1016/j.lungcan.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Igawa S, Gohda K, Fukui T, et al. Circulating tumor cells as a prognostic factor in patients with small cell lung cancer. Oncol. Lett. 2014;7(5):1469–1473. doi: 10.3892/ol.2014.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton G, Rath B, Holzer S, Hochmair M. Second-line therapy for small cell lung cancer: exploring the potential role of circulating tumor cells. Transl. Lung Cancer Res. 2016;5(1):71–77. doi: 10.3978/j.issn.2218-6751.2015.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorsey JF, Kao GD, MacArthur KM, et al. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: pilot study results. Cancer. 2015;121(1):139–149. doi: 10.1002/cncr.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin. Cancer Res. 2011;17(4):827–835. doi: 10.1158/1078-0432.CCR-10-0445. [DOI] [PubMed] [Google Scholar]
- 23.Bayarri-Lara C, Ortega FG, Cueto Ladron De Guevara A, et al. Circulating tumor cells identify early recurrence in patients with non-small cell lung cancer undergoing radical resection. PLoS ONE. 2016;11(2):e0148659. doi: 10.1371/journal.pone.0148659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofman V, Ilie M, Long E, et al. Detection of circulating tumor cells from lung cancer patients in the era of targeted therapy: promises, drawbacks and pitfalls. Curr. Mol. Med. 2014;14(4):440–456. doi: 10.2174/1566524014666140414205455. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Guan Y, Sun Y, Ai D, Guo Q. Tumor heterogeneity and circulating tumor cells. Cancer Lett. 2016;374(2):216–223. doi: 10.1016/j.canlet.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Marchetti A, Del Grammastro M, Felicioni L, et al. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS ONE. 2014;9(8):e103883. doi: 10.1371/journal.pone.0103883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Punnoose EA, Atwal S, Liu W, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a Phase II clinical trial of pertuzumab and erlotinib. Clin. Cancer Res. 2012;18(8):2391–2401. doi: 10.1158/1078-0432.CCR-11-3148. [DOI] [PubMed] [Google Scholar]
- 28.Sawada T, Watanabe M, Fujimura Y, et al. Sensitive cytometry based system for enumeration, capture and analysis of gene mutations of circulating tumor cells. Cancer Sci. 2016;107(3):307–314. doi: 10.1111/cas.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hata AN, Niederst MJ, Archibald HL, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat. Med. 2016;22(3):262–269. doi: 10.1038/nm.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Acquired resistance caused by the EGFR T790M gatekeeper mutation in lung cancer can occur either by selection of pre-existing EGFR T790M-positive clones or via genetic evolution of initially EGFR T790M-negative drug-tolerant cells. This provides evidence that clinically relevant drug-resistant cancer cells can both pre-exist and evolve from drug-tolerant cells and they point to therapeutic opportunities to prevent or overcome resistance in the clinic.
- 30.Douillard JY, Ostoros G, Cobo M, et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J. Thorac. Oncol. 2014;9(9):1345–1353. doi: 10.1097/JTO.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pailler E, Adam J, Barthelemy A, et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J. Clin. Oncol. 2013;31(18):2273–2281. doi: 10.1200/JCO.2012.44.5932. [DOI] [PubMed] [Google Scholar]
- 32.Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J. Cell Sci. 2009;122(Pt 18):3203–3208. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- 33.Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115(17):3427–3436. doi: 10.1182/blood-2009-10-247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berezovskaya O, Schimmer AD, Glinskii AB, et al. Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Res. 2005;65(6):2378–2386. doi: 10.1158/0008-5472.CAN-04-2649. [DOI] [PubMed] [Google Scholar]
- 35.Luzzi KJ, MacDonald IC, Schmidt EE, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998;153(3):865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glinsky VV, Glinsky GV, Glinskii OV, et al. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63(13):3805–3811. [PubMed] [Google Scholar]
- 37.Im JH, Fu W, Wang H, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004;64(23):8613–8619. doi: 10.1158/0008-5472.CAN-04-2078. [DOI] [PubMed] [Google Scholar]
- 38.Gabriel MT, Calleja LR, Chalopin A, Ory B, Heymann D. Circulating tumor cells: a review of non-EpCAM-based approaches for cell enrichment and isolation. Clin. Chem. 2016;62(4):571–581. doi: 10.1373/clinchem.2015.249706. [DOI] [PubMed] [Google Scholar]
- 39.Naito T, Tanaka F, Ono A, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J. Thorac. Oncol. 2012;7(3):512–519. doi: 10.1097/JTO.0b013e31823f125d. [DOI] [PubMed] [Google Scholar]
- 40.Scheel C, Weinberg RA. Cancer stem cells and epithelial–mesenchymal transition: concepts and molecular links. Semin. Cancer Biol. 2012;22(5–6):396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karabacak NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 2014;9(3):694–710. doi: 10.1038/nprot.2014.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin HK, Zheng S, Williams AJ, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin. Cancer Res. 2010;16(20):5011–5018. doi: 10.1158/1078-0432.CCR-10-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hofman V, Long E, Ilie M, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology. 2012;23(1):30–38. doi: 10.1111/j.1365-2303.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- 44.Ozkumur E, Shah AM, Ciciliano JC, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Transl. Med. 2013;5(179):179ra147. doi: 10.1126/scitranslmed.3005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandel P, Metais P. [Les acides nucléiques du plasma sanguin chez l'homme] C. R. Seances Soc. Biol. Fil. 1948;142(3–4):241–243. [PubMed] [Google Scholar]
- 46.Diehl F, Li M, Dressman D, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl Acad. Sci. USA. 2005;102(45):16368–16373. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J. Clin. Oncol. 2009;27(12):2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 48.Alix-Panabieres C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu. Rev. Med. 2012;63:199–215. doi: 10.1146/annurev-med-062310-094219. [DOI] [PubMed] [Google Scholar]
- 49.Perkins G, Yap TA, Pope L, et al. Multi-purpose utility of circulating plasma DNA testing in patients with advanced cancers. PLoS ONE. 2012;7(11):e47020. doi: 10.1371/journal.pone.0047020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Figg WD, 2nd, Reid J. Monitor tumor burden with circulating tumor DNA. Cancer Biol. Ther. 2013;14(8):697–698. doi: 10.4161/cbt.25361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diaz LA, Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J. Clin. Oncol. 2014;32(6):579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Recent advances in the sensitivity and accuracy of DNA analysis have allowed for genotyping of cell-free DNA for somatic genomic alterations found in tumors. The ability to detect and quantify tumor mutations has proven effective in tracking tumor dynamics in real-time as well as serving as a liquid biopsy that can be used for a variety of clinical and investigational applications not previously possible.
- 52.Mouliere F, Robert B, Arnau Peyrotte E, et al. High fragmentation characterizes tumour-derived circulating DNA. PLoS ONE. 2011;6(9):e23418. doi: 10.1371/journal.pone.0023418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mauger F, Dulary C, Daviaud C, Deleuze JF, Tost J. Comprehensive evaluation of methods to isolate, quantify, and characterize circulating cell-free DNA from small volumes of plasma. Anal. Bioanal. Chem. 2015;407(22):6873–6878. doi: 10.1007/s00216-015-8846-4. [DOI] [PubMed] [Google Scholar]
- 54.Benesova L, Belsanova B, Suchanek S, et al. Mutation-based detection and monitoring of cell-free tumor DNA in peripheral blood of cancer patients. Anal. Biochem. 2013;433(2):227–234. doi: 10.1016/j.ab.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 55.Diehl F, Li M, Dressman D, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl Acad. Sci. USA. 2005;102(45):16368–16373. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xue X, Teare MD, Holen I, Zhu YM, Woll PJ. Optimizing the yield and utility of circulating cell-free DNA from plasma and serum. Clin. Chim. Acta. 2009;404(2):100–104. doi: 10.1016/j.cca.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 57.Fong SL, Zhang JT, Lim CK, Eu KW, Liu Y. Comparison of 7 methods for extracting cell-free DNA from serum samples of colorectal cancer patients. Clin. Chem. 2009;55(3):587–589. doi: 10.1373/clinchem.2008.110122. [DOI] [PubMed] [Google Scholar]
- 58.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer. 2011;11(6):426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 59.Norton SE, Luna KK, Lechner JM, Qin J, Fernando MR. A new blood collection device minimizes cellular DNA release during sample storage and shipping when compared to a standard device. J. Clin. Lab. Anal. 2013;27(4):305–311. doi: 10.1002/jcla.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sherwood JL, Corcoran C, Brown H, Sharpe AD, Musilova M, Kohlmann A. Optimised pre-analytical methods improve KRAS mutation detection in circulating tumour DNA (ctDNA) from patients with non-small cell lung cancer (NSCLC) PLoS ONE. 2016;11(2):e0150197. doi: 10.1371/journal.pone.0150197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Messaoudi S, Rolet F, Mouliere F, Thierry AR. Circulating cell free DNA: preanalytical considerations. Clin. Chim. Acta. 2013;424:222–230. doi: 10.1016/j.cca.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 62.Norton SE, Lechner JM, Williams T, Fernando MR. A stabilizing reagent prevents cell-free DNA contamination by cellular DNA in plasma during blood sample storage and shipping as determined by digital PCR. Clin. Biochem. 2013;46(15):1561–1565. doi: 10.1016/j.clinbiochem.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014;6(224):224ra224. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brevet M, Johnson ML, Azzoli CG, Ladanyi M. Detection of EGFR mutations in plasma DNA from lung cancer patients by mass spectrometry genotyping is predictive of tumor EGFR status and response to EGFR inhibitors. Lung Cancer. 2011;73(1):96–102. doi: 10.1016/j.lungcan.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goto K, Ichinose Y, Ohe Y, et al. Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a Phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J. Thorac. Oncol. 2012;7(1):115–121. doi: 10.1097/JTO.0b013e3182307f98. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura T, Sueoka-Aragane N, Iwanaga K, et al. Application of a highly sensitive detection system for epidermal growth factor receptor mutations in plasma DNA. J. Thorac. Oncol. 2012;7(9):1369–1381. doi: 10.1097/JTO.0b013e31825f2821. [DOI] [PubMed] [Google Scholar]
- 67.Chen YM, Fan WC, Tseng PC, et al. Plasma epidermal growth factor receptor mutation analysis and possible clinical applications in pulmonary adenocarcinoma patients treated with erlotinib. Oncol. Lett. 2012;3(3):713–717. doi: 10.3892/ol.2011.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer. 2015;90(3):509–515. doi: 10.1016/j.lungcan.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Marchetti A, Palma JF, Felicioni L, et al. Early prediction of response to tyrosine kinase inhibitors by quantification of EGFR mutations in plasma of NSCLC patients. J. Thorac. Oncol. 2015;10(10):1437–1443. doi: 10.1097/JTO.0000000000000643. [DOI] [PubMed] [Google Scholar]; •• This is the first study showing a strong correlation between the EGFR copy number mutations in the first days of treatment and clinical response with relevant implications for patient management.
- 70.Fenizia F, De Luca A, Pasquale R, et al. EGFR mutations in lung cancer: from tissue testing to liquid biopsy. Future Oncol. 2015;11(11):1611–1623. doi: 10.2217/fon.15.23. [DOI] [PubMed] [Google Scholar]
- 71.Luo J, Shen L, Zheng D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis. Sci. Rep. 2014;4:6269. doi: 10.1038/srep06269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bordi P, Del Re M, Tiseo M. Crizotinib resensitization by compound mutation. N. Engl. J. Med. 2016;374(18):1790. doi: 10.1056/NEJMc1601366. [DOI] [PubMed] [Google Scholar]
- 73.Liang W, He Q, Chen Y, et al. Metastatic EML4–ALK fusion detected by circulating DNA genotyping in an EGFR-mutated NSCLC patient and successful management by adding ALK inhibitors: a case report. BMC Cancer. 2016;16:62. doi: 10.1186/s12885-016-2088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jamal-Hanjani M, Wilson GA, Horswell S, et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann. Oncol. 2016;27(5):862–867. doi: 10.1093/annonc/mdw037. [DOI] [PubMed] [Google Scholar]
- 75.Begum S, Brait M, Dasgupta S, et al. An epigenetic marker panel for detection of lung cancer using cell-free serum DNA. Clin. Cancer Res. 2011;17(13):4494–4503. doi: 10.1158/1078-0432.CCR-10-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wielscher M, Vierlinger K, Kegler U, Ziesche R, Gsur A, Weinhausel A. Diagnostic performance of plasma DNA methylation profiles in lung cancer, pulmonary fibrosis and COPD. EBioMedicine. 2015;2(8):927–934. doi: 10.1016/j.ebiom.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y, Wang R, Song H, et al. Methylation of multiple genes as a candidate biomarker in non-small cell lung cancer. Cancer Lett. 2011;303(1):21–28. doi: 10.1016/j.canlet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 78.Nie K, Jia Y, Zhang X. Cell-free circulating tumor DNA in plasma/serum of non-small cell lung cancer. Tumour Biol. 2015;36(1):7–19. doi: 10.1007/s13277-014-2758-3. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Wang R, Song H, et al. Methylation of multiple genes as a candidate biomarker in non-small cell lung cancer. Cancer Lett. 2011;303(1):21–28. doi: 10.1016/j.canlet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 80.Lee SM, Park JY, Kim DS. Methylation of TMEFF2 gene in tissue and serum DNA from patients with non-small cell lung cancer. Mol. Cells. 2012;34(2):171–176. doi: 10.1007/s10059-012-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang YW, Miao YF, Yi J, Geng J, Wang R, Chen LB. Transcriptional inactivation of secreted frizzled-related protein 1 by promoter hypermethylation as a potential biomarker for non-small cell lung cancer. Neoplasma. 2010;57(3):228–233. doi: 10.4149/neo_2010_03_228. [DOI] [PubMed] [Google Scholar]
- 82.Hoffmann AC, Vallbohmer D, Prenzel K, et al. Methylated DAPK and APC promoter DNA detection in peripheral blood is significantly associated with apparent residual tumor and outcome. J. Cancer Res. Clin. Oncol. 2009;135(9):1231–1237. doi: 10.1007/s00432-009-0564-x. [DOI] [PubMed] [Google Scholar]
- 83.Li M, Chen WD, Papadopoulos N, et al. Sensitive digital quantification of DNA methylation in clinical samples. Nat. Biotechnol. 2009;27(9):858–863. doi: 10.1038/nbt.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The methyl-BEAMing technology was developed to enable absolute quantification of the number of methylated molecules in a sample and was applied to detect methylation in DNA from plasma.
- 84.Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin. Chem. 2015;61(1):112–123. doi: 10.1373/clinchem.2014.222679. [DOI] [PubMed] [Google Scholar]
- 85.Murtaza M, Dawson SJ, Tsui DW, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497(7447):108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 86.Stewart EL, Tan SZ, Liu G, Tsao MS. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations – a review. Transl. Lung Cancer Res. 2015;4(1):67–81. doi: 10.3978/j.issn.2218-6751.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Denis MG, Vallee A, Theoleyre S. EGFR T790M resistance mutation in non small-cell lung carcinoma. Clin. Chim. Acta. 2015;444:81–85. doi: 10.1016/j.cca.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 88.Zheng D, Ye X, Zhang MZ, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci. Rep. 2016;6:20913. doi: 10.1038/srep20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 2015;21(6):560–562. doi: 10.1038/nm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The cell-free plasma DNA collected from subjects with advanced lung cancer whose tumors had developed resistance to the EGFR-tyrosine kinase inhibitor AZD9291 was studied. A new acquired EGFR C797S mutation was identified as new mechanism of acquired resistance to AZD9291.
- 90.Imamura F, Uchida J, Kukita Y, et al. Monitoring of treatment responses and clonal evolution of tumor cells by circulating tumor DNA of heterogeneous mutant EGFR genes in lung cancer. Lung Cancer. 2016;94:68–73. doi: 10.1016/j.lungcan.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 91.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 92.Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. (Berl.) 2013;91(4):431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review highlights the functional relevance of exosomes in cancer, as related to tumor microenvironment, tumor immunology, angiogenesis and metastasis.
- 94.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl Acad. Sci. USA. 2016;113(8):E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 96.Balaj L, Lessard R, Dai L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nolte-'t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, T Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40(18):9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 100.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20(9):1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 101.De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. doi: 10.3389/fimmu.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weidle UH, Birzele F, Kollmorgen G, Ruger R. Molecular basis of lung tropism of metastasis. Cancer Genomics Proteomics. 2016;13(2):129–139. [PubMed] [Google Scholar]
- 104.Qin X, Xu H, Gong W, Deng W. The tumor cytosol miRNAs, fluid miRNAs, and exosome miRNAs in lung cancer. Front. Oncol. 2015;4:357. doi: 10.3389/fonc.2014.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang HG, Zhuang X, Sun D, Liu Y, Xiang X, Grizzle WE. Exosomes and immune surveillance of neoplastic lesions: a review. Biotech. Histochem. 2012;87(3):161–168. doi: 10.3109/10520291003659042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am. J. Pathol. 2014;184(1):28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He M, Crow J, Roth M, Zeng Y, Godwin AK. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab. Chip. 2014;14(19):3773–3780. doi: 10.1039/c4lc00662c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jakobsen KR, Paulsen BS, Baek R, Varming K, Sorensen BS, Jorgensen MM. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J. Extracell. Vesicles. 2015;4:26659. doi: 10.3402/jev.v4.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jorgensen M, Baek R, Pedersen S, Sondergaard EK, Kristensen SR, Varming K. Extracellular vesicle (EV) array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J. Extracell. Vesicles. 2013;18:2. doi: 10.3402/jev.v2i0.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen C, Skog J, Hsu CH, et al. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab. Chip. 2010;10(4):505–511. doi: 10.1039/b916199f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Witwer KW. Circulating microRNA biomarker studies: pitfalls and potential solutions. Clin. Chem. 2015;61(1):56–63. doi: 10.1373/clinchem.2014.221341. [DOI] [PubMed] [Google Scholar]
- 112.Eitan E, Zhang S, Witwer Kw, Mattson MP. Extracellular vesicle-depleted fetal bovine and human sera have reduced capacity to support cell growth. J. Extracell. Vesicles. 2015;4:26373. doi: 10.3402/jev.v4.26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thakur BK, Zhang H, Becker A, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Balaj L, Lessard R, Dai L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thakur BK, Zhang H, Becker A, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nilsson RJ, Karachaliou N, Berenguer J, et al. Rearranged EML4–ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget. 2016;7(1):1066–1075. doi: 10.18632/oncotarget.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin. Lung Cancer. 2009;10(1):42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 118.Sharma A, Khatun Z, Shiras A. Tumor exosomes: cellular postmen of cancer diagnosis and personalized therapy. Nanomedicine (Lond.) 2016;11(4):421–437. doi: 10.2217/nnm.15.210. [DOI] [PubMed] [Google Scholar]; • This review provides an updated information in exosomes isolation strategies, presence of exosomes biomarkers and importance of tumor-derived exosomes in gauging tumor heterogeneity for their potential use in cancer diagnosis, therapy.
- 119.Fontana S, Saieva L, Taverna S, Alessandro R. Contribution of proteomics to understanding the role of tumor-derived exosomes in cancer progression: state of the art and new perspectives. Proteomics. 2013;13(10–11):1581–1594. doi: 10.1002/pmic.201200398. [DOI] [PubMed] [Google Scholar]