Abstract
The synapse is the focus of experimental research and theory on the cellular mechanisms of nervous system plasticity and learning, but recent research is expanding the consideration of plasticity into new mechanisms beyond the synapse, notably including the possibility that conduction velocity could be modifiable through changes in myelin to optimize the timing of information transmission through neural circuits. This concept emerges from a confluence of brain imaging that reveals changes in white matter in the human brain during learning, together with cellular studies showing that the process of myelination can be influenced by action potential firing in axons. This Opinion article summarizes the new research on activity-dependent myelination, explores the possible implications of these studies and outlines the potential for new research.
Myelin, the multilaminar sheath on axons formed in the CNS by multipolar glial cells called oligodendrocytes (FIG. 1), greatly speeds neurotransmission by fundamentally changing the way action potentials are propagated. Rather than sweeping down an axon as a relatively slow wave of sequential membrane depolarization and repolarization, the action potential is transmitted rapidly between electrogenic nodes of Ranvier (FIG. 2), which are approximately 1-μm axonal segments (FIG. 2b,d,e) at intervals separated by comparatively long stretches of inexcitable axon insulated by myelin (FIG. 2c). In the peripheral nervous system (PNS), spindle-shaped Schwann cells, which are derived from neural crest cells, form the myelin sheath.
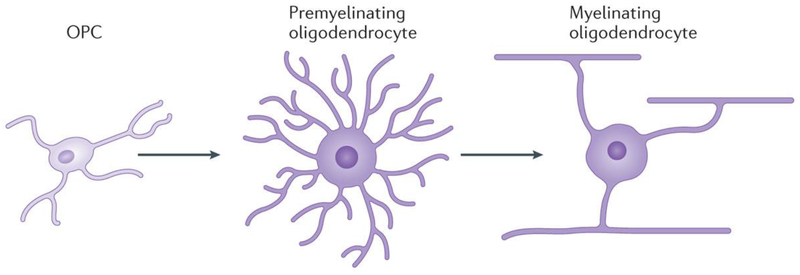
Figure 1 |. Development of oligodendrocytes.
Oligodendrocyte progenitor cells (OPCs) differentiate into multipolar premyelinating oligodendrocytes, which mature into myelinating oligodendrocytes. OPCs constitute the majority of mitotic cells in the adult brain, and mature oligodendrocytes can form myelin segments on multiple axons simultaneously.
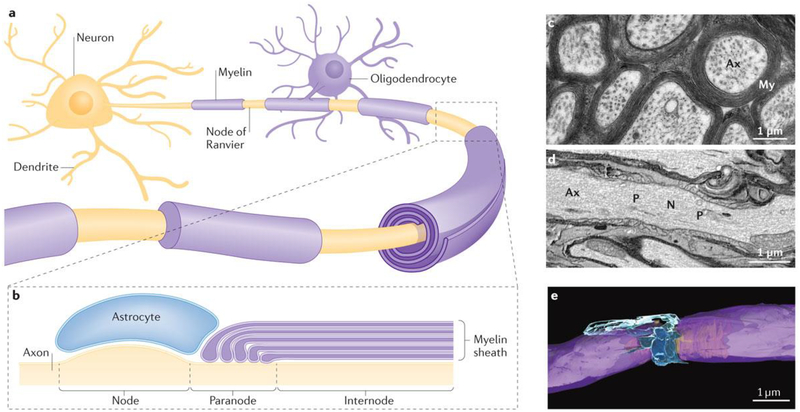
Figure 2 |. Myelin and the node of Ranvier.
a | Oligodendrocytes form myelin on axons to enable high-speed impulse transmission via saltatory conduction. b | Action potentials are generated at nodes of Ranvier situated periodically along the axon between internodal segments of axons insulated by compact myelin. Perinodal astrocytes are in contact with the axon at nodal regions. c | Transmission electron micrograph of mouse optic nerve axons in cross section, showing the multiple layers of compact myelin (My) and wrapping axons (Ax). d | Longitudinal section through an axon through the node of Ranvier, illustrating the axon perinodal astrocyte, nodal (N) paranodal (P) and internodal axon regions as shown in part b. e | Three-dimensional reconstruction of the node of Ranvier from serial blockface electron microscopy (compact myelin is shown in purple and perinodal astrocytes are shown in blue, with the axon being depicted in yellow).
The possibility that myelin could be regulated by functional activity and participate in nervous system plasticity is a departure from the traditional view of myelin as being static, modifiable only by damage. However, neuroanatomists of the nineteenth century did associate nervous system function and plasticity with myelination, in part because synapses are difficult to resolve with a light microscope but myelination is easily studied using lipophilic stains. A clear correlation between myelination of different fibre tracts and the time at which these become functionally active supported the early concept that myelin facilitated neural circuit function and behavioural performance, and that functional activity stimulated myelination1. Myelination of appropriate brain regions coincides with the development of specific behaviours and cognitive functions2–5, such as reading6, development of vocabulary7 and proficiency in executive decision making8,9. Myelination continues for decades in the human brain and, like synaptogenesis and synapse elimination, the postnatal context of this process provides the opportunity for myelin to be influenced by functional activity and to participate in learning and neural circuit plasticity. With the development of electrophysiology and electron microscopy in the twentieth century, the synapse completely displaced myelin as the cellular mechanism of nervous system plasticity.
Interest in myelin plasticity was revived when new MRI techniques unexpectedly revealed changes in white matter in the human brain during learning and in association with cognitive development2. At the same time, cellular studies began to show that the process of myelination can be influenced by action potential firing in axons10–19. Both diffusible and cell-surface signalling molecules regulated by action potential firing have been identified that influence the development of myelinating glia or the initiation of myelination, as discussed below. The widely acknowledged role of synaptic plasticity in learning is supported by a rich foundation of theory and neural modelling, whereas how changes in white matter might operate to promote learning is less evident.
Theoretical considerations — the importance of timing.
Forming and breaking connections between neurons and modifying the strength of synapses are of fundamental importance in nervous system function and plasticity but, at a network level, additional considerations arise that have profound effects on information transmission and processing: notably, the speed and timing of information transmitted between relay points. The fundamental theories of learning and memory rely on the simultaneous occurrence of two signals: the conditioned stimulus and the unconditioned stimulus; for example, the sound of a bell and the presentation of food in Pavlov’s experiments. At a cellular level, this translates into the coincidence of activity between two neurons converging onto a common target. Thus, spike-time arrival is of fundamental importance in neural coding, neuronal integration and synaptic plasticity, and myelination is the most-effective mechanism for determining maximal conduction velocity.
A high degree of precision is required for appropriate spike-time arrival. Conduction delays and processing time in neural networks reach hundreds of milliseconds in many complex co gnitive tasks, but synaptic integration and synaptic plasticity require action-potential arrival times with a precision of a few milliseconds20. For example, by spike-timing-dependent synaptic-plasticity mechanisms, synaptic strength is increased by synaptic input arriving simultaneously or only a few milliseconds before the postsynaptic neuron fires an action potential, but synapses are weakened if the input arrives a few milliseconds after the postsynaptic neuron fires. Thus, conduction time is critical for synaptic plasticity, but this is largely overlooked21.
It is important to emphasize that optimal network function is not achieved by simply maximizing the speed of conduction through every axon. Optimal synchrony of spike-time arrival through nodes in a network is what maximizes performance, just as arriving too soon at relay points along a transportation network is not optimal in transportation systems, and indeed has negative consequences. This important concept is largely lacking from neural modelling, plasticity and learning theory, and the cellular mechanisms that ensure appropriate conduction delays are scarcely explored.
Conduction velocities do range widely, from a fraction of a metre per second to approximately 200 metres per second in different vertebrate axons. Achieving the proper conduction delay in each link of the neuronal connectome, which comprises billions of axons, would seem difficult by genetic instruction alone. A mechanism of adjusting conduction velocity by myelinating unmyelinated axons, or by modulating the thickness of the myelin sheath or the structure of nodes of Ranvier through activity-dependent feedback, would seem advantageous.
Another important consideration of information processing at a network level is that, in contrast to sensory-evoked activity, much of the intrinsic activity in the mammalian brain is oscillatory. Brain rhythms of various frequencies are associated with selective attention, arousal, sleep, information processing, sensory gating of information, memory formation, emotion, perception and consciousness22,23. The frequency, propagation and coupling of oscillatory neuronal activity in the larger and more-complex neural networks of vertebrates would be strongly influenced by conduction delays and thus by myelin24. Recent computational analysis suggests that conduction delays between coupled oscillators in the brain would be highly sensitive to even small changes in conduction velocity that would result from subtle changes in myelin25. Conduction delays of 1 ms can shift the phase of gamma oscillations (~100 Hz) by 30°, significantly affecting signal amplitude and constructive and destructive interference between coupled oscillators25. Thus, a mechanism of nervous system plasticity acting through myelination would be particularly relevant to the complex nervous systems of animals with larger brains, and to more-complex cognitive tasks. It is known from human studies that, when conduction velocity becomes suboptimal, the deleterious effects of conduction delays on oscillatory activity in the brain contribute to a wide range of dysfunctions, and these disorders are often associated with white matter abnormalities2.
White matter plasticity — current status and major questions.
Research from a wide range of human brain-imaging studies in the past decade has shown that white matter structure can change after learning and functional experience. This research has been reviewed elsewhere2,26 but includes, for example, learning complex skills, such as playing the piano27, learning to read28, mastering juggling29 and many others30. However, these studies do not show that myelin has changed, because the voxel size (2 μm × 2 μm × 2 μm) does not enable resolution of the contributions of different cellular components to white matter changes.
Current research is attempting to determine whether myelin is modified in learning; if it is, there are numerous questions that must be addressed to embark on this new field of research, which include the types of learning in which activity-dependent myelination might participate. In addition, it is now firmly established that myelination can be influenced by functional activity10–19,31–33, but it is necessary to distinguish whether this is a homeostatic response to the overall level of neural activity of a circuit (a use–disuse phenomenon) or whether the modifications are driven by learning specifically. Also, current research is addressing whether activity-dependent changes in myelination are primarily mechanisms for regulating the development of neural circuits during critical periods of early life or instead operate throughout life as a complement to synaptic plasticity. Importantly, it is necessary to link the anatomical and physiological changes associated with myelination to behavioural outcomes showing that myelin plasticity contributes to improved cognitive performance. Finally, if myelination is modified during learning, it will be essential to determine the cellular and molecular mechanisms that are responsible for this modification. These mechanisms are likely to differ from those known to regulate synaptic transmission.
By analogy to synaptic plasticity, synaptogenesis and synapse elimination during learning, myelin could be influenced by functional activity through a plethora of mechanisms that affect the proliferation, migration, differentiation and survival of myelinating glia, and also influence the complex cell biological processes involved in synthesizing and wrapping multiple layers of compact membrane around axons and forming the specialized nodes of Ranvier. In this regard, it will be important to identify how functional activity in axons is detected by the myelinating glia situated along axons. This major question expands consideration of activity-dependent communication in the brain well beyond synaptic transmission.
On theoretical grounds, mechanisms altering the structure of myelin after it has formed would seem necessary for adaptive changes in conduction velocity during learning25. This could be achieved by changing the thickness of the myelin sheath. The large variation in myelin sheath thickness relative to axon diameter that is typically observed could reflect adjustments in sheath thickness for optimal conduction velocity in individual axons. Alterations in the structure of nodes of Ranvier and adjustments in internodal distance could also modify conduction velocity adaptively. Addressing these questions is technically challenging, but new methods of serial-section electron microscopy and in vivo confocal microscopy are being used to explore these possibilities.
Nevertheless, myelination of unmyelinated axons, which are present in many fibre tracts throughout life, would have significant effects on network function that could contribute to learning. For example, the conduction time between the left and right cerebral cortex through the corpus callosum is 30 ms through a myelinated axon and 300 ms through an unmyelinated axon34, so myelination of unmyelinated axons would have a profound effect on neural integration.
Recent experimental evidence for myelin plasticity in learning.
Several recent studies have investigated the consequences of environmental experience on white matter structure and development. Most of these were performed during early life, when myelination is actively progressing, or analysed in regions of the brain that are still actively myelinating in young adults, such as the prefrontal cortex35,36. Environmental enrichment does increase proliferation of oligodendrocyte progenitor cells (OPCs) in the amygdala37, and the number of oligodendrocytes in the corpus callosum increases in studies on middle-aged rats38. Physical exercise also increases OPC proliferation in the adult cerebral cortex39. For example, there is increased production of OPCs and oligodendrocytes in the corpus callosum of mice trained on a running wheel with irregularly spaced rungs40. However, this increase was also found in control animals running on a normal running wheel (that is, control animals did not need to learn how to negotiate missing rungs). Thus, proliferation of OPCs may be induced by the novelty of the wheel-running experience itself, relative to rearing in normal cages without wheels. Importantly, impairing oligodendrocyte production in adult mice without affecting pre-existing oligodendrocytes was found to impair motor skill learning in mice gaining proficiency in running on wheels with irregularly spaced rungs. This shows that new myelin formation does promote motor learning40. The next experimental task is to determine whether continuous myelination throughout life repairs or maintains the infrastructure for network communication that is necessary for motor skill performance or whether learning a new motor skill requires de novo myelination.
The majority of evidence from human and animal studies on white matter plasticity during learning has come from studies associated with motor skill learning that requires prolonged practice (over several weeks). Current emphasis is on determining whether activity-dependent myelination participates in other forms of learning and, if so, on determining how quickly myelination can respond during learning. Rats trained for 5 days in the Morris water maze were found by MRI to have changes in grey matter and white matter structure in specific brain regions involved in maze learning. Histological analyses identified cellular changes in neurons, astrocytes and oligodendrocytes41. In the corpus callosum, immunofluorescence staining of myelin basic protein (MBP) was increased, as was fractional anisotropy (FA), in the learning group as compared to control rats that performed the equivalent motor activity of swimming in the maze but without a learning task (increased FA indicates restricted diffusion of protons in one orientation in tissue, as occurs with increased myelination or increased bundling of axons into organized tracts). Thus, spatial learning is accompanied by changes in cellular structure and MRI signals (thus changes in FA) in white matter tracts necessary for the behavioural task, and increased MBP staining is consistent with increased myelination. In another study using the same training method that has been used to document functional and structural changes in grey matter of motor cortex controlling forelimb movements in rats learning to grasp a food pellet reward42, Sampaio-Baptista et al.43 recently detected changes in white matter microstructure using MRI (FIG. 3). FA increased in the trained animals in white matter tracts beneath the motor cortex contralateral to the trained limb 11 days after training (FIG. 3a,b). As in studies of synaptogenesis in grey matter in this model of motor learning, there were no changes in the white matter tracts of the ipsilateral cortex or in control animals that underwent an equivalent grasping activity but without learning. Histological analysis showed increased staining for MBP in the appropriate fibre tracts, which is indicative of myelination (FIG. 3d). The intensity of MBP staining correlated directly with the animal’s learning performance (FIG. 3e), as did the increase in FA (FIG. 3c), lending support for myelination contributing to white matter changes during learning in studies that have used MRI. Electron microscopy will be required to determine whether myelin thickness increased in these studies or whether more axons in the fibre tract had become myelinated. Together, these studies suggest that structural changes in white matter and increased myelination are associated with acquisition of new motor skills and spatial learning.
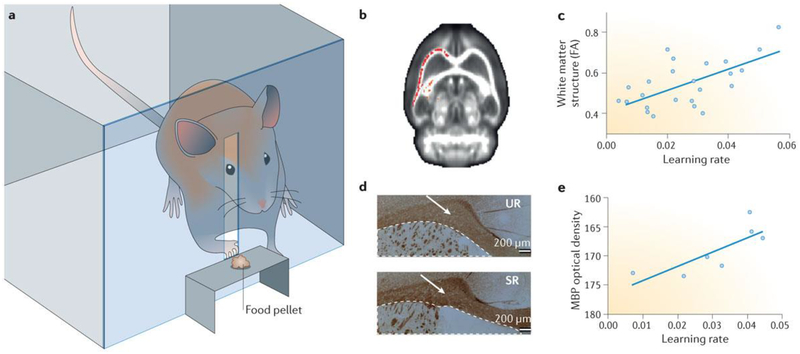
Figure 3 |. Changes in white matter tracts after learning.
a | Apparatus used to train rats to grasp a food pellet prior to analysis of brain structure by MRI to detect possible cellular changes during learning. b | After training rats to grasp a food reward, brain imaging by MRI showed higher fractional anisotropy (FA) in white matter tracts in the somatomotor areas of the hemisphere contralateral to the trained paw (higher FA indicated in red). Higher FA represents more highly polarized water diffusion in tissue as a consequence of more highly organized cellular structure. FA is increased by myelination, which restricts water diffusion more linearly along axons. c | Learning rate correlated significantly with the extent of white matter structure (FA). d | The intensity of immunocytochemical staining for myelin basic protein (MBP; brown staining, indicated by white arrows) was significantly higher in animals that had received training (skilled reaching (SR)) than in animals undergoing an equivalent amount of grasping activity but without a learning task (unskilled reaching (UR)), consistent with increased myelination of axons after motor learning. e | The increase in MBP staining was in proportion to the rate of learning, suggesting that learning proficiency was directly correlated with the increase in the level of myelination after motor training. Parts b–e are adapted with permission from REF. 43, Society for Neuroscience.
Recent research has reported that learning induces white matter structural changes in the fornix, the major white matter tract from the hippocampus, after only 2 hours of learning44. These studies used both human and animal subjects. Humans training for only 2 hours on a car-racing video game showed changes by diffusion tensor MRI in the hippocampus and parahippocampal gyrus and in the fornix. Significantly, the magnitude of changes in white matter structure correlated with behavioural proficiency. Rats trained for only 1 day in a water maze also showed changes in white matter by MRI indicative of increased FA during learning. An increase in FA could reflect a reduction in extracellular volume, cell swelling, blood vessel volume changes or a rapid increase in myelination. Although such rapid changes seem unlikely to be a result of myelination, recent cellular studies show that myelin can be initiated very rapidly by action potentials in axons by promoting the formation of an axo-glial signalling complex and stimulating local synthesis of MBP on axons firing action potentials16. Whether this could alter MRI signals within a period of 2 hours is uncertain.
This recent research from multiple laboratories is beginning to provide answers to many questions, and has provided support for the ideas that myelin changes during learning contribute to the white matter alterations seen by MRI and that activity-dependent myelination is not strictly restricted to early life when fibre tracts are actively being myelinated. Aged rats (14 months of age) given a 4-month period of environmental enrichment were found to have delayed loss of white matter during normal ageing. Electron microscopic analysis shows that myelin is increased in these animals compared with controls reared in standard cages45. The total volume of myelin and the total length of all myelinated fibres for both middle-age and old-age rats increased after environmental enrichment.
Similarly, in human studies, MRI analysis of white matter in older adults (61 years mean age) before and after 8 weeks of memory training showed significant increases in FA in left anterior hemisphere white matter tracts (the uncinated fasciculus)46. This study, among others, also shows that white matter plasticity is not restricted to motor learning. This fibre tract projects to the orbitofrontal cortex, where training-related changes in grey matter structure were reported previously by MRI analysis using the same experimental learning paradigm47. An association between uncinate FA and episodic and working memory performance has been reported previously, supporting a causal relationship between the white matter changes and improved memory scores after training48.
Degradation of cerebral white matter is the primary cellular change in the human brain with age seen in post-mortem analyses49. White matter degrades in the cerebral cortex in an anterior–posterior gradient50, which is consistent with a decrease in working memory and executive function with age, and the cortical association areas are particularly vulnerable to loss of myelin integrity during ageing51. The effect of memory training on FA in the study under discussion46 was found to be driven primarily by a significant decrease in FA in the control group during the training period, which was abated in the experimental group. So, although the study shows that white matter structure is influenced by mental activity during learning in adults and that cognitive performance is directly linked to white matter structure in terms of FA, it is not yet clear whether learning in this older population was dependent on these changes rather than functional activity being beneficial to myelin maintenance during ageing, which would better maintain cognitive performance.
Although many questions remain, these results together support the conclusions that environmental enrichment, motor training and other learning in middle-age and older animals induces recovery of oligodendrocyte numbers and that this contributes to an increase in newly myelinated fibres. In aged animals, this seems to include both remyelination of demyelinated fibres and myelination of new fibres sprouting after environmental enrichment. A process promoting learning by maintaining myelin integrity is not incompatible with a process of myelination promoting learning specifically. Mice in the running-wheel study were relatively young (postpartum day 60 (P60)–P90)40. No detectable loss of oligodendrocytes is seen at this age, suggesting that de novo myelination occurs during motor learning.
Signalling between axons and glia
An important question is, how do myelinating glia ‘know’ that the animal is in an enriched environment or undergoing learning? Oligodendrocytes have membrane receptors for a wide range of growth factors, but interestingly they also have ion channels and neurotransmitter receptors52–54. As oligodendrocytes are not excitable, ion channels and neurotransmitter receptors on oligodendrocytes may provide a way to inform these glial cells of functional activity in axons. Although growth factors and cell-adhesion molecules (CAMs) are the traditional focus of research on extrinsic factors regulating the development of myelinating glia, axon-derived neurotransmitters, including ATP54, adenosine13, glutamate55,56 and GABA57, were found to strongly regulate the development of Schwann cells and oligodendrocytes. How neurotransmitters could be released from axons to activate receptors on myelinating glia must be determined, because these cells are situated along the axon at great distances from the synapse.
Vesicular release of neurotransmitters at extrasynaptic sites.
Although the synapse is a specialization for rapid focal release of neurotransmitters, neurotransmitters can also be released by vesicles fusing with the axonal membrane without a specialized synaptic apparatus58. For example, non-synaptic transmission by vesicle fusion along axons and at axonal varicosities (vesicle-filled axonal swellings) is characteristic of autonomic transmission in the enteric nervous system59 and cholinergic transmission in the neocortex60. Thus, neurotransmitter release from vesicle fusion along the length of axons could activate neurotransmitter receptors on myelinating glia. This was first shown in activity-dependent communication between axons and myelinating glia in the PNS and Schwann cells54, and was later documented in oligodendrocytes13. Non-synaptic signalling between axons and astrocytes had been well documented previously by calcium imaging in excised nerves61, and astrocytes along axons in optic nerves are depolarized in response to action potentials generated by retinal stimulation62.
Non-vesicular release of neurotransmitters.
In addition to activity-dependent vesicular release, neurotransmitters can be released along axons through membrane channels. Activity-dependent release of the neurotransmitter ATP through volume-regulated anion channels in axons has been shown following trains of action potentials63, and these channels are permeable to small amino acids, including glutamate. Evidence suggests that specializations in the axon can cluster non-vesicular release of the neurotransmitter ATP from discrete regions of axons, as shown by imaging single photons generated by luciferin–luciferase detection of ATP64.
Such non-synaptic vesicular release of neurotransmitter could operate as a means of axo-glial communication at all stages of oligodendrocyte development and perhaps even in mature myelin65, possibly to maintain or remodel the myelin sheath in accordance with functional activity in the axon. AMPA, NMDA and kainate receptor subunits are expressed in mature CNS myelin sheaths66.
Other mechanisms.
Neurotransmitters can also be released from axons by reversal of neurotransmitter-reuptake mechanisms under certain conditions. However, this seems to be more relevant to stressful or pathological conditions, such as hypoxia, hyperexcitation and energy depletion67. Other categories of activity-dependent signalling between axons and oligodendrocytes include release of growth factors, nitric oxide signalling68, insertion or removal of CAMs in the axon membrane12,69,70, and activation of other cells, including astrocytes15 and blood vessels71, which in turn influence myelinating glia.
Surprisingly, functional synapses can form on some oligodendrocytes during their OPC stage72, but the function of these synapses is unknown. It is intriguing that OPCs are the largest population of dividing cells in the adult brain73,74. This large population of dividing cells, with their potential to form oligodendrocytes, may support a continuous process of myelination to renew senescent or damaged oligodendrocytes or to myelinate unmyelinated axons in response to functional activity or learning40,41,75.
Synaptic coupling from axons onto these glial cells via glutamate and GABA neurotransmitters72,76–79 raises the intriguing possibility that functional activity could influence the fate and activity of OPCs to promote myelination according to functional requirements. However, synaptic coupling to OPCs can be transient, vary in different brain regions, and not all OPCs are synaptically coupled to axons. As OPCs differentiate into premyelinating oligodendrocytes and ultimately mature oligodendrocytes, the synapses are lost. As myelin is formed by mature oligodendrocytes, synaptic communication may not be available to promote the initiation of myelin at the stage when oligodendrocytes are capable of myelinating axons.
The hypothesis that synapses on OPCs are necessary for myelin formation and for activity-dependent myelination have recently been tested. This was achieved by co-culturing OPCs on mouse dorsal root ganglion (DRG) axons. No synapses from axons onto OPCs could be detected by electrophysiology or electron microscopy80. However, myelin forms readily and myelination is regulated by electrical activity in these preparations12–16. Thus, neither myelination nor activity-dependent regulation of myelination requires axo-glial synaptic communication. Nevertheless, such synapses in vivo could promote myelination indirectly by influencing proliferation or differentiation of progenitor cells, and thus could increase the pool of myelinating glia associated with functionally active axons.
Synaptic communication is a specialization for high-speed (millisecond) serial communication, which appears to be unnecessary to regulate the development of OPCs or to orchestrate other cellular interactions of OPCs with axons to form myelin. Release of neurotransmitters through ion channels and via exocytosis of vesicles along the axolemma could be sufficient for activity-dependent regulation of myelination, and current evidence best-supports these non-synaptic modes of neurotransmitter release in intercellular signalling initiating and maintaining myelin preferentially on electrically active axons (discussed further below). Synapses on some populations of OPCs may have functions that are unrelated to myelination. For example, NG2 proteoglycan (also known as CSPG4) expressed by OPCs is enzymatically cleaved into the extracellular matrix in an activity-dependent manner, thereby altering glutamatergic synaptic communication between cortical pyramidal neurons81.
Recently, exosomes from oligodendrocytes have been shown to influence myelination in vitro82. Exosomes are extracellular vesicles released from cells that contain cargoes of protein and RNA that elicit physiological responses in target cells. Activity-dependent release of glutamate from axons triggers exosome release from oligodendrocytes83, and environmental enrichment is reported to generate exosomes and to promote remyelination84.
Mechanisms of myelin plasticity
Activity-dependent myelination of unmyelinated axons.
Induction of myelination requires cell–cell recognition, the formation of a specialized intercellular signalling complex between the appropriate axon and glial cell, the targeted synthesis of specialized myelin membrane and the ensheathment of the axon by the first wrap of membrane. When formed, the myelin sheath must be maintained for a prolonged period; perhaps for the lifetime of the animal. Electrical activity in axons influences all of these aspects of myelination.
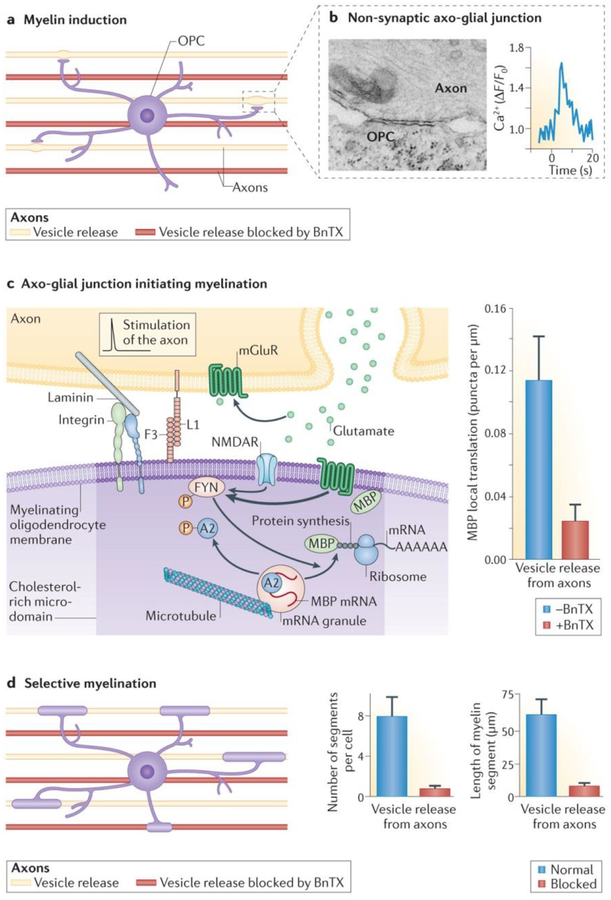
The process of myelination (FIG. 4) begins when an oligodendrocyte cell process contacts an axon and forms a specialized membrane junction (FIG. 4b), or “spot weld”, as described by Luse in 1959 in the earliest electron micrographic studies of CNS myelination85. This specialized intercellular junction between the oligodendrocyte and the axon membrane serves as scaffolding for cytoskeletal elements that traffic membrane proteins and membrane receptors, and it organizes intracellular signalling molecules that control the local synthesis of myelin proteins and their insertion into the membrane (FIG. 3c). This signalling complex involves the SRC family kinase FYN86, L1CAM and integrin87,88, concentrated in cholesterol-rich microdomains, and regulates the subcellular events necessary for induction of myelination on appropriate axons. Vesicular release of the neurotransmitter glutamate from electrically active axons stimulates the formation of this axo-glial signalling domain16. Electrical stimulation of axons causes the release of glutamate from vesicles, activating NMDA receptors and metabotropic glutamate receptor 5 (mGluR; also known as GRM5) on oligodendrocyte cell processes, causing a localized increase in intracellular calcium. This leads to increased surface expression of L1CAM and phosphorylation of FYN, and this axo-glial signalling complex stimulates local MBP translation from mRNA in oligodendrocyte cell processes16 (FIG. 3).
Figure 4 |. Non-synaptic junctions on myelinating glia promote preferential myelination of electrically active axons.
a | Oligodendrocyte progenitor cells (OPCs) were co-cultured with a mixture of mouse dorsal root ganglion neurons in which vesicle release from axons was blocked by botulinum toxin (BnTX; red axons) or was not blocked (yellow axons). The experiments show that myelination by oligodendrocytes is preferentially induced on electrically active axons releasing vesicles. b | Specialized contacts form between axons and oligodendrocyte cell processes, which lack the morphological and electrophysiological features of synapses but signal via release of neurotransmitters (glutamate and ATP) from axons. Neurotransmitters released at non-synaptic axo-glial junctions signal electrical activity in axons to oligodendrocytes and cause an increase in intracellular Ca2+ in the glial cell. c | Electrical stimulation of axons causes release of glutamate from vesicles that activate NMDA receptor (NMDAR) and metabotropic glutamate receptor (mGluR) on oligodendrocyte cell processes. This in turn triggers formation of the axoglial signalling complex (involving the SRC family kinase FYN, cell adhesion molecules (CAMs) L1CAM) and F3 (also known as contactin), laminin and integrin) and phosphorylation of FYN. Activated FYN phosphorylates heterogeneous nuclear ribonucleoprotein A2, resulting in local myelin basic protein (MBP) translation from mRNA in oligodendrocyte cell processes. The graph in the right-hand panel demonstrates that formation of the axo-glial signalling complex and local synthesis of MBP are inhibited by axonal firing when NMDAR and mGluR activation are blocked by BnTX. d | Three weeks after stimulating action potentials in axons, the number and length of myelin segments formed on axons releasing synaptic vesicles (yellow axons in diagram on the left and blue bars in graphs on the right) was much higher than on axons in which vesicle release was not blocked by BnTX. AAAAAA, poly(A) tail of mRNA. Parts b–d are from REF. 80, Nature Publishing Group. Part c is also from Wake, H., Lee, P. R. & Fields, R. D. Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011). Reprinted with permission from AAAS.
Neuronal L1CAM is necessary for the induction of myelination by oligodendrocytes89, and axonal expression of L1CAM is regulated by action potentials69,70. L1CAM is essential for the initial wrapping of Schwann cell membrane around the axon, after which the expression of L1CAM decreases in both the axon and the Schwann cell90,91. After stimulating neurons at different frequencies for 5 days in vitro, only one-third of the normal number of myelin segments formed on axons stimulated at 0.1 Hz compared with on unstimulated controls12. Stimulation at 0.1 Hz lowers L1CAM expression, but stimulation at other frequencies that had no effect on L1CAM expression also had no effect on myelination. This indicates that specific frequencies of impulse activity can regulate the initial wrapping of myelin around PNS axons by regulating the expression of specific CAMs. This is an important finding with respect to myelin plasticity in learning, because information is coded in the pattern of impulse firing. Other cell culture studies, in which neurons are stimulated electrically before OPCs are plated onto them, show enhanced myelination that is probably the result of activity-dependent changes in unknown cell surface molecules on the axon18.
Ion channels influence proliferation in many cell types, providing a mechanism for axonal firing to influence the proliferation of myelinating glia. A pioneering 1966 study, which provided the first evidence of activity-dependent neuron–glia communication, showed that astrocytes in the optic nerve respond to visual stimulation in response to K+ released from the axon in firing trains of action potentials62. In addition, modulation of ion channel activity can alter proliferation of OPCs54,92–94, and could thus influence myelination.
Neurotransmitters can influence the development of myelinating glia in the PNS and CNS in several ways. Adenosine released from axons firing action potentials inhibits OPC proliferation, although the same experiment showed that ATP does not13. Interestingly, ATP inhibits Schwann cell proliferation and differentiation54, but not oligodendrocyte proliferation13,14, presumably because of different types of purinergic receptors on Schwann cells and oligodendrocytes, and differences in intracellular signalling. Instead, adenosine released by axons firing action potentials stimulates OPC development and myelination13. ATP released from axons firing action potentials also stimulates the release of leukaemia inhibitory factor (LIF) from astrocytes, increasing myelination after development beyond the progenitor stage, when adenosine promotes myelination15. In addition to its action in the initial events of myelination, glutamate signalling through AMPA95 and NMDA receptors96 directs OPC migration. Therefore, it is possible that neuronal activity has a role in OPC migration, targeting the cells to sites of glutamate release during development, and in postnatal activity-dependent myelination.
In summarizing the experimental results obtained thus far, it is important to emphasize that the same neurotransmitter can exert multiple biological effects on the same cell type, depending on the developmental stage and many other factors. For example, glutamate can regulate cell proliferation or cause cell death. Different experimental paradigms are likely to reveal different effects of neurotransmitters on OPC development and myelination. Much more data will be required to form a comprehensive understanding, but it is clear that neurotransmitters are a primary signalling mechanism for modulating myelination according to functional activity in neurons. Glutamatergic signalling is particularly important in regulating myelination of excitatory axons, but purinergic signalling is also especially important. Purinergic signalling involves a much more diverse set of membrane receptors97, and ATP can be released by all types of cells; thus, purinergic signalling has the potential to participate more universally in activity-dependent myelination, regardless of what neurotransmitter is used for synaptic transmission.
Activity-dependent regulation of growth factor secretion can also influence the proliferation of OPCs. Blocking impulse activity in optic nerves using tetrodotoxin (TTX) can reduce the proliferation rate of OPCs, and this can be reversed by platelet-derived growth factor98. Brain-derived neurotrophic factor (BDNF) directly affects oligodendrocyte proliferation and differentiation99 and myelination100,101. Recent work in vitro implicates neuregulin and BDNF signalling in NMDA activity-dependent myelination by action potentials19. Interestingly, social experience in mice has been shown to affect myelination in the prefrontal cortex and may be controlled in part through a mechanism involving neuregulin signalling36.
Recent studies in cell culture show that, when given a choice, oligodendrocytes form myelin selectively on electrically active axons80. This is significant because selective myelination of electrically active axons would greatly affect neural network function and thus modify neural circuits for optimal performance in the environment experienced in early life. This preferential myelination of electrically active axons was found by co-culturing mouse DRG neurons that had been treated previously with botulinum toxin (BnTX) to prevent the release of vesicles from axons together with normal axons, and finding that individual processes of oligodendrocytes associated preferentially with the electrically active axons (FIG. 4a). Oligodendrocytes initiated local translation of MBP preferentially on those axons releasing vesicles (FIG. 4c), and they ultimately formed compact myelin predominantly on the electrically active axons80 (FIG. 4d).
These studies show that vesicular release of neurotransmitters (in this case, glutamate) from non-synaptic regions of axons stimulates formation of the axo-glial signalling complex to initiate synthesis of myelin and wrapping of the myelin membrane preferentially on electrically active axons. These points of vesicular release are not synapses (FIG. 4b). AMPA currents cannot be detected in OPCs in response to electrical stimulation of axons in this preparation, and blocking AMPA receptors, which normally mediate fast synaptic transmission, does not inhibit the activity-dependent increase in local MBP translation caused by vesicular release of glutamate from axons stimulated to fire action potentials80. In these studies, electrical stimulation was provided briefly during the period of myelin induction, and myelin was assessed 3 weeks later without action potential firing in axons during the intervening period (DRG neurons do not form synapses in monoculture, and they do not fire action potentials unless they are stimulated). Thus, the effects of action potentials are on the initial events of myelin initiation in these studies of mammalian neurons. Remarkably, the long-term consequences are a large (roughly eightfold) increase in the number of myelin segments formed, a much larger size of individual myelin segments that are formed and the preferential myelination of axons that were electrically active during the period of myelin induction (FIG. 4d).
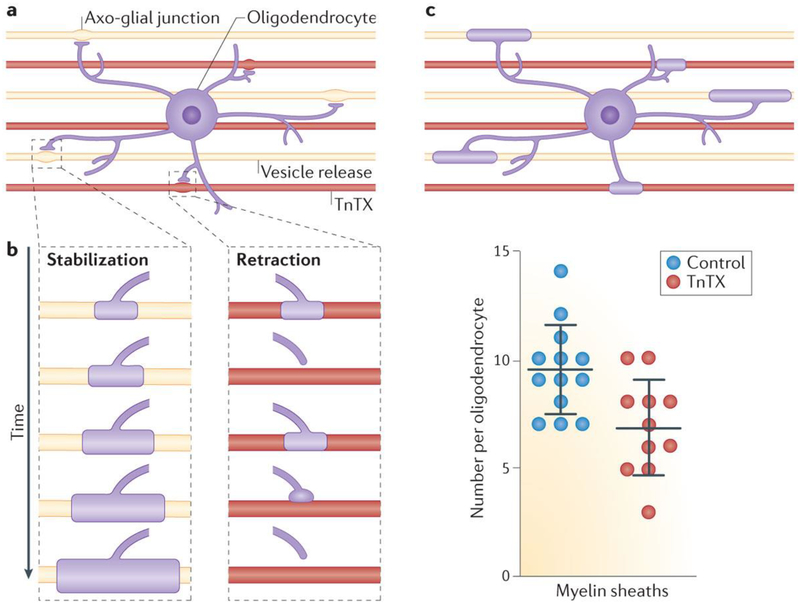
In zebrafish, myelin formation takes place during a narrow 5-hour window during development102, and electrical activity was found to promote the long-term maintenance of myelin sheaths, but the authors conclude that induction of myelination is activity-independent in zebrafish32. In these studies in zebrafish, 75% of the initial myelin wrappings of axons are lost within 90 minutes of observation by confocal imaging. When release of vesicles from axons is inhibited by tetanus toxin (TnTX), the initial wrappings of myelin are less stable and retract more frequently (FIG. 5). These failures to myelinate result in fewer myelin segments on axons in which exocytosis is inhibited by TnTX, and the myelin segments that are maintained are shorter in length. Another study in zebrafish using comparable methods failed to find a difference in retraction of myelin33, but this apparent discrepancy may be explained by the authors monitoring only myelin segments larger than 5 μm33. Hines et al.32 found that myelin segments that had reached 10 μm in length were stable, and because of differences in the sampling rate and period of imaging, the study by Mensch et al.33, might not have captured the retractions of the initial wrappings of myelin. However, in both zebrafish studies, the number of myelin segments was reduced on axons in which exocytosis was inhibited. The number of OPCs in zebrafish is also increased by increasing electrical activity and decreased by inhibiting electrical activity33, but this effect on oligodendrocyte numbers cannot fully account for the differences in myelin observed under these two conditions. This suggests that at least two processes are involved in zebrafish: activity-dependent regulation of OPC proliferation and/or development and maintenance of myelin, but not induction of myelination. The signalling molecules released by TnTX-sensitive vesicles that mediate activity-dependent myelin stabilization in zebrafish are not yet known. In addition to inhibiting release of neurotransmitters, suppressing exocytosis using BnTX or TnTX could inhibit delivery of CAMs and membrane receptors to the axolemma.
Figure 5 |. Myelin stabilization is promoted by vesicle release from axons in zebrafish.
a | Studies in zebrafish show that 75% of initial myelination attempts on axons (‘nascent myelin sheaths’) fail within 90 minutes. b | More frequent retraction of newly formed myelin is observed on axons in which vesicle release is inhibited by tetanus toxin (TnTX; red). c | Decreased stabilization of myelin on axons with vesicle release inhibited by TnTX leads to fewer myelin sheaths being retained on axons expressing TnTX (red) compared with controls (blue). Data for this figure combine information from REFS 32,33. The graph in part c is from REF. 33, Nature Publishing Group.
AIS.
The most-critical segment of the axon is the unmyelinated axon initial segment (AIS), which can be viewed as half of a node of Ranvier, as it is myelinated on only the distal end. The 5–80 μm-long unmyelinated section of axon between the cell body and first myelin segment is the electrogenic axonal membrane where action potentials are triggered. The morphological features of the AIS and types of ion channels present in it regulate the excitability of the neuron. The AIS also regulates the shape of the action potential, which affects the amount of neurotransmitter released from the synapse, the frequency of firing and other aspects of neuronal signalling103. All of these effects would influence information processing and could participate in learning.
The length and position of the AIS changes based on functional activity in the axon to homeostatically regulate neuronal activity104,105. In theory, the types of structural plasticity seen at the AIS could operate at each subsequent node of Ranvier, to modify action potential conduction velocity and firing frequency along the axon. A recent report shows that the nodal gap length increases in nodes of Ranvier in the auditory nerve in response to damaging noise that causes hearing loss106. Presumably, the lengthening of nodes from about 1.5 μm to 6 μm would reduce axonal firing and provide a homeostatic response to hyperexcitation.
Discussion
Technological advances increasing the exploration of nervous system function at the network level (termed ‘connectomics’) are motivating interest in plasticity mechanisms that operate beyond the synapse and even beyond grey matter. At the network level, propagation of signals, signal integration and coherence, and coupling oscillating signals become paramount. Thus, the conduction time through large complex networks becomes a critical factor, but mechanisms regulating impulse propagation time are not well studied, and this could represent an important form of neural circuit plasticity (FIG. 6).
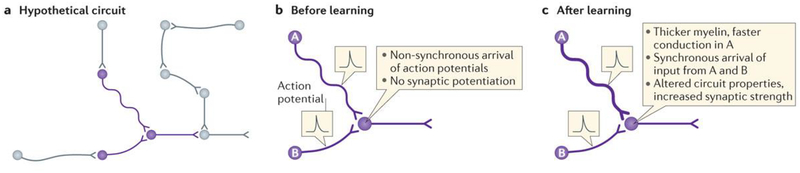
Figure 6 |. Activity-dependent myelination in nervous system plasticity and learning.
Information processing, synaptic plasticity and learning are highly dependent on precise spike-time arrival at relay points in neural circuits, and thus the optimal conduction velocity in individual axons in a circuit is critical. a | Hypothetical circuit. Neurons shown in purple are the subject of parts b and c. b | Before learning, non-synchronous arrival of action potentials would inhibit processes that strengthen synaptic transmission in response to coincident firing of the pre- and postsynaptic membrane. c | After learning, activity-dependent effects on myelination could adjust conduction velocity to optimize synchronous arrival of action potential input from converging circuits.
The dogma that myelin cannot be modified outside the context of disease is being overturned by new evidence. Environmental experience and functional activity in axons can influence myelination of unmyelinated axons. This is most evident during periods of development, when axons are being actively myelinated, but the process continues into adulthood and old age. The molecular mechanisms for activity-dependent myelination are being identified. From these early experiments, it is clear that the diversity of mechanisms involved in activity-dependent myelination is at least as vast as the range of mechanisms regulating synaptogenesis, synaptic elimination and synaptic plasticity.
Exploration of white matter plasticity is only beginning, but current research is lending support to various hypotheses: that many types of learning, not just motor skill learning, are accompanied by changes in myelination; that these changes are directly linked to improved behavioural performance; that myelination of unmyelinated axons is influenced by action potentials throughout life; and that changes in white matter structure can occur remarkably rapidly. The cellular basis for these rapid changes in white matter is not yet known, but this suggests the possibility of different mechanisms of white matter plasticity contributing to early events in learning and to later consolidation of learning. That mature myelin also can be remodelled non-destructively to alter conduction delays and improve performance remains an intriguing possibility for further study. These conclusions are best characterized as working hypotheses, based on compelling observations and a foundation of evidence that must be further investigated and confirmed.
Answering the many questions confronting researchers in the new field of activity- dependent myelination will be far more difficult than studying synaptic plasticity, for several reasons. Myelin formation and myelin structure are more complex than synapse formation and structure. Myelin is among the largest and most complex of known intercellular junctions. Formation of myelin requires remarkable orchestration of a complex set of cell biological processes to recognize the appropriate membrane to be myelinated, to synthesize vast quantities of specialized membrane, to coordinate elaborate cytokinesis to wrap multiple layers of highly compacted membrane around axon segments, to ensure the intricate assembly of appropriate ion channels and structural specializations that form the nodal apparatus and to maintain this structure throughout life.
Myelin spans an extremely broad range of spatial scales, challenging the limits of current techniques. The exquisite structure of myelin and the nodes of Ranvier is submicroscopic, requiring electron microscopy for analysis, but myelin operates over a macroscopic scale of networks spanning the entire brain. Synaptic plasticity is readily studied by electrophysiological analyses, but myelin plasticity requires morphological analyses, combined with behavioural, electrophysiological and molecular analyses. Synaptic plasticity often involves structural changes, which can be resolved with exacting electron microscopic analyses and by high-resolution confocal and two-photon imaging in live animals, but if morphological methods had been the only approaches available to study synaptic plasticity during the last century, progress on synaptic involvement in learning would have been extremely limited and slow.
This new research is expanding consideration of intercellular communication in the brain beyond synaptic transmission, to include many modes of activity-dependent cell–cell communication between neurons and glia, including vesicular and non-vesicular mechanisms of neurotransmitter release. These activity-dependent, non-synaptic signalling mechanisms have more-general significance in neuronal communication with other neurons (axons, dendrites and cell bodies), with vasculature and with all types of glia (astrocytes, microglia, oligodendrocytes and undifferentiated progenitor cells). Current research is also expanding studies of myelin beyond white matter tracts into grey matter tracts, in which myelin has an additional role in organizing the connectivity of neural networks through the inhibitory action of myelin on axon sprouting and synapse formation107–109. Tomassy et al.108 found that axons of pyramidal neurons in the cerebral cortex can have long segments lacking myelin interspersed with myelinated segments, and that these features differ in different cortical regions; for example, the AIS is longer in cortical layers III and IV than in layers V and VI. Inhibitory proteins in myelin prevent axon sprouting and the formation of synapses on axons109. Backpropagation of the action potential from the initial segment into the cell body and dendrites is influenced by AIS morphology103, and backpropagating action potentials reduce synaptic strength and couple neurons into transient functional assemblies during learning110.
The study by Tomassy et al.108 illustrates how the overly simplistic view of myelin that currently prevails can be misleading. A superficial conceptualization of myelin arises from the lack of detailed information about myelin as a consequence of tools that have been too blunt to probe the intricate structure of myelin over long-distance networks. Local activity-dependent regulation of myelin along a single axon, and independently in different axonal branches, would provide more exquisite control of conduction delays in complex neural networks. It appears that this level of intricacy is required for activity- dependent myelination to support nervous system plasticity in learning.
Myelin is present only in vertebrates111, and activity-dependent regulation of myelin in learning and information processing seems especially relevant to the human brain. Myelin is not found in hagfish, which are cordate forerunners of vertebrates, and it is not found in lampreys, the earliest living vertebrate on the phylogenetic tree. Many of the established preparations and animal models that have enabled remarkable advances in research on synaptic plasticity are not appropriate for studies of myelin plasticity as a potential cellular mechanism of learning. For example, learning in invertebrates like Aplysia spp., and local circuits in hippocampus and cortical grey matter, can be achieved fully by synaptic plasticity. Activity-dependent myelination becomes increasingly important in larger brains with more complex networks, in which conduction delays are substantial and brainwave rhythms are critical. This implicates myelin plasticity in learning beyond simple reflexes to encompass the more complex cognitive processes in larger-brained vertebrates, and especially in the primate brain. Aberrant or suboptimal synchrony of impulse traffic contributes to neuropsychiatric and neurological dysfunctions that are uniquely human, suggesting new insights and new potential therapies for human mental disorders from a better understanding of activity- dependent myelination. The mouse brain, which contains only 10% white matter, relative to 50% in the human brain, may be a poor model for studying activity-dependent myelination. In zebrafish, conduction delays in the small brain are much shorter, and activity-dependent myelination may provide a functional cue to guide development, regeneration, and homeostasis in neural networks, but may have little or no importance in learning. Here, activity-dependent effects on myelin may be more relevant to use–disuse regulation of maintenance and atrophy of myelin.
Synaptic plasticity has been guided by a beautifully simple theory of coincident activity between two neurons strengthening the connection between them. The concept of the Hebbian synapse has illuminated the most intricate molecular mechanisms of synaptic plasticity and learning theory but, at present, an equivalent theory guiding research on activity-dependent myelin plasticity has not yet been articulated. If myelin is modifiable to improve synchrony and to optimize the transmission of information through a neural circuit during learning, what are the guiding principles and rules, and what feedback mechanism to oligodendrocytes informs them that myelination has reached an optimal functional state? Expanding consideration of the cellular mechanisms of learning beyond the synapse is opening promising new avenues for research and stimulating new concepts for how the brain operates as a complex network and learns from experience. Connectomics in the twenty-first century returns us to seminal ideas of the nineteenth-century neuroanatomists, who in looking at the brain could see white matter but not synapses. Paradoxically, this is exactly the converse of what twentieth-century neuroscientists saw.
Acknowledgements
This work was supported by funds for intramural research from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Electron micro-graphs in Figure 2 are courtesy of Louis Dye, Microscopy Imaging Core, NICHD. 3D reconstruction is courtesy of Emily Benson and Grahame Kidd, Renovo, Inc.
Glossary
- Axolemma
The cell membrane of an axon.
- Fractional anisotropy
(FA). A measure of the symmetry of water diffusion in tissue analysed by MRI. Increased FA reflects more restricted diffusion of water, as occurs when axons become myelinated or more highly compacted, thus restricting water diffusion parallel to the axons.
- Non-synaptic transmission
Intercellular communication in the nervous system that does not involve synapses. This includes neurotransmitters released from vesicles fusing with the neuronal membrane outside synapses, as well as the release of neurotransmitters through membrane channels and other mechanisms.
- Synaptic coupling
A specialized junction between cells in the nervous system for rapid communication by electrical signalling. Neurotransmitters released from synaptic vesicles in the presynaptic neuron in response to electrical depolarization activate neurotransmitter receptors on the membrane of the postsynaptic cell to depolarize or hyperpolarize the postsynaptic membrane potential.
- Uncinate fasciculus
A white matter tract that connects the hippocampus, amygdala and temporal lobe with the orbitofrontal cortex of the brain.
Footnotes
Competing interests statement
The author declares no competing interests.
References
- 1.Fields RD in Neuroglia 3rd edn (eds Kettenmann H & Ransom BR) 573–585 (Oxford Univ. Press, 2013). [Google Scholar]
- 2.Fields RD White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31, 361–370 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yakovlev PI & Lecours A-R in Regional Development of the Brain in Early Life (ed. Minkowski A) 3–70 (Blackwell Scientific, 1987). [Google Scholar]
- 4.Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S & Rockel C White matter growth as a mechanism of cognitive development in children. Neuroimage 33, 936–946 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Nagy Z et al. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci 16, 1227–1233 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Kraft RH, Mitchell OR, Languis ML & Wheatley GH Hemispheric asymmetries during six-to eight-year-olds performance of Piagetian conservation and reading tasks. Neuropsychologia 18, 637–643 (1980). [DOI] [PubMed] [Google Scholar]
- 7.Pujol J et al. Myelination of language-related areas in the developing brain. Neurology 66, 339–343 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Liston C et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb. Cortex 16, 553–560 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Giedd JN Structural magnetic resonance imaging of the adolescent brain. Ann. NY Acad. Sci 1021, 77–85 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Schrager P & Novakovic SD Control of myelination, axonal growth, and synapse formation in spinal cord explants by ion channels and electrical activity. Brain Res. Dev. Brain Res 88, 68–78 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Demerens C et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl Acad. Sci. USA 93, 9887–9892 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens B, Tanner S & Fields RD Control of myelination by specific patterns of neural impulses. J. Neurosci 18, 9303–9311 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens B, Porta S, Haak LL, Gallo V & Fields RD Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–868 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens B, Ishibashi T, Chen JF & Fields RD Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol 1, 23–34 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishibashi T et al. Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–823 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wake H, Lee PR & Fields RD Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang IH et al. Axon myelination and electrical stimulation in a microfluidic compartmentalized cell culture platform. Neuromolecular Med 14, 112–118 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Malone M et al. Neuronal activity promotes myelination via a cAMP pathway. Glia 61, 843–854 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Luundgard L et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol 11, e1001743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dan Y & Poo MM Spike timing-dependent plasticity: from synapse to perception. Physiol. Rev 86, 1033–1048 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Fields RD Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist 11, 528–531 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buzsáki G Rhythms of the Brain (Oxford Univ. Press, 2006). [Google Scholar]
- 23.Ainsworth M et al. Rates and rhythms: a synergistic view of frequency and temporal coding in neuronal networks. Neuron 75, 572–583 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Nunez PL Srinivasan R & Fields RD EEG functional connectivity, axon delays and white matter disease. Clin. Neurophysiol 126, 110–120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pajevic S, Basser PJ & Fields RD Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 135–147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zatorre RJ, Fields RD & Johansen-Berg H Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci 15, 528–536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bengtsson SL et al. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci 8, 1148–1150 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Carreiras M et al. An anatomical signature for literacy. Nature 461, 983–986 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Scholz J, Klein MC, Behrens TE & Johansen-Berg H Training induces changes in white-matter architecture. Nat. Neurosci 12, 1370–1371 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fields RD Imaging learning: The search for a memory trace. Neuroscientist 17, 185–196 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson EM et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hines HH, Ravanelli AM, Schwindt R, Scott EK & Appel B Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci 18, 683–689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mensch S et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci 18, 628–630 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swadlow HA Physiological properties of individual cerebral axons studied in vivo for as long as one year. J. Neurophysiol 54, 1346–1362 (1985). [DOI] [PubMed] [Google Scholar]
- 35.Liu J et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci 15, 1621–1623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makinodan M, Rosen KM, Ito S & Corfas G A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okuda H et al. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J. Neurosci. Res 87, 3546–3553 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Zhao UY et al. Enriched environment increases the total number of CNPase positive cells in the corpus callosum of middle-aged rats. Acta Neurobiol. Exp 71, 322–330 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Simon C, Gotz M & Dimou L Progenitors in adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia 59, 869–881 (2011). [DOI] [PubMed] [Google Scholar]
- 40.McKenzie I et al. Motor skill learning requires central myelination. Science 346, 318–322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blumenfeld-Katzir T, Pasternak O, Dagan M & Assaf Y Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS ONE 6, e20678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleim JA et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol. Learn. Mem 77, 63–77 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Sampaio-Baptista C et al. Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci 33, 19499–19503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofstetter S, Tavor I, Moryosef ST & Assaf Y Short-term learning induces white matter plasticity in the fornix. J. Neurosci, 33, 12844–12850 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang S et al. Effects of an enriched environment on myelin sheaths in the white matter of rats during normal aging: A stereological study. Neuroscience 234, 13–21(2013). [DOI] [PubMed] [Google Scholar]
- 46.Engvig A et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum. Brain Mapp 33, 2390–2406 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engvig A et al. Effects of memory training on cortical thickness in the elderly. Neuroimage 52, 667–1676 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Kennedy KM & Raz N Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive funcitons, and speed. Neuropsychologia 47, 916–927 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marner L, Nyengaard JR, Tang Y & Pakkenberg B Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol 462, 144–152 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Salat DH et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol. Aging 26, 1215–1227 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Bartzokis G Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiol. Aging 25, 5–18 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Ransom BR & Orkand RK Glial-neuronal interactions in non-synaptic areas of the brain: studies in the optic nerve. Trends Neurosci 19, 352–358 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Káradóttir R, Cavelier P, Bergersen LH & Attwell D NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevens B & Fields RD Response of Schwann cells to action potentials in development. Science 287, 2267–2271(2000). [DOI] [PubMed] [Google Scholar]
- 55.Gallo V et al. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K1 channel block. J. Neurosci 16, 2659–2670 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mangin JM, Li P, Scafidi J & Gallo V Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci 15, 1192–1194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zonouzi M et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci 18, 674–682 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fields RD Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron–glia signaling. Semin. Cell Dev. Biol 22, 214–219 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burnstock G Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev 87, 659–797 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Zhang ZW, Kang JI & Vaucher E Axonal varicosity density as an index of local neuronal interactions. PLoS ONE 6, e22543 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kriegler S & Chiu SY Calcium signaling of glial cells along mammalian axons. J. Neurosci 13, 4229–4245 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orkand RK, Nicholls JG & Kuffler SW Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J. Neurophysiol 29, 788–806 (1966). [DOI] [PubMed] [Google Scholar]
- 63.Fields RD & Ni Y Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci. Signal 3, ra73 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fields RD Imaging single photons and intrinsic optical signals for studies of vesicular and nonvesicular ATP release from axons. Front. Neuroanat 5, 32 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Micu I et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Butt AM, Fern RF & Matute C Neurotransmitter signaling in white matter. Glia 62, 1762–1779 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Fern RF, Matute C & Stys PK White matter injury: Ischemic and nonischemic. Glia 62, 1780–1789 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Garthwaite G, Hampden-Smith K, Wilson GW, Goodwin DA & Garthwaite J Nitric oxide targets oligodendrocytes and promotes their morphological differentiation. Glia 63, 383–399 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Itoh K, Stevens B, Schachner M & Fields RD Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science 270, 1369–1372 (1995). [DOI] [PubMed] [Google Scholar]
- 70.Itoh K, Ozaki M, Stevens B & Fields RD Activity-dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J. Neurobiol 33, 735–748 (1997). [DOI] [PubMed] [Google Scholar]
- 71.Yuen TJ et al. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158, 383–396 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bergles DE, Roberts JD, Somogyi P & Jahr CE Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Horner PJ et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci 20, 2218–2228 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dawson MRL, Polito A, Levine JM & Reynolds R NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci 24, 476–488 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Young KM et al. Oligodendrocyte dynamics in healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–853 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin SC et al. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron 46, 773–785 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Kukley M, Capetillo-Zarate E & Dietrich D Vesicular glutamate release from axons in white matter. Nat. Neurosci 10, 311–320 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Kukley M et al. Glial cells are born with synapses. FASEB J 22, 2957–2969 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Maldonado PP, Velez-Fort M & Angulo MC Is neuronal communication with NG2 cells synaptic or extrasynaptic? J. Anat 219, 8–17 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wake H et al. Non-synaptic junctions on myelinating glia promote preferential myelination of electrically-active axons. Nat. Commun 6, 7844 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sakry D et al. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of NG2. PLoS Biol 12, 1001–1092 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bakhti M, Winter C & Simons M Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem 286, 787–796 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frühbeis C et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 11, e1001604 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pusic AD & Kraig RP Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 62, 284–299 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luse SA in The Biology of Myelin (ed. Korey SR) 59 (Paul B. Hoeber, 1959). [Google Scholar]
- 86.Krämer EM, Klein C, Koch T, Boytinck M & Trotter J Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J. Biol. Chem 274, 29042–29049 (1999). [DOI] [PubMed] [Google Scholar]
- 87.Laursen LS, Chan CW & ffrench-Constant C An integrin-contactin complex regulates CNS myelination by differential Fyn phosphorylation. J. Neurosci 29, 9174–9185 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.White R et al. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J. Cell Biol 181, 579–586 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barbin G et al. Axonal cell-adhesion molecule L1 in CNS myelination. Neuron Glia Biol 1, 65–72 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Seilheimer B, Persohn E & Schachner M Neural cell adhesion molecule expression is regulated by Schwann cell-neuron interactions in culture. J. Cell Biol 108, 1909–1915 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wood PM, Schachner M & Bunge RP Inhibition of Schwann cell myelination in vitro by antibody to the L1 adhesion molecule. J. Neurosci 10, 3635–3645 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chiu SY & Wilson GF The role of potassium channels in Schwann cell proliferation in Wallerian degeneration of explant rabbit sciatic nerves. J. Physiol 408, 199–222 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pappas CA, Ullrich N & Sontheimer H Reduction of glial proliferation by K+ channel blockers is mediated by changes in pH. Neuroreport 6, 193–196 (1994). [DOI] [PubMed] [Google Scholar]
- 94.Knutson P et al. K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells. J. Neurosci 17, 2669–2682 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gudz TI, Komuro H & Macklin WB Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J. Neurosci 26, 2458–2466 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiao L et al. NMDA receptor couples Rac1-GEF Tiam1 to direct oligodendrocyte precursor cell migration. Glia 61, 2078–2099 (2013). [DOI] [PubMed] [Google Scholar]
- 97.Fields RD & Burnstock G Purinergic signaling in neuron-glia interactions. Nat. Rev. Neurosci 7, 423–436 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barres BA & Raff MC Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260 (1993). [DOI] [PubMed] [Google Scholar]
- 99.Van’t Veer A et al. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J. Neurosci. Res 87, 69–78 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fulmer CG et al. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J. Neurosci 34, 8186–8196 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiao J, Wong AW, Willingham MM van den Buuse M, Kilpatrick TJ & Murray SS Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals 18, 186–202 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Czopka T, ffrench-Constant C & Lyons DA Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kole MHP & Stuart GJ Signal processing in the axon initial segment. Neuron 73, 235–247 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Kuba H, Oichi Y & Ohmori H Presynaptic activity regulates Na+ channel distribution at the axon initial segment. Nature 465, 1075–1078 (2010). [DOI] [PubMed] [Google Scholar]
- 105.Grubb MS & Burrone J Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465, 1070–1074 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tagoe T, Barker M, Jones A, Allcock N & Hamann M Auditory nerve perinodal dysmyelination in noise-induced hearing loss. J. Neurosci 34, 2684–2688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fields RD Myelin — more than insulation. Science 344, 264–266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tomassy GS et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 334, 319–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwab ME & Strittmatter SM Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol 27, 53–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bukalo O & Fields RD Synaptic plasticity by antidromic firing during hippocampal network oscillations. Proc. Natl Acad. Sci. USA 110, 5175–5180 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bullock TH, Moore JK & Fields RD Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci. Lett 48, 145–148 (1984). [DOI] [PubMed] [Google Scholar]