Abstract
To evaluate the steady-state pharmacokinetics of prednisone and its metabolite prednisolone in pregnant and lactating female subjects, nineteen subjects received prednisone (4–40 mg/day orally) in early- (n=3), mid- (n=9) and late- (n=13) pregnancy as well as postpartum with (n=2) and without (n=5) lactation. Serial blood and urine samples were collected over one dosing interval. Prednisone and its metabolite, prednisolone, steady-state non-compartmental PK parameters were estimated. During pregnancy, prednisone apparent oral clearance (CL/F) increased with dose (35.1 ± 11.4 L/h with 5 mg, 52.6 ± 5.2 L/h with 10 mg, and 64.3 ± 6.9 L/h with 20 mg, p=0.001). Similarly, unbound prednisone apparent oral clearance increased with dose. In addition, prednisolone renal clearance increased with dose (0.3 ± 0.3 L/h with 5 mg, 0.5 ± 0.4 L/h with 10 mg, and 1.3 ± 1.1 L/h with 20 mg, p=0.002). Higher prednisone (r=0.57, p≤0.05) and prednisolone (r=0.75, p≤0.05) concentrations led to higher percentage of unbound drug. Breast milk/plasma AUC ratios were 0.5–0.6 for prednisone and 0.02–0.03 for prednisolone. Relative infant doses were 0.35–0.53% and 0.09–0.18%, for prednisone and prednisolone, respectively. Prednisone and prednisolone exhibit dose- and concentration-dependent pharmacokinetics during pregnancy and infant exposure to these agents via breast milk is minimal.
Keywords: prednisone, prednisolone, pharmacokinetics, pregnancy, breast milk
Introduction
The prevalence of prednisone use during early pregnancy is approximately 1.3%, making prednisone one of the most commonly utilized medication during early pregnancy.1 Prednisone is a synthetic corticosteroid used to treat or prevent a variety of conditions during pregnancy, such as autoimmune disease, asthma, solid organ transplant rejection and others.2,3 Because many of these conditions warrant long-term use, understanding the pharmacokinetics (PK) of prednisone during pregnancy and lactation is important for its safe and effective use.
Prednisone and prednisolone are primarily metabolized in the liver by conjugation and hydroxylation. Oral prednisone is rapidly absorbed and converted into its active metabolite, prednisolone, by 11β-hydroxysteroid-dehydrogenase (11β-HSD).4 In the non-pregnant population, prednisone and prednisolone interconvert, with this process favoring prednisolone formation. Two forms of 11β-HSD exists: Type 1 (11β-HSD1) converts prednisone to prednisolone and Type 2 (11β-HSD2) converts prednisolone to prednisone.4 In the non-pregnant population, prednisone and prednisolone PK are dose- and concentration-dependent, in part due to concentration-dependent plasma protein binding.5 Prednisone and prednisolone are highly bound to both corticosteroid binding globulin (CBG or transcortin) and albumin in plasma. CBG concentrations increase and albumin concentrations decrease during gestation.6 The effects of pregnancy on prednisone and prednisolone PK and plasma protein binding have not been evaluated. Therefore, the objective of this study was to evaluate the PK of prednisone and its active metabolite, prednisolone, during pregnancy and postpartum.
Methods
Subjects
The study protocol was approved by the institutional review boards at the University of Washington, University of Pittsburgh, Georgetown University, and University of Texas Medical Branch in Galveston. All subjects were enrolled after obtaining written informed consent. We examined steady-state PK of orally administered prednisone in the plasma of 19 female subjects, 16–41 years of age with hematocrits ≥ 28% and receiving prednisone for therapeutic reasons. Blood and urine samples were collected during early- (10–14 weeks gestation), mid- (22–26 weeks gestation), and late-pregnancy (34–38 weeks gestation), as well as ≥ 12 weeks postpartum with and without lactation, depending on the subject’s time of enrollment and availability.
Maternal dosing regimen
Prednisone was dosed according to clinical need, without regard to the study. Total daily doses ranged from 4 to 40 mg, given once or twice daily. Dosing administration times were recorded for the 3 days prior to study visits, and pill counts were conducted to assess adherence. Subjects fasted (except for clear liquids) for 5 hours before observed prednisone administration until 1 hour after dosing on each study day. Subjects avoided caffeine, grapefruit-containing foods and beverages, and alcohol for 24 hours prior to each study day and throughout the sampling period.
Sample collection
Serial blood samples were collected from an indwelling venous catheter pre-dose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 hours following prednisone dosing, truncated to the subject’s dosing interval. Blood was collected in green top tubes containing sodium heparin as an anticoagulant. Samples were processed and plasma frozen immediately. Urine was collected over one dosing interval for evaluation of prednisone and prednisolone urinary excretion as well as creatinine clearance. Venous (n=13) and arterial (n=6) umbilical cord plasma samples were collected at the time of delivery. Intrapartum maternal blood samples were collected immediately after delivery. Breast milk was collected from two subjects. Both breasts were completely emptied of milk every 2–3 hours using a Medela Classic® double electric breast pump (Medela Inc., McHenry, IL) over one dosing interval. Breastfeeding was not allowed during the postpartum study days. After each breast milk collection, volume measurement and sample collection, remaining breast milk was returned to the mother for infant feeding. Samples were stored at −80° C until analysis.
Prednisone and prednisolone concentrations
Plasma, urine and breast milk concentrations of prednisone and prednisolone were determined using reverse-phase high performance liquid chromatography (HPLC) with mass spectrometry (MS) detection. Assay conditions are reported in Supplemental Table S1. An Agilent 1200 HPLC and 1956B mass spectrometer were used for the analysis of prednisone and prednisolone. A Restek Allure Biphenyl. 2.1 mm x 100 mm, 3 micron (Restek Corp., Bellefonte, PA) column was used for plasma and breast milk samples. An Agilent Zorbax SB-phenyl 2.1 mm x 150 mm, 5 micron (Agilent Technologies, Santa Clara, CA) column was used for urine samples. Monitored ions included m/z 393 for M+Cl for prednisone, m/z 395 for M+Cl for prednisolone and m/z 401 for M+Cl for d6-prednisolone (Supplemental Figure S1 for plasma and Supplemental Figure S2 for urine).
Plasma and breast milk samples (250 µL) were combined with the internal standard (40 ng d6-prednisolone), methanol (100 µL) and acetonitrile (1000 µL) to precipitate the proteins. After centrifugation (20,800 x G, 10 minutes), the supernatant was evaporated to dryness and reconstitute in 100 µL of mobile phase. The reconstituted samples were centrifuged again and 5 µL injected onto the HPLC column. The dynamic range was 4 to 800 ng/mL. The inter-day variability was less than 6% for both compounds. Urine samples (50 µL) were combined with mobile phase (100 µL) containing internal standard (40 ng) and 2 µL was injected onto the HPLC column. The dynamic range was 20 to 4000 ng/mL. The inter-day variability was less than 8% for both compounds.
Unbound prednisone and prednisolone
The unbound fraction was concentration dependent, so protein binding was determined for each plasma and breast milk sample. The unbound fractions were determined by equilibrium dialysis using Pierce rapid equilibrium dialysis devices (Thermo Fisher, Waltham, MA). The sample chamber contained 400 µL of plasma or breast milk and the buffer contained 600 µL buffer (0.1 M sodium phosphate, 0.15 M sodium chloride, pH7.2). Incubation was 4 hours at 100 rpm and 37º C. After incubation, 300 µL aliquots of each side were extracted (15 minutes) using 3 mL ethyl acetate containing internal standard (40 ng d6-prednisolone). Steroid-free plasma or buffer was added to normalize contents. The organic layer was evaporated to dryness and reconstituted in 75 µL (1:1 5mM ammonium formate:methanol). After centrifugation, 2 µL were injected onto the HPLC column (Phenomenex Luna C-18, 150 mm x 3 mm, 3 micron, Phenomenex, Torrance, CA). The dynamic range was 0.2 to 100 ng/mL. The inter-day variability was less than 5% for both compounds. The HPLC-MS method used for determining the concentration of prednisone and prednisolone in biofluids was adapted for the analysis of the unbound fractions and the conditions can be found in Supplemental Table S1 and the gradient in Supplemental Table S2. Monitored ions were m/z 359.1 for prednisone, m/z 361 for prednisolone and 367 for d6-prednisolone (Supplemental Figure S3). The percentage of unbound drug was determined by the concentration in the buffer chamber divided by the concentration in the plasma chamber x 100.
Pharmacokinetic analyses
Prednisone steady-state non-compartmental PK parameters were estimated utilizing Phoenix WinNonlin software (Certara, L.P., Princeton, NJ) as previously described.7 Total plasma area under the concentration-time curve (AUC) over one dosing interval was estimated utilizing linear trapezoidal rule. Prednisone apparent oral clearance (CL/F) was estimated as CL/F = dose/AUC. Because separate administration of prednisolone was not performed, prednisolone oral clearance was estimated with reference to the prednisone dose as described by Rose et al.5 and estimated by prednisolone CL/F = prednisone dose/prednisolone AUC. Dose was not adjusted for molecular weight differences given the very small difference and to be consistent with previously published work. 5 Apparent oral volume of distribution (Vβ/F) was estimated as Vβ/F = (CL/F)/k, in which k was the terminal elimination rate constant determined by log-linear regression. The prednisolone/prednisone metabolic ratio (MR) was estimated as AUCprednisolone/AUCprednisone for plasma and Aeprednisolone/Aeprednisone for urine in which Ae was the amount of unchanged prednisone or prednisolone excreted in the urine over one dosing interval. Prednisone and prednisolone renal clearance was calculated by CLR, prednisone= Aeprednisone/AUCprednisone and CLR, prednisolone = Aeprednisolone/AUCprednisolone, respectively. The percentage of dose excreted in urine was calculated by (Aeprednisone/prednisone dose) x 100 and (Aeprednisolone/prednisone dose) x 100 for prednisone and prednisolone, respectively.5 Actual body weights were used for weight-adjusted parameters. When concentrations fell below the lower limit of quantitation before the end of the dosing interval, the concentration at the end of the dosing interval (Cend) was determined by extrapolating from the last measured concentration (Clast) to the end of the dosing interval by in which k was the elimination rate constant and t was the time interval between Clast and Cend.
The amount of prednisone and prednisolone excreted in the breast milk over the dosing interval was determined by summing the amount excreted during each breast milk collection (breast milk volume x concentration). The prednisone or prednisolone breast milk/plasma AUC ratio was determined by (breast milk AUC)/(maternal plasma AUC). The percent of maternal prednisone dose excreted unchanged in breast milk was determined by (amount of prednisone excreted in the breast milk over 1 dosing interval/maternal prednisone dose) x 100. The percent of maternal prednisolone excreted unchanged in breast milk was determined by (amount of prednisolone excreted in the breast milk over 1 dosing interval/maternal prednisone dose) x 100. Infant daily exposure to prednisone via breast milk was calculated by (amount of prednisone excreted in the breast milk over one dosing interval x number of prednisone doses per day)/(body weight of an age-matched 50th percentile infant girl according to the U.S. Centers for Disease Control growth chart).8 The relative infant dose was calculated as (infant daily exposure/(maternal daily dose/maternal actual body weight)) x 100.
One subject concurrently received cyclosporine, a substrate and a competitive inhibitor of cytochrome P450 3A (CYP3A) and P-glycoprotein9 during mid- (150 mg twice daily) and late- (250 mg twice daily) pregnancy. Data regarding potential drug interactions between prednisolone and cyclosporine are conflicting. In a number of studies, cyclosporine has been reported to decrease the clearance of prednisolone by 25–30%. 10–12 However, three smaller studies did not find an interaction between cyclosporine and prednisone and suggested that the drug interaction may depend on dose, route of administration, disease course, or other factors.13–15 Given that CYP3A only accounts for 18% of steroid metabolism and this subject’s PK parameters were all within the range of the other subjects, her PK was included in our analysis. No other subjects were taking medications known or suspected to interact with prednisone. Urine collection was incomplete for three subjects; therefore, their related PK parameters were not determined. Postpartum PK parameters were averaged for the subject who participated in both a lactating and non-lactating study days.
Statistical analyses
Statistical comparisons were made utilizing R16 and the lme417,18 package to complete linear mixed effects analyses evaluating the relationship between dose and pharmacokinetic parameters of interest and the differences in prednisone and prednisolone PK between pregnancy and postpartum. The linear mixed effect model analysis included all subjects (19 pregnant, 6 postpartum) and all doses (4–40 mg/day). In all cases, we did not attempt to account for any direct interaction between variables other than the relationship in question, and only random intercepts were used so that the degrees of freedom could be held to an acceptable level. The method of maximum likelihood was used to fit the linear mixed effect models. P-values (p≤ 0.05) were determined by analysis of variance (ANOVA); i.e., comparing a null model without the effect under investigation to the model including it as a fixed effect. In all cases, subject variability was included as a fixed effect to correct for repeated measures. No significant deviations from normality or homoscedasticity were noted by Shapiro-Wilk test and visual inspection of Q-Q plots, histograms and residual plots. One-way ANOVA was used to analyze prednisone and prednisolone PK during early-, mid-, and late pregnancy to determine whether gestational age had a significant effect on total and unbound prednisone and prednisolone PK parameters. Results are reported as mean ± standard error plus the 95% confidence interval for linear mixed effect model analysis and mean ± standard deviation for all other analyses.
Results
Nineteen female subjects (9 Black, 7 White, 1 Hispanic-White, 1 Hispanic-unknown, and 1 Asian) participated in the study. Subjects participated in early- (n=3), mid- (n=9), and late-pregnancy (n=14), postpartum with lactation (n=2), and postpartum without lactation (n=5). One subject participated in both postpartum studies, i.e. with and without lactation. Six subjects participated in both a pregnancy and postpartum study. Among those six subjects, two subjects received the same dose during pregnancy and postpartum. On study day 1, average age, height, and weight were 27 ± 6 years, 162 ± 11 cm, and 87 ± 21 kg, respectively. Prednisone indications included systemic lupus erythematosus (n=11), rheumatoid arthritis (n=3), asthma (n=1), transplant recipient (n=1), autoimmune hepatitis (n=1), myasthenia gravis (n=1), and Wegner’s granulomatosis (n=1). Only one patient was also receiving a biologic agent (adalimumab).
Dose-dependent pharmacokinetics
Estimated total and unbound prednisone and prednisolone steady-state PK parameters during pregnancy in a subset of female subjects receiving 5, 10, or 20 mg of prednisone are summarized in Table 1. All other dosage groups were limited to only one subject and were included in the linear mixed effect model analysis, but not included in the table. Total prednisone CL/F was dose-dependent during pregnancy so that for every 1 mg increase in dose, prednisone CL/F increased by 0.7 ± 0.2 L/h (χ2=10.8, p=0.001, 95% CI [0.3, 1.0]). Prednisone Vβ/F was similarly dose dependent so that for every 1 mg increase in dose, Vβ/F increased by 3.1 ± 1.0 L (χ2=7.5, p=0.006, 95% CI [1.0, 5.2]). Prednisone AUC increased with dose, so that for every 1 mg increase in prednisone dose, prednisone AUC increased by 7.7 ± 1.0 hr•ng/mL (χ2=29.7, p<0.001, 95% CI [5.6, 9.8]). Prednisone Tmax increased with dose, so that for every 1 mg increase in prednisone dose, prednisone Tmax decreased by −0.01 ± 0.01 (χ2=18.4, p<0.001, 95% CI [−0.03, 0.01]). Prednisolone CLR likewise increased with dose, so that for every 1 mg increase in prednisone dose, prednisolone CLR increased by 0.02 ± 0.01 L/h (χ2=9.6, p=0.002, 95% CI [0.01, 0.04]). Prednisolone AUC increased with dose, so that for every 1 mg increase in prednisone dose, prednisolone AUC increased by 44.9 ± 9.5 hr•ng/mL (χ2=15.9, p<0.001, 95% CI [24.8, 67.9]). Similarly, urine prednisolone/prednisone metabolic ratios were altered by dose so that for every 1 mg increase in dose, urine metabolic ratio increased by 0.1 ± 0.0 (χ2=5.5, p=0.02, 95% CI [0.0, 0.1]). In contrast, prednisone half-life, Cmax, CLR and percent of dose excreted unchanged in the urine as well as prednisolone half-life, Cmax, Tmax, CL/F, Vβ/F and percent of dose excreted in the urine did not exhibit significant dose-dependency.
Table 1.
Estimated mean (± standard deviation) steady-state pharmacokinetic parameters for total and unbound prednisone and prednisolone in female subjects receiving oral prednisone 5, 10, or 20 mg during pregnancy.
| Parameter | Dose (mg) |
P valuea | ||
|---|---|---|---|---|
| 5 (n=5) | 10 (n=5) | 20 (n=7) | ||
| Prednisone | ||||
| t ½ (h) | 3.4 ± 0.4 | 2.9 ± 1.0 | 2.7 ± 0.4 | 0.4 |
| t ½ unbound (h) | 3.0 ± 0.3 | 2.7 ± 0.7 | 2.4 ± 0.3 | 0.9 |
| Tmax (h) b | 1.6 ± 0.6 | 2.6 ± 0.8 | 1.3 ± 0.3 | <0.001 |
| Cmax (ng/mL) b | 25.2 ± 13.1 | 25.4 ± 3.5 | 37.9 ± 2.5 | 0.2 |
| Cmax unbound (ng/mL) b | 5.5 ± 3.1 | 5.7 ± 1.1 | 10.7 ± 0.8 | <0.001 |
| AUC (h*ng/mL) b | 142.1 ± 70.3 | 176.2 ± 21.4 | 281.9 ± 36.2 | <0.001 |
| AUC unbound (h*ng/mL) b | 29.4 ± 14.4 | 35.8 ± 5.0 | 65.6 ± 5.3 | <0.001 |
| CL/F (L/h) | 35.1 ± 11.4 | 52.6 ± 5.2 | 64.3 ± 6.9 | 0.001 |
| CL/F unbound (L/h) | 198.1 ± 61.3 | 289.1 ± 46.9 | 298.6 ± 33.3 | 0.02 |
| CLR (L/h)c | 1.6 ± 0.8 | 2.3± 0.9 | 2.9 ± 0.8 | 0.4 |
| Vβ/F (L) | 177.6 ± 71.3 | 219.2 ± 63.2 | 266.9 ± 51.3 | 0.006 |
| Vβ/F unbound (L) | 884.0 ± 334.4 | 1121.8 ± 270.3 | 1028.9 ±92.8 | 0.09 |
| % excreted in urinec | 3.8 ± 2.0 | 4.0 ± 1.8 | 4.4 ± 1.0 | 0.8 |
| Prednisolone | ||||
| t ½ (h) | 3.1 ± 0.5 | 2.9 ± 0.8 | 2.9 ± 0.6 | 0.9 |
| t ½ unbound (h) | 2.7 ± 0.2 | 2.7 ± 0.8 | 2.1 ± 0.4 | 0.4 |
| Tmax (h) | 1.5 ± 0.4 | 1.6 ± 0.2 | 0.8 ± 3.0 | 0.4 |
| Cmax (ng/mL) | 187.1 ± 14.2 | 252.9 ± 97.1 | 431.8 ± 3.0 | 0.05 |
| Cmax unbound (ng/mL) | 10.9 ± 1.8 | 14.8 ± 7.3 | 54.8 ± 34.7 | <0.001 |
| AUC (h*ng/mL) | 998.9 ± 50.5 | 1406.0 ± 553.4 | 2054.9 ± 3.0 | <0.001 |
| AUC unbound (h*ng/mL) | 39.9 ± 6.3 | 55.0 ± 22.7 | 86.5 ± 23.4 | 0.3 |
| CL/F (L/h) | 4.8 ± 0.3 | 7.6 ± 3.3 | 9.4 ± 4.1 | 0.2 |
| CL/F unbound (L/h) | 133.7 ± 21.2 | 213.1 ± 101.0 | 170.9 ± 60.0 | 0.1 |
| CLR (L/h)b | 0.3 ± 0.3 | 0.5 ± 0.4 | 1.3 ± 1.1 | 0.002 |
| Vβ/F (L) | 21.5 ± 3.8 | 29.0 ± 7.0 | 37.6 ± 13.8 | 0.07 |
| Vβ/F unbound (L) | 513.1± 93.4 | 766.3 ± 283.8 | 518.5 ± 175.0 | 0.4 |
| % excreted in urinec | 5.4 ± 5.1 | 6.9 ± 5.8 | 10.8 ± 7.5 | 0.1 |
| Urine pln/pn MRc | 1.2 ± 1.2 | 1.6 ± 1.1 | 2.6 ± 1.9 | 0.02 |
| Plasma AUC pln/pn MR | 7.7 ± 2.6 | 7.9 ± 3.1 | 6.3 ± 1.6 | 0.09 |
| Plasma AUC pln/pn unbound MR | 1.5 ± 0.4 | 1.5 ± 0.5 | 1.9 ± 0.7 | 0.1 |
n: number of subjects; CL/F: apparent oral clearance; t½: terminal half-life; CLR: renal clearance; Vβ/F: apparent volume of distribution; AUC: area under the curve; pln: prednisolone; pn: prednisone; MR: metabolic ratio
P-value reflects significant differences across all pregnancy data controlled for subject and total daily dose utilizing the linear mixed effects model analyses.
Reflects n=4 for 5mg, n=5 for 10mg, and n=3 for 20mg taking oral prednisone once daily.
Reflects n=4 for 5mg, n=5 for 10 mg, and n=6 for 20 mg due to incomplete urine collection in some subjects.
In addition to dose dependency of total prednisone PK, unbound prednisone CL/F also exhibited dose dependency during pregnancy so that for every 1 mg increase in dose, unbound prednisone CL/F increased 2.2 ± 0.9 L/h (χ2=5.6, p=0.02, 95% CI [0.4, 4.0]). Unbound prednisone AUC increased with dose, so that for every 1 mg increase in prednisone dose, prednisone AUC increased by 1.7 ± 0.2 hr•ng/mL (χ2=30, p<0.001, 95% CI [1.2, 2.2]). Unbound prednisone Cmax increased with dose, so that for every 1 mg increase in prednisone dose, prednisone Cmax increased by 0.29 ± 0.04 ng/mL (χ2=20.5, p<0.001, 95% CI [0.20, 0.37]). In contrast, unbound prednisolone PK did not change with dose, except for unbound prednisolone Cmax. For every 1 mg increase in prednisone dose, prednisolone Cmax increased by 1.7 ± 0.3 ng/mL (χ2=21.2, p<0.001, 95% CI [1.0, 2.4]).
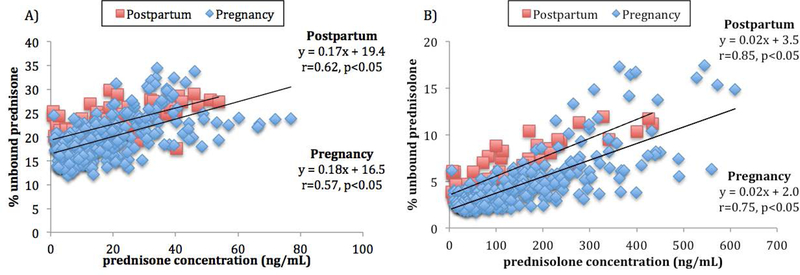
Higher prednisone (r = 0.57, p≤ 0.05) and prednisolone (r = 0.75, p≤ 0.05) concentrations were associated with higher percent unbound during pregnancy and postpartum. These data are consistent with concentration-dependent binding of prednisone and prednisolone within the therapeutic range (Figure 1). Pregnancy was associated with significantly lower percent unbound than postpartum for both prednisone (Figure 1A, p=0.003) and prednisolone (Figure 1B, p<0.001). Of note, there was a trend towards greater variability in the percent unbound as the concentration of prednisolone increased above 200 ng/mL.
Figure 1.
A) Correlation between prednisone concentrations and prednisone percent unbound during pregnancy (diamonds; early, mid-, and late pregnancy) receiving oral prednisone doses from 3.5 to 40 mg and ≥ 12 weeks postpartum (squares; with and without lactation) receiving doses from 2 to 20 mg. B) Correlation between prednisolone concentrations and prednisolone percent unbound during pregnancy (diamonds; early-, mid-, and late-pregnancy) receiving oral prednisone doses from 3.5 to 40 mg and ≥ 12 weeks postpartum (squares; with and without lactation) receiving doses from 2 to 20 mg.
Pharmacokinetics of prednisone and prednisolone in pregnancy vs postpartum
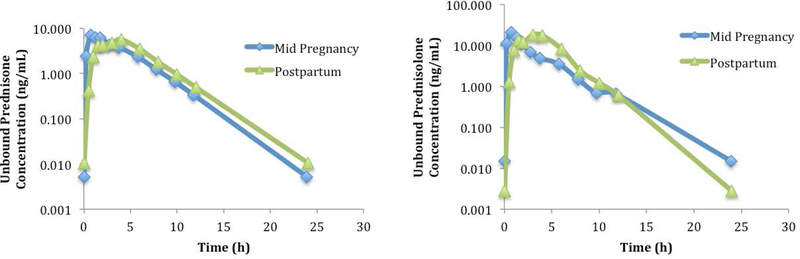
Figure 2 depicts the steady-state, unbound plasma concentration-time profiles of a representative subject receiving prednisone 10 mg once daily during mid-pregnancy and 12 weeks postpartum. The concentration-time profiles look similar except for earlier peak concentrations of both prednisone and prednisolone during pregnancy. When comparing pregnancy to postpartum, including all subjects and all doses, prednisone and prednisolone PK parameters did not show significant differences except prednisolone CL/F. Prednisolone apparent oral clearances, in a subset of subjects receiving prednisone doses of 5, 10, and 20 mg were 4.8 ± 0.3, 7.6 ± 3.3, and 9.4 ± 4.1 L/h during pregnancy, respectively and 11.3, 7.6, and 11.6 ± 2.0 L/h postpartum, respectively. Only one subject was available as a comparator for postpartum prednisone doses 5 and 10 mg. When controlling for dose and for intra-subject variability, prednisolone CL/F was significantly lower during pregnancy compared to postpartum (χ2=4.9, p=0.03, 95% CI [−1.8, −0.1]).
Figure 2.
A representative subject’s unbound steady-state plasma concentration-time profiles during mid-pregnancy and 12 weeks postpartum receiving prednisone 10 mg once daily.
Gestational Age Effects
Total and unbound prednisone and prednisolone half-life, CL/F, and Vβ/F were similar across gestational ages (early-, mid- and late-pregnancy), with no significant changes (data not shown).
Milk concentrations
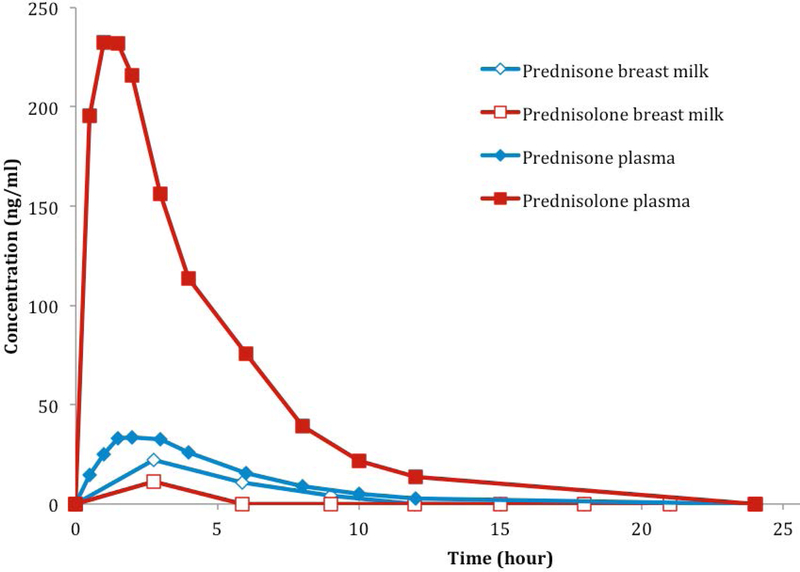
Figure 3 depicts a representative subject’s steady-state prednisone and prednisolone concentration-time curves in breast milk and maternal plasma at 12 weeks postpartum. In the two subjects who participated in the lactation portion of the study, prednisolone concentrations in plasma were 22-fold and 14-fold higher than in breast milk at 3 hours post-dose following 2 mg orally every 12 hours and 15 mg orally every 24 hours, respectively. In these same subjects, breast milk/plasma AUC ratios were 0.5 and 0.6 for prednisone, and 0.02 and 0.03 for prednisolone, respectively. The relative infant doses as a percent of the maternal body-weight-adjusted dose were 0.58% and 0.35% for prednisone and 0.18% and 0.09% for prednisolone. Prednisone and prednisolone were each below their limits of assay detection (4 ng/mL) in breast milk after 12 hours and 6 hours for these subjects, respectively. The unbound prednisone concentrations in the milk at 3 hours post dose were 2.4- to 3.8-fold higher than in maternal plasma. In contrast, unbound prednisolone concentrations were 1.3- to 1.5-fold lower in milk compared to maternal plasma.
Figure 3.
A representative concentration and time curve of prednisone and prednisolone in plasma and breast milk of a subject receiving prednisone 15 mg orally every 24 hours at 12 weeks postpartum.
Cord blood concentrations
Venous and arterial umbilical cord and maternal plasma samples were collected from 13 subjects at delivery, 1.2 to 32 h post-dose. Most concentrations at the time of delivery were too low to be quantified. We were able to quantify maternal prednisolone to prednisone ratio in 5 subjects, umbilical cord venous prednisolone to prednisone ratio in 4 subjects, umbilical cord to maternal prednisolone ratio in 5 subjects and umbilical cord to maternal prednisone ratio in 5 subjects. At the time of delivery, prednisolone to prednisone concentration ratios were 15.1 ± 12.4 (range 4.1–32.2) and 0.5 ± 0.5 (range 0.2–1.3) in the mother and umbilical cord venous samples, respectively. Umbilical cord to maternal plasma concentration ratios were 0.2 ± 0.2 (range 0.1–0.6) and 4.0 ± 4.3 (range 0.8–11.4) for prednisolone and prednisone, respectively. Arterial to venous concentration ratios were measurable in 3 subjects for prednisolone and 2 subjects for prednisone. Umbilical cord arterial to venous plasma concentration ratios were 1.4 ± 0.5 and 0.9 ± 0.0 for prednisolone and prednisone, respectively. In addition, there was a strong correlation between maternal prednisolone concentrations and umbilical cord prednisone concentrations (r2 = 0.97, data not shown).
Adverse events and outcomes
All reported adverse events related to the study days were minor and included bruising at venipuncture sites (n=4), mild dizziness (n=1), mild fatigue (n=1), and hives from adhesive tape (n=1). The hives resolved with diphenhydramine; the other events resolved without treatment. Labor and delivery information was available for 15 out of 19 subjects. Seven subjects delivered preterm, including 1 at 28 weeks, 1 at 34 weeks, 1 at 35 weeks, and 4 at 36 weeks of gestation. The rest of the subjects had unremarkable deliveries at term.
Discussion
We report, for the first time, the pharmacokinetics of oral prednisone and its active metabolite prednisolone during pregnancy. As described in the non-pregnant population5, prednisone also exhibits dose dependent pharmacokinetics during pregnancy. Prednisone apparent oral clearance, unbound apparent oral clearance, and apparent oral volume of distribution increased significantly with increasing dose (Table 1). In addition, unbound fraction increased with increasing concentration. The increased fraction unbound in plasma is likely due to saturation of plasma protein binding at higher concentrations. This provides a partial explanation for the increased prednisone apparent oral clearance and apparent oral volume of distribution. The increase in prednisone unbound apparent oral clearance (p=0.02) suggests additional changes as well, such as a decrease in bioavailability or increase in clearance with increasing dose. However, since prednisone is a low extraction ratio drug (<3%),19 its bioavailability would not be expected to change with protein binding. On the other hand, the increased unbound prednisone apparent oral clearance with increased dose could either be explained by dose-dependent increase in access to sites of metabolism or previously unrecognized dose-dependent intrinsic metabolic clearance process.
Although prednisolone fraction unbound also increased with increasing prednisolone concentrations (Figure 1), neither prednisolone CL/F nor unbound prednisolone CL/F exhibited statistically significant dose-dependency in our subjects, although there was a trend toward increasing prednisolone total CL/F with increasing prednisone dose. In line with this trend, previous work in the non-pregnant population has reported increased total prednisolone clearance with higher doses.5 Our data only showing a trend is likely a reflection of our small sample size. In addition, consistent with the previous study in non-pregnant subjects,5 unbound prednisolone AUC and unbound prednisolone CL/F did not change with dose.
Given the physiological changes that occur over the course of pregnancy, we explored the effects of gestational age on total and unbound prednisone and prednisolone half-life, CL/F, and Vβ/F. Gestational age (early-, mid- and late pregnancy) did not significantly alter any of these PK parameters. Given our limited sample size, additional work is needed to confirm these findings.
Protein binding of prednisone and prednisolone is quite complex even in the non-pregnant population. Both prednisone and prednisolone are known to be highly bound to both albumin (low affinity, high capacity) and corticosteroid binding globulin (high affinity, low capacity), both of which change during pregnancy.6 Albumin concentrations are known to decrease by ~15% and corticosteroid binding globulin concentrations increase by 2- to 3-fold during normal pregnancy. The increase in corticosteroid binding globulin concentrations during pregnancy increased the percent bound of both prednisone and prednisolone as shown in Figure 1 (p=0.003, p<0.001, respectively). These results differ from those for most highly bound drugs, which are bound to albumin and α1-acid glycoprotein and have decreased plasma protein binding during pregnancy. The increased variability in percent unbound for prednisolone concentrations above 200 ng/mL is likely explained by variability in corticosteroid binding globulin20 and albumin concentrations. In addition, binding of other endogenous competitors such as cortisol and cortisone might also affect saturation of binding sites.
Prednisone and prednisolone undergo inter-conversion. Type 1 11β-Hydroxysteroid-dehydrogenase (11β –HSD1) primarily converts prednisone to prednisolone in the liver. Outside the liver, in organs such as the placenta, kidney and intestine, 11β-hydroxysteroid-dehydrogenase type 2 (11β -HSD2) primarily converts prednisolone to prednisone.4 Pregnancy increased the apparent oral clearance of prednisolone. This could occur with either increased metabolism or decreased formation of prednisolone. Either increased 11β –HSD2 activity or decreased activity of 11β –HSD1 would increase prednisolone apparent oral clearance during pregnancy. Placental 11β-HSD2 appears to increase as pregnancy progresses, though further research on both enzyme activities is needed.21,22 Prednisolone is further metabolized by CYP3A, accounting for approximately 18% of its overall metabolism in the non-pregnant population.23 CYP3A activity is markedly increased during pregnancy, potentially contributing to the observed increase in prednisolone CL/F.7 Another possible factor would be a decrease in protein binding, but this is unlikely because protein binding actually increased during pregnancy. The lack of change in the unbound prednisolone apparent oral clearance during pregnancy suggests that dose adjustments may not be needed based on the pharmacokinetic changes alone. However, this requires confirmation with a larger study. In addition, pharmacodynamic changes may or may not require dosage adjustment.
The relative concentrations of prednisone and prednisolone are highly dependent on 11β-HSD1 and 11β-HSD2 activities. At the time of delivery, 11β-HSD1 dominance would explain our observed prednisolone to prednisone ratio in maternal plasma of 15 ± 12 (range 4–32), slightly higher but consistent with previously reported values ranging from 3–9. 24,25 However, when considering umbilical cord concentrations, placental 11β-HSD2 activity appears more important. Both 11β-HSD1 and 11β-HSD2 are expressed in the placenta, but 11β-HSD2 activity is 7- to 8-fold higher than 11β-HSD1.26 This differential activity is most likely responsible for the differing prednisolone to prednisone ratios in umbilical cord and maternal samples. We observed an umbilical cord plasma prednisolone to prednisone ratio of 0.5 ± 0.5 (range 0.2–1.3), consistent with previous reports (0.4 ± 0.2 and 0.5 ± 0.1).24,25 Differences in maternal and umbilical cord prednisolone to prednisone ratios might also be affected by differences in their placental P-glycoprotein affinity, though differences in transport efficiency in a stably-transfected LLC-PK cell model are insufficient to explain our results.27 Taken together, high relative expression and activity of 11β-HSD2 likely limits fetal exposure to the active prednisolone by back-metabolism to inactive prednisone.
Prednisolone umbilical cord venous to maternal plasma concentration ratio was 0.2 ± 0.2 (range 0.1–0.6) in our study, similar to previous reports (0.1 ± 0.0).24,25 In contrast, our prednisone umbilical cord venous to maternal plasma concentration ratio was 4.0 ± 4.3 (range 0.8–11.4), which is higher and more variable than in previous reports (range 0.8–2.7).24,25 The high variability in the prednisone and prednisolone umbilical cord to maternal concentration ratios are potentially due to the high variability in placental 11β-HSD activity. The activities of 11β-HSD1 and 11β-HSD2 are dependent not only on differences in genetics, but also on several external factors such as gestational age, preeclampsia, chorioamnionitis and asthma.21,22,28 This study had multiple subjects with pre-term delivery that likely affected these ratios as well. The variability in dosing in our study likely contributed to the increased variability in prednisone umbilical cord to maternal concentration ratios.
Although the fetus does not contribute significantly to overall metabolic drug clearance by the maternal-fetal unit during pregnancy, its ability to metabolize drugs can affect the local, fetal concentrations of drug and metabolites.29 In the few subjects with quantifiable prednisone and prednisolone concentrations in both the umbilical arterial and venous plasma, the dominant fetal effect appeared to be conversion of prednisone to prednisolone, presumably by 11β-HSD1. With the drug passing through the fetus, this translated into umbilical cord arterial to venous concentration ratio of 1.4 ± 0.5 for prednisolone and 0.9 ± 0.0 for prednisone. This is consistent with previously published work reporting the presence of 11β-HSD1 near term in fetal organs.30 Prior to mid-gestation, expression of 11β-HSD2 is dominant and 11β-HSD1 has little to no expression in fetal tissues,30 limiting exposure to active steroids until term. In a previous prospective study, glucocorticoid exposure during the first trimester of pregnancy (70% received prednisone) did not result in increased incidence of oral cleft or congenital anomalies.31 However, one study found prednisone doses of >20 mg/day during pregnancy to be associated with an increased risk for cleft palate.32 Evaluation of a dose-related effect would require further study. Interestingly, in a small controlled study, normal adrenal reserve was found in infants exposed in utero to long-term corticosteroid use.33
Only 0.02% and 0.04% of the maternal prednisone dose was recovered in breast milk from each of 2 study participants. In a previous study, prednisone recovery in breast milk after a two-hour collection was also very low (0.26% of the maternal dose).34 The relative infant doses of prednisone (0.35 and 0.53%) and prednisolone (0.09 and 0.18%) appear clinically insignificant. Interestingly, unbound prednisone in breast milk at 3 hours post dose was 2.4- to 3.8-fold higher than the unbound prednisone in maternal plasma, whereas unbound prednisolone was 1.3- to 1.5-fold lower. Prednisone and prednisolone, both neutral and non-ionizable drugs, are unlikely to be affected by the pH gradient between plasma and breast milk. Both are moderately lipophilic, low-molecular-weight compounds and are thus expected to readily cross the mammary epithelia. The slightly longer half-life of prednisone versus prednisolone (3.6 ± 0.4 h vs. 2.2 ± 0.5, respectively) is also an unlikely explanation for this difference. Both prednisone and prednisolone are substrates of the organic anion transporters (OATs).35 However, whether transport of prednisone or prednisolone via OATs occurs in the mammary gland is not known. While further research is required, this surprising concentration of unbound prednisone in breast milk may be explained by prednisolone back conversion mediated by mammary 11β-HSD2. This possibility is supported by elevated mammary 11β-HSD activity in the pregnant rat.36
Short-term use of prednisone has been shown to be associated with maternal and fetal complications such as preterm delivery.37 In addition, patients with autoimmune diseases or solid organ transplant recipients are at increased risk for preterm delivery and low birth weight infants. 38–40 Consistent with this data, approximately half of the females participating in this study had preterm deliveries. The combination of prednisone and the patients’ underlying medical conditions both may have contributed to the preterm deliveries observed in approximately half of our study subjects. It is not possible to differentiate the contributions by prednisone from those of the indications for its use in this relatively small study.
Conclusion
In conclusion, we report for the first-time detailed pharmacokinetics of prednisone and its active metabolite prednisolone during and after pregnancy. Prednisone exhibits dose-dependent pharmacokinetics during pregnancy, but dose adjustment of prednisone may not be needed during pregnancy for pharmacokinetic reasons given the lack of change in unbound prednisolone apparent oral clearance. However, a larger sample size is needed to verify our results. In addition, gestational changes in prednisolone pharmacodynamics require further study and might affect dosage requirements. Both prednisone and prednisolone are found in breast milk in very low concentrations such that oral prednisone use appears compatible with breastfeeding.
Supplementary Material
Acknowledgments
We gratefully acknowledge the expert technical assistance of Linda Risler and Brian Phillips from the University of Washington Department of Pharmaceutics Bio Analytical Laboratory.
Footnotes
Disclosure:
None of the authors have conflicts of interest.
Reference
- 1.Andrade SE1, Gurwitz JH, Davis RL, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol 2004. August;191(2):398–407. [DOI] [PubMed] [Google Scholar]
- 2.Pacheco LD, Ghulmiyyah LM, Snodgrass WR, et al. Pharmacokinetics of corticosteroids during pregnancy. Amer J Perinatol 2007; 24(2): 079–082 [DOI] [PubMed] [Google Scholar]
- 3.Bergmann TK, Barraclough KA, Lee KJ, et al. Clinical pharmacokinetics and pharmacodynamics of prednisolone and prednisone in solid organ transplants. Clin Pharmacokinet 2012; 51(11): 711–741. [DOI] [PubMed] [Google Scholar]
- 4.Diederich S, Eigendorff E, Burkhardt P, et al. 11Beta-hydroxy-steroid dehydrogenase types 1 and 2: an important pharmacokinetic determinant for the activity of synthetic mineralo- and glucocorticoids. J Clin Endocrinol Metab 2002;87(12):5695–701. [DOI] [PubMed] [Google Scholar]
- 5.Rose JQ, Yurchak AM, Jusko WJ. Dose dependent pharmacokinetics of prednisone and prednisolone in man. J Pharmacokinet Biopharm 1981;9(4):389–417. [DOI] [PubMed] [Google Scholar]
- 6.Potter JM, Mueller UW, Hickman PE. Corticosteroid binding globulin in normotensive and hypertensive human pregnancy. Clin Science 1987. June;72(6):725–35. [DOI] [PubMed] [Google Scholar]
- 7.Hebert MF, Easterling TR, Kirby B, et al. Effects of Pregnancy on CYP3A and P-glycoprotein Activities as Measured by Disposition of Midazolam and Digoxin: A University of Washington Specialized Center of Research Study. Clin Pharmacol Ther 2008. August;84(2):248–53. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics and the National Center for Chronic Disease Prevention and Health Promotion. Birth to 36 months: girls, length-for-age and weight-for-age percentiles May 30, 2000 (modified 4/20/01). http:www.cdc.gov/growthcharts. [Google Scholar]
- 9.Lam S, Partovi N, Ting LS, et al. Corticosteroid interactions with cyclosporine, tacrolimus, mycophenolate, and sirolimus: fact or fiction? Ann Pharmacother 2008. July;42(7):1037–47. [DOI] [PubMed] [Google Scholar]
- 10.Ost L Impairment of Prednisolone Metabolism by Cyclosporine Treatment in Renal Graft Recipients. Transplantation 1987. October;44(4):533–5. [PubMed] [Google Scholar]
- 11.Langhoff E, Madsen S. Rapid metabolism of cyclosporin and prednisone in kidney transplant patients on tuberculostatic treatment. Lancet 1983. December 3;2(8362):1303. [DOI] [PubMed] [Google Scholar]
- 12.Langhoff E, Madsen S, Flachs H, et al. Inhibition of Prednisolone Metabolism by Cyclosporine in Kidney-Transplanted Patients. Transplantation 1985. January;39(1):107–9. [PubMed] [Google Scholar]
- 13.Frey FJ, Schnetzer A, Horber FF, et al. Evidence that cyclosporine does not affect the metabolism of prednisolone after renal transplantation. Transplantation 1987. April;43(4):494–8. [DOI] [PubMed] [Google Scholar]
- 14.Rocci ML Jr, Tietze KJ, Lee J, et al. The effect of cyclosporine on the pharmacokinetics of prednisolone in renal transplant patients. Transplantation 1988. March;45(3):656–60. [DOI] [PubMed] [Google Scholar]
- 15.Arnold JC, O’Grady JG, Tredger JM, et al. Effects of low-dose prednisolone on cyclosporine pharmacokinetics in liver transplant recipients: radioimmunoassay with specific and non-specific monoclonal antibodies. Eur J Clin Pharmacol 1990;39(3):257–60. [DOI] [PubMed] [Google Scholar]
- 16.R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: 2015. Available from: http://www.R-project.org. [Google Scholar]
- 17.Bates D, Maechler M, Bolker B, et al. lme4: Linear mixed-effects models using Eigen and S4. R package version 1 1–8, 2016. April 16 Available from: http://CRAN.R-project.org/package=lme4.
- 18.Bates D, Maechler M, Bolker BM, et al. “Fitting Linear Mixed-Effects Models using lme4.” ArXiv e-print; in press, Journal of Statistical Software, 2015. Available from: http://arxiv.org/abs/1406.5823.
- 19.Delcò F, Tchambaz L, Schlienger R, et al. Dose adjustment in patients with liver disease. Drug Saf 2005;28(6):529–45. [DOI] [PubMed] [Google Scholar]
- 20.Doe R, Fernandez R, Seal US. Measurements of corticosteroid-binding globulin in man. J Clin Endocrinol Metab 1964; 24 (10): 1029–1039. [DOI] [PubMed] [Google Scholar]
- 21.Schoof E, Girstl M, Frobenius W, et al. Course of placental 11 β-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase mRNA expression during human gestation. European Journal of Endocrinology 2001. August;145(2):187–92. [DOI] [PubMed] [Google Scholar]
- 22.Shams M, Kilby MD, Somerset DA, et al. 11Beta-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Hum Reprod 1998. April;13(4):799–804. [DOI] [PubMed] [Google Scholar]
- 23.Frey FJ, Frey BM. Urinary 6 beta-hydroxyprednisolone excretion indicates enhanced prednisolone catabolism. J Lab Clin Med 1983. April;101(4):593–604. [PubMed] [Google Scholar]
- 24.van Runnard Heimel PJ, Schobben AF, Huisjes AJ, et al. The transplacental passage of prednisolone in pregnancies complicated by early-onset HELLP syndrome. Placenta 2005. November;26(10):842–5. [DOI] [PubMed] [Google Scholar]
- 25.Beitins IZ, Bayard F, Ances IG, et al. The transplacental passage of prednisone and prednisolone in pregnancy near term. J Pediatr 1972. November;81(5):936–45. [DOI] [PubMed] [Google Scholar]
- 26.Mericq V, Medina P, Kakarieka E, et al. Differences in expression and activity of 11 β-hydroxysteroid dehydrogenase type 1 and 2 in human placentas of term pregnancies according to birth weight and gender. Eur J Endocrinol 2009. September;161(3):419–25. [DOI] [PubMed] [Google Scholar]
- 27.Yates CR, Chang C, Kearbey JD, et al. Structural determinants of P-glycoprotein-mediated transport of glucocorticoids. Pharmaceut Res 2003. November;20(11):1794–803. [DOI] [PubMed] [Google Scholar]
- 28.Edwards CRW, Benediktsson R, Lindsay RS, et al. 11β-Hydroxysteroid dehydrogenases: Key enzymes in determining tissue-specific glucocorticoid effects. Steroids 1996. April;61(4):263–9. [DOI] [PubMed] [Google Scholar]
- 29.Szeto HH, Umans JG, Rubinow SI. The contribution of transplacental clearances and fetal clearance to drug disposition in the ovine maternal-fetal unit. Drug Metab Dispos 1982. 10:382–6 [PubMed] [Google Scholar]
- 30.Speirs HJ, Seckl JR, Brown RW. Ontogeny of glucocorticoid receptor and 11beta-hydroxysteroid dehydrogenase type-1 gene expression identifies potential critical periods of glucocorticoid susceptibility during development. J Endocrinol 2004. April; 181(1):105–16. [DOI] [PubMed] [Google Scholar]
- 31.Gur C, Diav-Citrin O, Shechtman S, Arnon J, Ornoy A. Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reprod Toxicol 2004. Jan-Feb;18(1):93–101. [DOI] [PubMed] [Google Scholar]
- 32.Ostensen M, Khamashta M, Lockshin M, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther 2006;8(3):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arad I, Landau H. Adrenocortical reserve of neonates born of long-term, steroid-treated mothers. Eur J Pediatr 1984;142:279–80. [DOI] [PubMed] [Google Scholar]
- 34.Katz FH, Dubcan BR. Entry of Prednisone into human milk. N Engl J Med 1975. 27;293:1154. [DOI] [PubMed] [Google Scholar]
- 35.Bossuyt X, Müller M, Hagenbuch B, et al. Polyspecific drug and steroid clearance by an organic anion transporter of mammalian liver. J Pharmacol Exp Ther 1996. March;276(3):891–6. [PubMed] [Google Scholar]
- 36.Quirk SJ, Slattery J, Funder JW. 11 beta-hydroxysteroid dehydrogenase activity in the mammary gland. J Steroid Biochem 1990. April;35(5):623–5. [DOI] [PubMed] [Google Scholar]
- 37.Cowchock FS, Reece EA, Balaban D, et al. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am. J. Obstet. Gynecol 1992. May;166(5):1318–23. [DOI] [PubMed] [Google Scholar]
- 38.Repke JT. Hypertensive disorders of pregnancy. Differentiating preeclampsia from active systemic lupus erythematosus. J Reprod Med 1998. April;43(4):350–4. [PubMed] [Google Scholar]
- 39.Erkan D, Sammaritano L. New insights into pregnancy-related complications in systemic lupus erythematosus. Curr Rheumatol Rep 2003. October;5(5):357–63. [DOI] [PubMed] [Google Scholar]
- 40.Brosens I, Brosens JJ, Benagiano G. The risk of obstetrical syndromes after solid organ transplantation. Best Pract Res Clin Obstet Gynaecol 2014. November;28(8):1211–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.