Supplemental digital content is available in the text.
Key Words: SINGLE NUCLEOTIDE POLYMORPHISM, ESTROGEN RECEPTOR, MUSCLE STIFFNESS, INJURY PREDICTION, ATHLETES
ABSTRACT
Purpose
Muscle injury is the most common sports injury. Muscle stiffness, a risk factor for muscle injury, is lower in females than in males, implying that sex-related genetic polymorphisms influence muscle injury associated with muscle stiffness. The present study aimed to clarify the associations between two genetic polymorphisms (rs2234693 and rs9340799) in the estrogen receptor 1 gene (ESR1) and muscle injury or muscle stiffness.
Methods
In study 1, a questionnaire was used to assess the muscle injury history of 1311 Japanese top-level athletes. In study 2, stiffness of the hamstring muscles was assessed using ultrasound shear wave elastography in 261 physically active young adults. In both studies, rs2234693 C/T and rs9340799 G/A polymorphisms in the ESR1 were analyzed using the TaqMan SNP Genotyping Assay.
Results
In study 1, genotype frequencies for ESR1 rs2234693 C/T were significantly different between the injured and noninjured groups in a C-allele dominant (CC + CT vs TT: odds ratio, 0.62; 95% confidence interval, 0.43–0.91) and additive (CC vs CT vs TT: odds ratio, 0.70; 95% confidence interval, 0.53–0.91) model in all athletes. In study 2, hamstring muscle stiffness was lower in subjects with the CC + CT genotype than in those with the TT genotype; a significant linear trend (CC < CT < TT) was found (r = 0.135, P = 0.029). In contrast, no associations were observed between ESR1 rs9340799 G/A and muscle injury or stiffness.
Conclusions
Our results suggest that the ESR1 rs2234693 C allele, in contrast to the T allele, provides protection against muscle injury by lowering muscle stiffness.
The incidence of sports-related injuries is associated with athletic success in both team and individual sports and may result in termination of an athlete’s career in some situations. Muscle injury, especially hamstring strain, is the most common type of injury, and the incidence is increasing in sports involved in sprinting and jumping (1–4). Susceptibility to muscle injury is, at least in part, causally associated with impaired joint flexibility (5,6), and muscle stiffness is one of the main components of joint flexibility (7,8). Although it is well known that joint flexibility is influenced by environmental factors, a recent meta-analysis showed that 50% of the variability in joint flexibility is explained by genetic factors (9). Taken together, certain genetic polymorphisms are associated with muscle injury related to joint flexibility and/or muscle stiffness.
There are sex-based differences in joint flexibility and muscle stiffness; females exhibit higher joint flexibility and lower muscle stiffness than males (10). These sex-related differences may be related to circulating levels of sex hormones and their specific receptors such as estrogen and androgen receptors. The effects of estrogen, a female sex hormone, on skeletal muscle are mediated via estrogen receptors in both males and females (11–13). Indeed, previous studies have demonstrated that circulating levels of estrogen are negatively associated with muscle stiffness (14,15), attributable to suppression of collagen synthesis (16). In addition, estrogen exerts anti-inflammatory and antioxidants effects on skeletal muscles (17–19), which may protect against muscle injury. Thus, it is possible that estrogen receptors functionally influence muscle stiffness and injury.
We focused on two functional polymorphisms, namely, rs2234693 C/T (defined by restriction enzyme PvuII) and rs9340799 G/A (defined by restriction enzyme XbaI), in the estrogen receptor 1 gene (ESR1). These polymorphisms are in the first intron of ESR1, positioned 397 and 351 base pairs upstream of exon 2, respectively. It has been suggested that these polymorphisms influence expression and activation of the ESR1 product and alter the action of estrogen. The rs2234693 C and rs9340799 G alleles in the ESR1, for example, are associated with higher gene expression and more favorable estrogen-induced actions (20–22). Based on these considerations, we hypothesized that these alleles provide a protective effect against muscle injury by lowering muscle stiffness. Thus, we aimed to clarify the association of two genetic polymorphisms in the ESR1 with a history of muscle injury (study 1) and muscle stiffness (study 2).
METHODS
Study 1
We obtained data from a total of 2181 Japanese athletes from March 2015 through November 2017. We assessed the history of up to three sports-related injuries (e.g., hernia, fracture, dislocation, ligament damage, muscle injury, tendon injury, meniscal injury, concussion, etc.) in descending order of severity using questionnaires, according to a previous study (23). In the present study, we focused on noncontact muscle injuries diagnosed by medical practitioners. This information was collected using a questionnaire and thus relied on athlete recall. In addition, the primary sport, playing years, competition level, and other factors were assessed using questionnaires. We excluded athletes with no Japanese ancestry (n = 29) and a lack of questionnaire data (n = 159). Athletes with regional level (n = 669) and less than 3 yr competition in their main sport (n = 13) were also excluded. After exclusion, we used data from 1311 top-level (those competing on a national or international level) Japanese athletes for further analysis (see Figure, Supplemental Digital Content 1, Flow diagram for the selection of subjects in study 1, http://links.lww.com/MSS/B373). This study was included in The Japanese Human Athlome Project (J-HAP) in “Athlome Project Consortium” (24). Written informed consent was obtained from each athlete in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Ethics Committees of Juntendo University, Nippon Sports Science University, and Tenri University.
Study 2
A total of 261 physically active young adults participated in study 2 (males, n = 152; females, n = 109). We excluded subjects who took part in sports requiring high flexibility of the hip joint such as gymnastics and rhythmic gymnastics. There were no apparent neurological, orthopedic, or neuromuscular problems in any of the subjects. Subjects came to the laboratory at least 20 min before measurements were taken. The laboratory room temperature was maintained at a constant 24°C ± 2°C to minimize temperature-induced effects. The subjects were not allowed to perform warm-up or stretching exercises before testing. Written informed consent was obtained from each subject in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Ethics Committee of Juntendo University.
Passive straight-leg raise test
All straight-leg raise (SLR) tests were performed on both legs by the same examiner. The subjects lay in a supine position with their legs straight on an examining bed. The pelvis and nontesting leg were secured on the bed with nonelastic straps to avoid posterior pelvic tilt. The examiner held one hand gently on the knee of the testing leg to maintain it straight and raised the leg with the other hand placed near the ankle until tightness was felt by the examiner. The hip flexion angle from the resting position was measured using a digital inclinometer (MLT-100; Sakai Medical, Japan) attached 3 to 4 cm proximal to the lateral malleolus and adopted as the score.
Muscle stiffness
The shear modulus (an index of stiffness; expressed in kilopascals) of the biceps femoris long head, as well as semitendinosus and semimembranosus of both legs was assessed using an ultrasound shear wave elastography system (Aixplorer ver. 10; Supersonic Imagine, France) with a linear probe (SL10-2; Supersonic Imagine) and preset for musculoskeletal analysis (persistence, med; smoothing, 5). The subjects were seated on a bench with their hip flexed at 70° (0°, anatomical position) and the knee fully extended. This hip flexion angle was chosen according to a recent study (25) that aimed to determine an angle where the hamstring could be stretched to a tensioned state without pain and the shear modulus could be quantified for all subjects. The subjects were requested to fully relax the leg throughout the measurements. Measurements for each muscle were performed at 50% of the thigh length (the distance between the greater trochanter and the lateral epicondyle of the femur). The probe orientation was adjusted to visualize fascicles within the B-mode image for each muscle. Care was taken not to press and deform the muscles while scanning. The images were then acquired after ensuring a stable color distribution for a few seconds. The probe location was slightly adjusted before image acquisition when a defocused image with a large variation of shear modulus was observed.
Values for the SLR and hamstring muscle stiffness tests are shown as an average of both legs. The measurement orders for SLR/muscle stiffness and right/left were randomized across subjects. For each variable, three measurements were performed and averaged. The shear modulus of three muscles was averaged to evaluate passive stiffness of the overall hamstring. The averaged SLR scores and hamstring stiffness values were used for subsequent analyses.
Genotyping Analysis
Total DNA was isolated from saliva with an Oragene® DNA collection kit (DNA Genotek, ON, Canada) in accordance with the manufacturer’s instructions. The concentration of DNA was quantified using a NanoDrop 8000 UV-Vis spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). DNA samples were stored at 4°C until use. Two ESR1 polymorphisms (rs2234693 and rs9340799) were genotyped using a real-time thermocycler in the endpoint analysis mode (LightCycler 480; Roche Applied Science, Mannheim, Germany) using the TaqMan® SNP Genotyping Assay (assay ID: C___3163590_10 and C___3163591_10). A genotyping mixture (final volume of 5 μL) containing 2.5-μL TaqMan® GTXpress™ Master Mix (2×), 0.0625 μL TaqMan® SNP Genotyping Assay Mix (40×), 1.4375 μL sterilized water, and 1 μL genomic DNA (10 ng·μL−1) was used. Two to four negative controls were included on each plate. Genotypes were determined on the basis of the TaqMan® assay results using LightCycler® 480 SW, version 1.5 (Roche Molecular Systems). Approximately 100 samples were genotyped in duplicate for the polymorphisms analyzed in the present study, and we confirmed that the genotyping results perfectly agreed between duplicates.
Statistical Analysis
All data are expressed as means ± SD. Hardy-Weinberg equilibrium (HWE) of the ESR1 rs2234693 and rs9340799 polymorphisms was tested using χ2 test. In study 1, characteristics of subjects were analyzed using unpaired t-test or χ2 test between muscle injured and nonmuscle injured groups as appropriate. Logistic regression analysis was applied to investigate the associations between ESR1 genotype and incidence of muscle injury. We adjusted for sex, the main sport (athletics or other), and playing years. Odds ratios (OR) and 95% confidence intervals (CI) were calculated under the dominant, recessive, and additive (allele counting) genetic models to estimate the degree of contribution to muscle injury for optimal genotype. Further, we analyzed the minimum Akaike’s information criterion (AIC), which evaluates the best fitting genetic model. In study 2, anthropometric measurements were analyzed by using unpaired t-tests between males and females. A comparison of each parameter among three genotypes (rs2234693: CC vs CT vs TT, rs9340799: GG vs GA vs AA) was assessed using one-way ANOVA, and linear trends were assessed using the Spearman correlation coefficient. Dominant and recessive models were assessed using unpaired t-tests (rs2234693: CC + CT vs TT or CC vs CT + TT, rs9340799: GG + GA vs AA). When there were fewer than five subjects (e.g., for the ESR1 rs9340799 GG genotype), statistical analyses were not conducted in the present study. Statistical significance was set at P < 0.05. Statistical analyses were performed using JMP Pro version 12 (SAS Institute).
RESULTS
Study 1
Characteristics of the studied athletes are shown in Table 1. There were no significant differences in age, height, and body mass between the muscle injured and nonmuscle injured groups. There were significantly fewer years spent playing a primary sport, on average, in the muscle injured group than in the nonmuscle injured group (P = 0.017). The rate of genotyping success was 1283 (97.9%) of 1311 and 1286 (98.1%) of 1311 for rs2234693 and rs9340799, respectively. Both polymorphisms were in HWE (P = 0.928 for rs2234693 and P = 0.330 for rs9340799). Results of the logistic regression analyses for rs2234693 and rs9340799 are shown in Tables 2 and 3, respectively. Genotype frequencies for the ESR1 rs2234693 C/T polymorphism were significantly different between the injured group and noninjured group in a C-allele dominant (CC + CT vs TT: OR, 0.62; 95% CI, 0.43–0.91; AIC, 815.6) and an additive (CC vs CT vs TT: OR, 0.70; 95% CI, 0.53–0.91; AIC, 814.5) genetic model for all athletes (Table 2). Based on AIC, the additive model showed the best fit to explain the association between the rs2234693 polymorphism and muscle injury. Similarly, in subgroup analyses for sex-based differences, genotype frequencies were significantly different between groups in a C-allele dominant genetic model (CC + CT vs TT: OR, 0.62; 95% CI,0.39–0.98; AIC, 550.6) in male athletes, and a C-allele recessive (CC vs CT + TT: OR, 0.30; 95% CI, 0.07–0.88; AIC, 269.7) and an additive (CC vs CT vs TT: OR = 0.61; 95% CI = 0.36–0.98; AIC = 270.6) genetic models in female athletes. In contrast, no significant associations were observed for the ESR1 rs9340799 G/A polymorphism for any groups (Table 3).
TABLE 1.
Characteristics of athletes recruited in study 1.
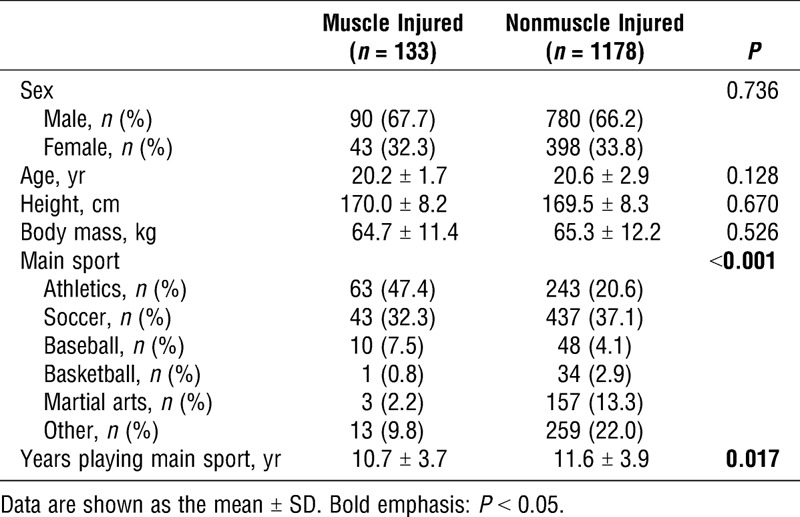
TABLE 2.
Associations of rs2234693 genotype with odds ratio of muscle injury with several adjustments for candidate confounding risk factors.

TABLE 3.
Associations of rs9340799 genotype with odds ratio of muscle injury with several adjustments for candidate confounding risk factors.

Study 2
The rate of genotyping success was 261 (100%) of 261 and 259 (99.2%) of 261 for the rs2234693 and rs9340799 polymorphisms, respectively. Both polymorphisms were in HWE (P = 0.574 for rs2234693 and P = 0.435 for rs9340799). Characteristics of the study subjects are shown in Table 4. Male subjects were taller (P < 0.001), heavier (P < 0.001), had lower SLR scores (P < 0.001), and had stiffer hamstring muscle (P = 0.012) than female subjects.
TABLE 4.
Characteristics of subjects recruited in study 2.

Fig. 1 shows stiffness of the hamstring muscle for the ESR1 rs2234693 C/T polymorphism. Subjects with the CC + CT genotype showed significantly lower stiffness of the hamstring muscle than subjects with the TT genotype (28.0 ± 7.2 vs 30.0 ± 8.6, P = 0.043), and the T allele of the ESR1 rs2234693 polymorphism was positively associated with stiffness of the hamstring muscle (r = 0.135, P = 0.029) (Fig. 1A). In males, stiffness of the hamstring muscle was significantly lower in subjects with the CC + CT genotype than in those with the TT genotype (28.9 ± 7.4 vs 31.8 ± 9.5, P = 0.046) (Fig. 1B). In females, there were no significant differences in muscle stiffness among rs2234693 C/T genotypes (Fig. 1C). Table, Supplemental Digital Content 2, shows the detailed characteristics of subjects with the ESR1 rs2234693 C/T polymorphism (see Table, Supplemental Digital Content 2, characteristics of subjects with the ESR1 rs2234693 genotype in accordance with the alleles present, http://links.lww.com/MSS/B374). The T allele of this polymorphism was positively associated with stiffness of the biceps femoris and semimembranosus muscles in all subjects, and with stiffness of the semimembranosus muscle in male subjects.
FIGURE 1.

Stiffness of hamstring muscles in all subjects (A), male subjects (B), and female subjects (C) with the ESR1 rs2234693 genotype in accordance with the alleles present (i.e., CC, CT, or TT).
Fig. 2 shows stiffness of the hamstring muscle for the ESR1 rs9340799 G/A polymorphism in all subjects. There were no significant differences in hamstring muscle stiffness among G/A genotypes for any groups (Fig. 2A, B, C). Table, Supplemental Digital Content 3, shows the detailed characteristics of subjects with the ESR1 rs9340799 G/A polymorphism (see Table, Supplemental Digital Content 3, characteristics of subjects with the ESR1 rs9340799 genotype in accordance with the alleles present, http://links.lww.com/MSS/B375). The A allele of this polymorphism was positively associated with stiffness of the semitendinosus muscle in female subjects.
FIGURE 2.
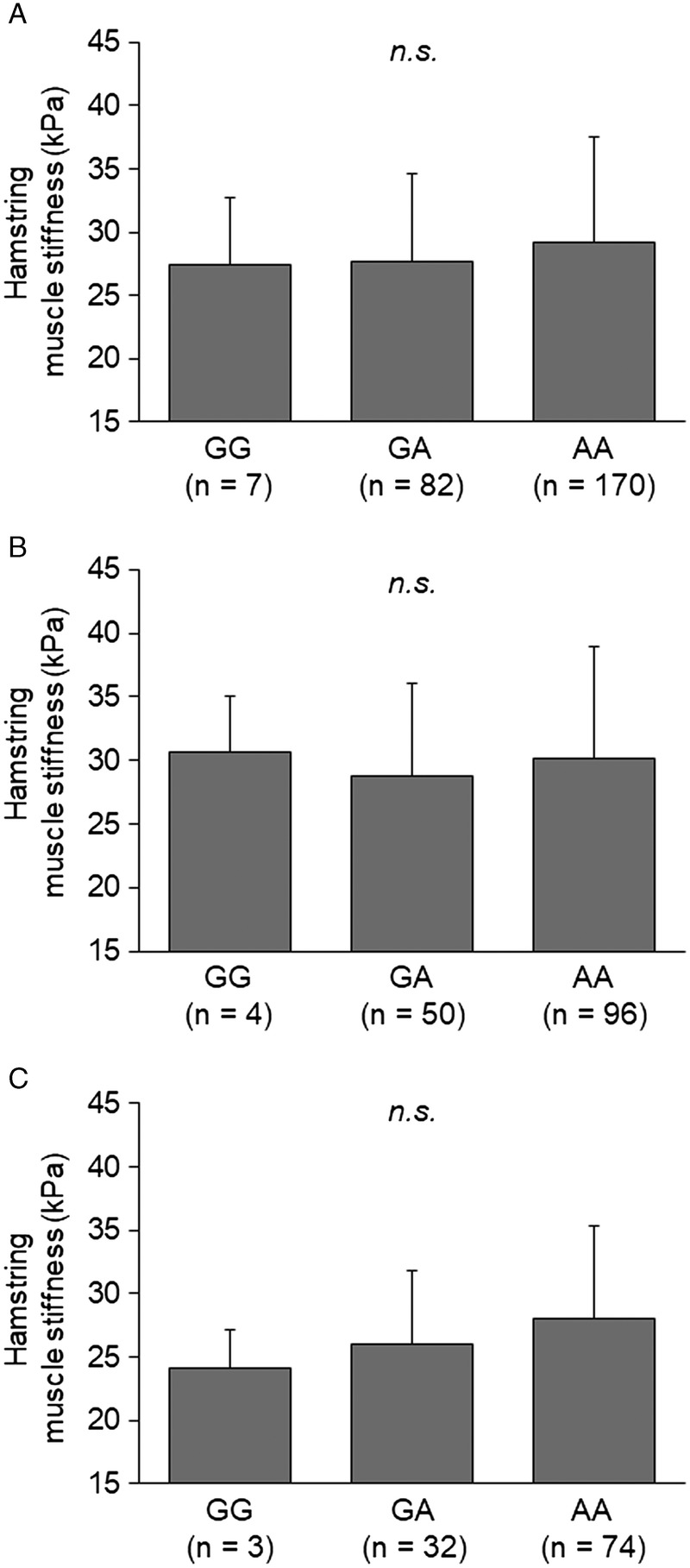
Stiffness of hamstring muscles in all subjects (A), male subjects (B), and female subjects (C) with the ESR1 rs9340799 genotype in accordance with the alleles present (i.e., GG, GA, or AA).
DISCUSSION
Preventing muscle injury is crucial in both professional and amateur sports, and muscle injury occurs as a consequence of intrinsic (i.e., age, sex, genetic polymorphisms, etc.) and extrinsic factors (i.e., exercise intensity, exposure time to practice and play matches, practice environments such as surface, etc.). Several previous studies have indicated the importance of intrinsic factors for preventing sports-related injuries (26,27). Indeed, regarding musculoskeletal soft tissue injuries such as anterior cruciate ligament injury and Achilles tendon injury, several studies have reported that the association between genetic polymorphisms and injuries in case-control association analysis (28–32). In addition to these case-control association studies, other studies have investigated the functional role of injury-related genetic polymorphisms (33–35). On the other hand, although the details of specific genetic polymorphisms as determinants for muscle injury risk are gradually being elucidated (36–44), the sample sizes of previous studies have been relatively small (54–257 athletes). In addition, there are no studies on the effect of muscle injury-related genetic polymorphisms on physiological functions (e.g., muscle stiffness). Therefore, it is difficult to draw firm conclusions regarding the associations between genetic polymorphisms and muscle injury. It is important to conduct replication and/or functional studies in addition to a case-control association study in large cohort to avoid false-positive results in Sports Genetics. In study 1 of the present report, we examined associations between two ESR1 genetic polymorphisms and the prevalence of muscle injuries in 1311 top-level Japanese athletes. In study 2 of the present report, we investigated physiological functions, such as muscle elasticity in relation to two ESR1 genetic polymorphisms in physically active subjects. We found that the ESR1 rs2234693 polymorphism was associated with muscle injury as well as muscle stiffness. Specifically, individuals with the C allele showed resistance against muscle injury and lower muscle stiffness than those with the T allele. As such, the present design, that is, the combined investigation of large case-control association and functional studies, will make a substantial contribution toward uncovering the associations between genetic polymorphisms and sports-related injuries.
A lack of joint flexibility and high muscle stiffness are risk factors for muscle injury (5,6) and can be reduced via the actions of estrogen (14,15). Estrogenic activity on muscles is mediated by means of estrogen-receptors in skeletal muscle tissue in both males and females (11–13). Moreover, previous studies have suggested that the ESR1 rs2234693 C/T polymorphism alters its own gene expression, with the C allele being associated with higher gene expression and more favorable estrogen-induced actions (20–22). In study 1 of the present report, the ESR1 rs2234693 C allele showed greater protective effect against muscle injury than the T allele in athletes. In study 2, there was significantly lower muscle stiffness in individuals with the ESR1 rs2234693 C allele than in individuals with the T allele in physically active subjects. Considering the previous and present findings, it is possible that the ESR1 rs2234693 polymorphism results in increased estrogenic activity and lower muscle stiffness, which is a possible physiological mechanism underlying the relation between the ESR1 rs2234693 polymorphism and muscle injury.
Muscle stiffness is mainly influenced by collagenous connective tissue (45), and it is well known that estrogen suppresses collagen synthesis (16). In the human α1 collagen gene, activator protein 1 binding sites are present on the first intron (46), and estrogen signaling regulates activator protein 1 binding sites (47). Collectively, these data suggest that estrogen lowers muscle stiffness by suppressing collagen synthesis. Indeed, in study 2 of the present report, we found that the subjects with the ESR1 rs2234693 C allele, which is reported to be associated with higher ESR1 gene expression and increased estrogen-induced actions (20–22), showed significantly lower muscle stiffness compared to the subjects with the TT genotype. Taken together, these data suggest that estrogen-associated suppression of collagen synthesis is a possible explanation for the relation between the ESR1 rs2234693 polymorphisms and muscle stiffness.
It has been suggested that estrogen has muscle protective effects, including anti-inflammatory and antioxidant properties (17–19). It is well known that strenuous and repeated contractions are associated with exercise-induced muscle damage and soreness (48,49). Williams et al. (18) reported that high levels of estrogen suppress the increases in creatine kinase levels observed after prolonged aerobic exercise in young females. Additionally, Feng et al. (19) suggested that estrogen increases antioxidant capacity, resulting in reduced muscle damage and accelerated muscle regeneration after muscle strain injury in rats. Thus, the biological activity of estrogen is associated with muscle injury. Taken together, these data suggest that variations in estrogen activity attributable to the ESR1 rs2234693 polymorphism may be associated with muscle injury through anti-inflammatory and anti-oxidant effects.
The present study has several limitations, including a cross-sectional study design; follow-up studies are needed to confirm these findings. In addition, the sample size of female subjects in study 1 was relatively small. In rs2234693 polymorphism in the ESR1, the statistical power to detect an association with an odds ratio of 2.0 and minor allele frequency of 0.43 in a group with 133 muscle injured and 1150 nonmuscle injured is 0.966. When subjects were divided into two groups, namely, males and females, statistical powers dropped to lower values of 0.874 for males and 0.570 for females. Thus, further replication and functional studies with larger sample size are necessary to confirm the present findings, especially in females because of the lack of statistical power. Further, we measured muscle stiffness in hamstrings under resting conditions; muscle injuries typically occur in situations where muscles are contracting. Thus, we need to investigate active muscle stiffness in addition to passive muscle stiffness in the future.
In study 1 of the present report, we found that the ESR1 rs2234693 C allele has more of a protective effect against muscle injury than the T allele in top-level Japanese athletes. In study 2 of the present report, the ESR1 rs2234693 C allele resulted in significantly less muscle stiffness than the T allele in physically active subjects. Our results suggest that the ESR1 rs2234693 C allele, in contrast to the T allele, provides protection against muscle injury by lowering muscle stiffness.
Acknowledgments
This study was supported in part by grants from the JSPS KAKENHI Scientific Research (B) (18H03155 to N. F. and 16H03233 to N. M.), Young Scientists (A) (17H04752 to E. M.), Young Scientists (18K17863 to H. K.), The Nakatomi Foundation (to H. K.), MEXT-Supported Program for the Strategic Research Foundation at Private Universities (to Juntendo University), and Tenri University Research Grant (N. Kamiya.). H. K. was a recipient of a Grant-in-Aid for Japan Society for Promotion of Science (JSPS) fellows from the JSPS (17 J10817).
The authors thank Dr. Kazuhiro Aoki, Dr. Koya Suzuki, Dr. Yoshimitsu Kohmura, and Yuko Shintake from the Juntendo University for their help recruiting the athletes, and Dr. Hiroaki Kanehisa from the National Institute of Fitness and Sports in Kanoya for his technical assistance. We would like to thank Editage (www.editage.jp) for English language editing.
No conflicts of interest, financial or otherwise, are declared by the authors. The results of the present study do not constitute endorsement by ACSM. All authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
REFERENCES
- 1.Orchard J, Marsden J, Lord S, Garlick D. Preseason hamstring muscle weakness associated with hamstring muscle injury in Australian footballers. Am J Sports Med. 1997;25(1):81–5. [DOI] [PubMed] [Google Scholar]
- 2.Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012; 42(3):209–26. [DOI] [PubMed] [Google Scholar]
- 3.Ekstrand J, Walden M, Hagglund M. Hamstring injuries have increased by 4% annually in men’s professional football, since 2001: a 13-year longitudinal analysis of the UEFA elite club injury study. Br J Sports Med. 2016;50(12):731–7. [DOI] [PubMed] [Google Scholar]
- 4.Ekstrand J, Hagglund M, Kristenson K, Magnusson H, Walden M. Fewer ligament injuries but no preventive effect on muscle injuries and severe injuries: an 11-year follow-up of the UEFA champions league injury study. Br J Sports Med. 2013;47(12):732–7. [DOI] [PubMed] [Google Scholar]
- 5.Watsford ML, Murphy AJ, McLachlan KA, et al. A prospective study of the relationship between lower body stiffness and hamstring injury in professional Australian rules footballers. Am J Sports Med. 2010;38(10):2058–64. [DOI] [PubMed] [Google Scholar]
- 6.Witvrouw E, Danneels L, Asselman P, D’Have T, Cambier D. Muscle flexibility as a risk factor for developing muscle injuries in male professional soccer players. A prospective study. Am J Sports Med. 2003;31(1):41–6. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto N, Hirata K, Kanehisa H. Effects of hamstring stretching on passive muscle stiffness vary between hip flexion and knee extension maneuvers. Scand J Med Sci Sports. 2017; 27(1):99–106. [DOI] [PubMed] [Google Scholar]
- 8.Magnusson SP, Simonsen EB, Aagaard P, Boesen J, Johannsen F, Kjaer M. Determinants of musculoskeletal flexibility: viscoelastic properties, cross-sectional area, EMG and stretch tolerance. Scand J Med Sci Sports. 1997;7(4):195–202. [DOI] [PubMed] [Google Scholar]
- 9.Schutte NM, Nederend I, Hudziak JJ, de Geus EJ, Bartels M. Differences in adolescent physical fitness: a multivariate approach and meta-analysis. Behav Genet. 2016;46(2):217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse CI. Gender differences in the passive stiffness of the human gastrocnemius muscle during stretch. Eur J Appl Physiol. 2011; 111(9):2149–54. [DOI] [PubMed] [Google Scholar]
- 11.Wiik A, Ekman M, Johansson O, Jansson E, Esbjornsson M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem Cell Biol. 2009;131(2):181–9. [DOI] [PubMed] [Google Scholar]
- 12.Lemoine S, Granier P, Tiffoche C, Rannou-Bekono F, Thieulant ML, Delamarche P. Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc. 2003;35(3):439–43. [DOI] [PubMed] [Google Scholar]
- 13.Brown M, Ning J, Ferreira JA, Bogener JL, Lubahn DB. Estrogen receptor-alpha and -beta and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. Am J Physiol Endocrinol Metab. 2009;296(4):E854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eiling E, Bryant AL, Petersen W, Murphy A, Hohmann E. Effects of menstrual-cycle hormone fluctuations on musculotendinous stiffness and knee joint laxity. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):126–32. [DOI] [PubMed] [Google Scholar]
- 15.Bell DR, Myrick MP, Blackburn JT, Shultz SJ, Guskiewicz KM, Padua DA. The effect of menstrual-cycle phase on hamstring extensibility and muscle stiffness. J Sport Rehabil. 2009;18(4): 553–63. [DOI] [PubMed] [Google Scholar]
- 16.Kwan G, Neugarten J, Sherman M, et al. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int. 1996;50(4):1173–9. [DOI] [PubMed] [Google Scholar]
- 17.Tiidus PM. Can oestrogen influence skeletal muscle damage, inflammation, and repair? Br J Sports Med. 2005;39(5):251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams T, Walz E, Lane AR, Pebole M, Hackney AC. The effect of estrogen on muscle damage biomarkers following prolonged aerobic exercise in eumenorrheic women. Biol Sport. 2015;32(3):193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng X, Li GZ, Wang S. Effects of estrogen on gastrocnemius muscle strain injury and regeneration in female rats. Acta Pharmacol Sin. 2004;25(11):1489–94. [PubMed] [Google Scholar]
- 20.Herrington DM, Howard TD, Brosnihan KB, et al. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E-selectin but not C-reactive protein. Circulation. 2002;105(16):1879–82. [DOI] [PubMed] [Google Scholar]
- 21.Nordstrom P, Glader CA, Dahlen G, et al. Oestrogen receptor alpha gene polymorphism is related to aortic valve sclerosis in postmenopausal women. J Intern Med. 2003;254(2):140–6. [DOI] [PubMed] [Google Scholar]
- 22.Ryan J, Canonico M, Carcaillon L, et al. Hormone treatment, estrogen receptor polymorphisms and mortality: a prospective cohort study. PLoS One. 2012;7(3):e34112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuller CW, Ekstrand J, Junge A, et al. Consensus statement on injury definitions and data collection procedures in studies of football (soccer) injuries. Br J Sports Med. 2006;40(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitsiladis YP, Tanaka M, Eynon N, et al. Athlome project consortium: a concerted effort to discover genomic and other “omic” markers of athletic performance. Physiol Genomics. 2016;48(3):183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto N, Hirata K, Kimura N, Miyamoto-Mikami E. Contributions of hamstring stiffness to straight-leg-raise and sit-and-reach test scores. Int J Sports Med. 2018;39(2):110–4. [DOI] [PubMed] [Google Scholar]
- 26.Vlahovich N, Fricker PA, Brown MA, Hughes D. Ethics of genetic testing and research in sport: a position statement from the Australian Institute of Sport. Br J Sports Med. 2017;51(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins M. Genetic risk factors for soft-tissue injuries 101: a practical summary to help clinicians understand the role of genetics and “personalised medicine.” Br J Sports Med. 2010;44(13): 915–7. [DOI] [PubMed] [Google Scholar]
- 28.Abrahams Y, Laguette MJ, Prince S, Collins M. Polymorphisms within the COL5A1 3’-UTR that alters mRNA structure and the MIR608 gene are associated with Achilles tendinopathy. Ann Hum Genet. 2013;77(3):204–14. [DOI] [PubMed] [Google Scholar]
- 29.Mokone GG, Schwellnus MP, Noakes TD, Collins M. The COL5A1 gene and Achilles tendon pathology. Scand J Med Sci Sports. 2006;16(1):19–26. [DOI] [PubMed] [Google Scholar]
- 30.Posthumus M, September AV, Keegan M, et al. Genetic risk factors for anterior cruciate ligament ruptures: COL1A1 gene variant. Br J Sports Med. 2009;43(5):352–6. [DOI] [PubMed] [Google Scholar]
- 31.Posthumus M, September AV, O’Cuinneagain D, van der Merwe W, Schwellnus MP, Collins M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. Am J Sports Med. 2009;37(11):2234–40. [DOI] [PubMed] [Google Scholar]
- 32.September AV, Cook J, Handley CJ, van der Merwe L, Schwellnus MP, Collins M. Variants within the COL5A1 gene are associated with Achilles tendinopathy in two populations. Br J Sports Med. 2009;43(5):357–65. [DOI] [PubMed] [Google Scholar]
- 33.Abrahams S, Posthumus M, Collins M. A polymorphism in a functional region of the COL5A1 gene: association with ultraendurance-running performance and joint range of motion. Int J Sports Physiol Perform. 2014;9(3):583–90. [DOI] [PubMed] [Google Scholar]
- 34.Foster BP, Morse CI, Onambele GL, Williams AG. Human COL5A1 rs12722 gene polymorphism and tendon properties in vivo in an asymptomatic population. Eur J Appl Physiol. 2014;114(7):1393–402. [DOI] [PubMed] [Google Scholar]
- 35.Foster BP, Morse CI, Onambele GL, Williams AG. Variants within the MMP3 gene and patellar tendon properties in vivo in an asymptomatic population. Eur J Appl Physiol. 2014;114(12):2625–34. [DOI] [PubMed] [Google Scholar]
- 36.Massidda M, Voisin S, Claudia C, et al. ACTN3 R577X polymorphism is associated with the incidence and severity of injuries in professional football players. Clin J Sport Med. 2017. [Epub ahead of print]. doi: 10.1097/JSM.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 37.Massidda M, Eynon N, Bachis V, et al. Influence of the MCT1 rs1049434 on indirect muscle disorders/injuries in elite football players. Sports Med Open. 2015;1(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massidda M, Corrias L, Bachis V, et al. Vitamin D receptor gene polymorphisms and musculoskeletal injuries in professional football players. Exp Ther Med. 2015;9(5):1974–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massidda M, Bachis V, Corrias L, Piras F, Scorcu M, Calo CM. Influence of the COL5A1 rs12722 on musculoskeletal injuries in professional soccer players. J Sports Med Phys Fitness. 2015;55(11): 1348–53. [PubMed] [Google Scholar]
- 40.Larruskain J, Celorrio D, Barrio I, et al. Genetic variants and hamstring injury in soccer: an association and validation study. Med Sci Sports Exerc. 2018;50(2):361–8. [DOI] [PubMed] [Google Scholar]
- 41.Pruna R, Ribas J, Montoro JB, Artells R. The impact of single nucleotide polymorphisms on patterns of non-contact musculoskeletal soft tissue injuries in a football player population according to ethnicity. Med Clin (Barc). 2015;144(3):105–10. [DOI] [PubMed] [Google Scholar]
- 42.Pruna R, Artells R, Ribas J, et al. Single nucleotide polymorphisms associated with non-contact soft tissue injuries in elite professional soccer players: influence on degree of injury and recovery time. BMC Musculoskelet Disord. 2013;14:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pruna R, Artells R, Lundblad M, Maffulli N. Genetic biomarkers in non-contact muscle injuries in elite soccer players. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3311–8. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto N, Miyamoto-Mikami E, Hirata K, Kimura N, Fuku N. Association analysis of the ACTN3 R577X polymorphism with passive muscle stiffness and muscle strain injury. Scand J Med Sci Sports. 2018;28(3):1209–14. [DOI] [PubMed] [Google Scholar]
- 45.Gajdosik RL. Passive extensibility of skeletal muscle: review of the literature with clinical implications. Clin Biomech (Bristol, Avon). 2001;16(2):87–101. [DOI] [PubMed] [Google Scholar]
- 46.Slack JL, Liska DJ, Bornstein P. Regulation of expression of the type I collagen genes. Am J Med Genet. 1993;45(2):140–51. [DOI] [PubMed] [Google Scholar]
- 47.Weisz A, Bresciani F. Estrogen regulation of proto-oncogenes coding for nuclear proteins. Crit Rev Oncog. 1993;4(4):361–88. [PubMed] [Google Scholar]
- 48.Newham DJ, Mills KR, Quigley BM, Edwards RH. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond). 1983;64(1):55–62. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc. 1984; 16(6):529–38. [PubMed] [Google Scholar]


