Abstract
Objective:
This study assessed the effect of varying prenatal protein levels on the development of homing behavior in rat pups.
Methods:
Long-Evans rats were fed one of the four isocaloric diets containing 6% (n = 7 litters), 12% (n = 9), 18% (n = 9), or 25% (n = 10) casein prior to mating and throughout pregnancy. At birth, litters were fostered to well-nourished control mothers fed a 25% casein diet during pregnancy, and an adequate protein diet (25% casein) was provided to weaning. On postnatal days 5, 7, 9, 11, and 13, homing behaviors, including activity levels, rate of successful returns to the nest quadrant and latencies to reach the nest over a 3-minute test period were recorded from two starting positions in the home cage. Adult body and brain weights were obtained at sacrifice (postnatal day 130 or 200).
Results:
Growth was impaired in pups whose mothers were fed a 6% or, to a lesser extent, a 12% casein diet relative to pups whose mothers were fed the 18 and 25% casein diets. The 6 and 12% prenatal protein levels resulted in lower activity levels, with the greatest reduction on postnatal day 13. However, only the 6% pups had reduced success and higher latencies in reaching the nest quadrant when compared with pups from the three other nutrition groups. Latency in reaching the nest quadrant was significantly and negatively associated with adult brain weight.
Discussion:
Home orientation is a sensitive measure of developmental deficits associated with variations in prenatal protein levels, including levels of protein deficiency that do not lead to overt growth failure.
Keywords: Homing behavior, Malnutrition, Prenatal protein, Protein deficiency, Rats
Introduction
Malnutrition in infancy and early childhood is a major public health problem in many parts of the developing world.1 While the global prevalence of malnutrition has declined over the past 20 years, in 2012, an estimated 25% of children under the age of 5 years had evidence of growth stunting.2 Stunted growth has been associated with adverse effects on learning and school achievement with major implications regarding lifelong earning potential and socioeconomic status.1 Our group has documented adverse effects of early malnutrition on cognitive and behavioral development over the life span, and have documented persisting deficits up to 40 years later, including impaired attention and cognitive functioning in middle adulthood.3,4 Parallel studies in animal models provide evidence that malnutrition during critical periods of early development results in permanent behavioral and brain changes.5 These deficits may continue into subsequent generations even after nutritional rehabilitation has been instituted.6–8 Given the difficulty of isolating the effects of childhood malnutrition independent of other conditions associated with poverty, including crowding, maternal depression, and infectious diseases, animal models have provided important insights into the independent effects of malnutrition on brain and behavioral development.9
Prenatal protein malnutrition has been commonly studied in animal models because of its relevance to the human condition.9,10 These studies have generally employed maternal diets with protein levels ranging between 6 and 25% protein. Maternal diets containing less than 5% protein result in almost complete resorption of the fetus at implantation sites.11 Thus, sustaining pregnancy requires a dietary protein level at or above 5%. Pups born to dams fed 5–6% protein have low birth weights, growth retardation and impaired brain and behavioral development in the postnatal period when compared to pups born to dams fed 18 and 25% protein diets during pregnancy.9,12 Although prenatal diets containing 8–12% protein do not typically result in lower birth weights, neurochemical and behavioral deficits,10 impaired metabolic and immune function,13 and elevated blood pressure have been reported.14 As a result, these intermediate levels of prenatal protein have been referred to as forms of ‘hidden’ malnutrition, because these deficits are present in the absence of significant growth deficits.15,16 However, only a few studies have directly compared the effects of moderate and mild forms of malnutrition.
Homing behaviors, or the ability to locate the nest after being displaced from it, are sensitive measures of early development that have been studied in both cats17 and rodents. In the rat, home orientation has been shown to be disrupted by a wide range of prenatal18–21 and postnatal insults,22–25 including exposures to potentially toxic agents during early development.26 Typically measured early in development and prior to the onset of eye opening, homing behaviors in rodents and other species are believed to be mediated by olfac-tory and thermal cues during the early postnatal period.17,26 After eyes are opened in the developing organism, however, visual cues are dominant,27 though all three sensory systems, olfactory, thermal, and visual, continue to play a role in the spatial search for the maternal odor and/or nest material.23
Homing behaviors have specifically been shown to be impaired following prenatal,28,29 postnatal,7,23,30 and intergenerational6–8 malnutrition. However, to our knowledge, the development of home orientation has not been studied in relationship to varying levels of prenatal protein levels. In this study, we have extended a rat model of prenatal malnutrition that has been extensively tested in our laboratory9,31,32 to evaluate the effects of four levels of prenatal protein in the maternal diet (6, 12, 18, or 25% casein) on the development of homing behaviors in well-nourished offspring during the postnatal period. We measured the ability of pups to initiate and maintain locomotion toward the nest using methods described previously by our group28,29 and similar to those of Fleischer and Turkewitz.33
Methods
All procedures described in this paper were approved by the University of New England Institutional Animal Care and Use Committee (20101005MOK) in accordance with guidelines outlined in the NIH Guide for the Care and Use of Laboratory Animals and the Society for Neuroscience Policies on the Use of Animals and Humans in Neuroscience Research. All personnel involved in collecting behavioral and weight data were blind to nutritional treatment during data collection.
Animals and housing conditions
Viral-free male and female Long-Evans hooded rats (Charles River, Wilmington, MA, USA) were used. All rats were pair housed with same-sexed littermates in polysulfonate microisolator cages (Tecniplast USA Inc., Exton, PA, USA) to control for exposure to microorganisms and given ad libitum access to water and laboratory diets, as specified (Nutritional Treatment). The animal quarters were maintained at a temperature of 22–24°C and a humidity of 40–60%. A reverse day/night light cycle (0700–1900 hour dark) was maintained to accommodate to behavioral observations during the active, waking period of the rat, i.e. during the dark portion of the cycle. During the dark portion of the light cycle, continuous dim illumination was provided by red fluorescent lighting. The health of the colony was routinely monitored using a sentinel system that consisted of taking blood for viral titers and select necropsies from non-experimental animals kept on the same racks as experimental animals.
Mating and nutritional treatment
Beginning 5 weeks prior to mating, virgin females (175–200 g) were randomly assigned to one of the four nutrition groups and provided with ad libitum access to pelleted diets containing 6, 12, 18, and 25% casein (Table 1; Teklad Lab Animal Diets, Harlan Laboratories, Madison, WI, USA), which they continued to receive throughout pregnancy until parturition. All experimental diets were isocaloric (4.2 kcal/g) and were supplemented with 3.7 g/kg L-methionine.
Table 1.
Dietary composition (g/Kg)
| 6% Casein (TD.91175) | 12% Casein (TD.110398) | 18% Casein (TD.110399) | 25% Casein (TD.86500) | |
|---|---|---|---|---|
| Casein | 60 | 120 | 180 | 250 |
| L-Methionine | 3.7 | 3.7 | 3.7 | 3.7 |
| Sucrose | 511.74 | 470.7 | 429.52 | 381.72 |
| Corn starch | 153.52 | 141.22 | 128.86 | 114.52 |
| Corn oil | 151.8 | 151.2 | 150.6 | 150 |
| Cellulose | 67.16 | 61.65 | 56.24 | 50.0 |
| Mineral mix, AIN-76 (170915) | 35.0 | 35.0 | 35.0 | 35.0 |
| Calcium phosphate, dibasic | 6.02 | 4.2 | 2.35 | — |
| Calcium carbonate | 0.73 | 2.0 | 3.4 | 5.06 |
| Vitamin mix, Teklad (40060) | 10.0 | 10.0 | 10.0 | 10.0 |
| Food color | 0.33 | 0.33 | 0.33 | — |
| kcal/g | 4.2 | 4.2 | 4.2 | 4.2 |
One week prior to mating, male rats (325–350 g) were provided with one of the four protein diets (Table 1). To ensure successful fertilization, one male and two females from the same diet group were housed together over a 7-day period. All females were monitored by once-daily vaginal smears to check for the presence of sperm or a vaginal plug. One week prior to the projected date of parturition, pregnant females were placed into individual polysulfone breeding cages, (39.5 × 34.6 × 21.3 cm; Tecniplast USA Inc., Exton, PA, USA) containing 2 cm of bedding (TEK-fresh Laboratory Animal Bedding, Harlan Laboratories, Madison, WI, USA). Litters were left intact for the first 4 days after parturition. Thereafter, half of the bedding was replaced on each testing day, commencing with postnatal day (PND) 5.
Fostering procedure
Beginning on the twenty-first day of pregnancy, cages were checked several times daily for the presence of new litters. At birth (PND 0), the number of pups delivered, sex, and weights were recorded. Litters from all nutrition groups were culled to eight pups (5–6 males and 2–3 females) when possible. In two instances, one 6% and one 18% litter, each containing four males and four females, were also studied. All litters were fostered as whole litters within 24 hours of birth to well-nourished lactating foster mothers from the 25% (control) group that had given birth within the previous 24 hours. At birth, pups were designated as 6/25, 12/25, 18/25, or 25/25 pups (Table 2), indicating both their prenatal (6, 12, 18, or 25% casein) and postnatal (25% casein) diets. Dams were given ad libitum access to the 25% casein diet during the litter period and at weaning (PND 21) pups were transferred to a standard laboratory chow diet containing 23% protein (Purina Mills Inc., Richmond, IN, USA; Formula 5001). As part of a behavioral protocol for an experiment described elsewhere,34 rats were food-restricted after PND 90 to prevent weight gain. They maintained at least 100% of their adult free feed weight until sacrificed at PND 130 or 200.
Table 2.
Experimental design showing protein content of the maternal diet and pup nutrition groups
| Pup nutrition group | Fostered litters n | Maternal diet before mating (5 weeks) (% casein) | Maternal diet during gestation (3 weeks) (% casein) | Maternal diet during litter period (3 weeks) (% casein) |
|---|---|---|---|---|
| 6/25 | 7 | 6 | 6 | 25 |
| 12/25 | 9 | 12 | 12 | 25 |
| 18/25 | 9 | 18 | 18 | 25 |
| 25/25 | 10 | 25 | 25 | 25 |
Growth and development
Pups were weighed at birth (Ohaus Triple beam Balance, Ohaus, Parsippany, NJ, USA) and were then left undisturbed until testing began on PND5. Weights and developmental landmarks (incisor eruption, presence of fur, and eye membrane disjunction) were recorded on PND 5–13.
Homing behaviors
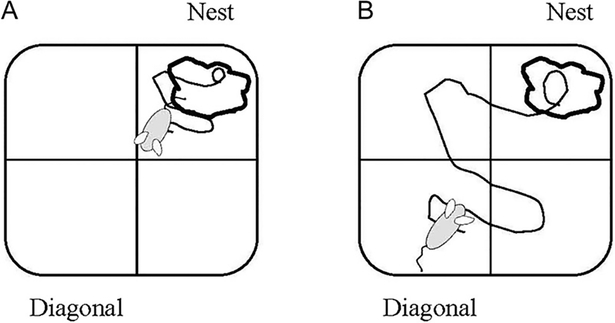
Pups were assessed on a homing test on PND 5, 7, 9, 11, and 13. The homing test, which measured the ability of pups to find the nest when displaced from it, was previously described.28 Briefly, at the beginning of each test session, the dam and pups were removed from the home cage. The dam was confined to a holding cage, and the pups were maintained in a separate cage and kept warm with a heating pad. Homing behaviors were tested in a fresh, clear polycarbonate cage (39.5 × 34.6 × 21.3 cm; Tecniplast USA Inc., Exton, PA, USA) that was placed on a grid, which divided the cage floor into four equal-sized quadrants. Nest materials from the home cage were placed into one of the cage quadrants (nest quadrant) and fresh nesting materials were sprinkled over the remaining three quadrants. Figure 1 illustrates the position of the nest quadrant, diagonal quadrant (diagonal to the nest quadrant), and two adjacent quadrants in the test cage.
Figure 1.
Cage orientation for the homing test. The cage was placed on a drawn grid that sub-divided the cage floor into four equal quadrants: the nest quadrant (where the nest was located), the diagonal quadrant (diagonal from the nest quadrant), and two adjacent quadrants. One pup from each litter was assigned to a start from the nest quadrant (A); a second pup was assigned to start from the diagonal quadrant (B). The pup’s path during a 3-minute test period was traced on a scale drawing of the grid, recording the position of the pup every 30 seconds.
Two male pups were tested per litter, each from a different starting position. Pups were randomly assigned to a start position from either the nest quadrant or the diagonal quadrant. At the beginning of each test session, individual pups were placed into the middle of the assigned quadrant with their heads facing the center of the cage, and their paths were then traced on a scale drawing of the grid during a 3-minute test period, noting their positions on the grid at 30-second intervals. The following outcomes were recorded: (1) activity – the number of quadrants traversed during the test period; (2) homing success – whether the pup was located in the nest quadrant at the end of the test period; and (3) latency (seconds) – time required to reach the nest quadrant for those pups starting from the diagonal quadrant.
Brain weights
Rats tested on the homing test and littermates were weighed and sacrificed by asphyxiation with CO2 at approximately PND 130 (mean ± SD: PND 134 ± 4, range 128–140, n = 24 rats) or at PND 200 (mean PND 199 ± 2, range 195–204, n = 23 rats), for a total of 47 rats derived from 23 litters. Brains were removed promptly and weighted to the nearest 0.01 g using a digital scale (Ohaus Analytic Plus, Ohaus, Parsippany, NJ, USA).
Statistical analyses
Statistical analyses were performed using SAS version9.3.35 For all analyses, the litter was considered the primary unit of analysis. The effect of nutrition group on pup body weights, activity, and latency to reach the nest were analyzed using mixed model regression analyses (MRA), implemented using PROC MIXED in SAS; these analyses include effects of nutrition and age (and start location for activity only), where litter was the repeated measures factor. Activity scores were log-transformed prior to analysis to minimize skewness. Post-hoc pairwise comparisons were undertaken using least-squares means. The day of appearance of developmental landmarks and homing success were compared across nutrition groups using Fisher’s exact tests. Student’s t-tests were used to compare pup weights associated with the presence of specific developmental landmarks. Finally, we examined the relationship between prenatal protein levels, homing behaviors, and adult brain weights using multiple regression analysis (implemented using PROC GLM). This model included nutrition group, age at sacrifice, and homing behaviors (activity, homing success, or latency recorded on PND 13) as independent variables and brain weight as the outcome.
Results
Litter characteristics
To control for potential effects of litter size on homing behaviors,33 only litters with six or more pups on PND 13 were included in the analyses. Three litters (two 6/25 litters and one 12/25 litter) did not meet this criterion and were excluded, leaving 35 litters for the homing study: 6/25 (n = 7; 14 pups tested), 12/25 (n = 9; 18 pups tested), 18/25 (n = 9; 18 pups tested), and 25/25 (n = 10; 20 pups tested) (Table 2). The distribution of male pups was not significantly different by nutrition group on PND 13 [% (95% CI) male: 6/25, 70 (56–81); 12/25, 72 (60–81); 18/25, 60 (54–77); 25/25, 71 (60–80); Chi-square P > 0.1].
Pup and adult weights
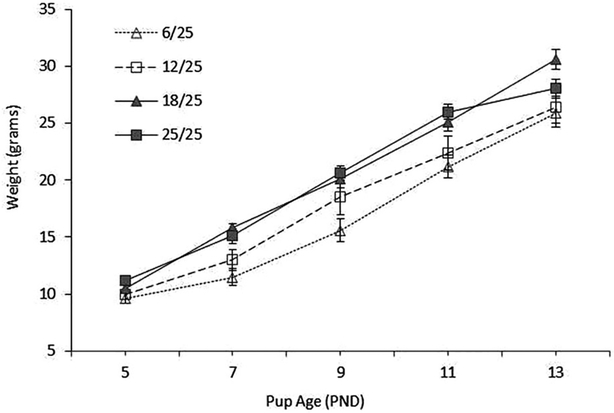
As shown in Fig. 2, pup weights increased over time and were related to the prenatal diet. Across all testing days, the 6/25 pups were consistently lighter than pups from the other nutrition groups, although weight differences became less pronounced over time. Results from the MRA confirmed a significant nutrition × pup age interaction (F(12,107) = 15.0; P < 0.0001) and significant independent effects of nutrition (F(3,31) = 5.2, P < 0.01) and pup age (F(4,107) = 476.7; P < 0.0001) on pup weight. Post-hoc tests confirmed that the weights of the 6/25 pups were significantly lower than those of the 25/25 pups on PND 5, 7, 9, 11, and 13 and lower than 18/25 pup weights on PND 7, 9, 11, and 13 (all P <0.05). Pups in the 12/25 group were intermediate in weight, between the 6/25 and the 18/25 and 25/25 pups. While 12/25 pups did not differ from the 6/25 pups on any day, they weighed significantly less than the 18/25 pups on PND 7 and 13 (P < 0.05). There were no significant differences in weight of 18/25 and 25/25 pups on any postnatal test day (P > 0.05).
Figure 2.
Pup weights (g) by nutrition group on PND 5, 7, 9, 11, and 13. The 6/25 pups were smaller than 18/25 pups (PND 7, 9, 11, and 13, P < 0.05) and 25/25 pups (PND 5, 7, 9, and 11, P < 0.05). The 12/25 pups were intermediate in weight; not different from 6/25 pups but smaller than 18/25 (days 7 and 13, P < 0.05) and 25/25 (days 7 and 11, P < 0.05) pups. The 18/25 and 25/25 pups did not differ in weight on any day tested.
By PND 130–200, there was no difference in weight across the four nutrition groups. A mixed model regression analysis (MRA) for adult body weight showed a significant effect of age (F(1,14) = 11.3, P < 0.01) but no significant effect of nutrition × age or nutrition group.
Developmental landmarks
The mean (mode) ± standard deviation day of appearance of fur was PND 6.3 (6) ± 1.1, incisor eruption was PND 6.6 (6) ± 1.3, and eye opening was PND13.1 (14) ± 0.9, with no significant differences between nutrition groups. Pup weight, however, was significantly associated with the appearance of fur and incisor eruption. On PND 7, pups whose incisors had erupted weighed significantly more than pups whose incisors had not yet erupted (15.0 g ± 0.4 vs.12.1 g ± 0.7; t = −3.6, P < 0.01). A similar relationship with weight was found for fur eruption (data not shown). On PND 13, there was a trend toward a difference in weight in pups whose eyes were open vs. pups whose eyes were closed (30.0 g ± 5.3 vs.27.8 g ± 4.1; t = −1.8, 0.05 < P < 0.01).
Activity level
Pup activity levels varied by start position and age. Multiple regression analyses (start position × age) confirmed that activity levels increased over testing days and were generally higher in pups that started in the diagonal quadrant as compared with pups starting in the nest quadrant (start position × age F(4,101) = 4.5, start position F(1,34) = 20.7, age F(4,110) = 67.7, all P < 0.01). Post-hoc tests showed that activity levels were significantly higher in pups starting from the diagonal quadrant compared with those starting from the nest quadrant on PND 11 and 13 (both P < 0.05). Similar effects of start position and age were present across all nutrition groups.
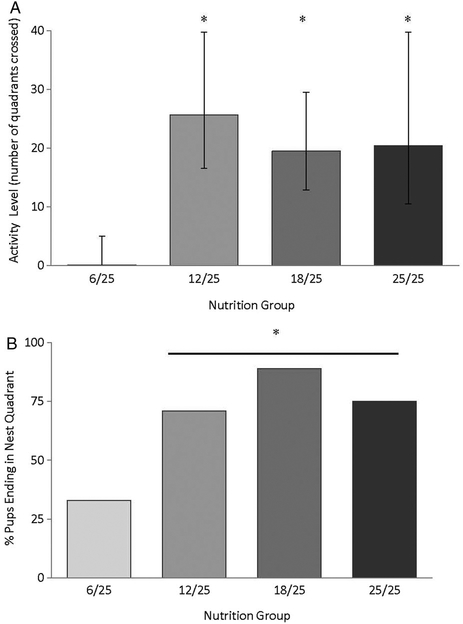
Next, we compared activity levels in pups by nutrition group and starting quadrant. In pups with a starting position in the diagonal quadrant, MRA revealed significant effects of both nutrition (F(3,31) = 3.6, P < 0.05) and age (F(4,95) = 56.8, P < 0.0001) and a trend for a nutrition × age interaction (F(12,95) = 1.8, 0.05 < P < 0.1). Post-hoc tests confirmed that overall activity levels were significantly lower in both 6/25 and 12/25 pups when compared with the 18/25 pups (both P < 0.05). On PND 13, 6/25 pups starting from the diagonal quadrant were found to be less active than pups from the three other groups (all P < 0.05, Fig. 3A), and by this date, the 12/25 pups resembled the well-nourished 18/25 and 25/25 pups. In contrast, activity levels in pups starting from the nest quadrant did not differ by nutrition group and there was no nutrition × age interaction (P > 0.1; data not shown).
Figure 3.
(A) Pup activity levels (quadrants crossed per3 minute) on PND 13 by nutrition group. On PND 13, the 6/25 pups were significantly less active relative to pups from all other nutrition groups (* vs. 6/25, P < 0.05). (B) Percentage of pups with a diagonal start position that ended in the nest quadrant on PND 13 by nutrition group. Pups from the 6/25 group had reduced homing success relative to pups from all other nutrition groups (Fisher’s exact test, P < 0.05).
Homing success
Percent of pups ending in the nest quadrant
The majority of pups that started in the nest quadrant remained in that quadrant across all testing days. Predictably, because most pups that started in the nest quadrant remained there, there were no nutrition group differences on homing success in those pups starting from this quadrant. A different pattern of homing behavior was observed among pups with the diagonal starting position. Initially, on PND 5, none of these pups ended in the nest quadrant during the 3-minute test period. However, homing success in pups with a diagonal start position increased progressively over test days, and by PND 13, 74% of all pups ended in the nest quadrant.
Next, we compared the percentage of pups that started in the diagonal quadrant and ended in the nest quadrant by nutrition group (Fig. 3B). As shown in the figure, pups from the 6/25 group were less likely to reach the nest quadrant than pups from the three other nutrition groups. On PND 13, success rates differed significantly by nutrition group, with only 33% of the 6/25 pups ending in the nest quadrant, in contrast to 71% of 12/25 pups, 89% of 18/25 pups, and 75% percent of 25/25 pups (Fisher’s exact tests, P < 0.05). Thus, although all pups had an increasing preference for the nest quadrant by PND 13, irrespective of prenatal protein diet, we documented significantly fewer returns to the nest quadrant by the 6/25 pups relative to the three other nutrition groups.
Latency to reach the nest quadrant
Latency to reach the nest quadrant from a diagonal start position declined with pup age in the 12/25, 18/25, and 25/25 groups, but this decline in latency was not present in the 6/25 group. MRA across all test days showed a significant nutrition × age interaction (F(12,95) = 7.8, P < 0.001) and a significant age effect (F(4,95) = 38.4, P < 0.001) but no main effect of nutrition group on latency. Post-hoc tests confirmed that PND 13 latencies to reach the nest quadrant were significantly shorter than latencies on PND 5 for 12/25, 18/25, and 25/25 pups (PND 5 vs. PND 13 all P < 0.05), whereas latencies did not significantly differ between PND 5 vs. PND 13 in 6/25 pups (P > 0.1).
Because it was possible that reduced activity levels in the 6/25 pups may have contributed to their greater latencies to reach the nest quadrant, we also analyzed the latency data adjusting for activity level. Results of the adjusted MRA showed a significant effect of activity on latency (F(1,94) = 12.3, P < 0.001). The nutrition and age effects were attenuated, although both the nutrition × age interaction (F(12,94) = 2.5, P < 0.01) and age effect (F(4,94) =3.8, P < 0.01) remained significant. Thus, differences in activity levels did not entirely explain the higher latencies observed in the 6/25 group.
Homing behavior and brain weight
Brain weights were compared across nutrition groups and age at sacrifice (Table 3). MRA confirmed a significant effect of age of sacrifice (F(1,14) = 11.3, P <0.01) on brain weight because of slightly heavier brain weights on PND 200 (2.00 ± 0.02 g) vs. PND 130 (1.92 ± 0.02 g). There was no significant effect of nutrition on brain weight and no nutrition × age interaction (P = 0.3). Next, relationships between brain weights and homing behaviors (with a start position from the diagonal quadrant) at PND 13, when the most striking group differences were present, were examined. MRA, adjusting for age at sacrifice, revealed that latency was significantly and negatively associated with adult brain weight (F(1,41) = 4.45, R = −0.28, P < 0.05). Homing success was also associated with heavier brain weights, although this relationship only approached but did not achieve significance (F(1,41) = 4.03, R = 0.31, 0.1 > P > 0.05). There was no significant association between activity level and adult brain weight, however (P > 0.1).
Table 3.
Mean brain weight (g) ± standard error by nutrition group at PND 130 and PND 200
| 6/25 | 12/25 | 18/25 | 25/25 | |
|---|---|---|---|---|
| n Litters (rats) | 6 (12) | 6 (11) | 6 (12) | 5 (12) |
| PND 130 | 1.93 ± 0.03 | 1.92 ±0.03 | 1.90 ±0.04 | 1.94± 0.02 |
| PND 200 | 2.01 ± 0.02 | 1.94 ±0.04 | 1.98 ±0.07 | 2.05± 0.03 |
Discussion
This study compared four levels of protein intake during the pre-gestational and prenatal periods of the pregnant rat on growth and homing behaviors of pups during the postnatal period. Our main finding was that a 6% protein diet prior to and during gestation resulted in impaired postnatal growth and deficits in home orientation that were most striking on PND 13. A prenatal diet of 12% casein was also associated with impaired growth and a reduction in activity levels, when compared with pups whose mothers were fed 18 or 25% casein during pregnancy. In contrast to the 6/25 group, other homing behaviors were not significantly impacted in the 12/25 pups. Latencies to reach the nest quadrant were significantly and negatively correlated with brain weights at PND 130 and 200 across all nutrition groups.
Home orientation in the postnatal period is thought to reflect an increasing attraction to the nest material and is mediated by olfactory responses to odors from the nest or by thermal cues due to the warmer temperature of the nest materials.17 In this study, pups in all four nutrition groups were able to discriminate between the nest quadrant and the diagonal quadrant, and all pups showed a preference for the nest quadrant from very early ages. This was evidenced by observations that pups from all four nutrition groups tended to remain in the nest quadrant, were less active when starting from the nest quadrant vs. the diagonal quadrant and displayed a progressive decline in the latency to reach the nest quadrant from the diagonal quadrant over the days of testing. Despite this preference for the nest quadrant, however, the 6/25 pups were the least likely of all four nutrition groups to reach the nest quadrant, a deficit that was not fully explained by a decrease in activity levels.
Deficits in homing behavior seen in prenatally mal-nourished 6/25 pups were consistent with findings from our previous papers, addressing the consequences of prenatal protein malnutrition in rodents.28,36,37 The findings were also similar to the effects of postnatal malnutrition secondary to restricting the diets of lactating dams, rearing pups in large litters containing 16 (vs. 8) pups and rotating pups between lactating and non-lactating females.7,30,33,38 Homing deficits were additionally impaired after inter-generational malnutrition, lasting up to two generations after nutritional rehabilitation from birth onwards.6–8 Impaired homing resulting from malnutrition has also been reported in other species, including protein-restricted cats.39 Other nutritional insults, not limited to protein deficiency, e.g. iron deficiency disorders in early life40 and excessive levels of vitamin A supplementation,41 have been reported as contributing to impaired homing behavior in the rodent. Thus, deficits in homing behavior result from a variety of nutritional disorders.
In this study, a maternal diet containing 12% casein resulted in slower growth of the young pups and in significant reductions in postnatal activity levels across all days of testing. Homing behaviors on PND 13 were not impaired relative to the 18/25 or 25/25 pups, however. While a 12% protein diet has been accepted as the minimum requirement to sustain pregnancy in the rat, other studies have also reported that this level of maternal protein intake may impair off-spring development. Langley-Evans and colleagues reported that 12% casein during pregnancy resulted in elevated systolic blood pressure in the offspring at 9 weeks of age in the absence of any growth deficits;14,42 however, behavior was not assessed in their study. Taken together, these results suggest that a prenatal diet of 12% casein may have metabolic or behavioral consequences for the developing rat fetus, and should therefore not be considered as being sufficient to sustain the central nervous system development of the rat fetus during gestation.
Finally, homing behaviors were found to be significantly associated with brain weights in adulthood. Higher latencies to reach the nest quadrant in the post-natal period were correlated with reduced brain weights recorded at PND 130 and 200, with a similar trend for homing success. Although these associations do not confirm a causal relationship, these results warrant further investigation. The absence of nutrition group differences in brain weight was consistent with previous reports showing that prenatal malnutrition impaired postnatal brain growth, but brain size was not reduced in prenatally malnourished animals that were nutritionally rehabilitated.43,44 A relationship between early life insults, activity level, and adult brain weight has been reported in studies of animal models of schizophrenia employing prenatal chemical exposure to methylazoxymethanol acetate or nitric oxide synthase inhibitor (NOS-1)45,46 in the early postnatal period.
While homing behaviors are dependent on olfactory and thermal cues during the early postnatal period,23 specific brain mechanisms underlying the acquisition of homing behaviors are not well understood. Prenatal malnutrition delays the structure and timing of brain development of several systems that are likely critical to the ontogeny of homing behaviors. Our group has reported that prenatal malnutrition significantly alters the pattern of granule cell neurogenesis and the birthdates of cells in the rat hippocampal formation.47 Moreover, in adult rats, there is an enduring reduction in the number of CA1 neurons in the hippocampus, which may reflect developmental alterations secondary to prenatal malnutrition.48 Although it is not known whether homing behaviors during early development (at PND 13 or earlier) are directly associated with the integrity of the hippocampus, spatial cognition, and firing of spatially tuned cells in the CA1 are present from PND 16 onward in the normal rat.49,50 Thus, one can speculate that malnutrition-related alterations in the CA1 may impede the development of spatial processes and homing behaviors in the rat.
The potential role of cholinergic innervation in the development of homing behaviors has also been considered.51 However, a reduction in cholinergic inner-vation at birth by selective lesions of cholinergic fibers in basal forebrain nuclei projecting to cortical areas did not impair homing responses or activity levels on PND 5 or 10.51 Cholinergic pathways are not altered in prenatally malnourished rats, but this insult produces defects in the GABAergic52,53 and glutamatergic receptors54 and changes in serotonin, dopa-mine, and tryptophan levels.55–57 The role of these neurotransmitter systems in the homing response is not known.
There are a number of advantages and disadvantages of this study. Because abrupt changes in diet can lead to increased levels of maternal stress, with resulting impact on the developing pups,58,59 an important advantage to this study was the prolonged exposure to the maternal diet beginning 5 weeks prior to mating and continuing through pregnancy. Another advantage of the present study was its developmental approach, documenting homing behaviors on successive postnatal days, which allowed us to test for nutrition × pup age interactions. In contrast, several published studies have limited their observations of homing behavior to a single postnatal day, which may be inadequate.24,25 In the current study, for example, a significant nutrition × pup age effect was present for latency, whereas the PND 13 observations alone did not reveal any significant nutrition group effect.
There are also limitations to this study. Our animal model examined the effects of a prenatal nutritional insult with consequent cross fostering at birth to well-nourished dams. However, cross fostering may have reduced but not fully eliminated changes in maternal behavior associated with variations in protein levels prenatally.60 Even small variations in maternal care can alter DNA methylation and result in behavioral changes later in the offspring at adult ages.61–63 Hence, developmental deficits in brain and behavior may be caused by changes in maternal behavior, as well as by the direct effects of variations in the prenatal protein levels on the pup themselves. Future studies in this series will report on the levels of maternal care using the current experimental design. Finally, because this was a developmental study, it would have been ideal to observe the same animal on different study days. However, to minimize disruption during the litter period, rats were not marked or uniquely identified until after weaning. While rat pups within the same litter should theoretically perform similarly across time, we were unable to document this in the current study.
Conclusions
Prenatal exposure to varying levels of protein during pregnancy resulted in differential effects on physical growth and homing behavior in the pre-weanling rat. Growth and homing behaviors were both impaired in pups whose mothers received a 6% casein diet prior to and during gestation. A 12% casein diet during gestation resulted in slower growth and reduced activity levels, but latency and success in reaching the nest quadrant were not impaired relative to pups in the 18 or 25% casein groups. Finally, higher latencies to reach the nest quadrant were associated with reduced brain weight in adult rats, but the underlying mechanism explaining this association is not known. The results of this study may have important implications for understanding mechanisms underlying developmental deficits in malnourished human populations.
Acknowledgments
This research was supported by NIH/MH 074811 (JRG). All authors confirm that there are no conflicts of interest and no financial arrangements with a company whose product figures in the submitted manuscript. The authors would like to acknowledge the contribution of the late Dr Peter Morgane who was an integral part of this research program.
Footnotes
Contributors None.
Funding None.
Conflicts of interest None.
Ethics approval None.
References
- 1.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet 2007; 369(9555):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNICEF Improving child nutrition: the achievable imperative for global progress: United Nations Children’s Fund; 2013.
- 3.Galler JR, Bryce CP, Zichlin ML, Fitzmaurice G, Eaglesfield GD, Waber DP. Infant malnutrition is associated with persisting attention deficits in middle adulthood. J Nutr 2012;142(4): 788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waber DP, Bryce CP, Fitzmaurice GM, Zichlin ML, McGaughy J, Girard JM, et al. Neuropsychological outcomes at midlife following moderate to severe malnutrition in infancy. Neuropsychology 2014;28(4):530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGaughy JA, Amaral AC, Rushmore RJ, Mokler DJ, Morgane PJ, Rosene DL, et al. Prenatal malnutrition leads to deficits in attentional set shifting and decreases metabolic activity in prefrontal subregions that control executive function. Dev Neurosci 2014;36(6):532–41. [DOI] [PubMed] [Google Scholar]
- 6.Galler JR. Home orientation in nursling rats: the effects of rehabilitation following intergenerational malnutrition. Dev Psychobiol 1979;12(5):499–508. [DOI] [PubMed] [Google Scholar]
- 7.Galler JR. Home-orienting behavior in rat pups surviving post-natal or intergenerational malnutrition. Dev Psychobiol 1980; 13(6):563–72. [DOI] [PubMed] [Google Scholar]
- 8.Galler JR, Seelig C. Home-orienting behavior in rat pups: the effect of 2 and 3 generations of rehabilitation following intergenerational malnutrition. Dev Psychobiol 1981;14(6):541–8. [DOI] [PubMed] [Google Scholar]
- 9.Galler JR, Shumsky JS, Morgane PJ. Malnutrition and brain development In: Walker WA, Watkins JB, (eds.) Nutrition in pediatrics: basic science and clinical application. 2nd ed. Neuilly-sur-Seine, France: J. B. Decker Europe, Inc.; 1996. p. 196–212. [Google Scholar]
- 10.Morgane PJ, Austin-LaFrance R, Bronzino J, Tonkiss J, Diaz-Cintra S, Cintra L, et al. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev 1993;17(1):91–128. [DOI] [PubMed] [Google Scholar]
- 11.Nelson MM, Evans HM. Relation of dietary protein levels to reproduction in the rat. J Nutr 1953;51(1):71–84. [DOI] [PubMed] [Google Scholar]
- 12.Tonkiss J, Shultz P, Galler JR. An analysis of spatial navigation in prenatally protein malnourished rats. Physiol Behav 1994; 55(2):217–24. [DOI] [PubMed] [Google Scholar]
- 13.Langley SC, Seakins M, Grimble RF, Jackson AA. The acute phase response of adult rats is altered by in utero exposure to maternal low protein diets. J Nutr 1994;124(9):1588–96. [DOI] [PubMed] [Google Scholar]
- 14.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86(2):217–22; discussion 121. [DOI] [PubMed] [Google Scholar]
- 15.Saez-Briones P, Soto-Moyano R, Burgos H, Castillo A, Valladares L, Morgan C, et al. Beta-adrenoceptor stimulation restores frontal cortex plasticity and improves visuospatial performance in hidden-prenatally-malnourished young-adult rats. Neurobiol Learn Mem 2014;pii:S1074–7427(14)00192–0. doi: 10.1016/j.nlm.2014.11.003. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Resnick O, Morgane PJ. Animal models for small-for-gestational-age (sga) neonates and infants-at-risk (iar). Brain Res 1983;312(2):221–5. [DOI] [PubMed] [Google Scholar]
- 17.Freeman NC, Rosenblatt JS. The interrelationship between thermal and olfactory stimulation in the development of home orientation in newborn kittens. Dev Psychobiol 1978;11(5):437–57. [DOI] [PubMed] [Google Scholar]
- 18.Antonelli T, Tomasini MC, Tattoli M, Cassano T, Tanganelli S, Finetti S, et al. Prenatal exposure to the cb1 receptor agonist win 55,212–2 causes learning disruption associated with impaired cortical nmda receptor function and emotional reactivity changes in rat offspring. Cereb Cortex 2005;15(12):2013–20. [DOI] [PubMed] [Google Scholar]
- 19.Pometlova M, Hruba L, Slamberova R, Rokyta R. Cross-fostering effect on postnatal development of rat pups exposed to meth-amphetamine during gestation and preweaning periods. Int J Dev Neurosci 2009;27(2):149–55. [DOI] [PubMed] [Google Scholar]
- 20.Baharnoori M, Bhardwaj SK, Srivastava LK. Neonatal behavioral changes in rats with gestational exposure to lipopolysaccharide: a prenatal infection model for developmental neuropsychiatric disorders. Schizophr Bull 2012;38(3):444–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galler JR, Tonkiss J. The effects of prenatal protein malnutrition and cocaine on the development of the rat. Ann N Y Acad Sci 1998;846(1):29–39. [DOI] [PubMed] [Google Scholar]
- 22.Mikulecka A, Subrt M, Stuchlik A, Kubova H. Consequences of early postnatal benzodiazepines exposure in rats. I. Cognitive-like behavior. Front Behav Neurosci 2014;8:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleischer SF, Turkewitz G, Finklestein H. Sensory influences on homing of stunted rat pups. Dev Psychobiol 1981;14(1):29–39. [DOI] [PubMed] [Google Scholar]
- 24.Stettner GM, Kubin L, Volgin DV. Antagonism of orexin 1 receptors eliminates motor hyperactivity and improves homing response acquisition in juvenile rats exposed to alcohol during early postnatal period. Behav Brain Res 2011;221(1):324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scattoni ML, Puopolo M, Calamandrei G, Ricceri L. Basal fore-brain cholinergic lesions in 7-day-old rats alter ultrasound vocalisations and homing behaviour. Behav Brain Res 2005;161(1): 169–72. [DOI] [PubMed] [Google Scholar]
- 26.Bignami G Economical test methods for developmental neuro-behavioral toxicity. Environ Health Perspect 1996;104(Suppl. 2): 285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenblatt JS, Turkewitz G., Schneirla TC Development of home orientation in newly born kittens. Trans NY Acad Sci 1969;31:231–250. [DOI] [PubMed] [Google Scholar]
- 28.Galler JR, Tonkiss J, Maldonado-Irizarry CS. Prenatal protein malnutrition and home orientation in the rat. Physiol Behav 1994;55(6):993–6. [DOI] [PubMed] [Google Scholar]
- 29.Tonkiss J, Harrison RH, Galler JR. Differential effects of prenatal protein malnutrition and prenatal cocaine on a test of homing behavior in rat pups. Physiol Behav 1996;60(3):1013–18. [DOI] [PubMed] [Google Scholar]
- 30.Altman J, Sudarshan K, Das GD, McCormick N, Barnes D. The influence of nutrition on neural and behavioral development. 3. Development of some motor, particularly loco-motor patterns during infancy. Dev Psychobiol 1971;4(2): 97–114. [DOI] [PubMed] [Google Scholar]
- 31.Tonkiss J, Galler J. Prenatal malnutrition alters diazepam-mediated suppression of ultrasonic vocalizations in an age dependent manner. Behav Brain Res 2007;182(2):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lister JP, Blatt GJ, Kemper TL, Tonkiss J, DeBassio WA, Galler JR, et al. Prenatal protein malnutrition alters the proportion but not numbers of parvalbumin-immunoreactive interneurons in the hippocampus of the adult sprague-dawley rat. Nutr Neurosci 2011;14(4):165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleischer SF, Turkewitz G. Effect of neonatal stunting on development of rats: large litter rearing. Dev Psychobiol 1979;12(2): 137–49. [DOI] [PubMed] [Google Scholar]
- 34.McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 1995;117(3): 340–57. [DOI] [PubMed] [Google Scholar]
- 35.SAS. 9.3 intelligence platform: system administration guide. Cary, NC: SAS Institute, Inc.; 2013. [Google Scholar]
- 36.Lister JP, Tonkiss J, Blatt GJ, Kemper TL, DeBassio WA, Galler JR, et al. Asymmetry of neuron numbers in the hippocampal formation of prenatally malnourished and normally nourished rats: a stereological investigation. Hippocampus 2006;16(11):946–58. [DOI] [PubMed] [Google Scholar]
- 37.Tonkiss J, Galler J, Morgane PJ, Bronzino JD, Austin-LaFrance RJ. Prenatal protein malnutrition and postnatal brain function. Ann N Y Acad Sci 1993;678:215–27. [DOI] [PubMed] [Google Scholar]
- 38.Fleischer SF, Turkewitz G. Behavioral effects of rotation between lactating and nonlactating females. Dev Psychobiol 1979;12(3): 245–54. [DOI] [PubMed] [Google Scholar]
- 39.Gallo PV, Werboff J, Knox K. Development of home orientation in offspring of protein-restricted cats. Dev Psychobiol 1984; 17(5):437–49. [DOI] [PubMed] [Google Scholar]
- 40.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr 1996;126(3):693–701. [DOI] [PubMed] [Google Scholar]
- 41.Schnorr CE, da Silva Morrone M, Simoes-Pires A, da Rocha RF, Behr GA, Moreira JC. Vitamin a supplementation in rats under pregnancy and nursing induces behavioral changes and oxidative stress upon striatum and hippocampus of dams and their offspring. Brain Res 2011;1369:60–73. [DOI] [PubMed] [Google Scholar]
- 42.Langley-Evans SC. Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proc Nutr Soc 2001;60(4):505–13. [DOI] [PubMed] [Google Scholar]
- 43.Angulo-Colmenares AG, Vaughan DW, Hinds JW. Rehabilitation following early malnutrition in the rat: body weight, brain size, and cerebral cortex development. Brain Res 1979;169(1):121–38. [DOI] [PubMed] [Google Scholar]
- 44.Muaku SM, Beauloye V, Thissen JP, Underwood LE, Fossion C, Gerard G, et al. Long-term effects of gestational protein malnutrition on postnatal growth, insulin-like growth factor (igf)-i, and igf-binding proteins in rat progeny. Pediatr Res 1996;39(4 Pt 1):649–55. [DOI] [PubMed] [Google Scholar]
- 45.Dec AM, Kohlhaas KL, Nelson CL, Hoque KE, Leilabadi SN, Folk J, et al. Impact of neonatal nos-1 inhibitor exposure on neurobehavioural measures and prefrontal-temporolimbic integration in the rat nucleus accumbens. Int J Neuropsychopharmacol 2013:1–13. [DOI] [PubMed] [Google Scholar]
- 46.Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on e17: implications for the neuro-pathology of schizophrenia. Biol Psychiatry 2006;60(3):253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Debassio WA, Kemper TL, Tonkiss J, Galler JR. Effect of prenatal protein deprivation on postnatal granule cell generation in the hippocampal dentate gyrus. Brain Res Bull 1996;41(6): 379–83. [DOI] [PubMed] [Google Scholar]
- 48.Lister JP, Blatt GJ, DeBassio WA, Kemper TL, Tonkiss J, Galler JR, et al. Effect of prenatal protein malnutrition on numbers of neurons in the principal cell layers of the adult rat hippocampal formation. Hippocampus 2005;15(3):393–403. [DOI] [PubMed] [Google Scholar]
- 49.Rudy JW, Stadler-Morris S, Albert P. Ontogeny of spatial navigation behaviors in the rat: dissociation of ‘proximal’- and ‘distal’-cue-based behaviors. Behav Neurosci 1987;101(1):62–73. [DOI] [PubMed] [Google Scholar]
- 50.Wills TJ, Cacucci F, Burgess N, O’Keefe J. Development of the hippocampal cognitive map in preweanling rats. Science 2010; 328(5985):1573–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kruger HS, Hanganu-Opatz IL. Neonatal cholinergic lesion alters the acoustic structure of infant rat vocalization but not the early cognitive development. Dev Psychobiol 2013;55(3): 294–308. [DOI] [PubMed] [Google Scholar]
- 52.Steiger JL, Alexander MJ, Galler JR, Farb DH, Russek SJ. Effects of prenatal malnutrition on gabaa receptor alpha1, alpha3 and beta2 mrna levels. Neuroreport 2003;14(13): 1731–35. [DOI] [PubMed] [Google Scholar]
- 53.Steiger JL, Galler JR, Farb DH, Russek SJ. Prenatal protein malnutrition reduces beta(2), beta(3) and gamma(2 l) gaba(a) receptor subunit mrnas in the adult septum. Eur J Pharmacol 2002;446(1–3):201–2. [DOI] [PubMed] [Google Scholar]
- 54.Fiacco TA, Rosene DL, Galler JR, Blatt GJ. Increased density of hippocampal kainate receptors but normal density of nmda and ampa receptors in a rat model of prenatal protein malnutrition. J Comp Neurol 2003;456(4):350–60. [DOI] [PubMed] [Google Scholar]
- 55.Chen JC, Turiak G, Galler J, Volicer L. Postnatal changes of brain monoamine levels in prenatally malnourished and control rats. Int J Dev Neurosci 1997;15(2):257–63. [DOI] [PubMed] [Google Scholar]
- 56.Mokler DJ, Torres OI, Galler JR, Morgane PJ. Stress-induced changes in extracellular dopamine and serotonin in the medial prefrontal cortex and dorsal hippocampus of prenatally mal-nourished rats. Brain Res 2007;1148:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manjarrez-Gutierrez G, Martinez-Radilla K, Boyzo-Montes de Oca A, Orozco-Suarez S, Hernandez-Rodriguez J. Increased expression of tryptophan-5-hydroxylase 1, but not 2, in brainstem as a result of intrauterine malnutrition. Int J Dev Neurosci 2012;30(6):445–50. [DOI] [PubMed] [Google Scholar]
- 58.Massaro TF, Levitsky DA, Barnes RH. Protein malnutrition induced during gestation: its effect on pup development and maternal behavior. Dev Psychobiol 1977;10(4):339–45. [DOI] [PubMed] [Google Scholar]
- 59.Purcell RH, Sun B, Pass LL, Power ML, Moran TH, Tamashiro KL. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav 2011;104(3):474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galler JR, Tonkiss J. Prenatal protein malnutrition and maternal behavior in sprague-dawley rats. J Nutr 1991;121(5):762–9. [DOI] [PubMed] [Google Scholar]
- 61.Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci 2007;121(6):1353–63. [DOI] [PubMed] [Google Scholar]
- 62.Bagot RC, Zhang TY, Wen X, Nguyen TT, Nguyen HB, Diorio J, et al. Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proc Natl Acad Sci USA 2012; 109(Suppl. 2):17200–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, et al. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS One 2011;6(2):e14739. [DOI] [PMC free article] [PubMed] [Google Scholar]