ABSTRACT
Purpose
Carbohydrate (CHO) ingestion 30 to 45 min before exercise results in transient hypoglycemia after starting the exercise in some, but not all, subjects. However, whether transient hypoglycemia is more likely to occur under fed or fasted condition remains unknown. This study aimed to directly compare the effects of fasting versus feeding on plasma glucose responses after preexercise CHO intake and to examine the relationship between insulin responses and onset of transient hypoglycemia.
Methods
Sixteen subjects performed 60-min cycle ergometer exercises at 75% maximal oxygen uptake (V˙O2max) under overnight fasted and fed (4 h after breakfast) conditions. In both conditions, they consumed 500 mL of beverage (150 g of glucose) 30 min before beginning exercise.
Results
The mean plasma glucose concentrations 15 min after starting the exercise did not fall below 4.0 mmol·L−1 (criteria for hypoglycemia) in both states; however, individual differences in the occurrence of transient hypoglycemia were noted. In the fasted state, plasma glucose levels transiently dropped below 4.0 mmol·L−1 in five subjects, who had substantially higher serum insulin levels at the start of exercise, compared with those who did not develop hypoglycemia. Although seven subjects developed transient hypoglycemia in the fed state, no relationship was observed between insulin responses and hypoglycemia. Three subjects developed hypoglycemia in both fasted and fed states.
Conclusions
These results suggest that transient hypoglycemia after preexercise CHO ingestion occurs in some, but not all, subjects, under both conditions. Furthermore, subjects with enhanced insulin responses seem to be more prone to transient hypoglycemia in the fasted condition.
Key Words: BREAKFAST, OVERNIGHT FAST, PLASMA GLUCOSE, INSULIN RESPONSE, SPORTS DRINK, CYCLING
Carbohydrates (CHO) are an important energy source for exercise. Depletion of muscle glycogen stores and a decrease in blood glucose during prolonged strenuous exercise are considered as major factors in the development of fatigue (1–3). Therefore, athletes are recommended to consume an adequate amount of CHO before exercise, to optimize muscle glycogen stores, and enhance exercise performance (4). However, several studies have reported that CHO ingestion 30 to 60 min before exercise results in hyperinsulinemia and hyperglycemia, which is followed by a rapid decrease in blood glucose at the start of exercise (5–14). This phenomenon is often called “rebound hypoglycemia” (12,15) or “transient hypoglycemia” (13). Additionally, some studies have reported that increased glycogenolysis and decreased lipolysis and fat oxidation during exercise can occur, which in turn affect exercise performance (5,6).
The preliminary article written by Costill et al. in the 1970s (5), and almost all other studies (7–10,12) that demonstrated rebound hypoglycemia after preexercise feeding, involved fasting for 6 to 14 h before the experiment. However, prolonged fasting before a competitive race is not recommended for the endurance of athletes (4), and this approach does not adequately reflect the real competitive situation. Although a few studies have examined the effects of preexercise ingestion of CHO after breakfast (6,11,13), no studies have directly compared the effects of preexercise glucose ingestion in both fed and fasted states within the same subjects. Therefore, this study aimed to directly compare the effects of fasting versus feeding on plasma glucose responses after preexercise CHO ingestion and to determine which condition is more likely to lead to tran-sient hypoglycemia.
In previous studies, transient hypoglycemia during exercise has been observed in some, but not all, subjects. Several studies reported that the severity of rebound hypoglycemia varies among individuals, and some individuals are prone to more severe rebounds (16–19). However, only a few studies have identified the characteristics of individuals prone to hypoglycemia (12,13), and consistent results have not been obtained for factors that contribute to individual differences. Hyperinsulinemia before exercise is one of the important factors in the occurrence of rebound hypoglycemia. Koivisto et al. (7) demonstrated that rapid decrease in plasma glucose caused by preexercise glucose ingestion might be at least in part mediated by hyperinsulinemia. In addition, fructose ingestion, which causes slight increase in plasma insulin level, did not result in hypoglycemia during exercise. These results indicate that higher insulin level is a key factor to induce a decrease in blood glucose during exercise. Therefore, subjects who have higher insulin responses are more likely to develop rebound hypoglycemia. However, whether insulin response is related to individual differences at the onset of hypoglycemia remains unclear. Therefore, the secondary purpose of this study was to examine the relationship between insulin responses and onset of transient hypoglycemia in both fed and fasted conditions.
METHODS
Subjects
Sixteen Japanese male college students participated in this study. Their physical characteristics were as follows (mean ± SD): age, 21.0 ± 1.0 yr; height, 173.7 ± 5.1 cm; body weight, 67.3 ± 8.2 kg; body fat, 13.1% ± 4.3%; maximal oxygen uptake (V˙O2max), 46.3 ± 7.4 mL·kg−1·min−1; and watt max (Wmax), 262 ± 25 W. All subjects signed a consent form after reading information about the study and having the procedures explained to them. This study was approved by the Ethical Committee of Waseda University (2015-102) and is registered with the University Hospital Medical Information Network in Japan, number UMIN000018343.
Preliminary testing
Before the start of the experiments, subjects were asked to perform a graded exercise test to exhaustion to determine their Wmax and V˙O2max, using a cycle ergometer (75XLII; COMBI Wellness, Tokyo, Japan). Initial exercise intensity was 60 to 90 W for 3 min, and then the exercise intensity was increased by 15 W·min−1 until the subject could no longer maintain the required pedaling frequency of 60 rpm. During the incremental stage of the exercise test, expired gas was collected from the participants, and oxygen uptake (V˙O2) and carbon dioxide production (V˙CO2) were measured and averaged with 30-s intervals using an automated gas analyzer (AE-310S; Minato Medical Science, Osaka, Japan). HR (BSM-2401; Nihon Kohden, Tokyo, Japan) and RPE were monitored every minute during exercise. Oxygen uptake was considered to be maximal (V˙O2max) when at least three of the following four criteria were met: 1) a leveling off of V˙O2 with increasing workload, 2) a HR within 10 bpm of predicted maximum (220; age, ± 5 yr), 3) a RER of greater than 1.1, and 4) an RPE of 19 or 20.
Height, body mass, and body fat percentage were also measured (InnerScan BC-612; TANITA, Tokyo, Japan) at this visit.
Experimental design
Each subject participated in two trials. They were instructed to fast from 9:00 pm, the day before the experiment, and then either to continue fasting (Fasted Trial), or to consume breakfast (Fed Trial). The two experimental trials were separated by at least 1 wk and were performed in a randomized order.
During both trials, a standardized dinner (808 kcal: 12% protein, 24% fat, 64% CHO), which consisted of rice, miso soup, Hamburg steak, coleslaw, braised pumpkin, and simmered seaweed, was consumed between 8:00 pm and 9:00 pm the day before the experiment. In the Fed Trial, breakfast (709 kcal: 10% protein, 29% fat, 61% CHO), which consisted of rice, pork miso soup, a thick omelet, and potato salad, was consumed between 6:30 am and 7:30 am.
Experimental protocol
Subjects reported to the laboratory at 10:30 am after an overnight fast, or after consuming breakfast 4 h before the start of exercise. They were instructed to abstain from drinking alcohol and strenuous exercise for 24 h before each trial day.
Thirty minutes after the subjects reported to the laboratory, a baseline blood sample was collected through a venipuncture from an antecubital vein. After providing a 15-mL blood sample, they consumed a 500-mL beverage containing 150 g of glucose within 2 min, at 30 min before the start of exercise. As Kodama et al. (20) reported that Asians, including Japanese, have lower insulin secretory capacity compared with whites and Africans, the large amount of glucose (150 g) was used in this study to maximize the serum insulin level. In the preliminary experiment, we confirmed that the ingestion of 150 g glucose increased the serum insulin level more than twofold compared with ingestion of 75 g glucose in Japanese subjects (data not shown). After consumption of the CHO drink, subjects rested quietly in the laboratory for 25 min. Subjects entered the experimental room (temperature, 22°C ± 1°C; humidity, 50% ± 1%) and then warmed up by cycling ergometer (75XLII; COMBI Wellness) at 60 W for 3 min. Exactly 30 min after the consumption of CHO drink, they started a cycling exercise at 75% V˙O2max for 60 min. Blood samples (15 mL) were collected before the start of exercise (after warm-up), and at 15, 30, and 60 min during exercise through a venipuncture from an antecubital vein without stopping the exercise. Expired air samples were collected at 12 to 15 min, 27 to 30 min, 42 to 45 min, and 57 to 60 min during exercise. HR and RPE were recorded at the same time. Subjects were given 100 mL of water every 15 min during exercise to prevent dehydration (Fig. 1).
FIGURE 1.
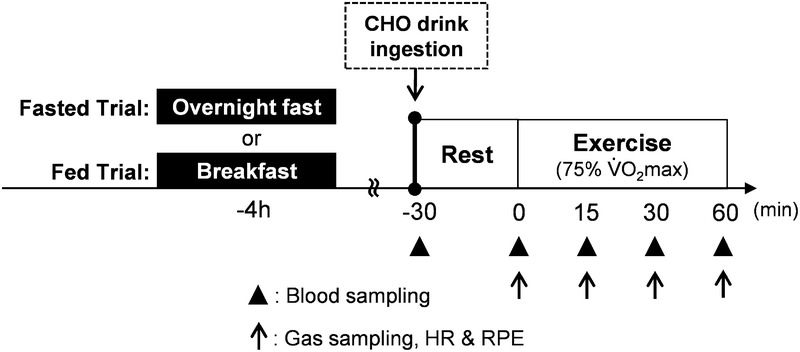
Experimental protocol and timing of measurements.
Blood sample analyses
Plasma and serum were separated by centrifugation at 3000 rpm for 15 min at 4°C and 10°C, respectively.Plasma glucose, serum insulin, and serum free fatty acids (FFA) were determined by BML Inc. (Tokyo, Japan).
The subjects were divided into two groups based on the plasma glucose responses: the “Hypo-group,” who had developed rebound hypoglycemia 15 min after starting the exercise (plasma glucose of ≤4.0 mmol·L−1) and the “Non-hypo group,” who did not develop rebound hypoglycemia. The criteria for hypoglycemia were set according to a previous study (21).
The insulinogenic index (IGI) was calculated as the ratio of increment between the serum insulin level (μU·mL−1) and plasma glucose level (mmol·L−1) during the first 30 min (before drinking and before the start of exercise) after glucose ingestion (22).
Statistical analyses
All data were expressed as mean ± SD. ANOVA for repeated measures on two factors (trial or group–time) was used to examine cardiovascular changes and blood-related parameters. When significant effects were revealed using ANOVA, then post hoc analyses were conducted with the Bonferroni t test. Associations between plasma glucose and each variable were determined using Pearson’s correlation coefficient. Data for IGI were log transformed to provide a normal distribution. All statistical analyses were performed using SPSS version 22.0 (SPSS, Inc., Chicago, IL). Statistical significance was set at P < 0.05.
RESULTS
Plasma glucose
Plasma glucose concentrations throughout the experiment were similar in the Fasted and Fed Trials (Fig. 2A). In the Fasted Trial, plasma glucose concentrations decreased from 7.1 ± 1.4 mmol·L−1 at the start of exercise to a glucose nadir of 4.9 ± 1.5 mmol·L−1 15 min after starting the exercise. On the other hand, plasma glucose concentrations in the Fed Trial decreased from 7.1 ± 1.5 mmol·L−1 at the start of exercise to a glucose nadir of 4.4 ± 1.3 mmol·L−1 15 min after starting the exercise.
FIGURE 2.
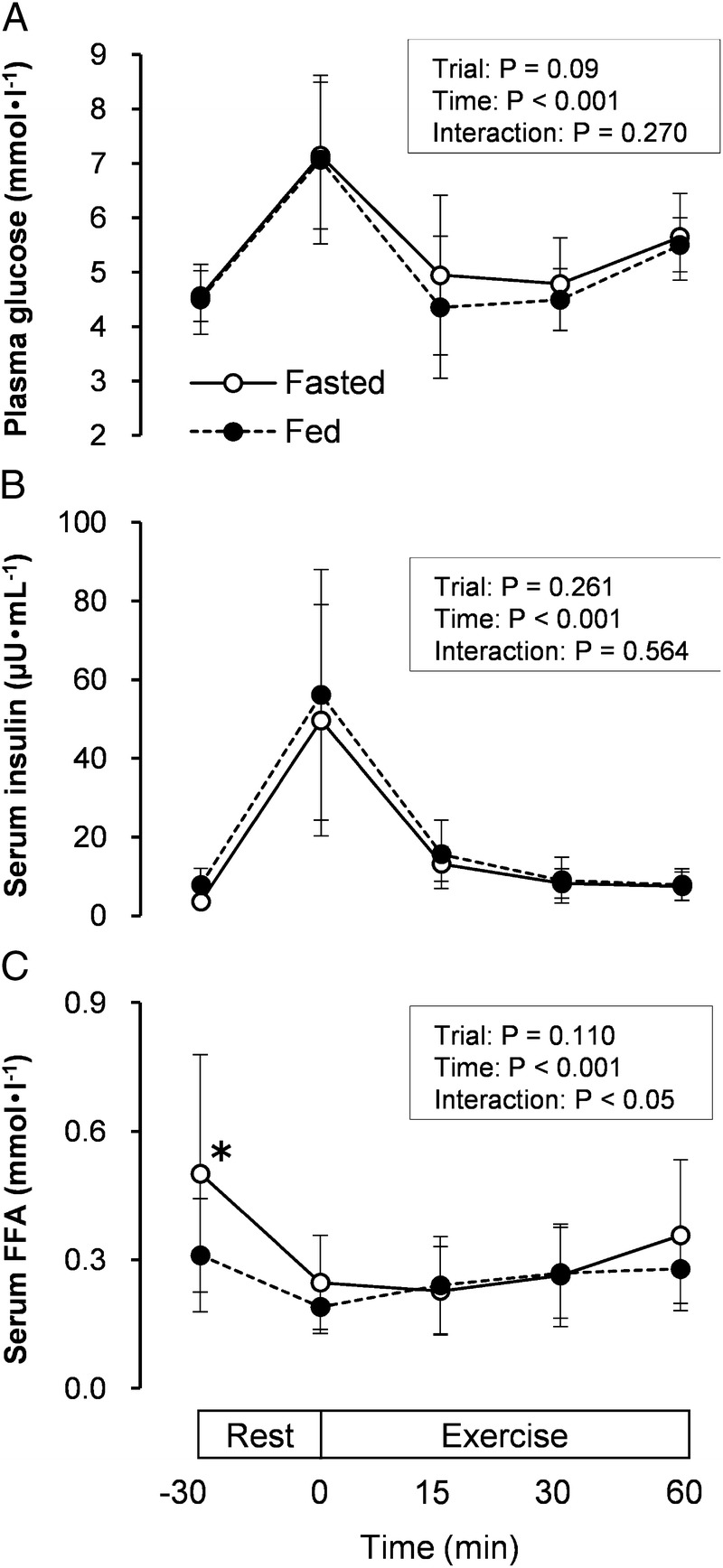
This figure illustrates the plasma glucose (A), serum insulin (B), and serum FFA (C) concentrations at rest and during exercise in the Fasted (○) and Fed (·) Trials. Fasted, overnight fast; Fed, 4 h after breakfast. The values are means ± SD. *Significantly higher than Fed Trial (P < 0.05).
The sharp decline in plasma glucose was observed 15 min after starting the exercise (45 min after glucose ingestion), but both trials did not exhibit a decline in the mean values of plasma glucose below 4 mmol·L−1. In addition, the plasma glucose concentration 15 min after starting the exercise was not significantly different between the Fasted and Fed Trials, although a considerable intersubject variability was found.
Serum insulin
Serum insulin concentrations are presented in Figure 2B, which were 14.0-fold and 7.2-fold higher at the start of exercise that that at the predrink baseline values (−30 min) in the fast and Fed Trials, respectively. However, serum insulin concentrations throughout the experiment were similar in the two trials.
Serum FFA
Serum FFA concentration before CHO ingestion was significantly higher (P = 0.027) in the Fasted Trial than in the Fed Trial (0.50 ± 0.28 mmol·L−1 vs 0.31 ± 0.13 mmol·L−1). However, differences in serum FFA concentrations were not significant between the two trials at any time point during exercise (Fig. 2C).
V˙O2, RER, HR, and RPE
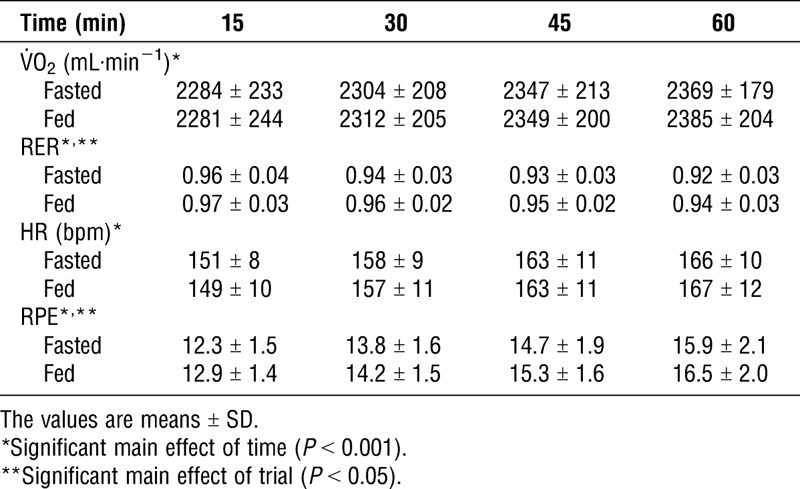
Respiratory data, HR, and RPE are provided in Table 1. No significant interaction was observed between trials and time for all measurement data. However, a main effect of the trial was observed for RER (P = 0.024) and RPE (P = 0.033). RER and RPE were significantly lower in the Fasted Trial than those in the Fed Trial, although we could not explain the reason for the decrease in RPE observed in the Fasted Trial.
TABLE 1.
Cardiorespiratory responses during exercise.
Factors associated with hypoglycemia
The individual plasma glucose responses from the start of exercise to 15 min after are shown in Figures 3A and B. Five of 16 subjects developed transient hypoglycemia (plasma glucose of ≤4.0 mmol·L−1) in the Fasted Trial, and 7 of 16 subjects developed transient hypoglycemia in the Fed Trial. Three subjects developed transient hypoglycemia in both Fasted and Fed Trials. The Hypo-group as well as the Non-hypo group did not show any symptoms (e.g., tiredness, irritability, hunger) during exercise, and no significant difference in RPE was observed between the Hypo-group and Non-hypo group in both Fasted and Fed Trials (Fasted, P = 0.072; Fed: P = 0.686).
FIGURE 3.
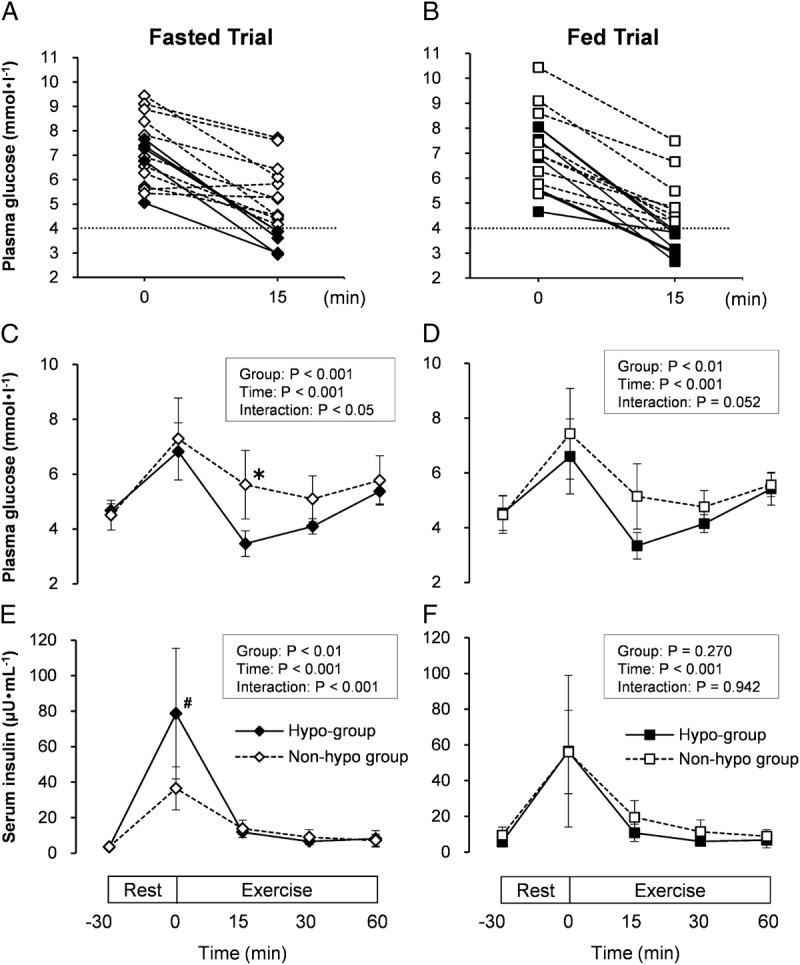
Individual subject’s plasma glucose at 0 and 15 min of exercise (A), plasma glucose (C), and serum insulin (E) concentrations at rest and during exercise in the Fasted Trials. Individual subject’s plasma glucose at 0 and 15 min of exercise (B), plasma glucose (D), and serum insulin (F) concentrations at rest and during exercise in the Fed Trials. Five or seven subjects developed transient hypoglycemia at 15 min after starting the exercise in the Fasted and Fed Trials, respectively. Hypo-group: subjects who had developed rebound hypoglycemia at 15 min after starting the exercise (plasma glucose of ≤4.0 mmol·L−1), Non-hypo group: subjects who did not develop rebound hypoglycemia. ♦, Hypo-group; ◊, Non-hypo group in the Fasted Trial; and ▪, Hypo-group; □, Non-hypo group in the Fed Trial. Fasted, overnight fast; Fed, 4 h after breakfast. The values are means ± SD. *Significantly lower than Non-hypo group (P < 0.05). #Significantly higher than Non-hypo group (P < 0.001).
Plasma glucose level 15 min after starting the exercise was significantly lower (P = 0.026) in the Hypo-group than Non-hypo group in the Fasted Trial (3.5 ± 0.5 mmol·L−1 vs 5.6 ± 1.2 mmol·L−1) (Fig. 3C). In addition, it was nearly significantly lower (P = 0.052) in the Hypo-group compared with the Non-hypo group in the Fed Trial (3.3 ± 0.5 mmol·L−1 vs 5.1 ± 1.2 mmol·L−1) (Fig. 3D).
The serum insulin concentrations of both Hypo-group and Non-hypo group are shown in Figures 3E and F. In the Fed Trial, no significant differences in serum insulin concentrations were observed between the Hypo and Non-hypo groups at any of the time points. In contrast, the serum insulin concentration at the start of exercise (30 min after glucose ingestion) in the Hypo-group was significantly higher (P < 0.001) than that in the Non-hypo group (78.6 ± 36.8 μU·mL−1 vs 36.5 ± 12.1 μU·mL−1) in the Fasted Trial.
As serum insulin concentrations at the start of exercise were significantly higher in the Hypo-group than in the Non-hypo group in the Fasted Trial (Fig. 3E), we have investigated the relationship between plasma glucose at 15 min after starting the exercise and IGI, which is an index of insulin secretory capacity. As a result, a significant negative correlation was found between plasma glucose at 15 min after starting the exercise and log-transformed IGI (r = −0.691; P = 0.003). This relationship was nonlinear, and the power law was the best fitting model (r2 = 0.65) (Fig. 4). Furthermore, the correlation coefficients were comparable between Hypo and Non-hypo groups (r = −0.801 and r = −0.757, respectively). In contrast, the difference in serum insulin concentrations was not found between the Hypo and the Non-hypo groups at the start of exercise in the Fed Trial (56.5 ± 42.5 μU·mL−1 vs 56.0 ± 23.4 μU·mL−1) (Fig. 3F).
FIGURE 4.
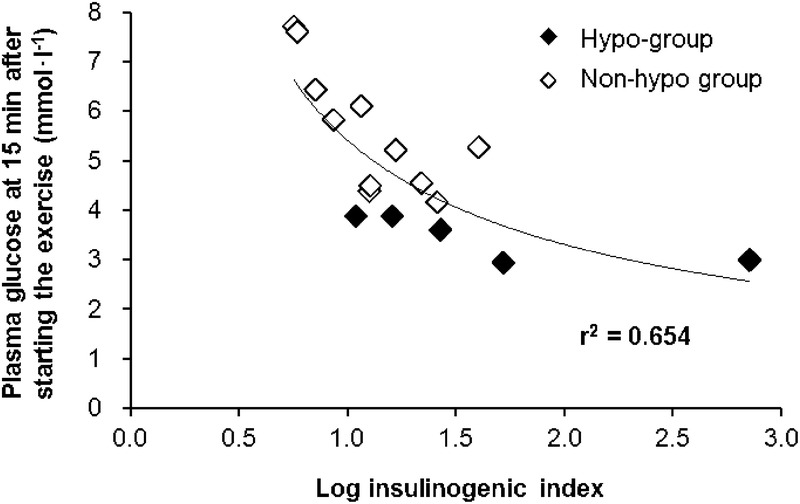
Relationship between log IGI and plasma glucose at 15 min after the onset of exercise in the Fasted Trial.
DISCUSSION
In this study, whether rebound hypoglycemia after preexercise CHO ingestion occurs not only in the fasted state but also in the fed state was first investigated. Consequently, we found that the mean values of plasma glucose concentration were similar in the Fasted and Fed Trials, and they did not fall below 4 mmol·L−1 (Fig. 2A). However, the individual plasma glucose responses have shown that five (Fasted Trial) and seven (Fed Trial) subjects developed transient hypoglycemia at 15 min after starting the exercise. These results indicate that transient hypoglycemia occurs not only in the fasted condition but also in the fed condition similar to a real-life situation as well as a sporting event.
In a series of studies that have investigated the effects of the amount of CHO feeding (16), timing of CHO intake (17), intensity of exercise (18), and type of CHO ingestion (19) on rebound hypoglycemia, the mean value of plasma glucose during exercise was not associated with rebound hypoglycemia in each study; however, some individuals developed rebound hypoglycemia, and the proportion was 25% to 65%. Consistent with the previous studies, our data showed that, not all, but some, subjects developed transient hypoglycemia in both Fasted (31%) and Fed (44%) Trials and provided additional evidence that factors such as individual differences were associated with the occurrence of rebound hypoglycemia. Subjects who developed hypoglycemia under the Fasted Trial showed significantly higher serum insulin levels at the start of exercise when compared with the Non-hypo group (Fig. 3E). Furthermore, a significant negative correlation was observed between the plasma glucose at 15 min after starting the exercise and log IGI. These data suggest that subjects with high insulin secretory capacity are more prone to develop rebound hypoglycemia in the fasted state. Skeletal muscle accounts for at least 80% of glucose uptake in humans (23). Insulin enhances glucose uptake in skeletal muscle via translocation of the intracellular glucose transporter GLUT-4 to the plasma membrane (24). Therefore, it is plausible that glucose uptake in skeletal muscle may have been substantially enhanced in subjects who had higher insulin responses at the start of exercise, resulting in the development of rebound hypoglycemia under the Fasted Trial.
Unlike the Fasted Trial, serum insulin at the start of exercise did not significantly differ between the Hypo-group and Non-hypo group in the Fed Trial (Fig. 3F). This result indicates that other factors rather than insulin responses may be involved in rebound hypoglycemia under the Fed Trial. In our results, the absolute value of V˙O2max (mL·min−1) was significantly higher in the Hypo-group than the Non-hypo group, which was negatively correlated with plasma glucose at 15 min after starting the exercise (data not shown), although we cannot explain the reason behind this phenomenon at present. Future studies will be required to determine why no difference was found in the serum insulin between the Hypo and Non-hypo groups in the Fed Trial and to clarify how higher V˙O2max is associated with rebound hypoglycemia. In any case, the fact that nearly half of the subject developed rebound hypoglycemia in the Fed Trial suggests that endurance athletes who generally run races after consuming a meal 3 to 4 h before exercise should carefully ingest a large amount of CHO 30 min before races.
One limitation of this experiment is the use of IGI as an indicator of insulin secretory capacity. Although IGI has been widely used as an index of insulin secretory capacity, it is an indirect measurement and may be influenced by various factors. To more accurately measure the insulin secretory capacity, the hyperglycemic clamp technique should be used, which is the gold standard method (25). Second, we did not control the meals, except for the dinner the day before the experiment. In addition, we also did not restrict strenuous exercise more than 24 h before starting the experiment. These factors may influence muscle and/or liver glycogen levels; therefore, we cannot rule out the possibility that these factors affect the incidence of rebound hypoglycemia. In future work, meals and exercise should be controlled for a longer period to minimize the potential influence on hypoglycemia.
In conclusion, the present study demonstrates that transient hypoglycemia occurring shortly after starting the exercise is not only observed in the overnight fasted state but also in the fed state. Furthermore, subjects with enhanced insulin responses seem to be more prone to developing transient hypoglycemia after preexercise CHO ingestion under fasted condition. However, because the difference in insulin concentration was not observed under the fed condition, further investigation will be required.
Acknowledgments
This work was supported by a Grant-in-Aid for Challenging Exploratory Research (Higuchi M. number 15K12673) from the Japan Society for the Promotion of Science. Beverages used in this study were manufactured and supplied by Asahi Soft Drinks Company, Ltd., Ibaraki, Japan.
There is no conflict of interest. The results of the present study do not constitute endorsement of the American College of Sports Medicine. All authors declare that the results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Holloszy JO, Kohrt WM, Hansen PA. The regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci. 1998;3:D1011–27. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71:140–50. [DOI] [PubMed] [Google Scholar]
- 3.Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol. 1986;61(1):165–72. [DOI] [PubMed] [Google Scholar]
- 4.Burke LM, Hawley JA, Wong SH, Jeukendrup AE. Carbohydrates for training and competition. J Sports Sci. 2011;29(1 Suppl):S17–27. [DOI] [PubMed] [Google Scholar]
- 5.Costill DL, Coyle E, Dalsky G, Evans W, Fink W, Hoopes D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(4):695–9. [DOI] [PubMed] [Google Scholar]
- 6.Foster C, Costill DL, Fink WJ. Effects of preexercise feedings on endurance performance. Med Sci Sports. 1979;11(1):1–5. [PubMed] [Google Scholar]
- 7.Koivisto VA, Karonen SL, Nikkilä EA. Carbohydrate ingestion before exercise: comparison of glucose, fructose, and sweet placebo. J Appl Physiol Respir Environ Exerc Physiol. 1981;51(4):783–7. [DOI] [PubMed] [Google Scholar]
- 8.Hargreaves M, Costill DL, Katz A, Fink WJ. Effect of fructose ingestion on muscle glycogen usage during exercise. Med Sci Sports Exerc. 1985;17(3):360–3. [PubMed] [Google Scholar]
- 9.Koivisto VA, Härkönen M, Karonen SL, et al. Glycogen depletion during prolonged exercise: influence of glucose, fructose, or placebo. J Appl Physiol. 1985;58(3):731–7. [DOI] [PubMed] [Google Scholar]
- 10.Short KR, Sheffield-Moore M, Costill DL. Glycemic and insulinemic responses to multiple preexercise carbohydrate feedings. Int J Sport Nutr. 1997;7(2):128–37. [DOI] [PubMed] [Google Scholar]
- 11.van Zant RS, Lemon PW. Preexercise sugar feeding does not alter prolonged exercise muscle glycogen or protein catabolism. Can J Appl Physiol. 1997;22(3):268–79. [DOI] [PubMed] [Google Scholar]
- 12.Jentjens RL, Jeukendrup AE. Prevalence of hypoglycemia following pre-exercise carbohydrate ingestion is not accompanied by higher insulin sensitivity. Int J Sport Nutr Exerc Metab. 2002;12(4):398–413. [DOI] [PubMed] [Google Scholar]
- 13.Kuipers H, Fransen EJ, Keizer HA. Pre-exercise ingestion of carbohydrate and transient hypoglycemia during exercise. Int J Sports Med. 1999;20(4):227–31. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell JB, Braun WA, Pizza FX, Forrest M. Pre-exercise carbohydrate and fluid ingestion: influence of glycemic response on 10-km treadmill running performance in the heat. J Sports Med Phys Fitness. 2000;40(1):41–50. [PubMed] [Google Scholar]
- 15.Jeukendrup AE, Killer SC. The myths surrounding pre-exercise carbohydrate feeding. Ann Nutr Metab. 2010;57(2 Suppl):18–25. [DOI] [PubMed] [Google Scholar]
- 16.Jentjens RL, Cale C, Gutch C, Jeukendrup AE. Effects of pre-exercise ingestion of differing amounts of carbohydrate on subsequent metabolism and cycling performance. Eur J Appl Physiol. 2003;88(4–5):444–52. [DOI] [PubMed] [Google Scholar]
- 17.Moseley L, Lancaster GI, Jeukendrup AE. Effects of timing of pre-exercise ingestion of carbohydrate on subsequent metabolism and cycling performance. Eur J Appl Physiol. 2003;88(4–5):453–8. [DOI] [PubMed] [Google Scholar]
- 18.Achten J, Jeukendrup AE. Effects of pre-exercise ingestion of carbohydrate on glycemic and insulinemic responses during subsequent exercise at differing intensities. Eur J Appl Physiol. 2003;88(4–5):466–71. [DOI] [PubMed] [Google Scholar]
- 19.Jentjens RL, Jeukendrup AE. Effects of pre-exercise ingestion of trehalose, galactose and glucose on subsequent metabolism and cycling performance. Eur J Appl Physiol. 2003;88(4–5):459–65. [DOI] [PubMed] [Google Scholar]
- 20.Kodama K, Tojjar D, Yamada S, et al. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gleeson M, Maughan RJ, Greenhaff PL. Comparison of the effects of pre-exercise feeding of glucose, glycerol and placebo on endurance and fuel homeostasis in man. Eur J Appl Physiol Occup Physiol. 1986;55(6):645–53. [DOI] [PubMed] [Google Scholar]
- 22.Seltzer HS, Allen EW, Herron AL, Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967;46(3):323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Ferrannini E, Sato Y, et al. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest. 1981;68(6):1468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol. 1997;273(6 Pt 1):E1039–51. [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. [DOI] [PubMed] [Google Scholar]


