Supplemental digital content is available in the text.
Key Words: gadolinium, gadolinium-based contrast agents, gadoterate meglumine, gadodiamide, juvenile rats, pediatric radiology, cerebellum, deep cerebellar nuclei
Abstract
Objectives
The main objective of the study was to assess the effect of age on target tissue total gadolinium (Gd) retention after repeated administration of gadodiamide (linear) or gadoterate (macrocyclic) Gd-based contrast agent (GBCA) in rats. The secondary objective was to assess the potential developmental and long-term consequences of GBCA administration during neonatal and juvenile periods.
Materials and Methods
A total of 20 equivalent human clinical doses (cumulated dose, 12 mmol Gd/kg) of either gadoterate or gadodiamide were administered concurrently by the intravenous route to healthy adult and juvenile rats. Saline was administered to juvenile rats forming the control group. In juvenile rats, the doses were administered from postnatal day 12, that is, once the blood-brain barrier is functional as in humans after birth. The tests were conducted on 5 juvenile rats per sex and per group and on 3 adult animals per sex and per group. T1-weighted magnetic resonance imaging of the cerebellum was performed at 4.7 T during both the treatment and treatment-free periods. Behavioral tests were performed in juvenile rats. Rats were euthanatized at 11 to 12 weeks (ie, approximately 3 months) after the last administration. Total Gd concentrations were measured in plasma, skin, bone, and brain by inductively coupled plasma mass spectrometry. Cerebellum samples from the juvenile rats were characterized by histopathological examination (including immunohistochemistry for glial fibrillary acidic protein or GFAP, and CD68). Lipofuscin pigments were also studied by fluorescence microscopy. All tests were performed blindly on randomized animals.
Results
Transient skin lesions were observed in juvenile rats (5/5 females and 2/4 males) and not in adult rats having received gadodiamide. Persisting (up to completion of the study) T1 hyperintensity in the deep cerebellar nuclei (DCNs) was observed only in gadodiamide-treated rats. Quantitatively, a slightly higher progressive increase in the DCN/brain stem ratio was observed in adult rats compared with juvenile rats, whereas no difference was noted visually. In all tissues, total Gd concentrations were higher (10- to 30-fold higher) in the gadodiamide-treated groups than in the gadoterate groups. No age-related differences were observed except in bone marrow where total Gd concentrations in gadodiamide-treated juvenile rats were higher than those measured in adults and similar to those measured in cortical bone tissue. No significant treatment-related effects were observed in histopathological findings or in development, behavior, and biochemistry parameters. However, in the elevated plus maze test, a trend toward an anxiogenic effect was observed in the gadodiamide group compared with other groups (nonsignificant). Moreover, in the balance beam test, a high number of trials were excluded in the gadodiamide group because rats (mainly males) did not completely cross the beam, which may also reflect an anxiogenic effect.
Conclusions
No T1 hyperintensity was observed in the DCN after administration of the macrocyclic GBCA gadoterate regardless of age as opposed to administration of the linear GBCA gadodiamide. Repeated administration of gadodiamide in neonatal and juvenile rats resulted in similar total Gd retention in the skin, brain, and bone to that in adult rats with sex having no effect, whereas Gd distribution in bone marrow was influenced by age. Further studies are required to assess the form of the retained Gd and to investigate the potential risks associated with Gd retention in bone marrow in juvenile animals treated with gadodiamide. Regardless of age, total Gd concentration in the brain and bone was 10- to 30-fold higher after administration of gadodiamide compared with gadoterate.
The safety profile of gadolinium (Gd)-based contrast agents (GBCAs) is generally considered excellent in most patients, including children from birth. However, GBCAs are occasionally associated with hypersensitivity reactions and the development of a severe delayed adverse reaction, nephrogenic systemic fibrosis (NSF), in patients with severe or end-stage kidney failure treated with linear GBCAs (L-GBCAs).1 It was suggested that a causal link could exist between the disease and GBCA administration in 2006.2 Health authorities and radiology learned societies therefore issued guidelines prohibiting administration of L-GBCAs (including gadodiamide) in patients with severe and end-stage renal failure.3–6 The European Medicines Agency has also contraindicated the use of those GBCAs with high risk for NSF in neonates under the age of 4 weeks.6 Almost no new cases have been reported since 2010.7
However, a new safety concern arose 4 years ago when Kanda et al8 demonstrated that Gd remained present in certain brain structures in patients with normal renal function after repeated administration of L-GBCAs. Several reports9 promptly confirmed increased signal intensity in the dentate nucleus (a deep cerebellar nucleus [DCN]) on unenhanced T1-weighted images after multiple administration of GBCAs, and Gd presence in brain tissues thereafter. The phenomenon was also reproduced in preclinical studies.10 The administered GBCA dose was found to be correlated with changes in T1 hyperintensity.11 Clinical and preclinical studies strongly suggest that this T1 effect depends on the molecular structure of the administered GBCA and thus its thermodynamic stability as previously observed with NSF. The clinical consequences of Gd retention are currently unknown. The European Medicines Agency recently recommended restricting the use of some L-GBCAs (gadobenate dimeglumine, disodium gadoxetate) used in magnetic resonance imaging (MRI) body scans or even suspending12 the marketing authorization of others (gadopentetate dimeglumine, gadodiamide, gadoversetamide). In Japan, health authorities restricted L-GBCAs as second-line agents while maintaining macrocyclic GBCAS (M-GBCAs) as first-line agents.13,14 In the United States, the Food and Drug Administration acknowledged that L-GBCAs result in more Gd retention in the body and retention for a longer time than M-GBCAs.15 Food and Drug Administration requested several actions: (1) changes to the labeling of GBCAs, (2) the development of a new Patient Medication Guide for GBCAs, and (3) requested manufacturers to conduct preclinical and clinical studies to further assess the safety of these agents.15
This phenomenon is usually reported in patients with normal renal function. However, renal failure obviously leads to longer Gd exposure and potentiates T1 signal hyperintensities in brain structures as demonstrated in dialyzed patients16 and in rats with moderate renal impairment.17,18 Patients with renal insufficiency, including elderly patients, therefore comprise an at-risk population for Gd retention after L-GBCA administration. Another population that can be considered vulnerable is the pediatric population. T1 hyperintensities have indeed been reported in children19–31 as well as Gd retention in the brain.32,33 It is currently unknown if children are more susceptible than adults to accumulation or retention of Gd in the brain and to its potential adverse effects. The morphological, physiological, and behavioral characteristics of children are different to those of adults,34,35 and many developmental processes occur during the neonatal and postnatal periods. These may affect drug pharmacokinetics and pharmacodynamics and potentially result in an increase or, conversely, a decrease in drug toxicity in the pediatric population. Several factors contribute to these potential differences in children and adults. For example, renal function immaturity in neonates and infants36 may result in increased systemic exposure of renally eliminated drugs such as GBCAs, leading to tissue retention of Gd in growing tissues. Furthermore, compared with adults, the developing brains of children are immature, increasing the risk of potential brain damage and later neurological disorders.37 For many years, it was firmly believed that the blood-brain barrier (BBB) was absent or immature in fetuses and newborns. However, new evidence shows that several adult protective mechanisms, including functionally effective tight junctions between endothelial and epithelial cells in the BBB and blood–cerebrospinal fluid (CSF; choroid plexus) interfaces as well as many transport mechanisms, are already present in the early stages of development.38,39 Certain transport mechanisms are unique to the developing brain, for example, transfer of plasma proteins to CSF, and may lead to increased drug entry in the brain and thus intensify their neurotoxic effects. Finally, the developing brain is vulnerable mainly due to potential drug or toxin interactions with neurodevelopmental processes.37,40 In the case of GBCAs, it is currently unknown whether these processes are affected by the presence of Gd. It appears essential to investigate whether Gd accumulation is higher in the developing brain than in adults receiving similar doses and to better assess the potential risks of repeated administration of GBCAs in children, a vulnerable population.
The first objective of this nonclinical study was to compare the T1 signal enhancement in the cerebellum and the degree of Gd retention in the tissues of juvenile and adult rats after repeated intravenous administration of equivalent clinical doses of GBCAs. The second objective was to assess the potential toxicological consequences of repeated administration of GBCAs during development and in adulthood of rats treated during the neonatal and juvenile periods. Two GBCAs from 2 different categories were compared, a M-GBCA, gadoterate meglumine, and an L-GBCA, gadodiamide.
MATERIALS AND METHODS
Materials
Two GBCAs were tested: gadoterate meglumine (Dotarem; Guerbet, Villepinte, France) and gadodiamide (Omniscan; GE Healthcare, Chalfont St Giles, United Kingdom). They were used in the form of the commercially available solution for injection, supplied at a concentration of 0.5 mmol/mL. Isotonic saline (Lavoisier, Paris, France) was used as the control.
Animals
All experimental procedures were performed in accordance with French regulations and in compliance with European Economic Community Directive 2010/63/EU on animal welfare.
The study was carried out on Sprague-Dawley rats (Crl: OFA) obtained from Charles River (Charles River Laboratories, L'Arbresle, France). Food and water were provided ad libitum. A 12-hour light/dark cycle was maintained. The average ambient temperature in the animal room ranged from 19°C to 25°C, and relative humidity ranged from 35% to 70%.
Regarding the juvenile rats, 3 lactating female rats, each with a litter of 5 male and 5 female pups, were received and housed on postnatal day 8 (PND 8). All pups from the same litter were allocated to the same product. Animals were weaned at PND 23. After weaning, animals were housed by sex and litter (and consequently, per group).
Regarding the adults rats, animals were randomized and allocated to treatment groups in a fully blinded manner. The rats were housed 2 or 3 per cage, irrespective of the treatment. The tests were conducted on 3 adult rats per sex and per group.
Experimental Design
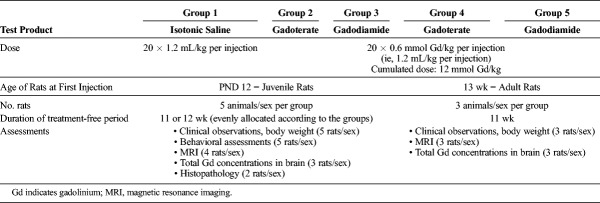
The study was conducted with the following 5 test groups (Table 1):
TABLE 1.
Test Groups
Group 1 (the control group) included juvenile rats (5 rats/sex), which were given 20 injections of saline (as the control) at the same volume as that used for the groups receiving the contrast agents (ie, 1.2 mL/kg).
Groups 2 and 3 included juvenile rats (5 rats/sex per group), which were administered 20 × 0.6 mmol Gd/kg per injection (in a volume of 1.2 mL/kg) of either gadoterate or gadodiamide, respectively.
Groups 4 and 5 included adult rats (3 rats/sex per group), which were administered 20 × 0.6 mmol/kg per injection (in a volume of 1.2 mL/kg) of either gadoterate or gadodiamide, respectively.
The same dosing scheme was used for both the juvenile and adult rats. Dosing started at 12 days of age (PND 12) for the juvenile groups and at 13 weeks old for the adult rats. The animals received 2 injections per week during the 2 first weeks (corresponding to the preweaning period) followed by 4 injections/week for 4 weeks. A total of 20 doses (cumulative dose, 12 mmol/kg) were administered.
The products were injected intravenously into the caudal vein in all rats, in conscious juveniles before weaning (at PND 12, 15, 19, and 22) and after isoflurane (IsoFlo; Axience, Pantin, France) anesthetization for subsequent administrations in weaned juveniles and in all adult rats.
The duration of the treatment-free period (11–12 weeks, ie, approximately 3 months) was comparable in both juvenile and adult rats.
The 0.6 mmol Gd/kg dose corresponds to the clinical dose (0.1 mmol Gd/kg) adjusted to the body surface area of the specific rat species according to US Food and Drug Administration guidelines.41
Magnetic Resonance Imaging Protocol
T1-weighted MRI was performed under general anesthesia (3%–3.5% isoflurane) at weeks 3, 4, 5, 6, and 7 (ie, after 4, 8, 12, 16, and 20 administrations, respectively) during the administration period (Fig. 1). During the treatment-free period, MRI was performed on weeks 9, 12, and 15. A preinjection MRI was performed only in adult rats. For logistic reasons, MRI was performed at random on 4 rats per sex and per group for the juvenile animals (groups 1, 2, and 3). Magnetic resonance imaging was performed with a dedicated phased-array quadrature head coil in a gradient/shims insert B-GA 12S HP (660 mT/m intensity and 4570 T/m per second maximum slew rate) on a 4.7 T preclinical magnet (Biospec 47/40; Bruker, Ettlingen, Germany). A T1-weighted 2D FLASH sequence was used: repetition time/echo time, 50/1.78 milliseconds; 48 averages; in-plane resolution, 164 × 164 μm2; slice thickness, 700 μm; acquisition time, 6 minutes 36 seconds. The scan range of the MRI sequence covered only the cerebellum (11 slices).
FIGURE 1.
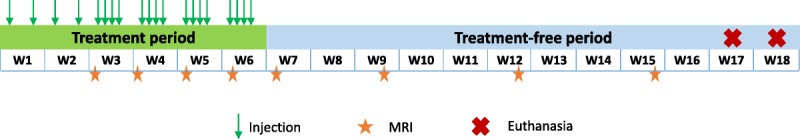
Experimental design.
Image Analysis
All T1-weighted image analyses were performed blinded (both test groups and time points) after randomization. Both qualitative and quantitative assessments of DCN T1 signal intensity were performed as described elsewhere.42
In brief, 3 different readers performed a qualitative visual assessment using a 3-point scoring scale: 0 was given for no detectable enhancement in the DCN region, 1 for doubtful enhancement, and 2 for definite enhancement. The images were analyzed quantitatively by positioning a region of interest (ROI) over the 2 DCN zones and over a reference zone in the brain stem and then by calculating the DCNmax/brain stem ratio.
Developmental and Behavioral Assessments
The methods are described in detail in the Supplementary methods section, Supplemental Digital Content, http://links.lww.com/RLI/A405.
Behavioral assessments were performed only in juvenile animals (groups 1, 2, and 3).
Before weaning, certain developmental reflexes were evaluated under white light (350–500 lux), that is, righting reflex, grasp reflex, cliff avoidance, negative geotaxis behavior,43 as well as some physical landmarks such as eye opening and fur development.
After weaning, behavioral assessments were performed between 8:00 am and 1:00 pm, under red light (100–160 lux), as follows:
The open-field test was used to assess locomotor activity and anxiety. At week 6 (end of the treatment period), week 9 (ie, 3 weeks after the last administration), and week 13 (ie, 7 weeks after the last administration), each rat was placed individually in a 1 × 1-m open-field box for 5 minutes. The number of visits to each zone (center and periphery), time spent in each zone (second) or immobility, distance moved (centimeter), and average speed (centimeter/second) were recorded using ActualTracks video tracking software (Actual Analytics, Edinburgh, United Kingdom). Visual examination was also used to record incidences of grooming/rearing.
The balance beam test was used to investigate motor coordination at week 8.44 The time needed to completely cross a narrow wooden beam (maximum authorized time 90 seconds) and the number and distances of foot faults (slips) were assessed. An alternative method (5-point scoring scale adapted from Metz et al45) was used to assess the ability of the rat to travel the beam. A score of 2 indicates normal walking on the beam, a score of 1 indicates that the rat was able to travel over more than half of the beam (but not the whole length) whereas a score of 0 indicates no walking on the beam. The scores of 2 trials were added, and a maximum score of 4 could be achieved.
The modified Irwin test was used to assess general behavior and physiological state46 at both week 9 and week 13.
The hidden pellet test was used to assess olfaction at week 10.47 It measures latency to find a hidden piece of food piece (chocolate chip).
The Elevated Plus Maze (EPM) was used to investigate anxiety at week 11.48 The time spent in the open/closed arms and the number of entries into the open/closed arms were recorded manually for 10 minutes. The percent of open arm time was calculated as follows: time spent in open arms/(time spent in open arms + time spent in closed arms) × 100.
The Morris water maze test was performed at week 12 (for males) or week 14 (for females) to test spatial learning and memory using a standard protocol.49
Blood and Tissue Collection
At study completion on week 17, 2 rats per sex and per group (groups 1, 2, and 3; Table 1) were euthanized by transcardiac perfusion (saline followed by formalin) under 5% isoflurane. The cerebellums were carefully harvested and post-fixed in 10% neutral buffered formalin at room temperature for subsequent histopathological analysis.
At week 17 or week 18, 3 rats per sex and per group (all groups; Table 1) were euthanized by exsanguination under 5% isoflurane. Plasma was collected after centrifugation and frozen at −20°C. The femurs were removed and divided as follows: bone marrow, epiphyses (corresponding to “trabecular bone”), and diaphyses (corresponding to “cortical bone”). The brains and skin were also collected. The forebrains were harvested. The following structures were dissected: the cortical forebrain (including the amygdala), the olfactory bulbs, the hippocampus, the “subcortical brain” (including the midbrain, the hypothalamus, and the thalamus), and the striatum. The cerebellums were sliced using a Brain Slicer Matrix (Stoelting Co, Wood Dale, IL) after freezing at −20°C, and the DCNs, cerebellums tissue apart from the DCNs (referred to as “cerebellum”), and the brain stems were carefully dissected as previously described.18 Choroid plexuses were also sampled (pooled from different ventricles). All tissues were frozen at −20°C for subsequent determination of total Gd concentrations.
Histopathological Analysis of Cerebellum
Histopathological analysis was performed blindly in 2 rats per sex and per group (randomized) in juvenile animals only.
After fixation of the cerebellums in 10% neutral buffered formalin, they were embedded in paraffin, and then sectioned (3–4 μm thick). They were trimmed transversally, cranially to the DCN, and sections were cut at the reference level of plates 124 to 130 in keeping with the fifth edition of Paxinos and Watson's atlas.50
One slide per cerebellum was stained with cresyl violet and hematoxylin and eosin, and assessed blindly by a pathologist to screen for any microscopic abnormalities.
One section per cerebellum was dewaxed, stained with DAPI (4′,6-diamidino-2-phenylindole), and mounted for direct fluorescence observation. Each slide was scanned using a Nanozoomer XT slide imager (Hamamatsu Photonics K.K., Shizuoka, Japan) and the TRITC (tetramethylrhodamine) and DAPI channels. Fluorescent pigments in the TRITC were detected in the neuronal perikarya and interpreted as lipofuscin pigments. Two methods of quantification were used: (1) classification of each neuron in the ROI according to pigment density and (2) quantification of signal area in the ROI using the Image J processing program (for determination of mean positive area fraction per neuron for the total neuron count).
Immunohistochemical staining was also performed to detect GFAP and CD68 using rabbit anti-GFAP antibody (diluted 1:1000, Abcam, Paris, France) and rabbit anti-CD68 antibody (diluted 1:200, Abcam) and anti-rabbit HRP-polymer (Diagomics, Blagnac, France) as secondary antibody. After immunostaining, the slides were counterstained in hematoxylin and cover-slipped with resin. Signal distributions and prevalence were assessed on a scale ranging from 0 (no signal) to 3 (high density of signal) in the DCN area.
Determination of Total Gadolinium Concentrations
Total Gd concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS; 7700x Agilent Technologies, Santa Clara, CA) after sample mineralization in 65% nitric acid (HNO3) for 8 hours at a temperature of 80°C. Gadolinium concentrations in plasma, bone, and brain tissues were analyzed by Kymos Pharma Services (Barcelona, Spain), whereas skin samples were analyzed in-house.
A standard curve of inorganic Gd (0.05–100 μg/L) in 6.5% HNO3 was used by monitoring the response of the 158Gd isotope. The lower limit of quantification (LLOQ) of Gd with the ICP-MS instrument was 0.32 nmol/L in HNO3 matrix, that is, approximately 0.02 nmol/mL for plasma, 0.41 nmol/g for DCN, 1.86 nmol/g for the plexus, 0.04 nmol/g for the striatum, 0.02 nmol/g for other brain tissues, 0.01 nmol/g for bone, and 0.2 nmol/g for skin.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD).
Shapiro-Wilk test was used to assess the normality of the data distributions in each group. Data with homogeneous variances and a normal distribution in all groups were analyzed using 1-way analysis of variance (ANOVA) or 2-way ANOVA followed by Dunnett test, Bonferroni test, or Tukey test for multiple comparisons when the ANOVA was significant. Data showing nonhomogeneous variances or a nonnormal distribution were analyzed using the Kruskal-Wallis test followed by Dunn test when the Kruskal-Wallis test was found to be significant.
For calculation of the means, SDs, and for statistical tests on Gd levels in tissues, values less than LLOQ were arbitrarily replaced by the LLOQ value.
Outliers for all data were detected and excluded from the analysis according to the Dixon test (5% risk).
Statistical analyses were carried out using GraphPad Prism 7 software (GraphPad Software Inc, San Diego, CA). Differences were considered significant at P ≤ 0.05.
RESULTS
Clinical Signs and Behavioral Assessments
Two rats died (1 juvenile male in the gadodiamide group and 1 adult female in the gadoterate group) due to anesthesia during the treatment period. These animals were therefore excluded from the study (no treatment-related effect).
One male rat from the juvenile gadodiamide group was found dead at week 15 (PND 113), that is, 9 weeks after the last administration.
Scabs and alopecia (Fig. 2) were observed in all juvenile female rats (5/5) treated with gadodiamide from week 9 (PND 70), that is, approximately 3 weeks after the last administration. Two of the 4 juvenile male rats treated with gadodiamide had scabs without alopecia. The lesions regressed spontaneously in all rats and complete recovery was observed at week 12 (PND 90). No skin effects were observed in adult gadodiamide-treated rats. No skin effects were observed in the control and gadoterate groups (neither in juveniles nor in adults).
FIGURE 2.

Typical dorsal skin lesions of a female juvenile rat treated with gadodiamide (PND 70; week 9).
No significant treatment-related effects were observed on mean body weight. Developmental reflexes and general behavior were not affected by treatment, irrespective of the test group. No behavioral test abnormalities (water maze, open-field, hidden pellet tests) were observed. However, in the EPM test, a trend toward a lower mean time in the time spent in the open (anxiogenic) arms (Fig. 3A) and in the percent of open arm entry (Fig. 3B) was observed in the gadodiamide group compared with the saline-treated group and the gadoterate group (nonsignificant).
FIGURE 3.
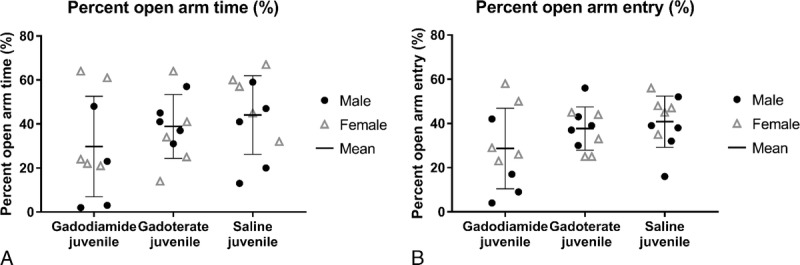
Behavior (at week 11) of juvenile rats treated with gadodiamide, gadoterate, or saline placed in the elevated plus maze. A, Percent time spent in open arms. B, Percent open arm entry. Data are presented as individual data (males vs females) and mean ± SD.
In the balance beam test, no differences in the number of foot slips, the distance travelled on the beam and mean duration to travel the beam (for successful trials) were observed between groups. However, several trials were excluded due to rats not walking on the beam 12/18 trials in the gadodiamide group, 7/20 trials in the gadoterate group, and 0/20 trials in the saline group). Using the alternative method (scoring system), the mean score of the gadodiamide group was lower (P < 0.05) than that of the saline group (Fig. 4). The effect was more marked in males (P < 0.01 vs saline) because all 4 male gadodiamide-treated rats were unable to completely cross the beam. The mean score in the gadoterate group was not significantly different from that of the control group.
FIGURE 4.
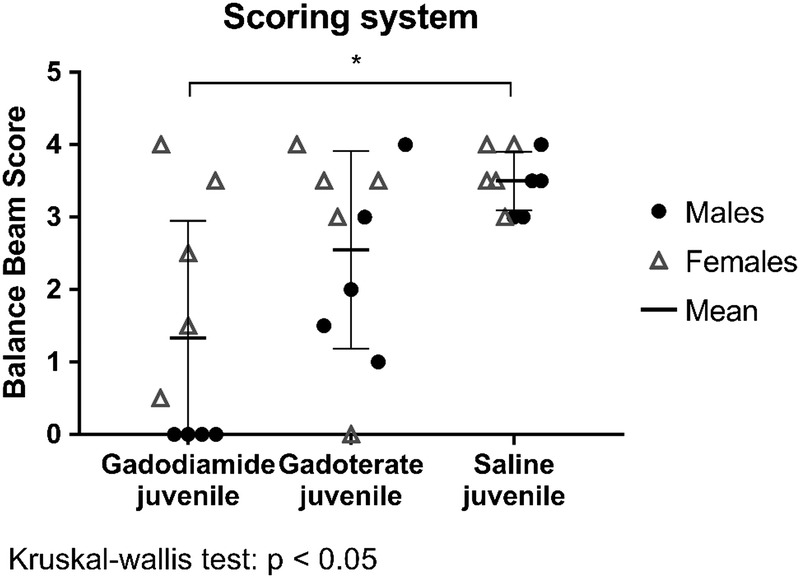
Balance beam score (0–4) at week 8 for juvenile rats treated with gadodiamide, gadoterate, or saline. Data are presented as individual data (males vs females) and mean ± SD.
Assessment of T1 Hyperintensity in the DCN
Figure 5 shows typical examples of brain T1-weighted MRI at the end of the treatment (week 7) and at the end of the treatment-free (week 15) periods.
FIGURE 5.
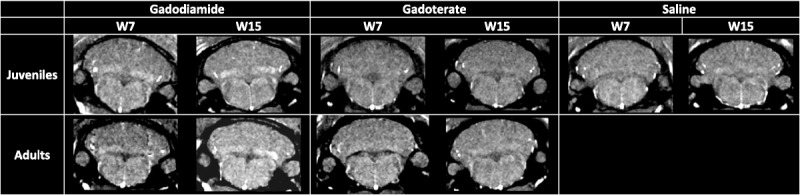
Examples of T1-weighted MRI images at week 7 (end of treatment period) and week 15 (end of treatment-free period).
Qualitatively, T1 hyperintensity was observed in juvenile and adult rats receiving gadodiamide only from week 5, that is, after administration of 12 doses (example of a qualitative analysis in Figure 6A) and remained stable until the end of the 3-month treatment-free period. There was no visually evident difference between juvenile and adult rats.
FIGURE 6.
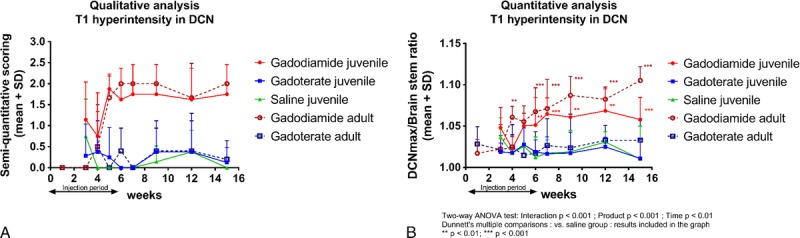
Qualitative (A, example of one reader) and quantitative (B) assessment of T1-weighted signal hyperintensity in the DCN area in juvenile and adult rats treated with gadodiamide, gadoterate, or saline. Data are presented as individual data and mean ± SD.
Quantitatively, a significant increase in the DCNmax/brain stem ratio compared with the saline group was observed in the gadodiamide groups from week 6 (Fig. 6B). No T1 hyperintensitity was observed in either of the gadoterate groups, irrespective of age and sex (Fig. 6B). A slight but significant age effect (P < 0.05) was observed in the gadodiamide groups with a higher DCNmax/brain stem ratio in adult versus juvenile rats at the end of the treatment-free period (P < 0.01). No sex-related difference was observed in any of the groups.
Histopathological Analysis of Cerebellum Samples
Conventional histopathology showed nothing of pathologic relevance in the DCNs area (n = 4/group). Regarding glial cell markers, no hypertrophic astrocytes were observed in any samples. The density and morphology of astrocytes in the cerebellar sections were similar in both treated and control rats. Rare activated CD68+ microglial cells were detected in the perivascular areas regardless of the group but were not observed deeper in the neural parenchyma. No treatment-related differences were observed.
Pigments were seen as small fluorescent granules in neuronal perikarya in the cerebellar sections with high heterogeneity among neurons. In a given animal, some neurons were lipofuscin-negative and some were highly positive, containing an elevated number of pigments. Comparison of class repartition profiles between the groups showed that pigment density was highest in 2 rats from the gadodiamide group and in 4 rats from the control group. No significant treatment-related differences in lipofuscin accumulation in neurons were therefore evidenced in rats. Similarly, no significant differences in pigment accumulation were observed between treated (gadodiamide or gadoterate) and control animals when the second method (quantification) was used.
Total Gd Concentrations in Tissues
No sex-related differences in total Gd concentrations were observed in any of the groups. Male and female data were therefore pooled per group for statistical analysis (n = 6/group).
After repeated administration, similar Gd concentrations were measured in all brain structures in juvenile and adult rats for both groups (Fig. 7), except in the DCN where a slightly lower but statistically significant Gd concentration mean was observed in juvenile rats compared with adult rats treated with gadodiamide only (8.5 ± 4.1 vs 12.0 ± 3.7 nmol/g; P = 0.04; Fig. 7). In the gadodiamide-treated juvenile rat group, 2 rats had lower total Gd concentration means than the other juvenile rats (3.2 and 3.7 vs 11.1 ± 1.7 nmol/g, respectively) in which mean total Gd concentrations were similar to those measured in adult rats (11.1 ± 1.7 vs 12.0 ± 3.7 nmol/g). These lower total Gd concentrations could be explained by nonselective dissection of DCN area because higher sample weights were observed for these 2 animals.
FIGURE 7.
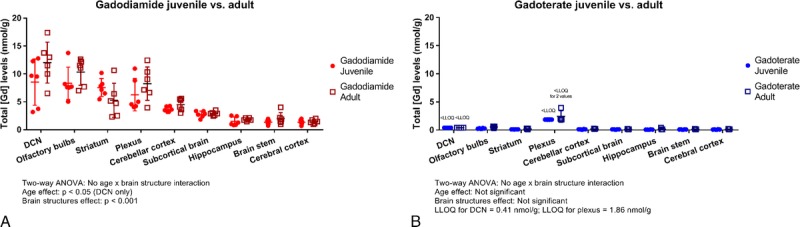
Comparative total Gd concentrations determined by ICP-MS in different brain structures after gadodiamide (A) or gadoterate (B) administration in juvenile or adult rats (n = 6/group) at week 17/18. Data are presented as individual data and mean ± SD.
After gadodiamide administration, the highest Gd concentrations were observed in the DCN, the olfactory bulbs, the striatum, and the plexus, and mean levels ranged between 5 and 15 nmol/g (Fig. 7A). In the gadoterate groups, total Gd concentrations were below the LLOQ in the DCN and the plexus (Fig. 7B) and below 0.2 nmol/g in the other brain structures, that is, at least 25 to 75 times lower than after gadodiamide administration. In all structures, total Gd concentrations were higher in the gadodiamide groups (P < 0.001) than in the gadoterate and saline groups irrespective of age. In the saline group, Gd concentrations were below the LLOQ except in the olfactory bulbs, the cerebellar cortex, and the brain stem where concentrations were below 0.05 nmol/g.
In dorsal skin, total Gd concentrations were not significantly different 3 months after the last administration of gadodiamide in both juvenile and adult rats in all treated groups (Fig. 8). However, variability was high in adult gadodiamide-treated rats. In both the gadoterate and saline groups, all values were below the LLOQ.
FIGURE 8.
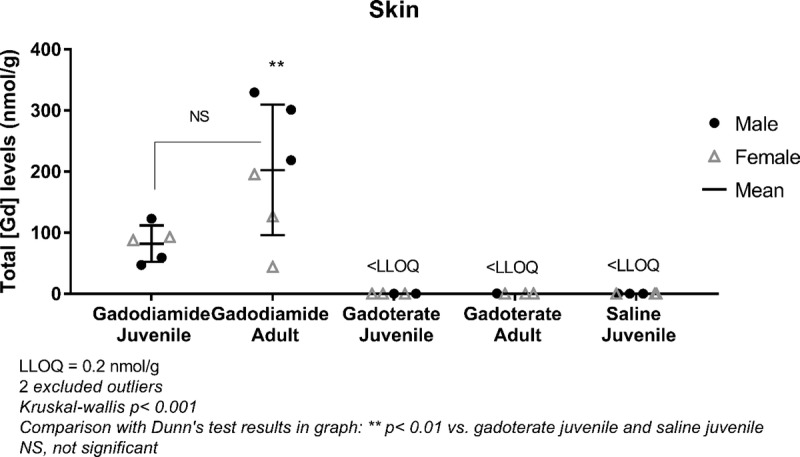
Total Gd concentrations determined by ICP-MS in dorsal skin after repeated administration of gadodiamide, gadoterate, or saline in juvenile or adult rats on week 17/18. Data are presented as individual data (males vs females) and mean ± SD.
In the femur, total Gd concentrations in all bone structures of rats from the gadodiamide groups (regardless of age) were higher than those measured in the bones of rats from the gadoterate and saline groups (Fig. 9A).
FIGURE 9.
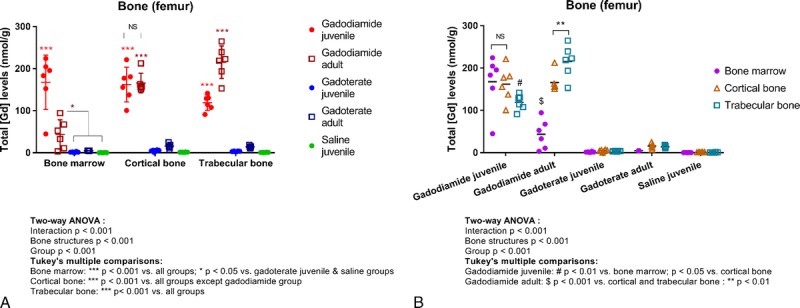
Total Gd concentrations determined by ICP-MS in different femur structures after repeated administration of gadodiamide, gadoterate, or saline in juvenile or adult rats at week 17/18. A, Representation per structure. B, Representation per group. Data are presented as individual data and mean ± SD.
In juvenile gadodiamide-treated rats, similar total Gd concentrations were observed in bone marrow and cortical bone tissue, whereas lower Gd concentrations were measured in trabecular bone tissue (Fig. 9B). In contrast, in adult gadodiamide-treated rats, the lowest Gd concentrations were observed in bone marrow and the highest in trabecular bone tissue (Fig. 9B).
DISCUSSION
The long-term consequences of Gd tissue retention are currently unknown. However, children could be more sensitive to the potential adverse effects of accumulated Gd after exposure equivalent to that of adults.37 Therefore, it appeared necessary to (a) investigate whether Gd accumulation and retention in the developing brain is higher than in the adult brain receiving similar doses and (b) to evaluate the potential risk of repeated administration of GBCAs in children during development and in adulthood.
Nonclinical studies are of great value in helping to understand the mechanism of action and potential safety consequences of Gd exposure.10 They have recently shown that part of the Gd remaining in the brain was irreversibly retained after repeated administration of L-GBCAs, but not after administration of M-GBCAs.51,52 A difference in the forms of residual Gd found in rat brains was also demonstrated52–54 between L-GBCAs (intact chelated Gd, soluble, and macromolecule-bound and insoluble Gd species) and M-GBCAs (only intact chelated Gd). Furthermore, it has been recently suggested that GBCAs penetrate the brain via CSF through the choroid plexus17,18,55 and are subsequently distributed into healthy CNS tissue and cleared via the glymphatic system.56–58
The rat was selected because it is the species recommended by health authorities for juvenile toxicity studies.59 It is also the most common species used in the nonclinical studies already performed on Gd presence in the brain.10 Furthermore, the sequence of key processes in brain development is very similar in humans and rats although time scales are considerably different.37,60 There is evidence that the BBB and choroid plexuses are already present and functional at birth in the human species.38,39 In rats, results are still conflicting regarding the BBB. Some authors report that the BBB is established at PND 1 to 3,60 whereas others indicate functionality at approximately PND 10.35,61 To avoid any bias regarding the BBB, we selected PND 12 as the start age for dosing. Furthermore, it must be stressed that rat organs, including the brain,62 are generally less mature compared with humans at birth and that a rat at PND 9 to 10 is roughly equivalent to a term human infant with respect to overall CNS34 and renal development.36 Romijn et al63 determined, based on several parameters including neurotransmitters such as gamma-aminobutyric acid, that the cerebral cortex of a newborn human is developmentally most comparable to that of a rat at PND 12 to 13.
We selected the classic dosing scheme,17,18,42,64,65 that is, 20 administrations of equivalent conventional human doses. Before weaning, it is not possible to perform daily intravenous injections to rats, so administrations were carried out twice a week during this period. Treatments were administered until PND 51 in juvenile rats, which correspond to adolescence in this species34 and an age range of 12 to 18 years in humans for brain development.60 The same dosing scheme was used in adult rats to compare Gd retention and toxicity in both populations under identical conditions.
This study confirms18,52,66 that T1 hyperintensity in the DCN occurs after repeated administration of an L-GBCA, gadodiamide (low thermodynamic and kinetic stabilities), and not after repeated administration of a high-stability M-GBCA, gadoterate, and that the effect depends on the number of administrations and thus the cumulative dose. This phenomenon was also observed in young rats. These findings are clinically relevant because T1 hyperintensity in the dentate nucleus has been reported in children and adults having received L-GBCAs.19–31,67,68 Visually, the occurrence and persistence of T1 hyperintensity was similar in juvenile and adult animals, suggesting similar wash-in and clearance of Gd regardless of the age of the animals. However, quantitatively, the DCN/brain stem ratio of T1 hyperintensity was lower in juvenile rats than in adult rats at the end of the treatment-free period. It is not clear whether this observation is related to different initial wash-out of Gd or to a modification of the form of Gd because it was not correlated with any variation of total Gd concentrations in the brain between juvenile and adult rats. Indeed, under our study conditions, after repeated administration of GBCA, age did not seem to affect total Gd distribution (same Gd concentration ranking) and retention in the brain. These results suggest that the routes of Gd entry into the brain and Gd clearance are probably influenced by a process that is already present during the postnatal development period of the brain. Higher Gd retention in juvenile animals could be expected because of lower CSF turnover and the larger extracellular space of the immature brain.69 Total Gd concentrations in brain structures were 15- (brain stem and cerebral cortex) to 30-fold (other brain structures including DCN) higher with gadodiamide than with the more stable gadoterate. These results are also consistent with those of the literature.18
Age and sex had no effect on Gd brain retention. Regarding systemic toxicity, no significant differences in bodyweight or mortality were observed (except one rat found dead in the juvenile gadodiamide group). However, almost all juvenile rats injected with gadodiamide showed transient macroscopic skin lesions associated with alopecia during the treatment-free period, whereas no scabs or alopecia were observed in any of the adult rats. Immaturity of renal function at the time of first administration in juvenile rats (before PND 21)36 could explain this effect, but similar skin lesions in healthy adult rats receiving higher dosage70 or in renally impaired rats with similar dosage17 have also been reported. This is consistent with a sensitizing role for immaturity of the renal function. The lesions were more severe in females than in males although the reasons for this are unclear. Morbimortality and severe skin lesions have been already reported in juvenile rats administered gadodiamide, whereas administration of gadoterate was well tolerated.71 The study protocol was different, but the cumulative dose was similar. In our study, the discrepancy in skin lesion occurrence was not associated with any sex- or age-related variations in Gd skin levels in the gadodiamide-treated groups, but the measurement was only performed at study completion.
In addition to its key role in motor coordination (regulation of the rate, force, rhythm, and accuracy of movements), the cerebellum is also involved in cognitive/affective operations.72 The dentate nucleus has a strategic position in the cerebellum, and it is involved in attention, working memory, procedural reasoning, salience detection, and planning task.73 To assess the potential long-term neurotoxicological consequences of Gd exposure in rats receiving GBCAs during the neonatal and juvenile periods, an exploratory behavioral study was performed using a hypothesis-free approach using a battery of conventional tests to cover a broad range of neurobehavioral functions.10 First, certain developmental reflexes and physical parameters were assessed before weaning, as proposed elsewhere.43 Repeated GBCA administration did not affect developmental milestones in unweaned rats. Then, after weaning, several behavioral tests were performed to assess general behavior, locomotion, motor coordination, olfaction, anxiety, and spatial learning/memory.74 No significant treatment related-effects were observed. In the balance beam test, which assesses motor balance and coordination,44 no between-group differences were observed for the usual end points (time, number/distance of paw slips). Only successful trials, that is complete crossing of the beam with a minimal amount of stopping, were taken into account. However, the number of successful trials per group was not homogenous, which induced a bias. We therefore used an alternative scoring system to assess the ability of rats to travel along the beam. In the gadodiamide-treated rats (mainly the males), the mean score was lower compared with the other groups due to the animals' inability to completely cross the beam. This may reflect stress or anxiety because the beam is elevated. However, confounding factors such as motivation/cooperation were identified. In the EPM test, a trend toward a lower percent of time spent in the open arms and a lower number of entries was also noted in the gadodiamide group compared with the saline and gadoterate groups. Further studies are required to investigate whether these findings are the first signs of anxiety. Nonclinical behavioral studies have also been recently performed in adult rats administered either gadobutrol or gadodiamide.75 A transient and dose-dependent attenuation in sensorimotor reflex of the acoustic startle response was observed only in gadodiamide group. No effect was noted in any other test.75 Our results were not consistent with another study performed in mice after perinatal exposure.76 Of note, the neurobehavioral battery of tests used here may be relatively insensitive to putative dentate nucleus toxicity (if one assumes that toxicity necessarily parallels the local Gd concentration).
No histopathological abnormalities were detected in the cerebellum samples of the rats irrespective of the group. These results are fully consistent with previous nonclinical65,77,78 and clinical studies.11 The quantity of lipofuscin pigments was also assessed because the link between Gd deposits and lipofuscin pigments has recently been demonstrated in renally impaired rats having received gadodiamide or gadobenate, two L-GBCAs, but not after gadoterate administration.58 No difference in the amount of lipofuscin pigments was noted among the groups in this study, which is consistent with our data.
It is well known that bone is a target organ for Gd, as for other lanthanides (Ln) and metals.79 It has been suggested that bone is a deep compartment for Gd storage.80,81 In patients, Gd was found to be retained in bone tissue for more than 8 years after GBCA administration.82 In the present study, Gd was still present 3 months after the last GBCA administration. However, Gd concentrations were significantly lower after injections of gadoterate than after injections of gadodiamide. These data are fully consistent with a recent study in mice.83
In contrast to distribution in the brain, Gd distribution in bone seemed to be related to age in the gadodiamide-treated rats. Gadolinium concentrations were higher in bone than in bone marrow of adult rats, whereas very similar concentrations were noted in juvenile rats. These findings are not in agreement with a meta-analysis81 where preferential distribution in bone marrow was reported in adult rats. However, in the analyzed studies, the Gd measurements were performed shortly after a single administration (up to 24 hours) unlike in our study (3 months after the last of 20 administrations). These differences may explain this discrepancy.
The International Commission on Radiological Protection established a biokinetics model to predict the behavior of Ln in the human body.84 Regarding the skeleton, Ln, including Gd, is initially distributed equivalently between trabecular and cortical surfaces.79,84 Subsequently, they are removed from bone surfaces at a rate proportional to the bone tissue turnover rate. A part is transferred into the bone volume and another part to the bone marrow. Then, they are removed from the bone volume according to the rate of bone remodeling and subsequently redistributed to the bone marrow. They are finally released into the circulation, giving rise to a new pool of “free” Ln. However, the process is slow because the removal half-life from bone marrow to blood is 0.25 years in humans.84 Distribution and retention of Ln in the bone matrix also depend on their interaction with bone components. Lanthanides can bind to both the mineral (eg, hydroxyapatite) and organic (eg, collagen) components of the bone matrix.79,85–88 Many interactions can occur simultaneously, and they are also driven by the form of the element in the blood. Speciation thus plays a critical role in bone distribution/retention, and further studies are required to investigate Gd speciation and its spatial distribution in the bone matrix. Moreover, immature bone consists of cartilage, whereas adult bone primarily consists of cortical bone.89,90 These differences could affect Gd distribution and retention during postnatal bone development as suggested in a previous juvenile toxicity study.71 The skeletal distribution of Ln, which depends on the remodeling activity of the bone, seems consistent with our data in gadodiamide-treated rats. The model assumes that L-GBCAs undergo in vivo dissociation as demonstrated earlier,91,92 and thus that dissociated Gd rather than chelated Gd is incorporated and sequestered in the bone. The faster formation of bone in young animals may explain the increased Gd retention in bone marrow (redistribution of Gd after bone remodeling) in juvenile gadodiamide-treated rats. High Gd levels could be a cause of concern because bone marrow is the primary site of hematopoiesis and lymphopoiesis. Signs of myelotoxicity with L-GBCAs have already been mentioned in the literature.93,94 The presence of Gd in bone marrow could also impair its developmental maturation, which occurs though the replacement of active hematopoietic red marrow by fatty yellow marrow that no longer produces hematopoietic cells. Furthermore, it is well known that NSF is characterized by infiltration of circulating fibrocytes in the dermis.95 These CD34+ fibrocytes have a medullar origin and experiments in rats have proven that bone marrow-derived cells accumulate in skin lesions in gadodiamide-treated rats.96,97 If these cells incorporate Gd in the bone marrow before their migration into the dermis, it could then induce or worsen dermal fibrosis. In the present study, it might explain why macroscopic skin lesions were observed only in juvenile gadodiamide-treated rats.
In the case of a thermodynamically stable GBCA, it can be anticipated that fixation of Gd onto the bone surface and its migration into bone tissue and bone marrow are negligible. The complex can then be easily eliminated by the kidneys. In the case of gadoterate, only very low levels of Gd were detected in the bone of juvenile and adult rats 3 months after the last administration while levels were 10- to 30-fold higher after administration of gadodiamide. The difference in bone retention between the two GBCAs is very likely due to the lower thermodynamic stability of the L-GBCA, gadodiamide.98 These findings are in agreement with those of numerous previous animal studies71,83,91,92,99–104 and human data105,106 in which higher bone Gd retention was noted with L-GBCAs than with M-GBCAs.
There are some limitations to our study. First, total Gd distribution and retention was investigated only in target organs, that is, skin, bone, and brain. It would be interesting to compare full tissue distribution and biospeciation of Gd in juvenile and adult rats. Second, the housing conditions of juvenile and adult animals were not strictly identical. Juvenile animals were housed per group, whereas adult rats from different groups were housed in the same cages, which likely caused some cross-contamination. It would have been more appropriate to perform the study with identical housing for all animals or to add a saline control group to the adult groups. Third, the present study included only a 3-month treatment-free period. It would be interesting to evaluate long-term Gd toxicity and tissue retention over at least 5 months or longer.10 Lastly, it would have been interesting to carry out myelogram studies in juvenile rats.
In conclusion, unlike with gadoterate, T1 hyperintensity in the deep cerebellar nuclei was observed in both juvenile and adult gadodiamide-treated rats. No differences in qualitative analysis and T1 signal onset were detected on MRI scans of juveniles compared with adult rats. Similar total Gd retention in skin and brain tissues was observed in animals receiving GBCAs irrespective of whether the doses were administered during the juvenile period or during adulthood. In contrast, Gd distribution in bone marrow was higher in juveniles than in adult rats but only in the gadodiamide groups. Further studies are needed to assess the form of retained Gd in bone marrow in juvenile rats receiving gadodiamide and to investigate the potential risks associated with this retention. Regardless of age and the tissues, Gd concentration was 10- to 30-fold higher after administration of the linear contrast agent gadodiamide than after administration of the macrocyclic contrast agent gadoterate.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Gaëlle Jestin-Mayer, Claire Wallon, and Cécile Factor for the Gd determination in tissues.
Footnotes
Conflicts of interest and sources of funding: All authors (except F.B.) were Guebert employees at the time of the study. This study was funded by Guebert through the Atlantic Bone Screen funding within the common research project.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.investigativeradiology.com).
REFERENCES
- 1.Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65:1095–1196. [DOI] [PubMed] [Google Scholar]
- 2.Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen HS, Morcos SK, Almén T, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR contrast medium safety committee guidelines. Eur Radiol. 2013;23:307–318. [DOI] [PubMed] [Google Scholar]
- 4.American College of Radiology. ACR manual on contrast media: Version 10.3. Am Coll Radiol. 2018. [Google Scholar]
- 5.Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based contrast agents by the food and drug administration. Radiology. 2012;265:248–253. [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency. Assessment report for gadolinium-containing contrast agents. EMA/740640/2010 European Medicines Agency. 2010. [Google Scholar]
- 7.Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines. Radiology. 2011;260:105–111. [DOI] [PubMed] [Google Scholar]
- 8.Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270:834–841. [DOI] [PubMed] [Google Scholar]
- 9.Runge VM. Safety of the gadolinium-based contrast agents for magnetic resonance imaging, focusing in part on their accumulation in the brain and especially the dentate nucleus. Invest Radiol. 2016;51:273–279. [DOI] [PubMed] [Google Scholar]
- 10.Robert P, Frenzel T, Factor C, et al. Methodological aspects for preclinical evaluation of gadolinium presence in brain tissue: critical appraisal and suggestions for harmonization—a joint initiative. Invest Radiol. 2018;53:499–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275:772–782. [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency. EMA's final opinion confirms restrictions on use of linear gadolinium agents in body scans. Available at: http://www.ema.europa.eu/docs/en_gb/document_library/referrals_document/gadolinium_contrast_agents_31/european_commission_final_decision/wc500240575.pdf. Accessed November 11, 2017.
- 13.Pharmaceuticals and Medical Devices Agency. Report on the investigation results. Available at: http://www.pmda.go.jp/files/000221379.pdf. Accessed November 11, 2017.
- 14.Pharmaceuticals and Medical Devices Agency. Revision of precautions, gadodiamide hydrate meglumine gadopentetate. Available at: http://www.pmda.go.jp/files/000221377.pdf. Accessed November 28, 2017.
- 15.Food and Drug Administration. FDA drug safety communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. Available at: https://www.fda.gov/drugs/drugsafety/ucm589213.htm 2018.
- 16.Cao Y, Zhang Y, Shih G, et al. Effect of renal function on gadolinium-related signal increases on unenhanced T1-weighted brain magnetic resonance imaging. Invest Radiol. 2016;51:677–682. [DOI] [PubMed] [Google Scholar]
- 17.Rasschaert M, Idee JM, Robert P, et al. Moderate renal failure accentuates T1 signal enhancement in the deep cerebellar nuclei of gadodiamide-treated rats. Invest Radiol. 2017;52:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasschaert M, Emerit A, Fretellier N, et al. Gadolinium retention, brain T1 hyperintensity, and endogenous metals: a comparative study of macrocyclic versus linear gadolinium chelates in renally sensitized rats. Invest Radiol. 2018;53:328–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamrazi B, Nguyen B, Liu CJ, et al. Changes in signal intensity of the dentate nucleus and globus pallidus in pediatric patients: impact of brain irradiation and presence of primary brain tumors independent of linear gadolinium-based contrast agent administration. Radiology. 2018;287:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renz DM, Kumpel S, Bottcher J, et al. Comparison of unenhanced T1-weighted signal intensities within the dentate nucleus and the globus pallidus after serial applications of gadopentetate dimeglumine versus gadobutrol in a pediatric population. Invest Radiol. 2018;53:119–127. [DOI] [PubMed] [Google Scholar]
- 21.Rossi Espagnet MC, Bernardi B, Pasquini L, et al. Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol. 2017;47:1345–1352. [DOI] [PubMed] [Google Scholar]
- 22.Schneider GK, Stroeder J, Roditi G, et al. T1 signal measurements in pediatric brain: findings after multiple exposures to gadobenate dimeglumine for imaging of nonneurologic disease. AJNR Am J Neuroradiol. 2017;38:1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flood TF, Stence NV, Maloney JA, et al. Pediatric brain: repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology. 2017;282:222–228. [DOI] [PubMed] [Google Scholar]
- 24.Roberts DR, Chatterjee AR, Yazdani M, et al. Pediatric patients demonstrate progressive T1-weighted hyperintensity in the dentate nucleus following multiple doses of gadolinium-based contrast agent. AJNR Am J Neuroradiol. 2016;37:2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts DR, Holden KR. Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev. 2016;38:331–336. [DOI] [PubMed] [Google Scholar]
- 26.Hu HH, Pokorney A, Towbin RB, et al. Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol. 2016;46:1590–1598. [DOI] [PubMed] [Google Scholar]
- 27.Miller JH, Hu HH, Pokorney A, et al. MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics. 2015;136:e1637–e1640. [DOI] [PubMed] [Google Scholar]
- 28.Young JR, Qiao J, Orosz I, et al. Gadolinium deposition within the paediatric brain: no increased intrinsic T1-weighted signal intensity within the dentate nucleus following the administration of a minimum of four doses of the macrocyclic agent gadobutrol. Eur Radiol. 2018. [Epub, ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasper E, Schemuth HP, Horry S, et al. Changes in signal intensity in the dentate nucleus at unenhanced T1-weighted magnetic resonance imaging depending on class of previously used gadolinium-based contrast agent. Pediatr Radiol. 2018;48:686–693. [DOI] [PubMed] [Google Scholar]
- 30.Dunger D, Krause M, Grafe D, et al. Do we need gadolinium-based contrast medium for brain magnetic resonance imaging in children? Pediatr Radiol. 2018;48:858–864. [DOI] [PubMed] [Google Scholar]
- 31.Kinner S, Schubert TB, Bruce RJ, et al. Deep brain nuclei T1 shortening after gadobenate dimeglumine in children: influence of radiation and chemotherapy. AJNR Am J Neuroradiol. 2018;39:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts DR, Welsh CA, LeBel DP, 2nd, et al. Distribution map of gadolinium deposition within the cerebellum following GBCA administration. Neurology. 2017;88:1206–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald JS, McDonald RJ, Jentoft ME, et al. Intracranial gadolinium deposition following gadodiamide-enhanced magnetic resonance imaging in pediatric patients: a case-control study. JAMA Pediatr. 2017;171:705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck MJ, Padgett EL, Bowman CJ, et al. Nonclinical juvenile toxicity testing. Dev Rep Toxicol. 2006;263–328. [Google Scholar]
- 35.De Schaepdrijver LM, Bailey GP, Coogan TP, et al. Juvenile animal toxicity assessments: decision strategies and study design. Pediatr Drug Dev. 2013;201–221. [Google Scholar]
- 36.Zoetis T, Hurtt ME. Species comparison of anatomical and functional renal development. Birth Defects Res B Dev Reprod Toxicol. 2003;68:111–120. [DOI] [PubMed] [Google Scholar]
- 37.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(suppl 3):511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liddelow SA. Development of the choroid plexus and blood-CSF barrier. Front Neurosci. 2015;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodier PM. Developing brain as a target of toxicity. Environ Health Perspect. 1995;103(suppl 6):73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Food and Drug Administration. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Center for Drug Evaluation and Research (CDER). 2005. [Google Scholar]
- 42.Robert P, Lehericy S, Grand S, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol. 2015;50:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heyser CJ. Assessment of developmental milestones in rodents. Curr Protoc Neurosci. 2004;8:8.18. [DOI] [PubMed] [Google Scholar]
- 44.Luong TN, Carlisle HJ, Southwell A, et al. Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp. 2011:2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metz GA, Merkler D, Dietz V, et al. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;883:165–177. [DOI] [PubMed] [Google Scholar]
- 46.Roux S, Sablé E, Porsolt RD. Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effects on behavior and physiological function. Curr Protoc Pharmacol. 2005; 10:10. [DOI] [PubMed] [Google Scholar]
- 47.Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009; 8:8.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, Qin J, Chen W, et al. Behavioral and pharmacological investigation of anxiety and maternal responsiveness of postpartum female rats in a pup elevated plus maze. Behav Brain Res. 2015;292:414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. 6th edition. London, England: Elsevier Academic Press; 2007.
- 51.Frenzel T, Jost G, Lohrke J, et al. Long-term study of residual Gd in brain after repeated injection of Gd based contrast agents in rats. Invest Radiol. 2017;52:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robert P, Fingerhut S, Factor C, et al. One-year retention of gadolinium in the brain: comparison of gadodiamide and gadoterate meglumine in a rodent model. Radiology. 2018;288:424–433. [DOI] [PubMed] [Google Scholar]
- 53.Frenzel T, Apte C, Jost G, et al. Quantification and assessment of the chemical form of residual gadolinium in the brain after repeated administration of gadolinium-based contrast agents: comparative study in rats. Invest Radiol. 2017;52:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gianolio E, Bardini P, Arena F, et al. Gadolinium retention in the rat brain: assessment of the amounts of insoluble gadolinium-containing species and intact gadolinium complexes after repeated administration of gadolinium-based contrast agents. Radiology. 2017;285:839–849. [DOI] [PubMed] [Google Scholar]
- 55.Jost G, Frenzel T, Lohrke J, et al. Penetration and distribution of gadolinium-based contrast agents into the cerebrospinal fluid in healthy rats: a potential pathway of entry into the brain tissue. Eur Radiol. 2017;27:2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taoka T, Naganawa S. Gadolinium-based contrast media, cerebrospinal fluid and the glymphatic system: possible mechanisms for the deposition of gadolinium in the brain. Magn Reson Med Sci. 2018;17:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taoka T, Jost G, Frenzel T, et al. Impact of the glymphatic system on the kinetic and distribution of gadodiamide in the rat brain: observations by dynamic MRI and effect of circadian rhythm on tissue gadolinium concentrations. Invest Radiol. 2018;53:529–534. [DOI] [PubMed] [Google Scholar]
- 58.Rasschaert M, Schroeder JA, Wu TD, et al. Multimodal imaging study of gadolinium presence in rat cerebellum: differences between Gd chelates, presence in the Virchow-Robin space, association with lipofuscin, and hypotheses about distribution pathway. Invest Radiol. 2018;53:518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrow PC, Schmitt G. Juvenile nonclinical safety studies in support of pediatric drug development. Methods Mol Biol. 2017;1641:25–67. [DOI] [PubMed] [Google Scholar]
- 60.Semple BD, Blomgren K, Gimlin K, et al. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watson RE, DeSesso JM, Hurtt ME, et al. Postnatal growth and morphological development of the brain: a species comparison. Birth Defects Res B Dev Reprod Toxicol. 2006;77:471–484. [DOI] [PubMed] [Google Scholar]
- 62.Schmitt G, Parrott N, Prinssen E, et al. The great barrier belief: the blood-brain barrier and considerations for juvenile toxicity studies. Reprod Toxicol. 2017;72:129–135. [DOI] [PubMed] [Google Scholar]
- 63.Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev. 1991;26:61–67. [DOI] [PubMed] [Google Scholar]
- 64.Bussi S, Coppo A, Botteron C, et al. Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. J Magn Reson Imaging. 2018;47:746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith AP, Marino M, Roberts J, et al. Clearance of gadolinium from the brain with no pathologic effect after repeated administration of gadodiamide in healthy rats: an analytical and histologic study. Radiology. 2017;282:743–751. [DOI] [PubMed] [Google Scholar]
- 66.Jost G, Lenhard DC, Sieber MA, et al. Signal increase on unenhanced T1-weighted images in the rat brain after repeated, extended doses of gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Invest Radiol. 2016;51:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tibussek D, Rademacher C, Caspers J, et al. Gadolinium brain deposition after macrocyclic gadolinium administration: a pediatric case-control study. Radiology. 2017;285:223–230. [DOI] [PubMed] [Google Scholar]
- 68.Radbruch A, Haase R, Kickingereder P, et al. Pediatric brain: no increased signal intensity in the dentate nucleus on unenhanced T1-weighted MR images after consecutive exposure to a macrocyclic gadolinium-based contrast agent. Radiology. 2017;283:828–836. [DOI] [PubMed] [Google Scholar]
- 69.Dziegielewska KM, Ek J, Habgood MD, et al. Development of the choroid plexus. Microsc Res Tech. 2001;52:5–20. [DOI] [PubMed] [Google Scholar]
- 70.Sieber MA, Pietsch H, Walter J, et al. A preclinical study to investigate the development of nephrogenic systemic fibrosis: a possible role for gadolinium-based contrast media. Invest Radiol. 2008;43:65–75. [DOI] [PubMed] [Google Scholar]
- 71.Fretellier N, Maazouz M, Luseau A, et al. Safety profiles of gadolinium chelates in juvenile rats differ according to the risk of dissociation. Reprod Toxicol. 2014;50:171–179. [DOI] [PubMed] [Google Scholar]
- 72.Koziol LF, Budding D, Andreasen N, et al. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum. 2014;13:151–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manto M, Oulad Ben Taib N. Cerebellar nuclei: key roles for strategically located structures. Cerebellum. 2010;9:17–21. [DOI] [PubMed] [Google Scholar]
- 74.Moser VC. Functional assays for neurotoxicity testing. Toxicol Pathol. 2011;39:36–45. [DOI] [PubMed] [Google Scholar]
- 75.Habermeyer J, von Hörste S, Schütz G, et al. Behavioral phenotyping in rats 5 to 30 weeks after multiple injections of gadobutrol and gadodiamide - c-2326. Vienna: ECR 2018; 2018. [Google Scholar]
- 76.Khairinisa MA, Takatsuru Y, Amano I, et al. The effect of perinatal gadolinium-based contrast agents on adult mice behavior. Invest Radiol. 2018;53:110–118. [DOI] [PubMed] [Google Scholar]
- 77.Lohrke J, Frisk AL, Frenzel T, et al. Histology and gadolinium distribution in the rodent brain after the administration of cumulative high doses of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol. 2017;52:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDonald RJ, McDonald JS, Dai D, et al. Comparison of gadolinium concentrations within multiple rat organs after intravenous administration of linear versus macrocyclic gadolinium chelates. Radiology. 2017;285:536–545. [DOI] [PubMed] [Google Scholar]
- 79.Vidaud C, Bourgeois D, Meyer D. Bone as target organ for metals: the case of f-elements. Chem Res Toxicol. 2012;25:1161–1175. [DOI] [PubMed] [Google Scholar]
- 80.Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(suppl 1):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lancelot E. Revisiting the pharmacokinetic profiles of gadolinium-based contrast agents: differences in long-term biodistribution and excretion. Invest Radiol. 2016;51:691–700. [DOI] [PubMed] [Google Scholar]
- 82.Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, et al. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics. 2009;1:479–488. [DOI] [PubMed] [Google Scholar]
- 83.Di Gregorio E, Iani R, Ferrauto G, et al. Gd accumulation in tissues of healthy mice upon repeated administrations of gadodiamide and gadoteridol. J Trace Elem Med Biol. 2018;48:239–245. [DOI] [PubMed] [Google Scholar]
- 84.Taylor DM, Leggett RW. A generic biokinetic model for predicting the behaviour of the lanthanide elements in the human body. Radiat Prot Dosimetry. 2003;105:193–198. [DOI] [PubMed] [Google Scholar]
- 85.Evans CH. Biochemistry Of The Lanthanides. Biochemistry Of The Elements. New York: Plenum Press; 1990. [Google Scholar]
- 86.Taupitz M, Stolzenburg N, Ebert M, et al. Gadolinium-containing magnetic resonance contrast media: investigation on the possible transchelation of Gd3+ to the glycosaminoglycan heparin. Contrast Media Mol Imaging. 2013;8:108–116. [DOI] [PubMed] [Google Scholar]
- 87.Drouven BJ, Evans CH. Collagen fibrillogenesis in the presence of lanthanides. J Biol Chem. 1986;261:11792–11797. [PubMed] [Google Scholar]
- 88.Nousiainen M, Derrick PJ, Kaartinen MT, et al. A mass spectrometric study of metal binding to osteocalcin. Chem Biol. 2002;9:195–202. [DOI] [PubMed] [Google Scholar]
- 89.Horton JA, Bariteau JT, Loomis RM, et al. Ontogeny of skeletal maturation in the juvenile rat. Anat Rec (Hoboken). 2008;291:283–292. [DOI] [PubMed] [Google Scholar]
- 90.Zoetis T, Tassinari MS, Bagi C, et al. Species comparison of postnatal bone growth and development. Birth Defects Res B Dev Reprod Toxicol. 2003;68:86–110. [DOI] [PubMed] [Google Scholar]
- 91.Fretellier N, Bouzian N, Parmentier N, et al. Nephrogenic systemic fibrosis-like effects of magnetic resonance imaging contrast agents in rats with adenine-induced renal failure. Toxicol Sci. 2013;131:259–270. [DOI] [PubMed] [Google Scholar]
- 92.Fretellier N, Idée JM, Dencausse A, et al. Comparative in vivo dissociation of gadolinium chelates in renally impaired rats: a relaxometry study. Invest Radiol. 2011;46:292–300. [DOI] [PubMed] [Google Scholar]
- 93.Harpur ES, Worah D, Hals PA, et al. Preclinical safety assessment and pharmacokinetics of gadodiamide injection, a new magnetic resonance imaging contrast agent. Invest Radiol. 1993;(28 suppl 1):S28–S43. [DOI] [PubMed] [Google Scholar]
- 94.Katsutani N, Sagami F, Tirone P, et al. General toxicity study of gadobenate dimeglumine formulation (e7155) (4)–4-week repeated dose intravenous toxicity study followed by 4-week recovery period in dogs. J Toxicol Sci. 1999;(24 suppl 1):41–60. [DOI] [PubMed] [Google Scholar]
- 95.Bucala R. Circulating fibrocytes: cellular basis for NSF. J Am Coll Radiol. 2008;5:36–39. [DOI] [PubMed] [Google Scholar]
- 96.Drel VR, Tan C, Barnes JL, et al. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30:3026–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181:1941–1952. [DOI] [PubMed] [Google Scholar]
- 98.Port M, Idée JM, Medina C, et al. Stability of gadolinium chelates and their biological consequences: new data and some comments. Br J Radiol. 2008;81:258–259. [DOI] [PubMed] [Google Scholar]
- 99.Fretellier N, Idée J, Bruneval P, et al. Hyperphosphataemia sensitizes renally impaired rats to the profibrotic effects of gadodiamide. Br J Pharmacol. 2012;165:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sieber MA, Lengsfeld P, Frenzel T, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008;18:2164–2173. [DOI] [PubMed] [Google Scholar]
- 101.Pietsch H, Lengsfeld P, Steger-Hartmann T, et al. Impact of renal impairment on long-term retention of gadolinium in the rodent skin following the administration of gadolinium-based contrast agents. Invest Radiol. 2009;44:226–233. [DOI] [PubMed] [Google Scholar]
- 102.Kartamihardja AA, Nakajima T, Kameo S, et al. Impact of impaired renal function on gadolinium retention after administration of gadolinium-based contrast agents in a mouse model. Invest Radiol. 2016;51:655–660. [DOI] [PubMed] [Google Scholar]
- 103.Erdene K, Nakajima T, Kameo S, et al. Organ retention of gadolinium in mother and pup mice: effect of pregnancy and type of gadolinium-based contrast agents. Jpn J Radiol. 2017;35:568–573. [DOI] [PubMed] [Google Scholar]
- 104.Haylor J, Schroeder J, Wagner B, et al. Skin gadolinium following use of MR contrast agents in a rat model of nephrogenic systemic fibrosis. Radiology. 2012;263:107–116. [DOI] [PubMed] [Google Scholar]
- 105.White GW, Gibby WA, Tweedle MF. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (Prohance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol. 2006;41:272–278. [DOI] [PubMed] [Google Scholar]
- 106.Gibby WA, Gibby KA, Gibby WA. Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (Prohance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol. 2004;39:138–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.