Abstract
Lung cancer is the leading cause of cancer-related deaths in worldwide, and NSCLC represents around 85% of all lung cancers. Squamous cell lung cancer (SqCLC) is the second most common subtype and it is challenging to treat. New options have been discovered but progresses are still limited for the lack of ‘druggable’ mutations. Specific resources for SqCLC are limited and this condition affects treatment and outcomes. This paper describes available and emerging therapeutic options and resources that may help patients to face their disease. We have also performed a monocentric survey collecting information about smoking habit and sense of guilty and analyzed the possibility for patients to find helpful sources for their disease. The results suggest that more materials focused on SqCLC are still needed.
Keywords: : squamous cell lung cancer, therapeutic options, unmet needs
Practice points.
Squamous cell lung cancer (SqCLC) is challenging to treat according to patients and disease characteristics.
SqCLC is strongly associated to smoking habit and this increases the stigma toward patients affected by this disease.
Immunotherapy has changed the treatment of squamous cell cancer after many years with no relevant changes.
Despite the identification of specific molecular alterations, progress in targeting oncogenic drivers still runs behind adenocarcinoma.
There are not enough resources exclusively dedicated to patients with SqCLC.
Background
Lung cancer is the leading cause of cancer death worldwide [1] and NSCLC accounts approximately 80–85% of cases, with the largest part of patients diagnosed in advanced stage of disease. In this setting, the treatment has a palliative intent aiming to control symptoms and prolong survival [2]. Squamous cell lung cancer (SqCLC) is the second most common subtype of NSCLC, representing approximately a third of all cases; it is a challenging subtype of cancer to treat as a result of concomitant-specific patient and disease characteristics, including older age that may limit chemotherapy use [3], advanced disease at diagnosis and a higher incidence of comorbidities (chronic obstructive pulmonary disease, cardiovascular disease and diabetes) with poor performance status (PS), that overall makes treatment decisions more complex compared with non-SqCLC. The vast majority of patients with SqCLC are current or former heavy smokers, in contrast, adenocarcinoma is more frequent in former, light or never smokers. The strong association between this disease and smoking contributes to increase negative feelings as stigma, shame, guilt and in turn, the illness burden [4]. Several clinical studies showed that shame and guilt negatively influence patient's lives. Berterö et al. showed that thoughts of death, shame and guilt reduce quality of life and these feelings led patients to taste a sense of social anguish, with a significant impact on interpersonal interactions [5]. Even after a diagnosis of lung cancer, clinicians should encourage smoking cessation in order to improve pulmonary function, radio-chemotherapy outcomes, response to chemotherapy, efficacy of targeted therapy, quality of life and reduce surgical complications, cancer-related and noncancer-related mortality, cancer recurrence, treatment toxicity, risk of developing second primary cancer (mainly for early stages) and risk of noncancer-related comorbidity [6]. A recent survey of Cancer National Institute found that only 62% of cancer centers routinely provide tobacco educational materials and 20% do not provide tobacco cessation services [7]. In 2017, Capelletto et al. published an Italian multicenter survey that investigated the level of smoking cessation counseling offered to patients by healthcare professionals [8]. From January 2013 to February 2016, 490 patients were evaluated with an anonymous survey developed by Women Against Lung Cancer in Europe (WALCE), a nonprofit organization. The results showed that the majority of patients enrolled (76%) stopped smoking after the diagnosis of a respiratory disease, 17% smoked less and 7% continued smoking. 38% of patients reported to have never received any smoking cessation counseling. Almost 73% reported a positive judgment about the quality of healthcare's intervention, but 83% of patients have stopped smoking overnight without any help. In conclusion, considering all the smoking-related side effects, greater efforts should be made in order to better support patients in smoking cessation.
Tumor biology & clinical presentation
According to the location of the primary site, SqCLC is classified into central (cSqCLC) and peripheral type SqCLC [9]. There have been few debates whether the location of SqCLC may increase any difference in biological features. Recently, a molecular classification of SqCLC, based on gene expression, has been proposed. Patients with SqCLC have been classified into four categories with different survival outcomes and biological behavior: the classical (37%), the basal (21%), the secretory (26%) and the primitive (16%) [10]. Genes related to smoking were overexpressed in classical subtype, genes related to cell adhesion in basal, the secretory subtype was associated with immune signature and the last one with genes involved in proliferation and DNA repair. Traditionally in literature, the cSqCLC has been reported to be the most common type accounting for nearly two-third of cases but it has been noted a trend toward an increase in the occurrence of peripheral type SqCLC. The cSqCLC is centrally located involving the main airways responsible for symptoms and signs such as cough, dyspnea, atelectasis, obstructive pneumonia and hemoptysis. Central tumor necrosis with or without cavitation remains a common radiological finding and SqCLC is still the most common histotype associated with Pancoast syndrome. This syndrome is clinically characterized by severe shoulder pain, weakness and muscle atrophy of the intrinsic muscles of the hand, Claude–Bernard–Horner syndrome (ptosis, miosis, enophthalmos and anhidrosis of the ipsilateral side of face) and upper arm oedema [11]. The standard treatment for this condition has become the combination of induction chemo-radiotherapy followed by surgery [12]. In the last 15 years, therapeutic advances for patients with metastatic NSCLC have been substantially limited to adenocarcinoma, while the treatment of SqCLC remained unchanged. For instance, the discovery of activated mutations in the EGFR, as well as translocation of anaplastic lymphoma kinase, and the introduction of maintenance treatment have changed the treatment of patients with nonsquamous carcinoma. This resulted in a tremendous disparity in the way patients with advanced NSCLC were treated, also considering that outcomes associated with conventional therapies remain poor. However, recent efforts to define the biology of SqCLC, together with the introduction of immunotherapy agents, have begun to change the landscape, including characterization of previously unknown genomic, signaling pathways and delineation of new potentially actionable molecular targets [13]. These alterations often do not appear to be mutually exclusive (in contrast to what frequently described in adenocarcinoma). This will likely complicate the interpretation of efficacy data of treatments directed against single targets.
In this article we are describing treatment's algorithm and future therapeutic strategies for advanced SqCLC and we are assessing the availability of materials that could help patients to increase the treatment adherence and face the disease. With this purpose, we have also performed a monocentric survey to identify and to compare the perspective of both patients with SqCLC and adenocarcinoma regarding the access to information useful to better cope their disease and to identify new resources that can help patients with SqCLC and their relationship with the clinicians. In the same group of patients, we have analyzed the smoking habit and their perception about stigma.
Current treatment for advanced SqCLC
Chemotherapy
The recommended treatment in first line for metastatic or recurrent SqCLC is still chemotherapy in many European countries, even if some changes will occur in the therapeutic algorithm with the introduction of immunotherapy (see below). Patients with good PS 0–1 are candidates to platinum-based doublets combined with third-generation agents. Patients with PS 2 and age <70 or PS 0–2 and age >70 are candidate to carboplatin-based chemotherapy or single agent chemotherapy. Treatment for patients with PS 3–4 is best supportive care (BSC) [14]. Two meta-analyses showed benefits of chemotherapy versus BSC in terms of reduction of death risk, median survival and quality of live irrespective of age, sex, histology and PS [15,16]. In 2004, a meta-analysis reported the survival benefit of two-agent over one-agent chemotherapy regimens and did not show a survival benefit of three-agent over two-agent regimens [17]. Another meta-analysis published in 2006 revealed a statistically significant reduction (equal to 22%) in the risk of death at 1 year for platinum over nonplatinum combinations, without an increase of toxicity. Ardizzoni et al. carried out a further meta-analysis looking at response by histological type in nine trials involving 2968 patients: although the cisplatin-based chemotherapy was superior to the carboplatin-based for nonsquamous cell carcinoma, both treatments were demonstrated to be equally effective for SqCLC [18]. Differences in terms of overall survival (OS) were not showed when six cycles of chemotherapy were compared with four cycles, although a longer progression-free survival (PFS) coupled with significantly higher toxicity was reported in patients receiving the highest number of cycles [19]. In 2008, a prospective Phase III study described for the first time in patients with NSCLC a survival difference based on histologic type. In particular, the combination of cisplatin/pemetrexed, compared with cisplatin/gemcitabine, demonstrated a statically significant increase of OS for patients with adenocarcinoma but not for patients with SqCLC, in which cisplatin/gemcitabine improved OS [20]. Recently, the association of carboplatin/nabpaclitaxel, a microtubular inhibitor conjugated with albumin, was compared with carboplatin/paclitaxel in advanced NSCLC patients [21]. The experimental arm pointed out a significant improvement in response rate (RR) of SqCLC (41 vs 24%; p < 0.001). This improvement was associated with a modest, but statistically nonsignificant improvement in PFS and OS. Based on these results, this combination was approved for the management of advanced NSCLC patients and is preferentially considered in SqCLC. EGFR amplification occurs in 7–10% of SqCLC tumors, overexpression of the EGFR protein is more common in SqCLC than in nonsquamous NSCLC but incidence of activating EGFR mutations is low in SqCLC [22]. Necitumumab is an IgG1 monoclonal antibody against EGFR. The Phase III trial (SQUIRE) evaluated cisplatin (CDDP)/gemcitabine plus necitumumab versus CDDP/gemcitabine in 1903 patients with untreated advanced SqCLC (Table 1) [23]. The experimental arm produced a significant improvement for OS (11.5 vs 9.9; HR: 0.84; 95% CI: 0.74–0.96; p = 0.01) and PFS, with a 1-year survival equal to 48% in the experimental arm versus 43% in the control arm. This improvement was more pronounced in the patient group with EGFR-expressing tumor. Based on these outcomes, necitumumab in combination with cisplatin/gemcitabine was approved by the US FDA as first-line treatment of patients with metastatic SqCLC and by the EMA for advanced EGFR-expressing SqCLC.
Table 1. . Available new agents for the treatment of squamous cell lung cancer.
| Study | Agent | Study (year) | Type of study | Line of treatment | Schedule | Findings |
|---|---|---|---|---|---|---|
| CheckMate 017 | Nivolumab | Brahmer et al. (2015) | Randomized, open-label, Phase III trial | Second line in SqCLC | Nivolumab 3 mg/kg every 14 days versus docetaxel 75 mg/mq every 21 days | Median OS: 9.2 versus 6.0 mo (HR: 0.59; 95% CI: 0.44–0.79; p < 0.001) Median PFS: 3.5 versus 2.8 mo (0.62; 95% CI: 0.47–0.81; p < 0.001) ORR (20% [95% CI: 14–28] vs 9% [95% CI: 5–15]; p = 0.008) |
| KEYNOTE010 | Pembrolizumab | Herbst et al. (2016) | Phase II–III | Second line with PD-L1 expression ≥1% | Pembrolizumab 2 mg/kg every 21 days versus pembrolizumab 10 mg/kg every 21 days versus docetaxel 75 mg/mq every 21 days | ORR: 19.4% Median OS: 10.4 versus 12.7 versus 8.5 mo Median PFS: 3.9 versus 4.0 versus 4.0 mo |
| KEYNOTE024 | Pembrolizumab | Reck et al. (2016) | Randomized, open-label, Phase III trial | First-line NSCLC with PD-L1 expression ≥50% | Pembrolizumab 200 mg every 21 days versus investigator's choice platinum-based chemotherapy | Median PFS 10.3 versus 6.0 mo (HR: 0.50; 95% CI: 0.37–0.68; p < 0.001) OS at 6 mo: 80.2 versus 72.4% (HR: 0.60; 95% CI: 0.41–0.89; p = 0.005) RR: 44.8 versus 27.8% |
| OAK | Atezolizumab | Rittmeyer et al. (2017) | Randomized, open-label, Phase III trial | Second line | Atezolizumab 1200 mg versus docetaxel 75 mg/mq every 21 days | ITT population: median OS: 13.8 versus 9.6 mo (HR: 0.73; 95% CI: 0.62–0.87; p = 0.003) SqCLC median OS (HR: 0.73; 95% CI: 0.59–0.96) versus nonsquamous (HR: 0.73; 95% CI: 0.60–0.89) |
| SQUIRE | Necitumumab | Tatcher et al. (2015) | Randomized, open-label Phase III trial | First line in SqCLC | Gemcitabine 1250 mg/mq days 1–8 plus cisplatin 75 mg/mq day 1 every 21 days plus necitumumab 800 mg days 1–8 versus gemcitabine 1250 mg/mq days 1–8 plus cisplatin 75 mg/mq day 1 every 21 days | Median OS: 11.5 versus 9.9 mo (HR: 0.84; 95% CI: 10.4–12.6; p = 0.01) |
| REVEL | Ramucirumab | Garon et al. (2014) | Randomized, double-blind, placebo-controlled Phase III trial | Second line in SqCLC and non-SqCLC | 10 mg/kg every 21 days versus docetaxel 75 mg/mq every 21 days | Median OS: 10.5 versus 9.1 mo (HR: 0.86; 95% CI: 0.75–0.98; p = 0.023) Median PFS: 4.5 versus 3.0 mo (HR: 0.76, 0.68–0.86; p < 0.0001) |
| Lux-Lung 8 | Afatinib | Soria et al. (2015) | Randomized, open-label Phase III trial | Second line in SqCLC | Afatinib (40 mg daily) or erlotinib (150 mg daily) until disease progression | Median OS: 7.9 versus 6.8 mo (HR: 0.81; 95% CI: 0.69–0.95; p = 0.0427) Median PFS: 2.6 versus 1.9 mo (HR: 0.81; 95% CI: 0.69–0.96; p = 0.0103) |
HR: Hazard ratio; mo: Month; ORR: Objective response rate; OS: Overall survival; PFS: Progression-free survival; RR: Response rate; SqCLC: Squamous cell lung cancer.
Maintenance therapy
Maintenance treatment is used to prolong the effect of first-line chemotherapy. Several studies, which included SqCLC patients, have examined maintenance treatment in patients with advanced NSCLC without progression after first line. Switch maintenance with docetaxel after treatment with carboplatin/gemcitabine compared with BSC followed by docetaxel at progression showed a significant PFS benefit (5.7 vs 2.7; p = 0.0001) and a trend an OS benefit (12.3 vs 9.7; p = 0.09) [24]. The study, that evaluated gemcitabine plus BSC versus BSC alone following cisplatin/gemcitabine treatment, demonstrated a significant improvement in TTP (6.6 vs 5.0; p < 0.001) and a nonsignificant improvement in OS (13.0 vs 11.0; p = 0.195) [25]. The IUNO study, that evaluated erlotinib maintenance versus erlotinib administered at disease progression in patients with advanced NSCLC without EGFR activating mutations, did not showed benefits in terms of OS (9.5 vs 9.7; HR: 1.02; p = 0.82) or PFS (13 vs 12 weeks; HR: 0.94; p = 0.48) [26]. Otherwise, the SATURN study, that evaluated erlotinib maintenance versus placebo in patients with advanced NSCLC, showed significant improvements in PFS (12.3 vs 11.1 weeks; HR: 0.71; p < 0.0001) and OS (12.0 vs 11.0; HR: 0.81; p = 0.0088), with OS benefits also observed in the SqCLC subgroup of patients who had stable disease after first-line chemotherapy (n = 190; HR: 0.67; 95% CI: 0.48–0.92; p = 0.0116) [27].
Immunotherapy
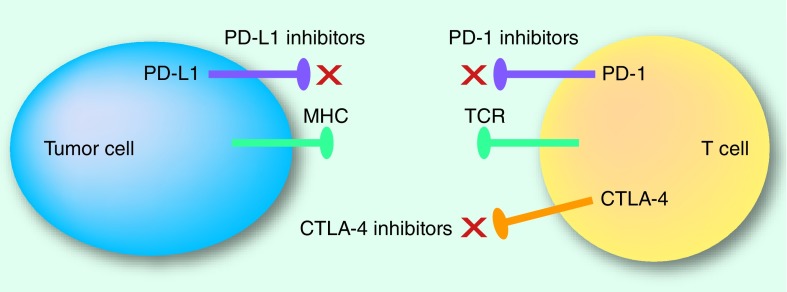
Lung cancer has been historically considered poorly immunogenic, with no established benefit from cytokine modulation or vaccines. In the recent years, immunotherapies have changed the treatment strategy of some types of tumor including NSCLC, in particular with the efficacy of immune checkpoint inhibitors [28]. Immune checkpoints are crucial for maintaining self-tolerance and modulating immune responses. It is now clear that tumors choose immune checkpoint pathways as a major mechanism of immune resistance. The treatment by immune checkpoint inhibitors targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4) and the programmed cell death protein-1 (PD-1) and programmed death-ligand 1 (PD-L1) pathways has led to significant clinical benefit either as monotherapy or in combination therapy. CTLA-4, the first immune checkpoint receptor to be targeted, is a CD28 homolog that is expressed exclusively on T cells. CTLA-4 leads to downregulation of T-cell responses through several mechanisms, including inhibiting IL-2 production and preventing cell cycle progression thereby acting as a negative regulator of T-cell response [29]. The role of PD-1 is limiting the activity of T cells in peripheral tissues at the time of an inflammatory response to infection and to limit autoimmunity [30]. PD-1 expression is induced by T-cells activation (in particular activated T cells, B cells and natural killer cells). PD-L1 is expressed across a range of tumors, including NSCLC. The PD-1/PD-L1 interaction inhibits T-cell response, induces apoptosis of tumor-specific T cells and promotes differentiation of CD4 T cells into Tregs and tumor cell resistance. Although PD-1 antibodies target the PD-1 receptor on activated immune cells, PD-L1 inhibitors block the interaction between PD-L1 and PD-1 and the interaction between PD-L1 and B7.1 (an inhibitory receptor on T cells) (Figure 1).
Figure 1. . Immune checkpoint blockade.
The most critical issue in the field of cancer immunotherapy is whether biomarkers can allow oncologists to better identify patients most likely to respond to these new therapeutic options. It is crucial find ‘enrichment factors’ that can identify responder patients and understand the mechanism of primary and acquired resistance. Unfortunately, current available data are still conflicting. One of the most analyzed factor is PD-L1 that has been evaluated in prospective and retrospective studies. In SqCLC patients, trial CheckMate 017 compared with CheckMate 057 has failed to demonstrate that PD-L1 plays a predictive role in terms of OS and PFS. These findings confirm the differences in the microenvironment between SqCLC versus nonsquamous tumors and the need of define the different role of biomarkers in these two histotypes.
Recent data suggest that tumor mutational burden (TMB), representing the total number of mutations per megabase of genome examined, plays a key role for the response to immunotherapy. In 2017, Chalmers et al. evaluated 100,000 human cancer genome and revealed that lung cancer has a high TMB with approximately 7.5 mutations/megabase compared with other cancer types, such as pediatric tumors or acute leukemia [31]. These results confirm that patients with high TMB better respond to immunotherapy. Furthermore, in 2016, Campbell et al. examined exome sequences and copy number profiles of 660 adenocarcinoma and 484 lung SqCLC tumors in order to identify similarities and differences in lung carcinogenesis and new therapeutic strategies. This study showed that recurrent alterations in SqCLC were more similar to those of other squamous carcinomas than to alterations in lung adenocarcinoma. Particularly, in SqCLC new significantly mutated genes and new amplification peaks included RASA1 and MIR205. Regarding neoantigens, 47% of adenocarcinoma (ADC) and 53% of SqCLC tumors had at least five predicted neoepitopes [32].
Pembrolizumab in first-line treatment
The randomized, Phase III trial (KEYNOTE 024) compared pembrolizumab with investigator's choice of platinum-based chemotherapy in patients with metastatic treatment-naive NSCLC and with a PD-L1 tumor proportion score of 50% or greater [33]. In this study, 154 patients were randomized to pembrolizumab and 151 received the investigator's choice of platinum-based chemotherapy. The results revealed that the 6-month OS was 80.2% with pembrolizumab versus 72.4% with chemotherapy (HR: 0.60; 95% CI: 0.41–0.89; p = 0.005). In addition, the median PFS was 10.3 versus 6 (HR: 0.50; 95% CI: 0.37–0.68; p < 0.001) and ORR was 44.8% with pembrolizumab versus 27.8% with chemotherapy. The median duration of the response was not reached with pembrolizumab (1.9–14.5 months) versus 6.3 (2.1–12.6 months) with chemotherapy. The benefit of pembrolizumab respect to PFS was evident in all subgroups. In particular, in the subgroup of patients with SqCLC, the HR for disease progression or death was 0.35 (0.17–0.71). Based on these outcomes, pembrolizumab was approved for the treatment of patients with metastatic treatment-naive NSCLC whose tumors express PD-L1 on at least 50% of tumor cells.
Second-line treatment for advanced SqCLC
Patients clinically or radiologically progressing after first-line chemotherapy and with PS 0–2 should be offered second-line therapy. Combination chemotherapy regimens failed to show any OS benefit over single-agent treatments [34]. The latest ESMO 2016 guidelines has recommended as therapeutic opportunities for advanced SqCLC patients with PS 0–2: nivolumab, pembrolizumab, docetaxel, docetaxel plus ramucirumab, erlotinib and afatinib. In the USA, NCCN guidelines state nivolumab, pembrolizumab and atezolizumab as the preferred treatments for advanced SqCLC and PS 0–2 based on the improved OS, longer duration of response and fewer adverse events compared with chemotherapy. Patients with PS 3–4 should be treated with BSC.
Nivolumab, pembrolizumab & atezolizumab
Nivolumab was the first PD-1 immune checkpoint inhibitor to be approved in advanced SqCLC after previous platinum-based chemotherapy (Table 1) [35]. The randomized, Phase III trial CheckMate 017 compared nivolumab (3 mg/kq every 14 days) versus docetaxel (75 mg/mq every 21 days) as second-line therapy in 272 patients with advanced or metastatic SqCLC already treated with platinum-based chemotherapy. The experimental arm experienced a 41% OS advantage over the docetaxel arm (9.2 vs 6.0; HR: 0.59; 95% CI: 0.44–0.79; p = 0.00025), significant improvements in PFS (3.5 vs 2.8; HR: 0.62; 95% CI: 0.47–0.81; p < 0.001), higher RR (20 vs 9%; p = 0.008) and a marked increase of 1-year survival (42 vs 24%). PD-L1 expression levels (1, 5 or 10% cutoffs) did not appear prognostic and predictive in terms of OS and PFS. The Phase II/III KEYNOTE-010 trial showed that in 1034 patients with advanced PD-L1-expressing NSCLC after first-line chemotherapy, pembrolizumab 2 and 10 mg/kg increased OS compared with docetaxel in patients with both TPS ≥50% (2 mg/kg: 14.9 vs 8.2; HR: 0.54; 95% CI: 0.38–0.77; p = 0.0002 and 10 mg/kg: 17.3 vs 8.2; HR: 0.50; 95% CI: 0.36–0.70; p < 0.0001) and TPS ≥1% (2 mg/kg: 10.4 vs 8.5; HR: 0.71; 95% CI: 0.58–0.88; p = 0.0008 and 10 mg/kg: 12.7 vs 8.5; HR: 0.61; 95% CI: 0.49–0.75; p < 0.0001) (Table 1) [36]. Atezolizumab is a humanized IgG1 monoclonal antibody targeting PD-L1. The randomized, Phase III trial (OAK study) evaluated atezolizumab versus docetaxel alone in 1225 patients with advanced NSCLC who had progressed during or after platinum-based chemotherapy. Prolonged OS was observed in the atezolizumab arm, regardless of PD-L1 expression levels on tumor cells (TC) or immune-infiltrating tumor cells (IC). OS was only slightly improved in patients with SqCLC receiving atezolizumab versus docetaxel (8.9 vs 7.7; HR: 0.73; 95% CI: 0.54–0.98; p = 0.038); however, there were fewer patients in the squamous group when compared with the nonsquamous group (222 vs 628) (Table 1) [37].
Antibody-targeting VEGF
Angiogenesis plays a key role in the process of tumor growth, cancer dissemination and survival of tumor cells [38]. Therefore, targeting the angiogenesis pathway has been identified as an attractive opportunity. One of the best characterized groups of protein factors includes VEGF and additional signaling molecules and pathways contribute to aberrant blood vessel formation. Bevacizumab, a recombinant humanized monoclonal antibody against VEGF, was approved for the first-line treatment of advanced NSCLC with nonsquamous cell histology in combination with carboplatin and paclitaxel [39]. Despite the efficacy demonstrated by bevacizumab in Phase II and III trials in patients with all NSCLC, clinically significant bleeding events, including major hemoptysis, delayed further evaluation of bevacizumab in patients with SqCLC.
Ramucirumab is a fully human monoclonal antibody that targets VEGFR2, preventing binding of all VEGF ligands. The combination of ramucirumab with docetaxel is a therapeutic option for patients with advanced NSCLC progressed after first-line treatment (Table 1) [40]. The registration study compared docetaxel plus ramucirumab or placebo in 1253 patients (25% with SqCLC) who had progressed during or after first-line chemotherapy. In overall population, experimental arm significantly improved median OS (10.5 vs 9.1; HR: 0.86; 95% CI: 0.75–0.98; p = 0.023) and PFS (HR: 0.76; p < 0.0001) and similar, but nonsignificant OS benefits were observed in the SqCLC subgroup (n = 328; 9.5 vs 8.2; HR: 0.88; 95% CI: 0.69–1.13). In terms of safety, no significant class effects were observed even if an increase of hematological toxicity was described in the combination arm. Nintedanib is an oral angiokinase inhibitor of VEGFR 1–3, FGFR 1–3 and PDGFR-α and -β. The registration study compared docetaxel versus docetaxel/nintedanib in 1314 NSCLC patients, 42% of whom were SqCLC [41]. PFS was significantly longer in the docetaxel plus nintedanib group compared with docetaxel alone (3.4 vs 2.7; HR: 0.79; 95% CI: 0.68–0.92; p = 0.0019). Similar results were noted both in adenocarcinoma and SqCLC patients. However, OS was significantly longer only in the subgroup of patients with adenocarcinoma, and the greatest advantage was observed in those who had progressed within 9 months after first-line chemotherapy (OS: 10.9 vs 7.9; HR: 0.75; 95% CI: 0.60–0.92; p = 0.0073). Based on this trial, FDA approved the combination of nintedanib plus docetaxel as second-line treatment for patients with advanced adenocarcinoma.
EGFR-targeted therapies
Erlotinib, a first generation EGFR TKIs, has demonstrated survival benefit of 2 months versus BSC in unselected patients with advanced NSCLC with progressive disease after one or two lines of treatment and not candidate for chemotherapy [42]. Different Phase III studies have also investigated the role of EGFR TKIs in comparison with docetaxel for the second- or third-line treatment of unselected for EGFR mutations NSCLC patient. The randomized, Phase III trial (TAILOR) comparing docetaxel versus erlotinib in patients with metastatic NSCLC and EGFR wild-type tumors (n = 222), demonstrated the superiority of docetaxel over erlotinib in terms of PFS and OS (8.2 vs 5.4; HR: 0.78; 95% CI: 0.51–1.05; p = 0.10) [43]. The randomized, Phase III trial (DELTA), that evaluated erlotinib versus docetaxel as second- or third-line therapy, demonstrated superior PFS but not OS for docetaxel treatment in wild-type (WT) EGFR [44]. In 2016, FDA modified the indication for erlotinib for treatment of NSCLC to limit use to patients whose tumors have specific EGFR mutations.
Most recently, the Phase III trial Lux-Lung 8 evaluated afatinib versus erlotinib in 795 patients with SqCLC progressing after four cycles of platinum-based chemotherapy. A modest, but significant improvement was observed for afatinib in terms of PFS (HR: 0.81; p = 0.0103) and OS (7.9 vs 6.8; HR: 0.81; 95% CI: 0.69–0.95; p = 0.0077) (Table 1) [45]. In conclusion, according to ESMO guideline, erlotinib still represents a potential second-line treatment option in pretreated patients with unknown or WT EGFR status and preferably in patients not suitable for chemotherapy, with, however, limited efficacy in WT EGFR patients compared with chemotherapy.
Emerging agents
SqCLC displays a somatic mutation rate comparable to that of patients with small cell lung cancer or other smoking-related cancers, different from what described in lung adenocarcinoma in which cancers from nonsmokers harbor one-fifth to one-sixth the genomic alterations of a smoker's cancer [46]. SqCLC is strongly associated to smoking, however, also occurs in a small number of never or light smokers. Several etiologic factors have been proposed to explain the development of lung cancer in never smokers. In recent years, there have been large technological advances in particular with the development of next-generation sequencing (NGS) [47]. NGS can sequence complete genomes, exomes and transcriptomes and identify novel chromosomal rearrangements and copy number alterations. International guidelines strongly advise molecular profiling to identify driver mutations to ensure that patients receive the most appropriate treatment. Based on these perspectives, smokers or light smokers with SqCLC should be characterized by NGS in order to evaluate molecular profile with important clinical implications.
In 2012, the Cancer Genome Atlas profiled 178 untreated SqCLC tumor specimens for genomic alterations by whole exome sequencing and mRNA sequencing. The study found statistically recurrent mutations in 18 genes including: TP53, NFE2L2/KEAP1 in 34%, squamous differentiation genes in 44%, PI3K/AKT in 47% and CDKN2A/RB1 in 72% of tumors with at least one potentially therapeutic target in each of the analyzed specimens [48]. The FGF and FGFR pathway is considered one of the most promising druggable targets in SqCLC. FGFR amplification is found in approximately 10–20% of SqCLC with subsequent activation of PI3K/AKT and RAS/MAPK pathways that stimulate growth and angiogenesis, while mutations are in approximately 0–8% of cases [49]. Several FGFR inhibitors have been evaluated, including dovitinib and AZD4547. In patients with SqCLC, a Phase II trial evaluated dovitinib, a multikinase inhibitor of FGFR 1–3, VEGFR 1–3, PDGFR-β, c-KIT and FLT3, and showed limited antitumor activity and side effects including gastrointestinal toxicity (nausea, diarrhea and anorexia), skin rash and fatigue [50]. AZD4547, a selective inhibitor of FGFR 1–3 and VEGFR2, remains largely under investigation and early phase trials have reported mixed results in terms of efficacy [51]. The PI3KCA pathway is an intracellular signaling involved in the development and progression of advanced lung cancer [48]. The PI3KCA gene amplification and mutations, which are both found predominantly in SqCLC, occur in a range of 35 and 6.5%, respectively [52]. Nowadays, various agents targeting this pathway are in development including dual PI3K/mTOR inhibitors, isoform-specific and pan-isoform PI3KCA inhibitors. DDR2 is a receptor expressed in normal cells and its binding with ligand promotes migration, differentiation, proliferation and survival [53]. DDR2 mutations occur in approximately 1–4% of SqCLC patients and dasatinib, a multityrosine kinase inhibitor that targets BCR-ABL, Src family, c-KIT and PDGFR-β, has emerged as a new therapeutic option. A Phase II trial evaluated the RR of dasatinib at the dose of 140 mg in patients with SqCLC previously treated with first-line chemotherapy. This study showed that dasatinib administered at 140 mg/day was associated with excess adverse events and consequently not recommended. Further studies to identify patients likely to benefit from dasatinib and to manage dasatinib-related toxicities are needed [54].
Other targets with active agents that are under investigation include PDGFR, AKT pathway and MET amplification that has been reported in 6–10% of SqCLC but, until now, several trials have failed to show any results in SqCLC subpopulation. The biomarker-driven Lung Cancer Master Protocol (S1400) (NCT02154490) is Phase II/III study, using a multidrug, targeted screening approach to match patients with substudies testing investigational new treatments based on their unique tumor profiles evaluated by NGS [55]. Patients are then randomized to one of several substudies, each evaluating standard of care versus an experimental targeted therapy, based on identification of candidate predictive biomarkers. Lung Cancer Master Protocol could change the way new drugs in lung cancer are developed.
In the last few years, immunotherapy has changed therapeutic landscape for patients with SqCLC; in order to maximize the benefit of this new approach, the combinations of immune checkpoint inhibitors with other treatment options such as chemotherapy, targeted therapy, radiotherapy and other immunotherapeutic agents are being evaluated. These combinations can obtain higher RRs, more durable responses, and may expand the number of patients treating with benefit. The association of immune checkpoint inhibitors is more effective, but also more toxic than single agent. The efficacy of chemotherapy combined with immunotherapy depends on the drug, and the relative timing of administration. A randomized, Phase II study evaluated the addition of pembrolizumab to carboplatin/pemetrexed for advanced nonsquamous NSCLC, with an ORR of 55% for experimental arm compared with 29% for chemotherapy [56]. Based on this trial, the FDA approved pembrolizumab in combination with pemetrexed and carboplatin for the first-line treatment of metastatic nonsquamous NSCLC, irrespective of PD-L1 expression.
An ongoing randomized, Phase III study is evaluating carboplatin/paclitaxel with or without pembrolizumab in patients with SqCLC and results are pending (NCT02775435). EGFR TKIs have significantly improved clinical outcomes compared with chemotherapy in NSCLC patients with sensitizing EGFR mutation. Almost all patients treated with EGFR TKIs develop acquired resistance during variable time. Preclinical studies have showed that activated mutations increases susceptibility of the lung tumors to PD-1 blockade, suggesting that the combination of PD-1 inhibitors with EGFR TKIs may be a promising therapeutic strategy. The study CheckMate 012 evaluated erlotinib plus nivolumab in 21 EGFR-mutated NSCLC patients (20 patients pretreated with erlotinib and 1 EGFR TKI naive) [57]. The ORR was 19%, PFS at 24 weeks was 51% and OS at 18 months was 64%, but the grade 3 toxicities were reported in 19% of patients. The combination of osimertinib plus durvalumab in pretreated or chemo-naive NSCLC patients showed encouraging clinical activity; however, this combination was associated with high incidence of interstitial lung disease (38%), leading to termination of further enrollment [58]. Considering the high incidence of treatment-related toxicities associated with combination of EGFR TKI and immunotherapy, further development of this approach remains controversial. Radiotherapy is used for local tumor control and palliation of symptoms. The abscopal effect, which is the regression of nonirradiated lesions after the irradiation of an index lesion, is an important observation and reflect secondary activation of antitumor immunity [59].
Coping with diagnosis of SqCLC
There has been an important statistical increase in oncological diseases in recent decades, and the problem of diagnostic information and communication between physician and patient has been at the center of an intense debate in the legal, ethical and psychological fields.
Telling or not telling the ‘truth’ to the cancer patient is an old, still current issue in Italy. On the other hand, the way in which an empathic relationship with the patient is established is certainly an integral part of the attitudes of the physician, certainly not less than the appropriate choice of the chemotherapy protocol and the quality of the supportive therapies ‘logistical-welfare organization’. In oncology, the strong burden of anxiety that tumor illnesses evoke, the persistence of treatments that have important side effects and the increase in survival require a continuity of communication and relationship between physician and patient that provides the person with adequate and personalized information on his/her disease. The condition of patients with advanced SqCLC still remains challenging for several reasons; they too would need to receive specific information to improve their understanding about treatment options, about the impact of the disease and the drugs in the daily life and about the way to face this condition. Although a lot of information is provided on adenocarcinoma and related issues, there are not so many resources dedicated to SqCLC and only recently has information focused on this NSCLC subtype become more available. About 3 years ago, a survey carried out worldwide and highlighted that there is still a severe lack of patient education and awareness of SqCLC, perhaps due to the strong association between this disease and smoking cigarette and the persistent stigma around it. This gap needed to be addressed and in late 2014 a pharma company initiated the SqCLC consortium with the aim to assess the unmet educational needs around this subtype of lung cancer and developing appropriate resources. It has brought together an international, multidisciplinary group of highly experienced clinicians, nurses and patient advocates, who have developed a series of educational initiatives and among the others, a patient booklet that directly addresses patients’ and caregivers’ need for information about SqCLC; it has been intentionally prepared as a printed booklet and has been made available from a range of sources, including patient Advocacy Groups, congress booths and symposia and in PDF format from the IASLC and Advocacy Group (www.iaslc.org/patient-resources/resources). The booklet titled ‘Living with Squamous Cell Lung Cancer’, containing 50 most frequent questions (with responses) and covering all aspects across the SqCLC patient journey was launched during the World Conference on Lung Cancer in December 2016 in Vienna and it is currently distributed in Europe and USA with the help of the local advocacy groups. In this scenario, advocacy groups like WALCE, Lung Cancer Europe, Bonnie J Addario Lung Foundation (ALCF) and LUNGevity that have actively contributed to the development and dissemination of the booklet may play a key role in order to help patients to increase the understanding of their disease and to foster patients to become more active participants in their decision making and care, because changes may happen whether patients understand why action is necessary.
Italian monocentric survey: patients with advanced SqCLC & their perception about available information for their disease comparing to patients with adenocarcinoma
Based on the above-mentioned evidences, we performed a prospective monocentric survey with the aim to investigate the possibility of patients with SqCLC to obtain useful information about their disease compared with patients with adenocarcinoma. Furthermore, we evaluated smoking habit of patients and their perception of stigma and blame. Patients with SqCLC and adenocarcinoma (comparing group) were recruited between April 2017 and May 2017 at a single institution (Thoracic Oncology Division, ‘San Luigi Gonzaga Hospital’, Orbassano, Turin, Italy). They have been assessed for their smoking habit and the level of useful information that has been obtained with available resources. Patients were evaluated before starting treatment with anonymous 20 questions, self-administered and compiled only once during the study. Main questions are listed in Box 1. The entire project was designed and supported by WALCE and approved by local ethical committee. 50 consecutive patients were enrolled in this study. Clinical and social characteristics are reported in Table 2. Totally, 15 patients (30%) have stopped smoking after diagnosis (36% SqCLCs and 25% adenocarcinomas), 14 (28%) before diagnosis (69% SqCLCs and 51% adenocarcinomas), 10 (20%) have continued smoking after diagnosis (23% SqCLCs and 16% adenocarcinomas) and 11 (22%) have never smoked (8% SqCLCs and 33% adenocarcinomas). Totally, 21 patients (42%) have decided to stop by themselves, 8 (16%) have been helped by family and physicians. Totally, 31 patients (62%) have thought that their disease was correlated to smoking habit and most of them (40%) have felt guilty, 26 (52%), 32 (64%) and 27 (54%) patients have denied being judged by their family, physicians and society, respectively. Regarding their disease, 27 (54%) patients have asked to their physicians if other treatments, beyond chemotherapy, were available and most of them were interested about immunotherapy (in particular eight patients with adenocarcinomas and three patients with SqCLCs) and targeted therapy (in particular seven patients with adenocarcinomas and one patient with SqCLC). Totally, 68% of SqCLC and 61% of adenocarcinoma patients have answered that available resources are enough to get information about their disease and the majority of them referred to their physicians they have used booklets or websites and blog/forum, as main sources. In squamous subgroup, 65% would need to have a dedicated materials for their histotype (e.g., a booklet) and 64% of adenocarcinoma patients have answered not to know about this possibility. None of the patients asked for help from advocacy group. Totally, 52% of patients think not to have specific ‘need’, but for 18 patients (36%) the most important need is clinical and 6 patients (12%) needed psychological support. This survey, with the limitation of the small sample of patients enrolled, has not shown great differences between patients with SqCLC and adenocarcinoma, at least in the items examined. Regarding smoking status after the diagnosis of lung cancer, it is important to highlight that patients often do not stop smoking, because they wrongly believe that is too late and not useful for the disease and treatments. Furthermore smoking may help patients to deal with the anxiety for their condition. Regarding the first objective, patients with SqCLC, as adenocarcinoma patients, have answered that available resources for their disease are sufficient and they have considered their physicians as main resource. These data confirm the importance of the relationship between patients and their clinicians. Patients trust in their physicians to provide the most reliable information, and they do not need to consider other sources in order to collect it. It could be intriguing to consider if in a multicentric observation or in other countries the results could be similar to this study. Looking at the data, patients with adenocarcinoma, comparing with SqCLC patients, have requested more information about immunotherapy. This is quite unexpected considering that immunotherapy has been first approved for the squamous setting, but it may be explained by the fact that patients with adenocarcinoma tend to make more questions about their disease and new therapeutic options. Results about secondary objective have evidenced that the majority of patients were former smokers and the percentage of never smokers was greater in the adenocarcinoma subgroup than in the squamous according to the known epidemiological data. Results about guilty and stigma were similar in the two subgroups. This survey has also highlighted that patients have not looked for help from advocacy groups and this can be due to cultural habit, but also to the fact that advocacy groups specifically focusing lung cancer are not so frequent as those for other malignancies (i.e., breast cancer or colorectal cancer), at least in Europe. This survey can offer a partial vision about the binding between SqCLC patients and resources for their disease, but further evaluations are needed to have more details.
Box 1. . Main questions of the anonymous survey.
-
Gender
Male
Female
-
Family status
Married
Unmarried
Separated/divorced
Widow/widower
-
Age (in years)
Up to 39
40–45
46–55
56–65
Above 65
-
Education level
Primary school
Secondary school
High level
Graduation
-
Working conditions
Employee
Self-employed
Unemployed for health reasons
Unemployed for other reasons
Retired
Other
-
Smoking habit
Never smoker
Former smoker (suspended several months/years before diagnosis of lung cancer)
Former smoker (suspended shortly before diagnosis of lung cancer)
Former smoker (suspended after diagnosis of lung cancer)
Still smoker
-
If you stopped smoking after diagnosis, who helped/encouraged you?
Family
Physicians
Family and physicians
Friends
No one, only for my own decision
-
If you are or have been a smoker, have you ever thought that smoking habit has contributed to causing your disease?
Yes
No
-
If you have answered yes to the previous question, have you ever felt guilty for having smoked?
Yes
No
-
As a smoker or former smoker, at the moment of diagnosis have you been judged by your family and/or friends?
Yes
No
-
As a smoker or former smoker, at the moment of diagnosis have you been judged by your physicians and/or nurses?
Yes
No
-
Have you ever felt judged by public information (TV, newspapers, social)?
Yes
No
-
Have you ever asked to your physician or looked for information about therapies beyond chemotherapy?
Yes
No
-
If you have answered yes to previous question, which alternative therapies have you thought?
Tablets (targeted therapy)
-
Immunotherapy
Other
-
Do you think that booklets, website and newspapers are enough to get information about your disease?
Yes
No
-
If you have answered yes to previous question, what tools have you used to get information about your disease and therapeutic options?
Websites
Physicians
Blog or forum
Paper materials (such as booklets)
Patient associations
Other patients
Other
-
If you have answered yes to previous question, on which histology (type) of tumor have you found more information using available tools?
Squamous cell lung cancer
Adenocarcinoma
Both
-
Do you think that it might be useful to have scientific material dedicated only to squamous subtype?
Yes
No
I do not know
-
At the time of diagnosis, have you looked for patient's association to have support and information?
Yes
No
-
Thinking to your disease, do you feel having any specific need?
Medical
Psychological
Social
Table 2. . Clinical characteristics of patients enrolled.
| N (%) | |
|---|---|
| Patients | 50 |
| Gender | |
| Male | 36 (72%) |
| Female | 14 (28%) |
| Age | |
| Up to 39 | 1 (2%) |
| 40–45 | 1 (2%) |
| 46–55 | 5 (10%) |
| 56–65 | 12 (24%) |
| >65 | 31 (62%) |
| Family status | |
| Married | 38 (76%) |
| Unmarried | 4 (8%) |
| Divorced | 4 (8%) |
| Widow/widower | 4 (8%) |
| Educational level | |
| Primary school | 19 (38%) |
| Secondary school | 13 (26%) |
| High level | 11 (22%) |
| Graduation | 9 (14%) |
| Working condition | |
| Employee | 6 (12%) |
| Self-employee | 4 (8%) |
| Unemployed for health reason | 3 (6%) |
| Unemployed for other reason | 1 (2%) |
| Retired | 36 (72%) |
| Other | 0 |
| Histology type | |
| Squamous cell carcinoma | 22 (44%) |
| Adenocarcinoma | 28 (56%) |
| Smoking status | |
| Never smoker | 11 (22%) |
| Former smoker (several years/months before diagnosis) | 11 (22%) |
| Former smoker (shortly before diagnosis) | 3 (6%) |
| Former smoker (after diagnosis) | 15 (30%) |
| Smoker | 10 (20%) |
Conclusion & future perspective
During the last 12 months, new therapeutic options have been introduced in the therapeutic algorithm of SqCLC. Immune checkpoint inhibitors have changed the therapeutic strategy for SqCLC and other options were developed in second line, including ramucirumab and afatinib. However, the lack of biomarkers, that predict which patients could better respond and benefit from each therapy, remains an unresolved issue that future studies could resolved. The situation is still evolving and it is important for patients affected by SqCLC to be aware of this change. For them, it is crucial to refer to reliable sources as their oncologists, and to scientific organizations like IASLC (www.iaslc.org/about-lung-cancer) or advocacy groups like WALCE (www.womenagainstlungcancer.eu/wp-content/uploads/2011/08/living_with_sqclc_-_a_guide_for_patients_nov2016_0.pdf).
Footnotes
Financial & competing interests disclosure
S Novello declared a role as Speaker Bureau for Roche, Boeringer Ingelheim, Eli Lilly, Astra Zeneca, MSD. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.National Cancer Institute; MD, USA: SEER Cancer Statistics Factsheets: lung and bronchus cancer. [Google Scholar]
- 2.Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J. Thorac. Oncol. 2008;3(8):819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CL, Chen JH, Chen KY, et al. Advanced non-small cell lung cancer in the elderly: the impact of age and comorbidities on treatment modalities and patient prognosis. J. Geriatr. Oncol. 2015;6(1):38–45. doi: 10.1016/j.jgo.2014.09.178. [DOI] [PubMed] [Google Scholar]
- 4.Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. BMJ. 2004;328(7454):1470. doi: 10.1136/bmj.38111.639734.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berterö C, Vanhanen M, Appelin G. Receiving a diagnosis of inoperable lung cancer: patients’ perspectives of how it affects their life situation and quality of life. Acta Oncol. 2008;47(5):862–869. doi: 10.1080/02841860701654333. [DOI] [PubMed] [Google Scholar]
- 6.Cataldo JK, Dubey S, Prochaska JJ. Smoking cessation: an integral part of lung cancer treatment. Oncology. 2010;78(5–6):289–301. doi: 10.1159/000319937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balogh EP, Dresler C, Fleury ME, et al. Reducing tobacco-related cancer incidence and mortality: summary of an institute of medicine workshop. Oncologist. 2014;19(1):21–31. doi: 10.1634/theoncologist.2013-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capelletto E, Rapetti SG, Demichelis S, et al. Final data of an Italian multicentric survey about counseling for 2 smoking cessation in patients with diagnosis of a respiratory disease. Clin. Respir. J. 2017 doi: 10.1111/crj.12644. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai H, Asamura H, Watanabe S, Suzuki K, Tsuchiya R. Clinicopathologic characteristics of peripheral squamous cell carcinoma of the lung. Ann. Thorac. Surg. 2004;78(1):222–227. doi: 10.1016/j.athoracsur.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Wilkerson MD, Yin X, Hoadley KA, et al. Lung squamous cell carcinoma mRNA expression subtypes are reproducible, clinically important, and correspond to normal cell types. Clin. Cancer Res. 2010;6(19):4864–4875. doi: 10.1158/1078-0432.CCR-10-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foroulis CN, Zarogoulidis P, Darwiche K, et al. Superior sulcus (Pancoast) tumors: current evidence on diagnosis and radical treatment. J. Thorac. Dis. 2013;5(4):342–358. doi: 10.3978/j.issn.2072-1439.2013.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marulli G, Battistella L, Perissinotto E, et al. Results of surgical resection after induction chemoradiation for Pancoast tumours. Interact. Cardiovasc. Thorac. Surg. 2015;20(6):805–811. doi: 10.1093/icvts/ivv032. [DOI] [PubMed] [Google Scholar]
- 13.Seidel D, Zander T, Heukamp LC, et al. Clinical Lung Cancer Genome Project (CLCGP); Network Genomic Medicine (NGM). A genomics-based classification of human lung tumors. Sci. Transl. Med. 2013;5(209) doi: 10.1126/scitranslmed.3006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016;27(Suppl. 5):v1–v27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 15.Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311(7010):899–909. [PMC free article] [PubMed] [Google Scholar]
- 16.Burdett S, Stephens R, Stewart L, et al. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J. Clin. Oncol. 2008;26(28):4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delbaldo C, Michiels S, Syz N, Soria JC, Le Chevalier T, Pignon JP. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysis. JAMA. 2004;292(4):470–484. doi: 10.1001/jama.292.4.470. [DOI] [PubMed] [Google Scholar]
- 18.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin-versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J. Natl Cancer Inst. 2007;99(11):847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 19.Rossi A, Chiodini P, Sun JM, et al. Six versus fewer planned cycles of first-line platinum-based chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2004;15(11):1254–1262. doi: 10.1016/S1470-2045(14)70402-4. [DOI] [PubMed] [Google Scholar]
- 20.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 21.Socinski MA, Bondarenko I, Kasareva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first line therapy in patients with advanced non-small cell lung cancer: final results of a Phase III trial. J. Clin. Oncol. 2012;30(17):2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 22.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled Phase III trial. Lancet Oncol. 2015;16(7):763–774. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 24.Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J. Clin. Oncol. 2009;27:591–598. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 25.Brodowicz T, Krzakowski M, Zwitter M, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a Phase III trial. Lung Cancer. 2006;52:155–163. doi: 10.1016/j.lungcan.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Cicènas S, Geater SL, Petrov P, et al. Maintenance erlotinib versus erlotinib at disease progression in patients with advanced non-small-cell lung cancer who have not progressed following platinum-based chemotherapy (IUNO study) Lung Cancer. 2016;102:30–37. doi: 10.1016/j.lungcan.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled Phase III study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 28.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 31.Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell JD, Alexandrov A, Kim J, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 2016;48(6):607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 34.Di Maio M, Chiodini P, Georgoulias V, et al. Meta-analysis of single-agent chemotherapy compared with combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer. J. Clin. Oncol. 2009;27(11):1836–1843. doi: 10.1200/JCO.2008.17.5844. [DOI] [PubMed] [Google Scholar]
- 35.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 37.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a Phase III, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):46–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 40.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised Phase III trial. Lancet. 2014;384(9944):665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 41.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a Phase III, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 42.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 43.Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14(10):981–988. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 44.Kawaguchi T, Ando M, Asami K, et al. Randomized Phase III trial of erlotinib versus docetaxel sd second or third-line therapy in patients with advanced non-small-cell-lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA) J. Clin. Oncol. 2014;32:1902–1908. doi: 10.1200/JCO.2013.52.4694. [DOI] [PubMed] [Google Scholar]
- 45.Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled Phase III trial. Lancet Oncol. 2015;16(8):897–907. doi: 10.1016/S1470-2045(15)00006-6. [DOI] [PubMed] [Google Scholar]
- 46.Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150(6):1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat. Rev. Genet. 2010;11:685–696. doi: 10.1038/nrg2841. [DOI] [PubMed] [Google Scholar]
- 48.Hammerman PS, Lawrence MS, Voet D, et al. Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heist RS, Sequist LV, Engelman JA. Genetic changes in squamous cell lung cancer: a review. J. Thorac. Oncol. 2012;7(5):924–933. doi: 10.1097/JTO.0b013e31824cc334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim SH, Sun JM, Choi YL, et al. Efficacy and safety of dovitinib in pretreated patients with advanced squamous non-small cell lung cancer with FGFR1 amplification: a single-arm, Phase II study. Cancer. 2016;122:3024–3031. doi: 10.1002/cncr.30135. [DOI] [PubMed] [Google Scholar]
- 51.Paik PK, Shen R, Ferry D, et al. Phase Ib open-label multicenter study of AZD4547 in patients with advanced squamous cell lung cancers: preliminary antitumor activity and pharmacodynamics data. J. Clin. Oncol. 2014;32(Suppl.) doi: 10.1158/1078-0432.CCR-17-0645. Abstract 8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGowan M, Hoven AS, Lund-Iversen M, et al. PIK3CA mutations as prognostic factor in squamous cell lung carcinoma. Lung Cancer. 2017;103:52–57. doi: 10.1016/j.lungcan.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 53.Leitinger B. Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2 identification of collagen binding sites in DDR2. J. Biol. Chem. 2003;278:16761–16769. doi: 10.1074/jbc.M301370200. [DOI] [PubMed] [Google Scholar]
- 54.Brunner AM, Costa DB, Heist RS, et al. Treatment-related toxicities in a Phase II trial of dasatinib in patients with squamous cell carcinoma of the lung. J. Thorac. Oncol. 2013;8(11):1434–1437. doi: 10.1097/JTO.0b013e3182a47162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.S1400 Lung-MAP: biomarker-targeted second-line therapy in treating patients with recurrent stage IV squamous cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT02154490 NCT02154490.
- 56.Langer CJ, Gadjeel SM, Borgheai, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, Phase II cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antonia SJ, Rivzi NA, Chow LQ, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (Pt-DC) or erlotinib in advanced non small cell lung cancer (NSCLC) J. Thorac. Oncol. 2014;32(5s) Abstr 8113. [Google Scholar]
- 58.Ahn MJ, Yang J, Yu H, et al. 136O: osimertinib combined with durvalumab in EGFR-mutant non small cell lung cancer: results from TATTON Phase Ib trial. J. Thorac. Oncol. 2016;11:S115. [Google Scholar]
- 59.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]