INTRODUCTION
Intrauterine growth restriction (IUGR) is linked to metabolic dysfunction in offspring, but the mediating mechanisms are still under investigation (Barker et al., 1993). IUGR fetuses adapt to their poor intrauterine environment by repartitioning nutrients to organs critical for survival (i.e., brain, heart) at the expense of tissues such as muscle (Yates et al., 2012c). These developmental adaptations help the fetus to survive in utero but have lifelong consequences in offspring; persistent reduction of highly metabolic muscle mass is detrimental to glucose homeostasis (DeFronzo et al., 1981). Glucose metabolism is regulated primarily by insulin, and nutrient depravation is associated with impaired β-cell mass, insulin secretion, and insulin action in the IUGR fetus (Limesand et al., 2006). Moreover, inflammation disrupts insulin action and aids in the development of insulin resistance (Bach et al., 2013). We recently showed that inflammatory cytokines acutely stimulate glucose metabolism despite their antagonistic effects on insulin signaling (Cadaret et al., 2017b). However, we hypothesize that chronic exposure alters responsiveness to cytokines and results in basal cytokine concentrations having a greater inhibitory tone. Furthermore, chronic maternal inflammation may induce fetal inflammatory adaptations that impair muscle growth and metabolism. Therefore, our objective was to determine the effects of sustained maternal inflammation on fetal growth, islet function, and muscle glucose metabolism.
MATERIALS AND METHODS
Animals and Experimental Design
Experiments were approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln, which is accredited by AAALAC International. Timed-mated Polypay ewes were administered saline (control, n = 9) or 0.1 µg/kg BW (i.v.) of bacterial lipopolysaccharide (LPS; n = 7; E. coli O55:B5; Sigma-Aldrich) every third day from 100 to 115 d of gestation (dGA) to produce maternal inflammation-induced IUGR (MI-IUGR) fetuses. At 118 ± 2 dGA, indwelling catheters and a perivascular flow probe were surgically placed in fetal femoral arteries and veins and exteriorized at the ewe’s flank as previously described (Limesand et al., 2007). Maternal and fetal blood samples were taken at 123 dGA and complete blood counts (CBC, HemaTrue Veterinary Chemistry Analyzer, Heska) were performed for total and differential white blood cell (WBC) analysis. Glucose-stimulated insulin secretion (GSIS) and hyperinsulinemic-euglycemic clamp (HEC) studies were performed at 123 and 124 dGA, respectively, and ewes were euthanized at 125 ± 2 dGA. At necropsy, fetal soleus muscles were isolated for ex vivo metabolic studies.
Fetal GSIS Studies
To determine β-cell function, a square-wave hyperglycemic clamp was performed at 123 ± 2 dGA in control (n = 6) and MI-IUGR fetuses (n = 5) as previously described (Yates et al., 2012b). Three baseline fetal blood samples were collected in 5-min intervals, and an intravenous fetal glucose bolus (3,000 mM; 1 mL/kg fetal BW) was then administered followed by continuous variable-rate glucose infusion in order to target steady-state glucose concentrations of ~2.5 mM. After 30 min of steady-state hyperglycemia, three fetal blood samples were collected at 5-min intervals. Blood collected in EDTA syringes was centrifuged (14,000 × g, 2 min, 4 °C) to isolate plasma to determine insulin concentrations via ELISA (Ovine Insulin; Alpco). Blood collected in heparin-coated syringes underwent blood gas, glucose, and lactate analysis using an ABL90 Flex (Radiometer).
Fetal HEC Studies
Hindlimb glucose utilization and oxidation rates were evaluated in control (n = 4) and MI-IUGR (n = 4) fetuses at baseline and during HEC at 124 ± 2 dGA. Fetal lambs were bolused (1 mL, i.v.) with U-[14C]-glucose tracer (18.75 µCi/mL; Perkin-Elmer) followed by infusion at a constant rate (2 mL/kg/h). After 40 min, venous and arterial blood samples were collected simultaneously in heparin-coated syringes at 5-min intervals (four total draws). Fetuses were bolused with glucose (3,000 mM, 2 mL/kg BW) and insulin (500 mU/mL; 0.5 mL/kg BW; Humulin-R, Eli Lilly) followed by infusion (glucose, variable; insulin, 0.5 mL/kg/h). After HEC was achieved for 2 h, venous and arterial blood samples were collected simultaneously in 5-min intervals (four total draws) as described above. Additional venous and arterial blood was collected at each time point to measure glucose oxidation. Blood containing glucose tracer was added to micro-centrifuge tubes containing 2 M HCl suspended inside sealed 20-mL scintillation vial with 1 M NaOH at the bottom. HCl releases CO2 from blood, which is then recaptured by the NaOH in the scintillation vial. After 24-h incubation at room temperature, the centrifuge tube was removed and UltimaGold scintillation fluid (Perkin-Elmer) was added to the scintillation vial. Concentrations of 14CO2 from each blood sample were quantified using a Beckman-Coulter 1900 TA LC counter. Hindlimb glucose utilization rate was calculated as the difference between arterial and venous samples normalized to femoral blood flow rate and hindlimb weight. Glucose oxidation rates were determined from the difference between venous and arterial 14C specific activities normalized to femoral blood flow rate and hindlimb weight. Millimoles of glucose oxidized were calculated from blood 14C using the specific activity of the infused radiolabeled glucose.
Primary Muscle Glucose Metabolism
Skeletal muscle glucose uptake and oxidation was determined in control (n = 8) and MI-IUGR (n = 6) fetuses as previously described (Cadaret et al., 2017b). Briefly, fetal soleus muscle was dissected longitudinally into ~500-mg strips and incubated in Krebs–Henseleit bicarbonate buffer (KHB) containing no additive (basal), insulin (5 mU/mL Humulin-R), or tumor necrosis factor alpha (TNFα) (20 ng/mL; Sigma-Aldrich). For glucose uptake studies, muscle was incubated in treatment-spiked KHB media containing 1 mM [3H]2-deoxyglucose for 20 min. For glucose oxidation studies, muscle strips were incubated in treatment-spiked KHB media containing [14C-U] D-glucose for 2 h, and 14CO2. was captured for 2 h.
Statistical Analysis
All data were analyzed using the Mixed procedure in SAS (SAS Institute, Cary NC) with fetus as the experimental unit. Variables in GSIS, HEC, and ex vivo studies were analyzed for effects due to treatment, period (or incubation condition), and interaction, with period/condition as a repeated variable. For GSIS and HEC studies, samples within each period were averaged for each fetus. Likewise, three technical replications/condition were averaged for each fetus in ex vivo studies. Fetal weights and dGA 123 CBC data were analyzed for effects due to treatment. Data are presented as means ± SEM.
RESULTS
Fetal Weights and CBC
Fetal weights at necropsy were ~22% less (P < 0.05) for MI-IUGR fetuses than for controls. At ~123 dGA, circulating WBC, lymphocytes, monocytes, and granulocytes were increased (P < 0.05) in dams treated with LPS compared to those given saline. However, circulating WBC, lymphocytes, and monocytes were decreased (P < 0.05) in MI-IUGR fetuses compared to controls. Circulating granulocytes were similar in all fetuses.
Fetal Insulin Secretion
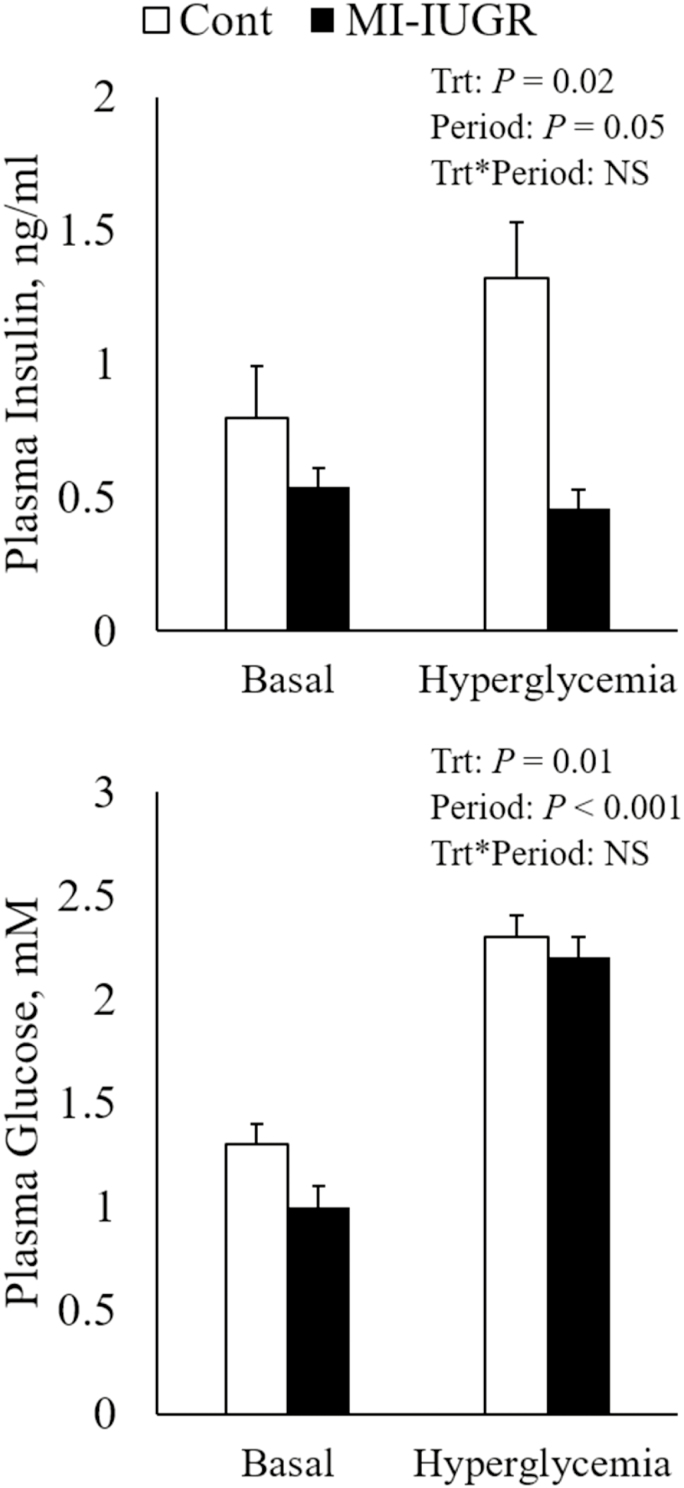
During square-wave hyperglycemic clamps, glucose was increased (P < 0.05) to similar concentrations in all fetuses by design. At baseline, however, plasma glucose concentrations were less (P < 0.05) in MI-IUGR fetuses compared to controls (Figure 1). In response to hyperglycemia, plasma insulin concentrations increased (P < 0.05) from baseline in all fetuses, but concentrations at baseline and hyperglycemia were substantially less (P < 0.05) in MI-IUGR fetuses compared to controls.
Figure 1.
Plasma insulin (A) and glucose (B) concentrations at basal and hyperglycemic states in control (n = 6) and IUGR (n = 5) fetuses during GSIS.
Fetal Hindlimb Glucose Utilization
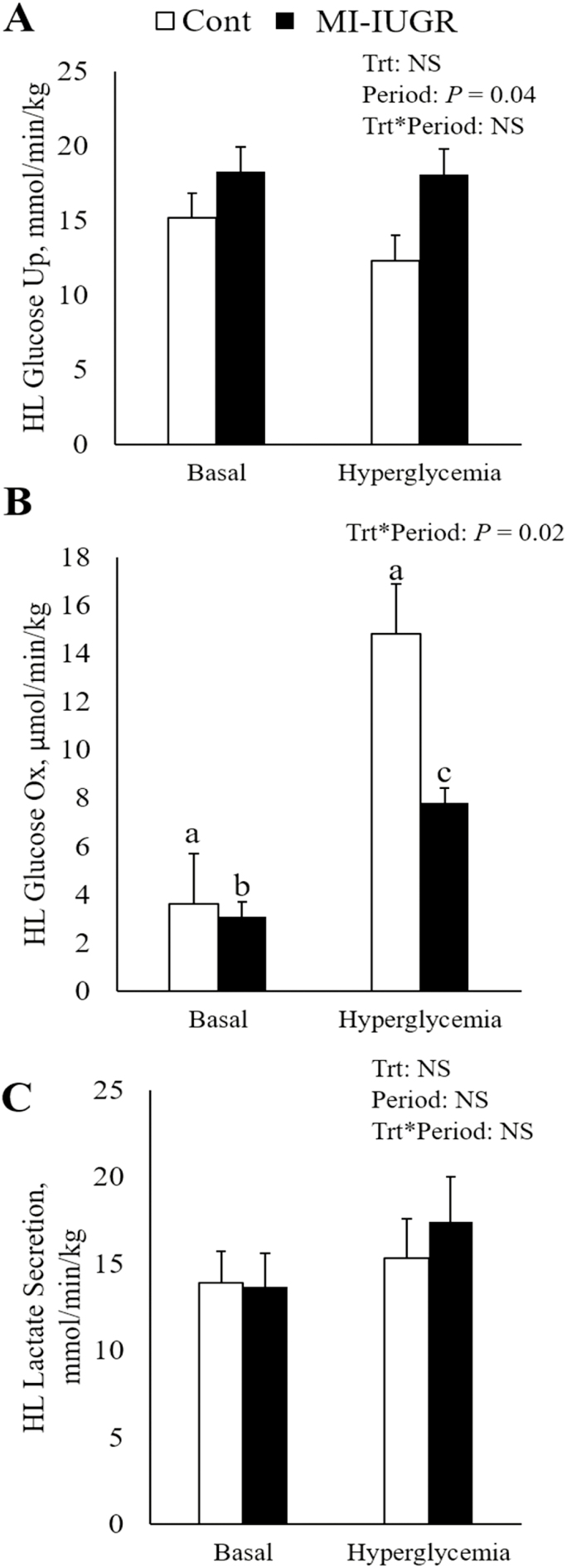
Baseline hindlimb glucose uptake and oxidation rates were similar between MI-IUGR and control fetuses (Figure 2). As plasma insulin was experimentally increased (P < 0.05) between baseline and HEC conditions, hindlimb glucose uptake likewise increased (P < 0.05) to similar rates in all fetuses. Hindlimb glucose oxidation was increased (P < 0.05) by HEC in all fetuses, but the change was substantially less (P < 0.05) in MI-IUGR fetus compared to controls. There were no differences between treatments or periods for hindlimb lactate secretion (Table 1). In response to HEC, arterial pH was increased (P < 0.05) and arterial CO2 tended to be increased (P < 0.10) in all fetuses. Arterial HCO3 content was reduced (P < 0.05) in MI-IUGR fetuses compared to controls regardless of condition.
Figure 2.
In vivo hindlimb specific glucose uptake (A), glucose oxidation (B), and lactate secretion (C) determined under basal and hyperinsulinemic clamp conditions in control (n = 4) and IUGR (n = 4) fetuses. a,b,cMeans with differing superscripts differ (P < 0.05).
Table 1.
Fetal blood analysis during HEC study
| Control | MI-IUGR | P value | |||||
|---|---|---|---|---|---|---|---|
| Basal | HEC | Basal | HEC | Treatment | Period | T * P | |
| n | 4 | 4 | 4 | 4 | |||
| Plasma insulin, ng/mL | 0.37 ± 1.9 | 0.64 ± 1.2 | 15.63 ± 1.9 | 14.39 ± 1.2 | NS | <0.001 | NS |
| Arterial pH | 7.38 ± 0.02 | 7.35 ± 0.01 | 7.33 ± 0.02 | 7.334 ± 0.01 | NS | 0.05 | NS |
| Arterial pCO2, mmHg | 52.9 ± 1.7 | 52 ± 0.5 | 57 ± 1.7 | 53.7 ± 0.5 | NS | 0.06 | NS |
| Arterial HCO3, mM | 30.3 ± 0.5 | 28.6 ± 0.2 | 29.4 ± 0.5 | 27.9 ± 0.3 | <0.001 | NS | NS |
Values are expressed as means ± SE.
Ex Vivo Skeletal Muscle Metabolism
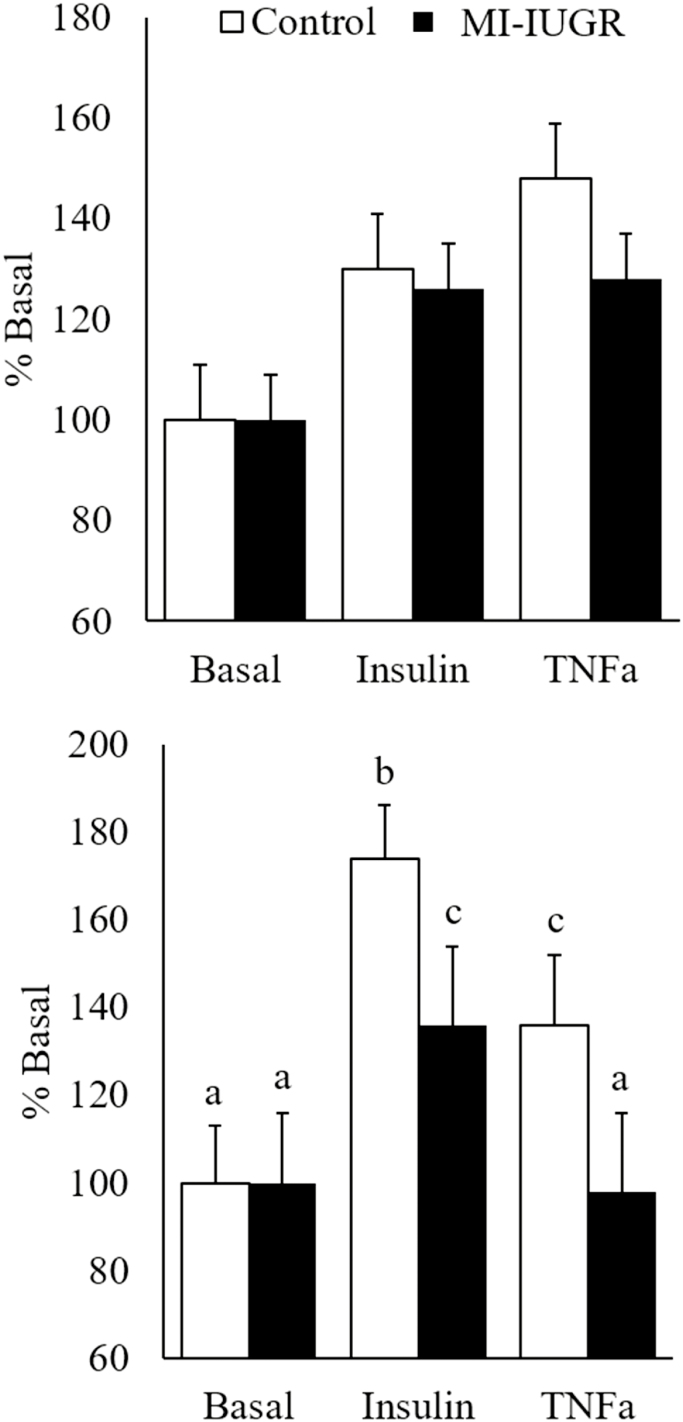
Glucose uptake in primary soleus muscle was similar in all fetuses at each condition (Figure 3). Glucose oxidation rates were similar in all fetuses under basal conditions, but insulin- and TNFα-stimulated glucose oxidation rates were decreased (P < 0.05) in soleus muscle from MI-IUGR fetuses.
Figure 3.
Ex vivo skeletal muscle glucose uptake (A) and oxidation (B) under basal, insulin, and TNFα-stimulated conditions measured in control (n = 8) and IUGR (n = 6) fetuses. a,b,cMeans with differing superscripts differ (P < 0.05).
DISCUSSION
In the present study, chronic maternal inflammation yielded IUGR and muscle-centric adaptations that shift glucose metabolism. Our MI-IUGR fetuses oxidized substantially smaller amounts of glucose within hindlimb tissues with no change in hindlimb glucose uptake. This was due to poor responsiveness to metabolic stimulators, as basal glucose oxidation in primary muscle strips from MI-IUGR fetuses was normal but insulin- and TNFα-stimulated glucose oxidation were decreased. Additionally, MI-IUGR fetuses exhibited poor β cell function that was related to reduced islet responsiveness to glucose. These findings indicate that chronic maternal inflammation restricts fetal growth and produces a metabolic phenotype that mirrors other IUGR models. Thus, inflammation is likely a key part of the adaptive fetal programming that increases the risk for metabolic dysfunction in IUGR-born offspring. We attribute reduced muscle glucose oxidation to a shift toward anaerobic glycolysis (Brown et al., 2015), which produces lactate that can be utilized by crucial organs such as the brain and heart (Yates et al., 2012a). Although circulating lactate was not different in the present study, it is likely that lactate was being cleared by the MI-IUGR liver for gluconeogenesis (Limesand et al., 2007). Impaired β cell function at hyperglycemia but not resting conditions indicates that the disruption is in stimulus-secretion coupling (i.e., the ability of β cells to respond to rising glucose). Previous studies show that other stress factors act similarly (Yates et al., 2012b; Chen et al., 2014). Leukocytes are potent cytokine producers, and reduced circulating WBC counts in MI-IUGR fetuses despite greater counts in ewes indicates a compensatory action by the fetus to moderate the heightened inflammatory environment. Although circulating cytokine concentrations were not determined in these fetuses, we previously observed increased plasma TNFα in rat fetuses well after maternal inflammation has subsided (Cadaret et al., 2017a).
IMPLICATIONS
Chronic maternofetal inflammation resulted in IUGR fetuses with altered muscle metabolic capacity and reduced β cell function. This phenotype is characteristic of low birth weight humans and livestock, and thus our findings indicate that fetal adaptations to chronic inflammation are a major factor in the development of low birthweight pathologies. Furthermore, muscle-centric adaptive fetal programming of inflammatory regulation may be a target for interventions to improve outcomes in low birthweight offspring.
Footnotes
This project is based on research that was partially supported by the National Institute of General Medical Sciences Grant 1P20GM104320 (J. Zempleni, Director), the Nebraska Agricultural Experiment Station with funding from the Hatch Act (NEB-26- 224) and Hatch Multistate Research capacity funding program (NEB-26- 226, NEB-26- 225) through the USDA National Institute of Food and Agriculture.
LITERATURE CITED
- Bach E., Nielsen R. R., Vendelbo M. H., Møller A. B., Jessen N., Buhl M., K-Hafstrøm T., Holm L., Pedersen S. B., Pilegaard H., et al. 2013. Direct effects of TNF-α on local fuel metabolism and cytokine levels in the placebo-controlled, bilaterally infused human leg: increased insulin sensitivity, increased net protein breakdown, and increased IL-6 release. Diabetes 62:4023–4029. doi:10.2337/db13-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J., Hales C. N., Fall C. H., Osmond C., Phipps K., and Clark P. M.. 1993. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36:62–67. [DOI] [PubMed] [Google Scholar]
- Brown L. D., Rozance P. J., Bruce J. L., Friedman J. E., Hay W. W. Jr, and Wesolowski S. R.. 2015. Limited capacity for glucose oxidation in fetal sheep with intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309:R920–R928. doi:10.1152/ajpregu.00197.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadaret C. N., Beede K., Merrick E., Barnes T., Loy J., and Yates D.. 2017a. Maternal inflammation at mid-gestation in pregnant rats impairs fetal muscle growth and development at term. Proc. West. Sect. Am. Soc. Anim. Sci. 68:213–218. [Google Scholar]
- Cadaret C. N., Beede K. A., Riley H. E., and Yates D. T.. 2017b. Acute exposure of primary rat soleus muscle to zilpaterol HCl (β2 adrenergic agonist), TNFα, or IL-6 in culture increases glucose oxidation rates independent of the impact on insulin signaling or glucose uptake. Cytokine 96:107–113. doi:10.1016/j.cyto.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Green A. S., Macko A. R., Yates D. T., Kelly A. C., and Limesand S. W.. 2014. Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep. Am. J. Physiol. Endocrinol. Metab. 306:E58–E64. doi:10.1152/ajpendo.00517.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., and Felber J. P.. 1981. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30:1000–1007. doi:10.2337/diab.30.12.1000 [DOI] [PubMed] [Google Scholar]
- Limesand S. W., Rozance P. J., Smith D., and Hay W. W. Jr. 2007. Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am. J. Physiol. Endocrinol. Metab. 293:E1716–E1725. doi:10.1152/ajpendo.00459.2007 [DOI] [PubMed] [Google Scholar]
- Limesand S. W., Rozance P. J., Zerbe G. O., Hutton J. C., and Hay W. W. Jr. 2006. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147:1488–1497. doi:10.1210/en.2005-0900 [DOI] [PubMed] [Google Scholar]
- Yates D. T., Macko A. R., Chen X., Green A. S., Kelly A. C., Anderson M. J., Fowden A. L., and Limesand S. W.. 2012b. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J. Physiol. 590:5439–5447. doi:10.1113/jphysiol.2012.237347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. T., Macko A. R., Nearing M., Chen X., Rhoads R. P., and Limesand S. W.. 2012a. Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. J. Pregnancy 2012:631038. doi:10.1155/2012/631038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. T., Macko A. R., Nearing M., Chen X., Rhoads R. P., and Limesand S. W.. 2012c. Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. J. Pregnancy 2012:631038. doi:10.1155/2012/631038 [DOI] [PMC free article] [PubMed] [Google Scholar]