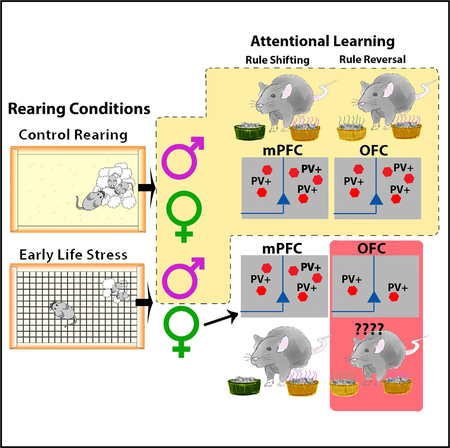
SUMMARY
Poverty, displacement, and parental stress represent potent sources of early life stress (ELS). Stress disproportionately affects females, who are at increased risk for stress-related pathologies associated with cognitive impairment. Mechanisms underlying stress-associated cognitive impairment and enhanced risk of females remain unknown. Here, ELS is associated with impaired rule-reversal (RR) learning in females, but not males. Impaired performance was associated with decreased expression and density of interneurons expressing parvalbumin (PV+) in orbitofrontal cortex (OFC), but not other inter-neuron subtypes. Optogenetic silencing of PV+ inter-neuron activity in OFC of control mice phenocopied RR learning deficits observed in ELS females. Localization of reversal learning deficits to PV+ interneurons in OFC was confirmed by optogenetic studies in which neurons in medial prefrontal cortex (mPFC) were silenced and associated with select deficits in rule-shift learning. Sex-, cell-, and region-specific effects show altered PV+ interneuron development can be a driver of sex differences in cognitive dysfunction.
In Brief
Goodwill et al. investigate the effect of early life stress (ELS) on cognitive development in a mouse model. Using a combination of genetic, histological, optogenetic, and behavioral techniques, they find that ELS leads to female-selective impairments in the ability to engage in rule reversal, but not other forms of attentional learning. Impairments are associated with diminished parvalbumin (PV) expression and a decreased density of PV+ interneurons in the orbitofrontal cortex (OFC).
Graphical Abstract
INTRODUCTION
Early life stress (ELS), increases the risk for, and severity of, neuropsychiatric disorders, including cognitive and affective pathology (Anda et al., 2006; Dube et al., 2001; Felitti et al., 1998). Females are nearly twice as likely as males to develop stress-associated disorders (Burt and Stein, 2002; Kessler, 2003). However, the neurobiological substrates of stress-associated effects on cognitive function, and the sex bias in risk for developing stress-associated pathology, are poorly understood.
The prefrontal cortex (PFC), crucial for the integration of cognition and emotion through cortical and subcortical pathways (Cotter et al., 2005; Frank and Claus, 2006; Minzenberg et al., 2015), is especially sensitive to ELS (Baudin et al., 2012; Monroy et al., 2010). Stress-linked pathologies, cognitive impairments, and inflexibility have been linked with functional and morphological alterations in the PFC (Cotter et al., 2005; Kunzler et al., 2015; Minzenberg et al., 2015; Thomas et al., 2016). Lesion studies have demonstrated regional specificity in PFC for select cognitive abilities, such as set-shifting versus rule-reversal learning (Birrell and Brown, 2000; Bissonette et al., 2008; Fox et al., 2003). Parvalbumin-positive (PV+) cells are the most prevalent type of inhibitory interneuron in the neocortex (Tremblay et al., 2016) and are integral in generating gamma oscillations that support cognition (Buzsáki and Wang, 2012; Cardin et al., 2009). Specifically, PV+ interneurons have been shown to regionally mediate set shifting through studies of optogenetic silencing and prenatal maternal immune activation (Canetta et al., 2016; Cho et al., 2015). PV+ interneurons are late maturing, and highly sensitive to stress (Grassi-Oliveira et al., 2016; Helmeke et al., 2008; Holland et al., 2014; Hu et al., 2010; Uchida et al., 2014), but little is known regarding how postnatal ELS affects regional PV+ interneuron expression and cognition.
Decreased PV+ interneuron densities have been found in the PFC of patients with stress-related disorders such as depression (Karolewicz et al., 2010; Rajkowska et al., 2007). Failure to develop a normal γ-aminobutyric acid-ergic (GABAergic) pheno-type (e.g., decreased PV+ cell counts and lower PV and glutamic acid decarboxylase-67 kDa [GAD67] mRNA levels) has been linked to cognitive impairments observed in schizophrenia (Lewis et al., 2012), which is comorbid with depression (Murrough et al., 2011; Zimmerman et al., 2006). Thus, cognitive function and flexibility may depend on appropriate levels of inhibition provided by PV+ interneurons in the PFC, and altered PV+ interneuron density or activity following ELS may be one mechanism by which stress causes impaired cognition (Bissonette et al., 2014).
Here, we used a mouse model of ELS in the form of fragmented maternal care, induced by limited bedding during a critical developmental period (postnatal day [P] 4–P11), to test whether altered maternal interaction leads to sex- and region-specific effects on attentional learning. Using a modified attentional set-shifting task (Cho et al., 2015; Dias-Ferreira et al., 2009), a select impairment in rule-reversal learning was found in female, but not male, ELS-reared mice. In female ELS mice, a decrease in PV+ interneurons was found in the orbitofrontal cortex (OFC). This effect was restricted to PV+ cells (not affecting expression of inhibitory cell markers in OFC). Deficits in rule-reversal learning could be recapitulated in healthy control animals through optogenetic silencing of PV+ interneurons in OFC, but not medial PFC (mPFC). We further showed that PV+ interneurons in mPFC are critical for rule shifting and are not affected by ELS. Thus, ELS may contribute to sex-specific effects on defined cognitive operations through effects on the development and functioning of PV+ inter-neurons in select regions of PFC.
RESULTS
ELS Leads to Selective Deficits in Rule-Reversal Learning in Female Mice
To test whether ELS affects cognition, we used a set-shifting task that assessed multiple aspects of attentional processing and flexibility. Cognitive flexibility, defined as the ability to adapt established patterns of behavior to changing circumstances or rules, depends critically on the PFC (Cho et al., 2015; Kim et al., 2016; Lapiz-Bluhm et al., 2008; McAlonan and Brown, 2003). Forms of cognitive flexibility include rule shifting, the capacity to switch attention from a dimension that was previously correct to a different dimension that has to be newly learned, and rule-reversal learning, learning a new rule that is opposite what was previously correct within the same dimension. Here, food-deprived mice were habituated to the testing arena and learned to dig for a food reward in a small container (see Experimental Timeline in Figure 1A). Mice were then presented with two containers on each trial and learned to associate food reward with either the odor of the container or the texture of a surface that surrounded the container (simple discrimination [SD]). Mice then learned to search in one container to find the reward when both texture and odor cues were present (compound discrimination [CD]). Once the mouse reached criterion (8 of 10 trials correct) on the initial association, the stimulus predicting reward changed, e.g., from odor to texture, or vice versa, and the new rule was learned (rule shift). Alternatively, mice learned that the other stimulus within the same sensory dimension predicted the reward, e.g., odor A instead of odor B (rule reversal) (Figure 1B). Animals that started with rule shift were subsequently tested for rule reversal, while animals that started with rule reversal were tested for rule shift. Order of testing and dimension (odor versus texture) were counterbalanced among mice to account for order effects.
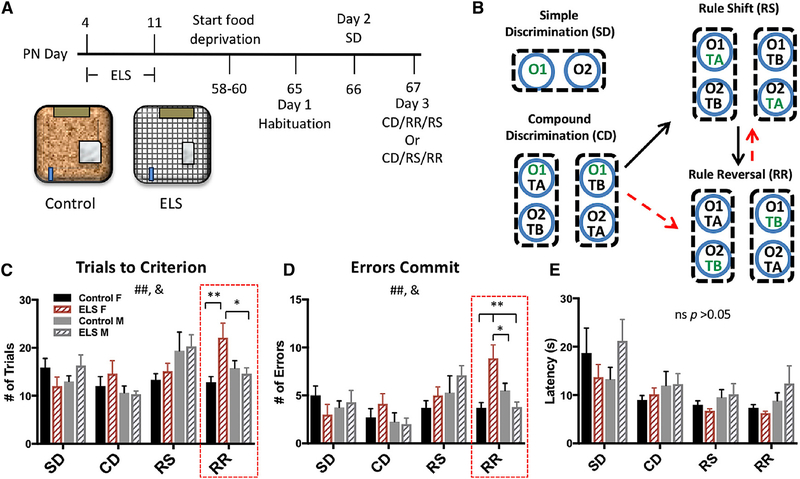
Figure 1. Females Exposed to ELS Demonstrated Significant Impairments in Rule-Reversal Learning.
(A) Timeline for ELS and behavioral testing. Schematic of control and ELS conditions from PN days 4–11. Control animals remain in their home cage with ample bedding, while ELS dam and pups are transferred into the ELS cage.
(B) Schematic of the attentional set-shifting task (black arrows, top) in which animals perform a simple discrimination (SD), followed by a compound discrimination (CD), a rule shift (RS), and a rule reversal (RR). The schematic with red arrows (bottom) shows that half of the mice underwent testing in a counterbalanced order, with RR preceding RS. Green text indicates the rewarded stimulus.
(C) A significant difference in the number of trials to reach criterion between task phases (repeated measure [RM] ANOVA; F(3,34) = 5.145, p = 0.002) and a significant interaction between task phase × group (F(3,34) = 2.470, p = 0.014) were found. A significant effect of group was found specifically in RR learning (F(3,34) = 5.106, p = 0.005). ELS females (n = 8) took significantly more trials to reach criterion than ELS males and control females (n = 10) (Bonferroni multiple comparisons post hoc test; p = 0.029 and p = 0.004, respectively).
(D) There was also a significant difference in the number of errors committed between task phases (RM ANOVA; F(3,34) = 6.635, p < 0.001) and an interaction between group and task phase (F(3,34) = 2.675, p = 0.008). A significant difference between groups was again only observed in RR learning (F(3,34) = 8.413, p < 0.001), with ELS females committing significantly more errors than any other group (Bonferroni multiple comparisons post hoc test; ELS female [F] versus unhandled control (UHC) male [M], p = 0.038; ELS M, p = 0.001; and UHC F, p = 0.001).
(E) No significant differences in latency were observed among groups in any task phase. For all plots, data show means ± SEM. Significance is denoted as follows: # for main effects or interaction effects, and * for significant post hoc comparisons; *p < 0.05, **p < 0.005. In the case of a significant one-way ANOVA, F statistics are reported, but only the significant post hoc Bonferroni comparisons are shown.
Select impairments in rule-reversal learning were observed in ELS female mice relative to unstressed controls (n = 8, p < 0.01) (Figures 1C and1D), as evidenced by more trials to criterion and more errors committed in the rule-reversal task phase (Figures 1C and 1D). No effect of ELS was observed on any other phase of testing or on latency to make a decision (Figure 1E), suggesting that deficits were not due to altered motivation or locomotion. No effects of ELS on cognitive functioning were observed in male mice during any phase of testing (Figures 1C–1E).
ELS Decreases PV Expression and PV Cell Density in the OFC of Female Mice, but Not Male Mice
Previous lesion, physiology, and functional imaging work in primates, humans, and rodents have identified the OFC as critical for rule-reversal learning (Bissonette and Powell, 2012;Dias et al., 1996;van der Schaaf et al., 2013;Nakayama et al., 2018). To assess the effects of ELS on expression of neural markers in OFC and control regions (mPFC and primary somato-sensory cortex), tissue punches were collected from control and ELS-reared mice. Using quantitative real-time PCR, a significant reduction in PV and GAD67 mRNA were found in the OFC of ELS-reared females (Figure 2A). These effects were not observed for PV in the mPFC (Figure 2B) or the primary somato-sensory cortex (Figure S1). No effect of ELS was found for other interneuron markers in the OFC, suggesting that PV+ cells were uniquely influenced (Figures 2A and 2B). To determine whether reduced gene expression was indicative of altered PV+ inter-neuron development, survival, or normal expression patterns, immunohistochemical labeling and stereological counting were used. ELS significantly reduced the density of PV+ interneurons in the OFC of ELS female mice (Figure 2C). Males showed only a slight decrease in PV expression in the OFC, with no effects on GAD67 (Figure 2D) or changes in PV+ cell density in this region (Figure 2F).
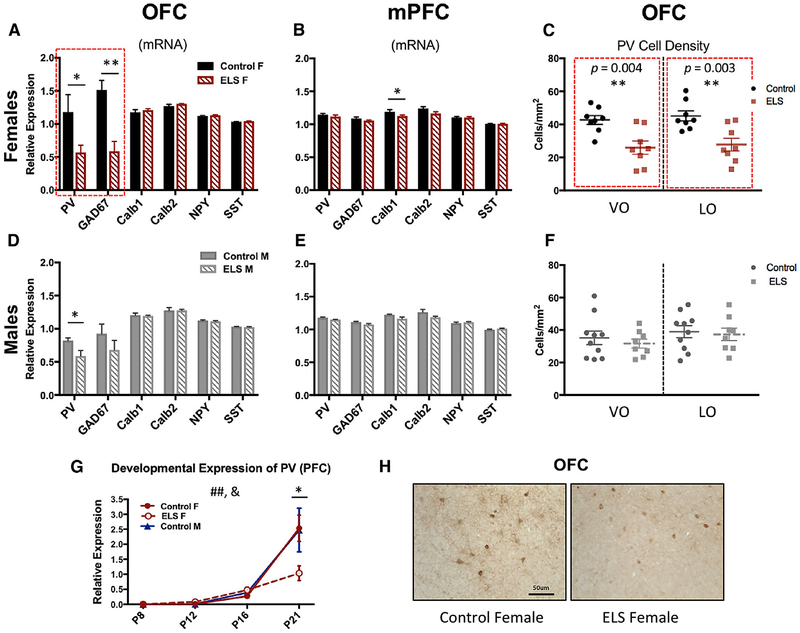
Figure 2. ELS Females Showed Significant Reductions in PV and GAD67 mRNA Expression and PV Cell Density in the OFC.
Quantitative real-time PCR analysis of relative mRNA expression of several interneuron markers—parvalbumin (PV), calbindin (Calb1), calretinin (Calb2), and somatostatin (SST), as well as neuropeptide Y (NPY) and GABA-synthesizing enzyme, and glutamic acid decarboxylase-67 kDa (GAD67)—was performed relative to control gene β-actin in the orbitofrontal cortex (left panels) and medial prefrontal cortex (middle panels). Top panels show female data (A–C); bottom panels show male data (D–F).
(A) In the OFC, female ELS-exposed mice had a significant and selective reduction of PV (t(1,11) = 2.282, p = 0.043) and GAD67 (t(1,11) = 2.429, p = 0.003) in comparison with control females. No other interneurons were affected by stress.
(B) In the mPFC of female animals, no effects of ELS were observed on PV, GAD67, NPY, or any other interneuron except a slight reduction in calbindin expression in ELS females (t(1,9) = 2.302, p = 0.047).
(C) Cell counts in the OFC show ELS females had a significant decrease in PV+ density in both the ventral orbitofrontal (VO) cortex (t(1,14) = 3.480, p = 0.004) and lateral orbitofrontal (LO) cortex (t(1,14) = 3.533, p = 0.003) regions of the OFC in comparison with control females.
(D–F) In males, we observe(D) a significant reduction in PV mRNA gene expression in the OFC (t(1,8) = 2.451, p = 0.040) of ELS mice, (E) no change in gene expression in the mPFC, and (F) this effect was not associated with changes in PV cell density in this region. No other interneuron markers were affected, nor were any changes detected in the mPFC.
(G) PV expression in the prefrontal cortex (PFC) increases across development in all groups (F(3,53) = 36.404, p < 0.000) but is significantly affected by ELS (treatment × day interaction; F(6,50) = 2.725, p = 0.024). Specifically, by P21, ELS females showed a significant reduction in PV expression in comparison with control animals (F(1,13) = 6.020, p = 0.029).
(H) Images from the OFC of adult control and ELS females show PV+ immunohistochemical labeling (images at 203 magnification; scale bar, 50 ~M ). For all plots, data show means ± SEM and are analyzed using two-tailed Student’s t tests or ANOVA. Significance is denoted as follows: # for main effects or interaction effects, and * for post hoc or pairwise comparisons; *p < 0.05, **p < 0.005.
To determine whether ELS altered the development of PV+ cells and/or led to a loss of PV expression in PFC, total PFC tissue was collected across several early developmental time points from a separate cohort of animals. ELS led to delayed onset of expression of PV mRNA in ELS female mice (Figure 2G), suggesting that decreased PV+ cell density in adulthood is a result of failed PV+ cell development.
Inhibition of PV+ Cell Activity in OFC Leads to Selective Deficits in Rule-Reversal Learning
The selective reduction of PV+ cell density and PV mRNA in OFC suggests a role for PV+ interneuron dysfunction in the effects of ELS on rule-reversal learning in females (Bissonette et al., 2008; Lodge, 2011; Mar et al., 2011). To determine whether diminished PV+ interneuron function in OFC is sufficient to induce impairments in rule-reversal learning, we employed optogenetic modulation. A PV-Cre mouse line was crossed with a line that would selectively express the inhibitory optogenetic construct halorhodopsin (NpHR) in Cre+ cells to suppress PV+ interneurons. Optic fibers were implanted bilaterally over either the OFC or the mPFC in control-reared male and female mice. The timeline of surgical manipulation and behavioral testing are presented in Figure 3A.
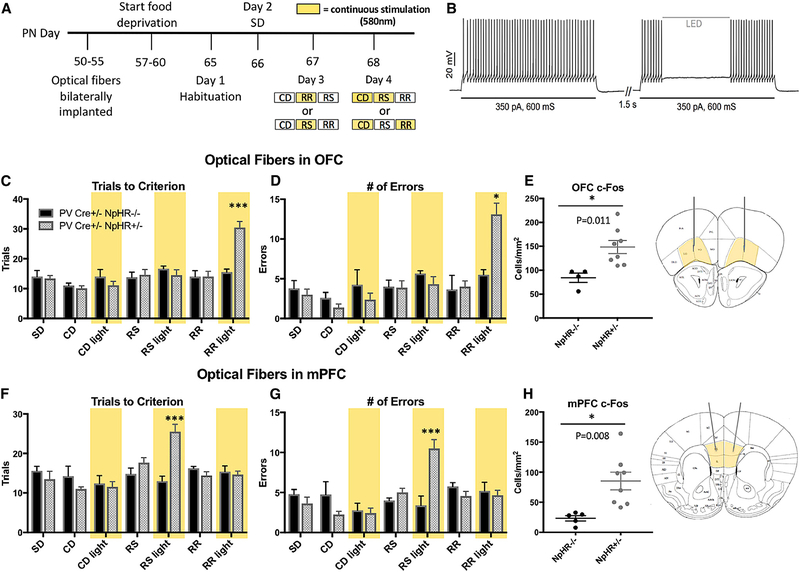
Figure 3. Optogenetic Inhibition of PV Interneurons in the OFC or the mPFC Selectively Impaired Rule-Reversal Learning or Rule Shifting, Respectively.
(A) Surgical and behavioral timeline for animals that underwent optogenetic stimulation in the OFC or mPFC. Examples of testing and illumination protocols on days 3 and 4 are shown, where yellow indicates continuous photostimulation with 620-nm light.
(B) In slice preparation from PV-Cre NpHR+/− mice, we demonstrate optogenetic suppression of stimulus current-induced spiking in a NpHR+/−-expressing PV interneuron. For slice experiments, a 545 ± band-pass excitation filter on white light emitting diode (LED) light was used. The light power at the plane of focus was 28.2 μW/mm2. A more in-depth analysis of the effects of optogenetic stimulation is presented in Figure S4.
(C) The effects of continuous illumination (yellow bars) over the OFC during various test phases of the attentional set-shifting task were assessed in NpHR−/− mice (shown in black, n = 4) and NpHR+/− mice (shown in gray, n = 8). There is a significant interaction among light stimulation, genotype, and task phase (three-way ANOVA; F(2,69) = 7.963, p = 0.001). Specifically, NpHR+/− mice take significantly more trials to reach criterion (t(3,19) = 5.053, p = 0.0002) than NpHR−/− littermates under light stimulation during RR learning.
(D) Similarly, under continuous illumination over the OFC, NpHR+/− mice commit significantly more errors during RR learning than NpHR−/− animals (t(3,19) = 3.541, p = 0.0065). A significant three-way interaction among light stimulation, genotype, and task phase on errors commit during the task was also reported (F(2,69) = 4.208, p = 0.019).
(E) Postmortem c-Fos analysis revealed continuous optical inhibition of PV interneurons in the OFC leads to an increase in c-Fos expression in this region t(1,10) = 3.108, p = 0.011). Data shown for lateral orbitofrontal (LO) cortex; ventral orbitofrontal (VO) expression was also significant t(1,10) = 2.841, p = 0.018) and can be found in STAR Methods (Figure S3).
(F) Effects of continuous photostimulation with 570-nm light over the mPFC. A significant interaction among light stimulation, genotype, and trial phase was reported (three-way ANOVA; F(2,69) = 3.232, p = 0.045). Specifically, NpHR+/− animals take significantly more trials to meet criterion during the RS phase than NpHR−/− mice t(8,31) = 5.686, p < 0.0001). These effects were not present in any other task phase or in the absence of light.
(G) Optogenetic inhibition of mPFC interneurons also affected errors committed during the task, with a three-way interaction found among light stimulation, genotype, and task phase (F(2,69) = 4.590, p = 0.013). NpHR−/+ mice (n = 6) committed significantly more errors than NpHR−/− mice (n = 5) (t(8,31) = 5.374, p < 0.0001) committed selectively during the RS task phase. Again, effects were only observed in the presence of photostimulation.
(H) Postmortem c-Fos analysis revealed that optically silencing PV interneurons in the mPFC led to an increase in c-Fos expression (t(1,11) = 3.216, p = 0.008). Data shown for the infralimbic cortex (IL); expression in the prelimbic cortex (PL) was also significant (t(1,11) = 2.883, p = 0.015) and can be found in STAR Methods (Figure S3).
For all plots, data show means ± SEM. Significant main effects and main interactions are reported, but only significant Bonferroni comparisons are shown. For c-Fos expression, data are analyzed using two-tailed Student’s t tests. Significance is denoted as follows: *p < 0.05, ***p < 0.001
Whole-cell recordings from NpHR-enhanced yellow fluores-cent protein (EYFP)-expressing cells in slices of OFC in vitro demonstrated that photostimulation could strongly suppress current-evoked action potential firing (Figure 3B; Figure S4). Optical silencing of OFC PV+ cells (yellow bars) in vivo resulted in a selective impairment in rule-reversal (RR) learning (Figures 3C and 3D) but did not influence other phases of the attentional set-shifting task or initial learning of the paradigm. There were no effects of photostimulation on performance in control animals during any task phase and no impairments in PV-Cre+/− ×NpHR+/− mice in the absence of photostimulation (non-yellowed bars, Figures 3C and 3D). Multiple controls were used to confirm that effects were not due to order of testing, genotype, and locomotion or the presentation of light during testing. Neither Cre+/−× NpHR+/− mice nor Cre+/− × NpHR−/− control mice showed motivational or locomotor deficits as a result of optical modulation of OFC, measured by latency to decision during the task (Figure S2A). Locomotion was measured in an open field after cognitive testing, and no effects of genotype or photostimulation were observed (Figure S2). Furthermore, order of photostimulation was counterbalanced within groups, with some animals receiving stimulation during RR first while others received stimulation during rule shift (RS) first. Testing order was also counter-balanced (Figure 1B).
To confirm that the optogenetic manipulation successfully modulated neuronal activity, a subset of mice received 20 min of photostimulation to mimic stimulation during behavioral testing and were sacrificed 45 min later. Brains were processed for c-Fos immunohistochemistry and verification of fiber placement (Figures S2C and S3). PV-Cre+/− × NpHR+/− mice showed enhanced c-Fos expression, an effect that was not observed in PV-Cre+/− × NpHR−/− mice (Figure 3E; Figure S3).
Suppression of PV+ Cell Activity in mPFC Causes Select Deficits in Rule-Shift Learning
To determine whether the OFC PV+ population is crucial for RR learning or disruption of other frontal PV+ cells can cause broader behavioral deficits, a separate group of mice was implanted with optical fibers bilaterally over mPFC (Figures 3F–3H). In previous studies, loss or silencing of PV+ interneurons in mPFC impaired rule-shift learning (Bissonette et al., 2008; Cho et al., 2015; Nakayama et al., 2018). Consistent with those results, PV-Cre+/− × NpHR+/− mice, but not PV-Cre+/− × NpHR−/− control mice, receiving optical stimulation of mPFC had select deficits in rule-shift learning (Figures 3F and 3G). Silencing of PV interneurons in mPFC did not affect RR learning or performance during any other task phase (Figures 3F and 3G). Optical silencing of PV+ cells in mPFC did not affect locomotor activity in the open field (Figures S2E and S2F) or during the attentional learning (Figure S2B). Again, order of testing and photostimulation were counterbalanced across animals and did not contribute to the observed deficits. Optic fiber placement verified in the mPFC (Figure S2D), and c-Fos expression again showed increased neuronal activity following 20 min of optical stimulation, suggestive of successful PV+ cell suppression in PV-Cre+/− × NpHR+/− mice (Figure 3H; Figure S3B).
DISCUSSION
Here we found that ELS led to sex-selective loss of PV+ interneurons in the OFC of female mice, along with cognitive deficits in reversal learning in an attentional set-shifting task. Inhibiting OFC PV+ interneurons in control mice phenocopied the deficits observed in ELS-exposed females, consistent with a possible causal relationship between these observations. Conversely, inhibiting PV+ interneurons in mPFC impaired RS, but not RR learning, indicating a behavioral dissociation between these brain regions. This finding is consistent with past lesion and deletion studies demonstrating behavioral specificity of the OFC and mPFC (Bissonette et al., 2008, 2010; McAlonan and Brown, 2003). However, until now, this had not been shown through selective and reversible inhibition of PV+ interneurons. Our results imply that ELS specifically disrupts the development of PV+ interneurons in the OFC of female mice, causing the disruption in PFC-dependent cognition. They also indicate that stress vulnerability of PV+ interneurons is a potential mechanism for sex-selective cognitive disturbance observed after ELS.
ELS Female Mice Exhibit PV+ Cell Deficiencies and Specific Cognitive Deficits that Align with Impairments Observed in Patients with Stress-Associated Pathologies
ELS has life-long effects on neural and behavioral maturation and increases risk for developing anxiety or depression (Anda et al., 2006; Felitti et al., 1998). Females are particularly vulnerable to stress-linked mental illness and are nearly twice as likely as males to be affected (Gater et al., 1998; Kessler, 2003). We have shown that ELS, in the form of maternal bedding restriction, leads to a robust depression-like phenotype in adult females (Goodwill et al., 2018). Depression is highly associated with functional and morphological alterations in the PFC and limbic system and comorbid with cognitive impairments and inflexibility (Bondi et al., 2008; Elliott et al., 2011). Despite links between cognitive dysfunction and stress-related mental illness, few animal models of stress have explored this relationship (Dias-Ferreira et al., 2009; Minzenberg et al., 2015). Here, we shed light on how ELS might influence cognitive functioning in females. Impairments in the GABAergic system, including reduced PV+ interneurons in OFC, have been reported in clinically depressed patients (Cotter et al., 2005; Hasler et al., 2007; Rajkowska et al., 2007), consistent with the decreased PV+ cell density observed here in ELS female mice. More work will be required to determine whether this is a loss of protein expression or loss of inhibitory cells. Furthermore, decreased PV+ interneurons number and reduced PV and GAD67 in OFC of ELS females were present early and continued into adulthood, suggesting a disturbance in inhibitory development.
Several mechanisms may underlie the effects of ELS on attention and PV+ interneurons. ELS increases circulating stress hormones and pro-inflammatory cytokines via sex-dependent mechanisms associated with oxidative damage to PV+ interneurons in the PFC (Steullet et al., 2017). Damage may also involve failed development of perineuronal nets (PNNs), the extracellular matrix important for maturation and stabilization of synapses and networks of PV+ cells, as seen in patients and animal models of schizophrenia (Bitanihirwe and Woo, 2014). ELS leads to higher circulating stress hormones by postnatal (PN) day 16 (Bath et al., 2017) in mice; thus, the magnitude of the ELS effects of hypothalamic pituitary axis (HPA) function may also contribute to increased risk for altered PV+ cell development and cognitive impairments.
Sex hormones may play a role in the altered development or degradation of GABAergic networks. In a rat model of hippocampal neurodegeneration, administration of 17-beta-estradiol (E2) in ovariectomized animals induced upregulation of genes involved in neuroprotection and synaptogenesis, as well as GAD67, PV, and genes that regulate PV synthesis: Pgcα−1 and Sirtuin 1 (Corvino et al., 2015). There is evidence that during proestrus, a period of high estrogen levels, female mice are at heightened risk of stress-induced PFC dysfunction (Shansky et al., 2006). In male rats, testosterone has protective and anti-inflammatory effects, reducing circulating pro-inflammatory cytokines and increasing levels of anti-inflammatory cytokines (Zhang et al., 2007). There is evidence that ELS, in the form of maternal separation, reduces circulating testosterone at periadolescence (PN 35) in male rats and leads to a correlated decrease in interleukin-10 (IL-10) and PV+ cells in the mPFC (Grassi-Oliveira et al., 2016). In light of these possibilities, we took measures to account for such effects by randomly selecting animals from multiple litters and tested females from the same litter on different days to sample across the cycle. We have preliminary data from precycling animals (P36), indicating that the selective disturbance in RR learning emerges early and is unlikely to be specific to cycle stage (unpublished data).
Selectively Inhibiting PV+ Interneurons in the PFC Produces Persistent and Anatomically Specific Cognitive Deficits
Consistent with prior lesion and deletion studies, we demonstrate a behavioral dissociation between OFC and mPFC (Bissonette et al., 2008; Dias et al., 1996). Sohal et al. (2009) demonstrated that inhibiting PV+ interneurons in mPFC specifically disrupts RS (Cho et al., 2015), but effects on OFC and RR learning had not been shown. We tested this dissociation through optogenetic inhibition of PV+ interneurons, replicating rule-shift deficits in mPFC and inducing RR deficits through PV+ cell inhibition in OFC. Our results suggest that PV+ inter-neuron activity in OFC and mPFC are integral to RR and rule-shift learning, respectively.
There is evidence that chronic stress in rats impairs the generation of rhythmic spontaneous induced pluripotent stem cells (IPSCs) originating specifically from PV+ interneurons in the hippocampus (Hu et al., 2010). Perisomatic inhibition is pivotal in synchronizing activity of pyramidal neurons and the generation of some cortical gamma oscillations (Cardin et al., 2009; Sohal et al., 2009). In PFC, PV+ interneurons and gamma oscillations may be central to the control of attention (Kim et al., 2016). Thus, ELS-induced loss of PV+ interneurons in female mice may lead to abnormal network oscillations and the reported cognitive impairments often observed in patients with stress-related psychiatric disorders (Cho et al., 2015; Lodge et al., 2009). Here, we find that ELS effects on cognitive performance can be replicated through optogenetic inhibition of PV+ interneurons in the OFC.
In summary, ELS leads to female-specific deficits in reversal learning during adulthood. We uncovered a mechanism underlying ELS-induced impairments and demonstrated a behavioral dissociation between the OFC and the mPFC through optogenetic inhibition of PV+ interneurons. This is a significant advance in our understanding of how ELS affects the PFC in a sex-selective manner to cause cognitive dysfunction in adulthood that is highly comorbid with other stress-related pathologies. Our results could suggest new interventional strategies for populations with adverse early life experiences that may be at risk for developing neuropsychiatric disorders with attentional or cognitive components, such as major depressive disorder or schizophrenia.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Kevin G. Bath (Kevin_Bath@Brown.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Adult (P60–80) male and female C57BL/6N mice were bred in house, from breeding stock obtained from Charles River labs. Mice had ad libitum access to food and water, and were housed on a 12h:12h light cycle. For optogenetic studies, male and female mice were obtained by crossing a floxed Halorhodopsin (NpHR)-EYFP (JAX#014539) mouse with a mouse that contains a PV-directed Cre driver (PV-Cre, JAX#008069) to obtain animals that express the eNpHR3.0 fusion protein in all PV-interneurons, as well as their control littermates that did not express the protein. Genotyping of mouse lines was carried out though the third party vendor, Transnetyx. All animal procedures were approved by the Brown University Institutional Animal Care and Use Committee and consistent with the guide for the care and use of animals in research.
METHOD DETAILS
Early life stress: fragmented maternal care
Four days following birth of a litter, the dam and pups were transferred from their standard home cage, to a home cage with a wire mesh floor and were provided with a 2 × 4 cm cotton nestlet as their only source of bedding (Rice et al., 2008). Mice continued to have ad libitum access to food and water. Dam and litters remained in these modified housing conditions for seven days, and at P11 were returned to standard housing, containing cob bedding and a 4 × 4cm nestlet. Control mice were left undisturbed throughout these procedures. All pups were weaned and sex segregated at 21 days of age.
Attentional set-shifting task
To test cognitive flexibility in control and ELS male and female mice, we adapted a version of a previously established attentional set-shifting task (Figure 1B) (Birrell and Brown, 2000; Bissonette et al., 2008; Cho et al., 2015). Briefly, mice were food restricted to 80%–85% of their ad libitum feeding weight. At start of each trial, the mouse was placed in a large (~ 25cm × 40cm), empty test cage and presented with two bowls that it had to choose between, based on the current rule set (Figure 1B). A choice was indicated by the mouse starting to dig for the food reward, a small piece of Fruity Pebbles cereal. The bowls each contained one odor (Yankee Candle wax) and one texture (coaster surrounding container,) which served as the cues to predict reward. The small, glass bowls were all identical in color and size, as was the digging material (white odorless rat bedding provided by Animal Care, Brown University).
After mice reached their target weight, they were trained to dig for the bait in the small glass bowls in the testing arena during day one of habituation (Figure 1B). On this day, animals were given 8 consecutive trials with a baited bowl to ensure that they could reliably dig. During day 2, the mice were run through a simple discrimination (SD), where only one relevant dimension was present (e.g., odor or texture, but not both). Mice learned which of two odors predicted the reward and that only one bowl contained the food reward. This also served as an olfactory or texture discrimination test to determine that mice were able to reliably discriminate between cues without a distractor. On the following days (3 and sometimes 4), mice were tested in a series of trials where each bowl differed in both odor and texture. The determination of which odor and texture to pair as well as which side (front, back, right, left) contained the baited bowl was randomized (with the requirement that the same combination of pairings and side did not repeat on more than three consecutive trials). A mouse was considered to have learned the association and that task phase was complete when it achieved 80% correct in the prior 10 trials.
Mice first learned a compound discrimination (CD), where the second (irrelevant) dimension (e.g., texture) was introduced while the original dimension (e.g., odor) continued to predict reward. For half of the mice, this was followed by a rule-shift (RS), in which a stimulus in the previously irrelevant dimension (e.g., texture) now predicted reward. Following completion of this task phase, mice were tested in a RR, where the previously unrewarded stimulus within a dimension (e.g., previously un-rewarded texture), now predicted reward (Figure 3B). Across all experiments, the order of testing (stimulus dimension as well as test phase) was counterbalanced for practice effects and interference from the previous rule set or dimension on performance on subsequent tests. For instance, the other half of mice in each group were first tested on an RR, followed by a rule-shift. For each group of animals tested in the attentional set shifting task, n = 8–10.
Immunohistochemisty
Animals were transcardially perfused with buffered saline followed by buffered 4% paraformaldehyde fixative, post-fixed for one hour, immersed in 30% sucrose and then processed as previously described (Bath et al., 2008). For parvalbumin immunohistochemistry, free-floating sections were blocked with 10% normal goat serum and 0.1% triton-X and then incubated with mouse anti-PV antibody (1:1000; EMD Millipore MAB81572, lot no. 2718×12, Temecula, CA), followed by biotin-conjugated goat anti-mouse secondary antibodies (1:1000: Vector labs, BA-9200), avidin-biotin peroxidase complex (Vector labs Vectastain ELITE, PK-4000) and DAB peroxidase (Sigma Aldrich, Sigmafast DAB tablet set, D4293). Sections were then mounted, dehydrated, and coverslipped. Primary antibody was omitted as a negative control. For detection of c-Fos, we used an affinity purified rabbit polyclonal antibody raised against the N terminus of human c-Fos (1:1000 dilution; EMD Millipore, ABE457, lot no. 2610509, Temecula, CA).
Cell counting
To quantify cell density, PV+ cells and c-Fos positive cells were manually counted by an experimenter blind to the experimental condition, sex, and genotype of the mouse. Using an unbiased stereological technique, the optical fractionator method with Cavalieri estimator (Stereo Investigator), cell density of immunoreactive cells was randomly sampled across four representative 40 μm thick sections with 120 μm between sections within each region of interest (OFC- VO, LO and mPFC- IL, PL), as in our previous work (Bath et al., 2008, 2017).
Realtime qPCR
Brain tissue punches were collected from the OFC (VO and LO), the medial PFC (IL and PL), the motor cortex (M1), and primary somatosensory cortex (S1). Tissue was homogenized in RNAzol (Molecular Research Center; RN 190) and stored at 80C until RNA was isolated in accordance with manufacturers protocol, followed by cDNA synthesis (as described in previous work). Pre-validated Taqman assays were run in multiplex with housekeeping gene (Beta Actin, Life Technologies). Relative gene expression was calculated based on a standard curve included on each plate. CFX384 RT-qPCR system (Biorad) and associated software (Biorad CFX 3.1) was used for gene expression profiling. For each comparison, n = 5 − 8 animals per group.
Optical Fiber preparation
Optical fibers were prepared in house. A 200 μm core, 0.37 NA standard multimode fiber (Thorlabs, Newton, NJ) was stripped of cladding, passed through a 230 μm multimode ceramic zirconia ferrule (Precision Fiber Products, Milpitas, CA), and secured in place using epoxy. Ferrules were then polished and cut to length to target the OFC or mPFC. They were tested for light output using an optical sensor (ThorLabs, PM100D, Newton, NJ) and sterilized with 70% ethanol prior to implantation.
Surgery
Naive PV-Cre:Halo mice were anesthetized with isofluorane gas anesthesia (1.5%–2.0% in 1 |/min oxygen) and secured in a stereo-taxic apparatus. The scalp was shaved and cleaned with iodine solution and alcohol to minimize risk for infection. After intraperitoneal (IP) injection of Buprenex (0.1 mg/kg, as an analgesic) and local application of lidocaine, the skull was exposed with an incision along the midline. After the skull was cleaned, a ~1 mm–diameter craniotomy was drilled over orbitofrontal cortex. The cannula and fiber optic implant were aligned and lowered to the surface of the cortex, over the OFC. The cannula was implanted at a 0°angle 2.46 mm anterior, and 1.35 mm lateral from bregma at a depth of 2.0mm below skull. If notable bleeding occurred during implantation, the surgery was aborted. Once the implants were stable, a dental acrylic cap was placed around their base and mice were taken off of isoflurane. For mPFC implantation, identical procedures were used, but fibers were localized to coordinates 1.9 mm anterior and 0.7 mm lateral from bregma at a depth of 1.4 mm below the skull at a 10°angle. Mice were monitored until awake and alert and daily post-surgery for three days to ensure that no signs of distress or infection were present.
Optogenetic Study
Mice (male and female) were bred that express the eNpHR3.0-EYFP fusion protein in all PV-interneurons (Madisen et al., 2012; Ting and Feng, 2013). These mice were generated by crossing a floxed Halorhodopsin (NpHR)-EYFP (JAX#014539) mouse with a PV-directed Cre driver (PV-Cre, JAX#008069). Between P50–55, mice underwent surgery to bilaterally implant optical fibers over the OFC or mPFC and were allowed > 9 day recovery before testing (Figure 3A). Studies were carried out in independent groups of mice. Control (NpHR −/−) and experimental (NpHR ± ) mice were then tested on the attentional learning task (described above). During the attentional learning task, in a subset of mice, the PV-interneurons in the OFC were photo-inhibited with continuous administration of orange light (620 nm Plexbright LED, Plexon, Dallas, TX), using an LED driver (Plexon, Dallas, TX) during the total duration (all trials) of the RR learning phase of testing. A separate cohort of mice received optical inhibition during the rule-shift or initial rule acquisition task phases to ensure that any effects were phase-selective. The same protocol was followed for animals that underwent PV-cell photo-inhibition in the mPFC. Testing order and order of optogenetic silencing was counterbalanced across groups of mice to control for potential effects of order or previous photostimulation on learning. Approximate light power was < 1 mW/mm2, similar to previous successfully used sustained ‘DC’ stimulation (Cho et al., 2015).
In vitro slice physiology
Testing of NpHR efficacy in PV cells (as in Figure 3B) was achieved using targeted whole-cell recordings in parasagittal slices of OFC maintained in vitro. Methods were very similar to those of Neske and Connors (2016). Mice were identical to those used in behavioral experiments. NpHR-expressing PV interneurons were identified by their EYFP fluorescence, and verified by their fast-spiking pheno-type (Cruikshank et al., 2010). Photostimulation was done with collimated light from a white LED (cool white 5500K, Mightex) controlled by a Thorlabs LEDD1B driver reflected through a dichroic mirror (FF655-Di01, Semrock) and a 40x immersion objective (LUMPlanFl 40x/0.80 W, Olympus).
Open Field Testing (OFT).
To verify that optical stimulation did not impact general locomotor activity or risk for seizure, mice were placed in custom-made white plastic open field arena (50×50×40 cm) that were evenly illuminated (~10 Lux) in a dimly lit room. The day after mice finished the attention task, mice were acclimated to the testing room for 20 minutes, and then placed in an open field arena. Locomotor activity was measured as distance and velocity traveled during a 15-minute test using a video-tracking system (Noldus Ethovision 10.1). During the entire test the animals were bilaterally connected to optical fibers, but continuous stimulation was only administered from minute 5:00 to minute 10:00. Changes in locomotor activity prior to, during, and after stimulation were examined.
c-Fos activation
Animals were food and water deprived for a one-hour acclimation period in the lab. Mice then received bilateral light stimulation for 20 minutes, to mimic the stimulation during behavioral testing. Thirty minutes after the stimulation, animals were transcardially perfused, as described above for immunohistochemistry and verification of fiber placement.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data analyses were performed in SPSS. A detailed description of the statistical parameters used for each analysis can be found in the corresponding figure legend and/or methods section in the text. When conducting statistical analyses, we tested for normality, and confirmed a normal distribution of each dataset. For analysis of optogenetic studies, we also carried out non-parametric statistical comparisons, using Kruskal Wallis H-test to compare performance between groups (Halo+, Halo-, light+, light-) on trials to criterion for each phase of the task. Consistent with Analysis of Variance results, we found a significant effect of group for the rule reversal phase of testing, but no other phases of testing.
For the cognitive flexibility task in control and ELS C57BL/6N mice, we used repeated-measures one-way ANOVAs to compare trials to completion, errors and latency within groups (control male, ELS male, control female, ELS female) during the various task phases (SD, CD, RS, and RR). There was a significant effect of task phase, within groups, which we would expect with a task that is We followed up this analysis with a one-way ANOVA during each specific task phase and reported significant post hoc Bonferroni comparisons between groups (Figure 1).
For the RT-qPCR gene expression analysis in the OFC and mPFC, we used two-tailed Student’s t tests to compare groups (ELS versus control) for each specific gene and sex. The same was used when analyzing the difference in immunoreactive PV cell density between groups in each brain region (VO and LO). For developmental RT-qPCR PV gene expression analysis in the PFC, we ran a two-way ANOVA to determine effects of treatment (control or ELS) and age (P8, P12, P16 or P21) (Figure 2).
To analyze the effects on optogenetic silencing of PV+ interneurons in the OFC and mPFC of NpHR+/− and NpHR−/− animals during the cognitive flexibility task, we first used a three-way ANOVA (task phase (SD, CD, RS, or RR) * stimulation (on or off) * genotype (NpHR+/− or NpHR-/)) and found significant three-way interactions. We then checked for differences between genotype for each task phase with photostimulation and without photostimulation using Student’s two-tailed, unpaired t tests (Figure 3). To analyze the effects of photostimulation on c-Fos activation in NpHR+/− and NpHR−/− animals, we again used Student’s two-tailed, unpaired t tests to determine effects between genotypes in various brain regions (OFC- VO and LO; mPFC- IL and PL).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| mouse anti-PV antibody | EMD Millipore | RRID: MAB81572 (lot 2718312) |
| biotin-conjugated goat anti-mouse secondary antibodies | Vector labs | RRID: BA-9200 |
| rabbit polyclonal antibody raised against the N terminus of human c-Fos | EMD Millipore | RRID: ABE457 (lot 2610509) |
| Critical Commercial Assays | ||
| Taqman assay- parvalbumin | Life Technologies | Mm00443100_m1 |
| Taqman assay- somatostatin | Life Technologies | Mm00436671_m1 |
| Taqman assay- calbindin | Life Technologies | Mm00486647_m1 |
| Taqman assay- calretinin | Life Technologies | Mm00801461_m1 |
| Taqman assay- Gad-67 | Life Technologies | Mm04207432_g1 |
| Taqman assay- NPY | Life Technologies | Mm01410146_m1 |
| Taqman assay- Beta Actin | Life Technologies | 4352341E |
| Experimental Models: Organisms/Strains | ||
| floxed Halorhodopsin (NpHR)-EYFP mouse | Jackson Labs | JAX#014539 |
| PV-directed Cre driver mouse | Jackson Labs | JAX#008069 |
| C57BL/6N mouse | Charles River labs | C57BL/6N- #027 |
| Software and Algorithms | ||
| Noldus Ethovision 10.1 | Noldus | Ethovision XT 10.1 |
| SPSS | IBM | SPSS v.24 |
| Biorad CFX3.1 | Biorad | CFX3.1 |
| Other | ||
| Vectastain ELITE | Vector Labs | PK-4000 |
| Sigmafast DAB tablet set | Sigma Aldrich | D4293 |
| 0.37 NA standard multimode fiber | Thor Labs | 200um core |
| multimode ceramic zirconia ferrule | Precision Fiber Products | 230um |
| Optical sensor | Thor Labs | PM100D |
| white LED | Mightex | Cool white 5500K |
| Thorlabs LEDD1B driver reflected through a dichroic mirror | Thor Labs | FF655-Di01 (Semrock) |
| 40x immersion objective | Olympus | LUMPlanFl 40x/0.80 W |
| CFX384 RT-qPCR system | Biorad | CFX384 |
Highlights.
Early life stress leads to select deficits in reversal learning in female mice
Impaired rule-reversal learning is associated with decreased PV and GAD67 in OFC
Optogenetic silencing of OFC PV+ cells recapitulates ELS effects on reversal learning
Optogenetic silencing of mPFC PV+ cells impairs rule shifting, but not reversal learning
ACKNOWLEDGMENTS
This project was supported by a Robert and Nancy Carney Institute for Brain Sciences gift for scientific innovation (to K.G.B.), NIH (R01-MH115914 and R01-MH115049 to K.G.B.), NIH Ruth Kirchenstein NRSA (F31-MH1111×1 to H.L.G.), NIH (F99-NS105219 to G.M.-N.), and a NIH COBRE pilot award (P20-GM103645 to Jerome Sanes and K.G.B.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.11.010.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, and Giles WH (2006). The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci 256, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen Z, Khan T, Proenca CC, Kraemer R, Cleland TA, et al. (2008). Variant Brain-Derived Neurotrophic Factor (Val66Met) Alters Adult Olfactory Bulb Neurogenesis and Spontaneous Olfactory Discrimination. J. Neurosci 28, 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Nitenson AS, Lichtman E, Lopez C, Chen W, Gallo M, Goodwill H, and Manzano-Nieves G (2017). Early life stress leads to developmental and sex selective effects on performance in a novel object placement task. Neurobiol. Stress 7, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin A, Blot K, Verney C, Estevez L, Santamaria J, Gressens P, Giros B, Otani S, Daugé V, and Naudon L (2012). Maternal deprivation induces deficits in temporal memory and cognitive flexibility and exaggerates synaptic plasticity in the rat medial prefrontal cortex. Neurobiol. Learn. Mem 98, 207–214. [DOI] [PubMed] [Google Scholar]
- Birrell JM, and Brown VJ (2000). Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci 20, 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, and Powell EM (2012). Reversal learning and attentional set-shifting in mice. Neuropharmacology 62, 1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, and Powell EM (2008). Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci 28, 11124–11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Bae MH, Suresh T, Jaffe DE, and Powell EM (2010). Astrocyte-mediated hepatocyte growth factor/scatter factor supplementation restores GABAergic interneurons and corrects reversal learning deficits in mice. J. Neurosci 30, 2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Bae MH, Suresh T, Jaffe DE, and Powell EM (2014). Prefrontal cognitive deficits in mice with altered cerebral cortical GABAergic interneurons. Behav. Brain Res 259, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, and Woo TU (2014). Perineuronal nets and schizophrenia: The importance of neuronal coatings. Neurosci. Biobehav. Rev 45, 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, and Morilak DA (2008). Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33, 320–331. [DOI] [PubMed] [Google Scholar]
- Burt VK, and Stein K (2002). Epidemiology of depression throughout the female life cycle. J. Clin. Psychiatry 63 (Suppl 7), 9–15. [PubMed] [Google Scholar]
- Buzsáki G, and Wang X-J (2012). Mechanisms of gamma oscillations. Annu. Rev. Neurosci 35, 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta S, Bolkan S, Padilla-Coreano N, Song LJ, Sahn R, Harrison NL, Gordon JA, Brown A, and Kellendonk C (2016). Maternal immune activation leads to selective functional deficits in offspring parvalbumin inter-neurons. Mol. Psychiatry 21, 956–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, and Moore CI (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KKA, Hoch R, Lee AT, Patel T, Rubenstein JLR, and Sohal VS (2015). Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/−) mice. Neuron 85, 1332–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvino V, Di Maria V, Marchese E, Lattanzi W, Biamonte F, Michetti F, and Geloso MC (2015). Estrogen administration modulates hippocampal GABAergic subpopulations in the hippocampus of trimethyltin-treated rats. Front Cell Neurosci 9, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Hudson L, and Landau S (2005). Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord 7, 358–369. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, and Connors BW (2010). Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65, 230–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, and Roberts AC (1996). Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav. Neurosci 110, 872–886. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, and Sousa N (2009). Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325, 621–625. [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, and Giles WH (2001). Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA 286, 3089–3096. [DOI] [PubMed] [Google Scholar]
- Elliott R, Zahn R, Deakin JFW, and Anderson IM (2011). Affective cognition and its disruption in mood disorders. Neuropsychopharmacology 36, 153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, and Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Fox MT, Barense MD, and Baxter MG (2003). Perceptual attentional set-shifting is impaired in rats with neurotoxic lesions of posterior parietal cortex. J. Neurosci 23, 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, and Claus ED (2006). Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol. Rev 113, 300–326. [DOI] [PubMed] [Google Scholar]
- Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, and Olatawura MO (1998). Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch. Gen. Psychiatry 55, 405–413. [DOI] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, Gallo M, Lee HI, Oyerinde E, Serre T, and Bath KG (2018). Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology, Published online September 6, 2018. 10.1038/s41386-018-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, and Bren-house HC (2016). Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology 71, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, and Drevets WC (2007). Reduced prefrontal glutamate/glutamine and g-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 64, 193–200. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Ovtscharoff W Jr., Poeggel G, and Braun K (2008). Imbalance of immunohistochemically characterized interneuron populations in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience 152, 18–28. [DOI] [PubMed] [Google Scholar]
- Holland FH, Ganguly P, Potter DN, Chartoff EH, and Brenhouse HC (2014). Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neurosci. Lett 566, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czéh B, Flugge G, and Zhang W (2010). Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology 35, 1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, and Rajkowska G (2010). Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int. J. Neuropsychopharmacol 13, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (2003). Epidemiology of women and depression. J. Affect. Disord 74, 5–13. [DOI] [PubMed] [Google Scholar]
- Kim H,Ährlund-Richter S, Wang X, Deisseroth K, and Carlén M (2016). Prefrontal parvalbumin neurons in control of attention. Cell 164, 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzler J, Braun K, and Bock J (2015). Early life stress and sex-specific sensitivity of the catecholaminergic systems in prefrontal and limbic regions of Octodon degus. Brain Struct. Funct 220, 861–868. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bédard-Arana T, and Morilak DA (2008). Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J. Neuroendocrinol 20, 1115–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, and Volk DW (2012). Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ (2011). The medial prefrontal and orbitofrontal cortices differentially regulate dopamine system function. Neuropsychopharmacology 36, 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, and Grace AA (2009). A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J. Neurosci 29, 2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ III, Gu X, Mao Y, et al. (2012). A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci 15, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar AC, Walker ALJ, Theobald DE, Eagle DM, and Robbins TW (2011). Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J. Neurosci 31, 6398–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, and Brown VJ (2003). Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res 146, 97–103. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Lesh TA, Niendam TA, Yoon JH, Cheng Y, Rhoades RN, and Carter CS (2015). Control-related frontal-striatal function is associated with past suicidal ideation and behavior in patients with recent-onset psychotic major mood disorders. J. Affect. Disord 188, 202–209. [DOI] [PubMed] [Google Scholar]
- Monroy E, Hernández-Torres E, and Flores G (2010). Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J. Chem. Neuroanat 40, 93–101. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iacoviello B, Neumeister A, Charney DS, and Iosifescu DV (2011). Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol. Learn. Mem 96, 553–563. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Ibañez-Tallon I, and Heintz N (2018). Cell-type specific contributions of medial pregrontal neurons to flexible behaviors. J. Neurosci 38, 4490–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neske GT, and Connors BW (2016). Distinct Roles of SOM and VIP Inter-neurons during Cortical Up States. Front Neural Circuits 10, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, and Miguel-Hidalgo JJ (2007). GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, and Baram TZ (2008). A Novel Mouse Model for Acute and Long-Lasting Consequences of Early Life Stress. Endocrinology 149, 4892–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, and Arnsten AF (2006). The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav. Brain Funct 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, and Deisseroth K (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Coyle J, Didriksen M, Gill K, Grace AA, Hensch TK, LaMantia AS, Lindemann L, Maynard TM, et al. (2017). Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol. Psychiatry 22, 936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AW, Caporale N, Wu C, and Wilbrecht L (2016). Early maternal separation impacts cognitive flexibility at the age of first independence in mice. Dev. Cogn. Neurosci 18, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JT, and Feng G (2013). Development of transgenic animals for optogenetic manipulation of mammalian nervous system function: Progress and prospects for behavioral neuroscience. Behav. Brain Res 255, 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay R, Lee S, and Rudy B (2016). GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Furukawa T, Iwata S, Yanagawa Y, and Fukuda A (2014). Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offspring. Transl. Psychiatry 4, e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaaf ME, Zwiers MP, van Schouwenburg MR, Geurts DE, Schellekens AF, Buitelaar JK, Verkes RJ, and Cools R (2013). Dopami nergic drug effects during reversal learning depend on anatomical connections between the orbitofrontal cortex and the amygdala. Front. Neurosci 7, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-Z, Xing X-W, He B, and Wang L-X (2007). Effects of testosterone on cytokines and left ventricular remodeling following heart failure. Cell. Physiol. Biochem 20, 847–852. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, McGlinchey JB, Chelminski I, and Young D (2006). Diagnosing major depressive disorder V: applying the DSM-IV exclusion criteria in clinical practice. J. Nerv. Ment. Dis 194, 530–533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.