Abstract
LMB-100 is a recombinant immunotoxin being developed for cancer treatment that is composed of a Fab that binds to mesothelin and a portion of Pseudomonas exotoxin A. LMB-100 is in clinical trials for the treatment of mesothelioma and pancreatic cancer. To determine if check point modulating antibodies enhance the formation of anti-drug antibodies (ADA) against LMB-100, we treated mice with LMB-100 and four different immune modulating monoclonal antibodies that have different mechanisms of action; anti-CTLA4, anti-OX40, anti-PD-1 and anti-PDL-1. We found that anti-PD-1 and anti PDL-1 do not increase the formation of ADA, but anti-CTLA-4 and anti-OX-40 do increase the onset of ADA. These results indicate that combining anti-CTLA-4 and anti-OX-40 with antibodies and other protein-based therapeutics may enhance ADA formation in humans.
Keywords: Immunotoxin, Immunogenicity, anti-drug antibodies, check point inhibitor, immune modulation, immune-oncology
1. Introduction
LMB-100 is a recombinant immunotoxin composed of an antibody that targets mesothelin and a fragment of Pseudomonas exotoxin A that is designed to selectively kill tumor cells. Mesothelin is a cell surface glycoprotein that is highly expressed in mesothelioma, pancreatic cancer, and other cancers including lung, ovary and stomach [1, 2]. By combining the target specificity of an antibody with the cell killing activity of a powerful toxin, recombinant immunotoxins can kill cancer cells and spare normal organs that do not express the target antigen.
Immunotherapy has revolutionized the field of cancer treatment and recently Immune-modulating monoclonal antibodies (mAbs) have produced many durable cancer regressions in melanoma, colorectal cancers, non-small cell lung cancer (NSCLC) and other cancer types [3]. Immunotherapy mAbs have been subdivided into two major groups based on their mechanism of action. The first group includes antibodies that activate and prime T cells. These include anti-CTLA4, anti-OX-40, anti-CD137 and anti-CD-27. The second group involves mAbs that interfere with cell exhaustion mechanisms (“release the breaks”) such as anti-PD-1 and anti-PDL-1 [3]. While the specific mechanism of each of those mAbs varies, they all affect immune regulation, and their activity holds many risks including autoimmunity and cytokine storm [4, 5]. In cases when protein therapeutics are combined with these immune modulating mAbs, there is also a risk of increase in unwanted immunogenicity of the therapeutic protein. In addition, check point inhibitors are now being administered with other anti-cancer agents to increase their efficacy [6, 7] and there is a risk in alteration of the immune response against those agents.
Pseudomonas exotoxin A kills cells by ADP ribosylation of elongating factor II which leads to protein translation arrest and promotes apoptosis. This mechanism of killing has been recently suspected to promote immune engagement in the regressing tumor site [8, 9]. This immune engagement lead researchers to evaluate the effectiveness of combination therapy of immunotoxins with immune modulating mAbs in pre-clinical models [10] and in recent clinical trials (NCT02990416, NCT03258593). While the pre-clinical efficacy of such combinations seems promising, the effect of such combination on the immunogenicity and the formation of anti-drug antibodies (ADA) against the immunotoxin has not been studied. Understanding the effect of immune modulating mAbs on immunogenicity is critical to plan safer and more effective clinical studies.
We evaluated the formation of ADAs against LMB-100 given with four immune modulating mAbs that are favorable candidates for combination therapy and represent several immunotherapy mechanisms: T cell activator via agonism (anti-CTLA4), T cell activator via antagonism (anti-OX40), and T cell “release the breaks” (anti-PD-1 and anti-PDL-1).
2. Material and Methods
2.1. Compounds
LMB-100 was manufactured by Roche Diagnostics (Mannheim, Germany) as previously described [11] and provided through a Collaborative Research and Development Agreement with F. Hoffman-LaRoche Ltd. All monoclonal antibodies were purchased from BioXcell with inVivoPlus grade; anti-CTLA4 (mouse IgG2b, clone 9D9), anti-PD1 (Rat IgG2a, clone RMP1–14), anti-PDL-1 (Rat IgG2b, clone 10F.9G2) and anti-OX40 (Rat IgG1, clone OX-86). All mAbs were diluted in PBS to a concentration of 1mg/ml.
2.2. Mice and plasma samples
Female, wild-type BALB/c mice 8–10 wk of age were acquired from Charles River (Frederick, MD). All mice experiments followed National Institutes of Health guidelines approved by the Animal Care and Use Committee of the National Cancer Institute. Mice were injected with 50 μg of LMB-100 (I.V) twice a week on the first and third day of each week over the course of three weeks (total of six doses of LMB-100) and immune modulating mAbs (or vehicle) were injected I.P (100 μg/mouse) on the second and fifth day of every week (total of six doses). Blood samples were collected on days 0, 7, 14 and 21 into heparinized tubes. Samples were centrifuged at 3000 rpm for 5 min and plasma was collected and stored at −20°C. Mice weight was measured once a week and treatment withheld if mice experienced a weight loss of 10% of their initial body weight. One mouse in the group of LMB-100+anti-OX40 died prior to bleeding on week 2. Apart from this one mouse, no animals were excluded from statistical analysis. The experiment was performed twice, once with n=4 and once with n=8 with similar results. The results shown are a mean of all 12 mice in each group.
2.3. ELISA assays
Anti–LMB-100 titers were measured as previously described [12]. In brief, ELISA plates (Thermo Fisher) were coated with 100 μl of LMB-100 (91 μg/ml). Plates were blocked with 3% BSA and serial dilutions of plasma were incubated for 1 h. Anti-LMB-100 antibodies were detected with goat anti-mouse IgG (H+L) HRP (Jackson ImmunoResearch) (1:3000) and TMB substrate (Thermo Fisher). Optical density of the wells was read immediately after the addition of H2SO4 stop solution, at a wavelength of 450 nm with subtraction at 650 nm. Titers were calculated based on a four-parameter logistic curve-fit graph and interpolated on the half maximal value of the anti–LMB-100 (IP12) [13] (BioXcell, custom lot).
2.4. Statistical analysis
Statistical analysis and graphing were performed using GraphPad Prism software. Time for detectable titer (Fig. 1D) was tested for significance using two-way ANOVA with individual Dunnett’s multiple comparisons test. All other comparisons were tested using one Way ANOVA with Dunnett’s multiple comparisons test.
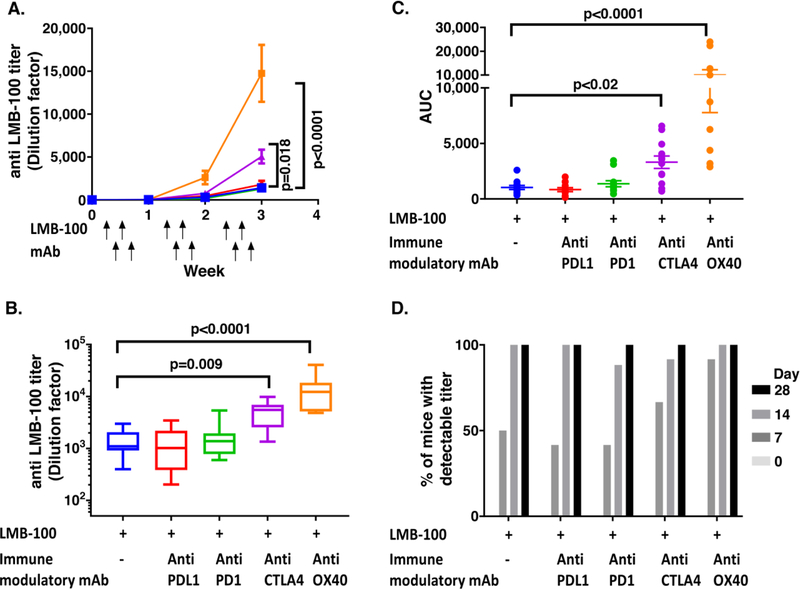
Fig. 1. ADA formation after combination therapy of LMB-100 with immune modulating antibodies.
BALB/c mice (n=12) were injected (I.V) with LMB-100 (2.5mg/kg) on days 1, 3, 8, 10, 15 and 17 and with immune modulating antibodies (anti-PDL1 (green), anti-PD1 (red), anti-CTLA4 (purple), anti-OX40 (orange) or vehicle (blue)) (5mg/kg I.P) on days 2, 5, 9, 12, 16 and Blood samples were taken on days 0, 7, 14 and 21 and plasma was isolated. A. Mean titer of anti-LMB-100 at each time point. P value indicates significant variance in AUC as shown in C. B. Titer of each mouse at the end of the experiment (week 4). C. AUC for each mouse in each treatment group. D. Onset time of detectable anti-LMB-100 titer for each treatment group.
Study was performed in two separate experiments (n=4 and n=8). Titers of both experiments are shown. Statistical significance was assessed using one-way ANOVA with Dunn’s multiple comparisons test. Error bars show SEM
3. Results
To study the effect of immune modulating antibodies on the immunogenicity of LMB-100, mice were injected with LMB-100 on days on days 1, 3, 8, 10, 15 and 17 and with anti-PDL1, anti-PD1, anti-CTLA4 or anti-OX40 on days 2, 5, 9, 12, 16 and 19. ADA formation against LMB-100 was evaluated on plasma samples (taken on days 0, 7, 14 and 21). The pilot study included 4 mice per group and was followed by a validation with eight mice per group. The results are shown for all 12 mice together.
3.1. ADA formation against LMB-100 are increased by anti-OX40 and anti-CTLA4 but not by anti-PD1 and anti-PDL-1
ADA against LMB-100 increased after each week of injections in all treatment groups (Fig. 1A). The mean titer on the final titer measurement at the end of week 3 for LMB-100 alone was 1,410 ± 776. When LMB-100 was given in combination with anti-PDL-1 and anti-PD-1, no change in mean titers were observed with a mean titer of 1,291 ± 1,058 and 1,806 ± 1,464, respectively. When LMB-100 was given in combination with anti-OX40 and anti-CTLA a 4–10-fold increase in the ADA titer was observed with a mean of 14,766 ± 11,026 and 5,054 ± 2,808 (p<0.0001 and p=0.009), respectively (Fig. 1B).
The cumulative area under the curve (AUC) of anti-LMB-100 titers for each mouse was calculated to compare the overall response (Fig. 1C). No difference in the AUC was observed between LMB-100 alone and LMB-100 with anti-PDL-1 or anti-PD-1 (mean AUC was 1,033 ± 618, 837 ± 622 and 1,375 ± 937, respectively). In contrast the AUC of mice titers after combinations of LMB-100 with anti-OX40 or LMB-100 with anti-CTLA4 was 3–10-fold higher with mean AUCs of 10,019 ±7,428 and 3,308 ± 1,938, respectively (p<0.0001 and p=0.02). This result shows that the increase in titer was occurring throughout the course of the treatment and not only in the last week.
3.2. ADA formation against LMB-100 are accelerated by anti-OX40
To evaluate if the immune modulating mAbs accelerate the onset of immunogenicity, we compared the number of mice that had detectable titers (an O.D higher than background that can fit on a four-parameter logistic curve-fit) after each week of treatment (Fig. 1D). After the first week of injections, 6/12 (50%) mice injected with LMB-100 alone had a detectable titer. Mice treated with a combination of LMB-100 and anti-PD-1 or anti-PDL-1 had a similar rate of 5/12 (42%) and anti-CTLA4 has a mild increase 8/12 (67%). However, a combination of LMB-100 with anti-OX40 had a higher number of mice with positive titer (11/12, 92%). This increase was significant when comparing the mean rates of the pilot and validation experiments (p=0.0006). This indicates that anti-OX40 accelerated the formation of anti-LMB-100 antibodies. After the second and third week of treatment most of the mice in all treatment groups had detectable titers.
3.3. Combination of LMB-100 with immune modulating mAbs does not affect mice weight
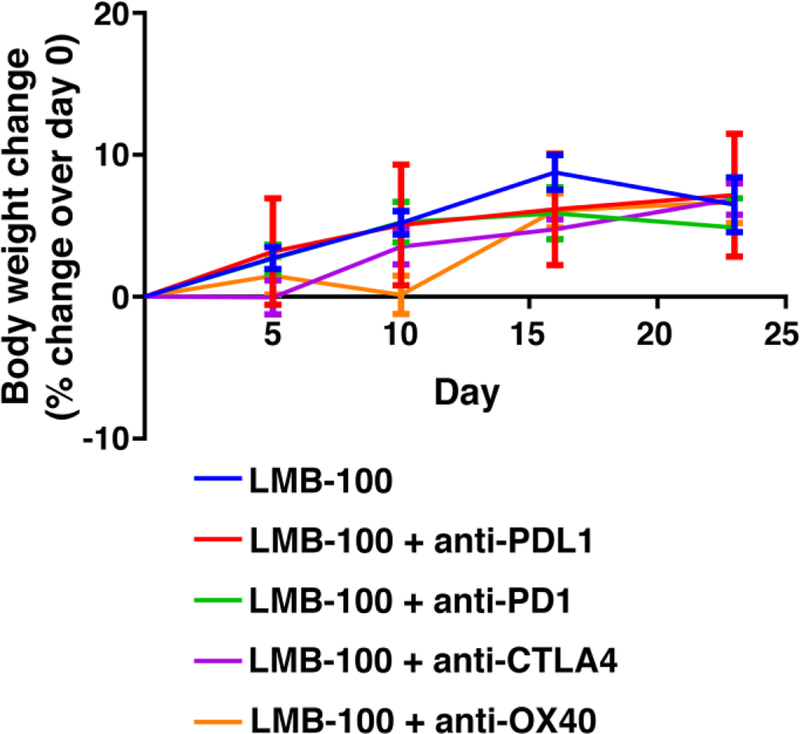
To ensure the treatment did not affect the health of the mice which could change the immune response, we measured the change in mice weight throughout six doses of LMB-100 and six doses of immune modulating mAbs. All mice in the study gained 6.6% of their body weight (from a mean of 18.4g to 19.6g) with no change among the different combination groups (Fig. 2). For no reason we could identify, one mouse from a group treated with LMB-100 + anti-OX-40 was found dead after the third LMB-100 dose. This death was not accompanied with any weight loss.
Fig. 2. Effect of combination treatment on body weight.
Body weight was measured weekly for all mice. Data is shown as percent change of body weight over the starting weight on day 0 (mean +-SEM).
4. Discussion
In this study, we used a mouse model to evaluate the effect of immune modulating mAbs on the immunogenicity of LMB-100. We found that anti-PD-1 and anti PDL-1 do not increase the ADA level against LMB-100, but anti CTLA-4 and anti-OX-40 significantly increase the ADA onset and titer against LMB-100.
Based on these results, we expect that it would be more beneficial to combine LMB-100 with anti-PD-1 or anti-PDL-1 than with anti-CTLA4 or anti-OX40, because ADA against immunotoxins have been shown to be highly neutralizing in vitro and in vivo and also accelerate their clearance [12, 14].
High ADA levels can be associated with serious adverse events like infusion related reactions, allergic reactions, anaphylaxis or delayed hypersensitivity. Patients who develop antibodies are more likely to show infusion-related reactions. Delayed hypersensitivity can be mediated by immune complexes that potentially could be deposited in tissues [5, 15]. Combination with anti-PD-1 or anti-PDL-1 should therefore be safer than with anti-CTLA4 or anti-OX40.
The finding that anti-CTLA4 therapy can induce formation of ADAs has been observed by Kverneland et al. that observed that Ipilimumab induced ADA formation against itself in 26% of patients [16]. Furthermore, Nivolumab (anti PDL-1) as a single agent is only mildly immunogenic (24/281, 8.5% patients were positive for ADA). However, when it was combined with Ipilimumab the immunogenicity of Nivolumab almost tripled to 23/105 (22%) (according to FDA package insert Reference ID: 3827356), indicating that ipilimumab may increase the immunogenicity of a drug it is combined with.
In this study we used a mouse model and mAbs that target the murine CTLA4, PD-1, PDL-1 and OX40. The corresponding human mAbs do not cross react with the mouse antigens and have a risk of mouse anti-human responses that could bias the results. The clones used in this study have been used in numerus studies with similar activity to the human antibodies used in patients. [17–23]. Our results suggest that combining antibodies to CTLA4 or to OX40 with other protein-based therapeutics may enhance ADA formation to them.
Highlights.
LMB-100 is a recombinant immunotoxin for cancer therapy
We studied the effect of immune modulatory mAbs on the immunogenicity of LMB-100
Mice were treated with LMB-100 and mAbs that target CTLA4, OX40, PD-1 or PDL-1.
anti-PD-1 and anti PDL-1 did not increase the immunogenicity of LMB-100
anti-CTLA-4 and anti-OX-40 did increase the immunogenicity of LMB-100
Acknowledgments
Funding
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (Project BC008753).
Abbreviations:
- ADA
anti-drug antibodies
- AUC
area under the curve
- mAbs
monoclonal antibodies
- NSCLC
non-small cell lung cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None
References
- [1].Hassan R, Kreitman RJ, Pastan I, Willingham MC, Localization of mesothelin in epithelial ovarian cancer, Appl. Immunohistochem. Mol. Morphol 13 (2005) 243–247. [DOI] [PubMed] [Google Scholar]
- [2].Hollevoet K, Mason-Osann E, Liu XF, Imhof-Jung S, Niederfellner G, Pastan I, In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer, Mol. Cancer Ther 13 (2014) 2040–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, Immunity, 39 (2013) 1–10. [DOI] [PubMed] [Google Scholar]
- [4].Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, Guminski A, Puzanov I, Lawrence DP, Buchbinder EI, Mudigonda T, Spencer K, Bender C, Lee J, Kaufman HL, Menzies AM, Hassel JC, Mehnert JM, Sosman JA, Long GV, Clark JI, Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders, JAMA Oncol 2 (2016) 234–240. [DOI] [PubMed] [Google Scholar]
- [5].Brennan FR, Morton LD, Spindeldreher S, Kiessling A, Allenspach R, Hey A, Muller PY, Frings W, Sims J, Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies, mAbs, 2 (2010) 233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lazzari C, Karachaliou N, Bulotta A, Vigano M, Mirabile A, Brioschi E, Santarpia M, Gianni L, Rosell R, Gregorc V, Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: is this the beginning of the end for cancer?, Ther. Adv. Med. Oncol 10 (2018) 1758835918762094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Emens LA, Middleton G, The interplay of immunotherapy and chemotherapy: harnessing potential synergies, Cancer Immunol. Res 3 (2015) 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, Kreitman RJ, Miettinen MM, Steinberg SM, Fowler DH, Pastan I, Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression, Sci. Transl. Med 5 (2013) 208ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rosado MM, Bennici E, Novelli F, Pioli C, Beyond DNA repair, the immunological role of PARP-1 and its siblings, Immunology, 139 (2013) 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leshem Y, O’Brien J, Liu X, Bera TK, Terabe M, Berzofsky JA, Bossenmaier B, Niederfellner G, Tai CH, Reiter Y, Pastan I, Combining local immunotoxins targeting mesothelin with CTLA-4 blockade synergistically eradicates murine cancer by promoting anti-cancer immunity, Cancer Immunol. Res 6 (2017) 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bauss F, Lechmann M, Krippendorff BF, Staack R, Herting F, Festag M, Imhof-Jung S, Hesse F, Pompiati M, Kollmorgen G, da Silva Mateus Seidl R, Bossenmaier B, Lau W, Schantz C, Stracke JO, Brinkmann U, Onda M, Pastan I, Bosslet K, Niederfellner G, Characterization of a re-engineered, mesothelin-targeted Pseudomonas exotoxin fusion protein for lung cancer therapy, Mol. Oncol 10 (2016) 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mazor R, Crown D, Addissie S, Jang Y, Kaplan G, Pastan I, Elimination of murine and human T-cell epitopes in recombinant immunotoxin eliminates neutralizing and anti-drug antibodies in vivo, Cell. Mol. Immunol 14 (2017) 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu W, Onda M, Lee B, Kreitman RJ, Hassan R, Xiang L, Pastan I, Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes, Proc. Natl. Acad. Sci. USA, 109 (2012) 11782–11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mazor R, King EM, Onda M, Cuburu N, Addissie S, Crown D, Liu XF, Kishimoto TK, Pastan I, Tolerogenic nanoparticles restore the antitumor activity of recombinant immunotoxins by mitigating immunogenicity, Proc. Natl. Acad. Sci. USA, 115 (2018) E733–E742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jahn EM, Schneider CK, How to systematically evaluate immunogenicity of therapeutic proteins-regulatory considerations, N. Biotechnol 25 (2009) 280–286. [DOI] [PubMed] [Google Scholar]
- [16].Kverneland AH, Enevold C, Donia M, Bastholt L, Svane IM, Nielsen CH, Development of anti-drug antibodies is associated with shortened survival in patients with metastatic melanoma treated with ipilimumab, Oncoimmunology, 7 (2018) e1424674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dai M, Yip YY, Hellstrom I, Hellstrom KE, Curing mice with large tumors by locally delivering combinations of immunomodulatory antibodies, Clin. Cancer Res 21 (2015) 1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Muller P, Martin K, Theurich S, Schreiner J, Savic S, Terszowski G, Lardinois D, Heinzelmann-Schwarz VA, Schlaak M, Kvasnicka HM, Spagnoli G, Dirnhofer S, Speiser DE, von Bergwelt-Baildon M, Zippelius A, Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells, Cancer Immunol. Res 2 (2014) 741–755. [DOI] [PubMed] [Google Scholar]
- [19].Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM, Kumari S, Kelly RL, Kwan BH, Abraham W, Hu K, Mehta NK, Kauke MJ, Suh H, Cochran JR, Lauffenburger DA, Wittrup KD, Irvine DJ, Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses, Nat. Med 22 (2016) 1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ, Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer, Nature, 520 (2015) 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zander RA, Obeng-Adjei N, Guthmiller JJ, Kulu DI, Li J, Ongoiba A, Traore B, Crompton PD, Butler NS, PD-1 Co-inhibitory and OX40 Co-stimulatory crosstalk regulates helper T cell differentiation and anti-plasmodium humoral immunity, Cell Host Microbe, 17 (2015) 628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX, Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice, J. Clin. Invest 124 (2014) 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guo Z, Wang X, Cheng D, Xia Z, Luan M, Zhang S, PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer, PLoS One, 9 (2014) e89350. [DOI] [PMC free article] [PubMed] [Google Scholar]