Abstract
Background:
Drug-induced liver injury (DILI) is a potentially severe adverse drug reaction especially in susceptible patients. But there are no sensitive or specific parameters to detecting DILI. The specific expression of miR-122 in the liver has been a hotspot in the evaluation of hepatic toxicity due to its high stability and sensitivity.
Methods:
We performed a systematic literature review through July 31, 2017 to identify studies which evolved DILI patients testing miR-122 without limiting a certain drug. According to the PRISMA statement, a meta-analysis: the diagnostic role of miR-122 in DILI was made. QUADAS-2 quality evaluation table was used to evaluate the quality of the documentary evidence, PRISMA flowchart and quality evaluation table were drawn with RevMan, use Stata to calculate the sensitivity and specificity of miR-122 in diagnosing DILI, ROC curve and Deeks funnel plot were also drawn by STATA.
Results:
Eleven studies involved 194 DILI patients and 251 controls, all were tested miR-122 (fold change). Sensitivity of miR-122 in diagnosing DILI was [0.85 (95% CI, 0.75–0.91), I2 = 53.46%] and specificity was [0.93 (95% CI, 0.86–0.97), I2 = 65.10%], the area under ROC curve was 0.95 (95% CI, 0.93–0.97). While in acetaminophen (APAP)-induced liver injury, the sensitivity was [0.82 (95%CI, 0.67–0.91), I2 = 65.77%] specificity was [0.96 (95%CI, 0.88–0.99), I2 = 31.46%], AUROC was 0.97 (95% CI, 0.95–0.98).
Conclusions:
In this systematic review and meta-analysis, we found miR-122 have a high specificity in DILI, and a modest positive diagnostic effects. On the basis of the limited evidence, further research is needed to evaluate the long-term observation and more clinical data to testify miR-122 in diagnosing DILI.
Keywords: diagnostic biomarker, drug-induced liver injury, miR-122
1. Introduction
Drug-induced liver injury (DILI) is a commonly encountered disease in those years. Although it accounts for <1% of acute liver injury cases, it is the most common reason lead to acute liver failure (ALF) in the United States and Europe.[1,2] In the United States, 13% of ALF patients are finally diagnosed idiosyncratic DILI, with more than half of the cases need liver transplantation.[3] Overall surveys in France and Iceland showed that DILI occurs with an annual incidence of about 14 to 19 per 100,000 inhabitants.[4,5] In China, only 50.65% DILI patients cured but 1.60% died.[6] Also, DILI is one of the potentially severe adverse drug reactions (ADR) which are a major concern for healthcare systems and pharmaceutical industry, with a cost of £1 billion in United Kingdom and $4 billion in the United States.[7]
Lacking of the objective diagnostic criteria, DILI is often diagnosed after exclusion from other diseases, causality scores such as the Roussel-Uclaf Causality Assessment Method (RUCAM) are intended to confirm or exclude the suspicion of DILI.[8] But limitations of such scoring algorithms are poor inter-rater reliability and arbitrary scoring, for example, for alcohol use.[9] During rule out diseases one by one, it may already lead to irreversible liver damage even ALF. New emerging biomarkers could be useful in diagnosing DILI include total keratin18 (K18), caspase-cleaved keratin18 (ccK18), high mobility group box 1 (HMGB1) and microRNA-122 (miR-122),but there are still no conclusive evidence to that.[10] The microRNAs are fine tuners of diverse biological responses and are expressed in various cell types of the liver.[11] Some miR species demonstrate high tissue enrichment, and miR-122 is specifically expressed by hepatocytes where it accounts for 70% of the total miRNA content found in the liver, making it potentially an ideal candidate as a DILI biomarker.[12] And it has been shown to be a more sensitive and specific biomarker for liver toxicity when compared with standard protein biomarkers used to date.[13,14]
While important for clinical decision-making, little is known about the overall and comparative diagnosing role of miR-122 in DILI patients. To address this knowledge gap, we conducted a systematic review and meta-analysis assessing the diagnosing effect of miR-122 in DILI patients.
2. Methods
2.1. Search strategy
The search strategy was designed and conducted by an experienced librarian with input from the primary investigators and utilizing various databases from inception to July 31, 2017. The databases included PubMed, Web of Science, Taylor&Francis Online, EMBASE, Springer Link, Wiley Online Library, Elsevier Science Direct and Cochrane Central Register of Controlled Trials. In addition, we searched clinical trial registries (www.clinicaltrials.gov), conference proceedings and performed a recursive search of published systematic reviews. Controlled vocabulary supplemented with keywords was used to search for miR-122 and drug-induced liver injury.
2.2. Study selection
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for meta-analysis.[15]
Selection criteria were: research type: publicated studies that testing miR-122 in DILI patients (human research); research object: case group was clinically diagnosed DILI with a definite history of drug-intake and ALT/AST elevation or experimentally caused DILI (no limited to a specific drug), the control group was healthy volunteers or patients enrolled without using any possible drugs; selecting method: serum or plasma miR-122 were tested in every group and can get a complete diagnostic four grid data—true positive (TP), false positive (FP), true negative (TN) and false negative (FN) from the literature.
Exclusion criteria were: animal studies; repeated publication of the literature; abstract, lecture and other non primitive research and basic research; dissertations, conference proceeding articles.
2.3. Data extraction
Two reviewers independently screened the titles and abstracts for potential eligibility. Full text versions of the included abstracts were retrieved and screened in duplicate. Disagreements were harmonized by consensus and if not possible by consensus through arbitration by a 3rd reviewer. We extracted the following variables from each study: study characteristics including primary author, time period of study/year of publication and country of study true positive, false positive, false negative and true negative values of the index tests. Data extraction was done in duplicate.
2.4. Risk of bias assessment
We used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool to assess the risk of bias and applicability of diagnostic accuracy studies.[16] We assessed the following domains: patient selection, index test, reference standard and flow and timing. Quality of evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.[17] Methodological quality graph is presented using Review Manager (RevMan) 5.3 (The Cochrane Collaboration, Copenhagen, Denmark).
2.5. Statistical analysis
We performed meta-analyses of diagnostic test accuracy (sensitivity, specificity, likelihood ratios and diagnostic odds ratio) using a bivariate regression model allowing for correlation between sensitivity and specificity. I2 > 50% suggests high heterogeneity. A bivariate-mixed model was used to estimate the receiver operating characteristic (ROC) curve and the area under the ROC curve (AUROC) were also estimated to optimize cut-off points. Statistical analyses were conducted using Stata version 14 (StataCorp, College Station, TX). Assessed small study effects including publication bias by examining Deeks funnel plot asymmetry and regression test also using STATA 14.
Furthermore, considering the large number of acetaminophen-induced liver injury (APAP-ILI) studies, we did a sub-analysis of those studies.
3. Results
Our search strategy identified 988 citations. After screening titles and abstracts, a total of 185 were deemed eligible for full text retrieval. We eventually included 11 studies, a total of 194 patients from these clinical sites were included in the final analysis (Fig. 1). Studies were published from 2012 to 2017, DILI patients were categorized by several drugs: acetaminophen,[13,18–22] heparin,[23] cholestyramine,[24] paraquat,[25] and 1 study contains 1 patient used paracetamol and 7 patients used nitrofurantoin,[26] 1 study did not mention the specific drug.[27] Clinical datas were showed in Table 1. Reference standards: APAP overdose and treatment of N-acetyl-cysteine (NAC),[18–20,22] APAP-induced acute liver injury,[13,21] scored as “definitive” in Roussel Uclaf Causality Assessment (RUCAM),[26] drug exposure and induced aminotransferase elevations.[23,24,27] Control group: suspected DILI,[13,18] some were healthy volunteers,[19–21,23–27] 1 contains healthy individuals and ischemic hepatitis.[22]
Figure 1.
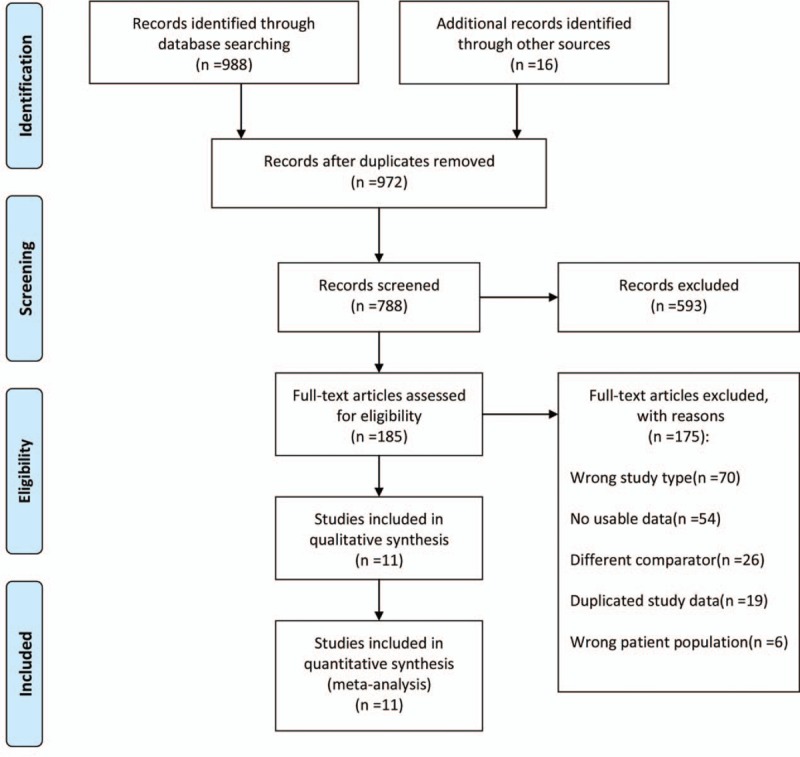
Flow diagram of study selection (PRISMA). PRISMA = preferred reporting items for systematic reviews and Meta-analyses.
Table 1.
The basic features of the inclusion of the studies.
3.1. Methodological quality of the included studies
The risk of bias applicability concerns of each study showed in Figure 2. Almost 50% of the studies were judged to have low risk of bias in terms of patient selection, index test, reference standard and flow and timing. The majority of the studies were considered low risk of bias on applicability to clinical practice in terms of reference standard, index test and patient selection.
Figure 2.
Methodological quality of the study enrolled (QUADAS-2). QUADAS-2 = quality assessment of diagnostic accuracy studies.
3.2. Pooled analysis of diagnostic accuracy
Eleven studies compared the diagnostic accuracy of miR-122 in detecting DILI.
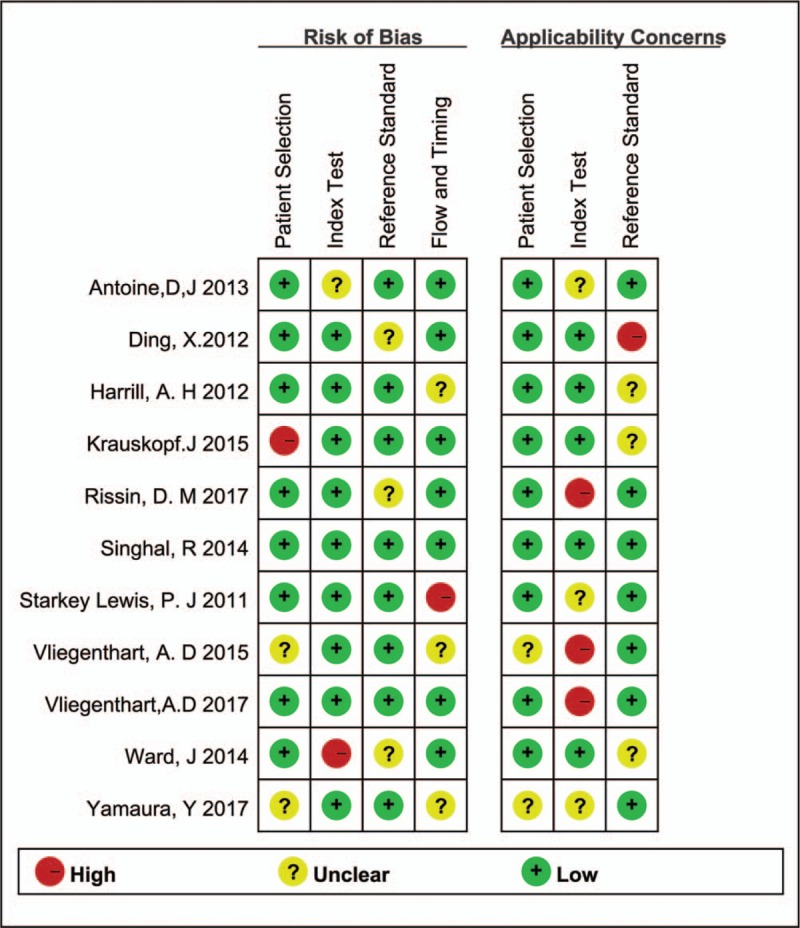
It shows a significantly high sensitivity [0.85 (95%CI, 0.75–0.91), I2 = 53.46%] and specificity [0.93 (95%CI, 0.86–0.97), I2 = 65.10%] in diagnosis of DILI, as showed in Figure 3A. Both of them indicated substantial heterogeneity. Figure 4A illustrates the analysis of ROC and AUC: the accuracy of miR-122 for detection DILI was 0.95 (95%CI, 0.93–0.97). The sub-analysis of miR-122 in APAP-ILI has been done, the sensitivity was 0.82 (95%CI, 0.67–0.91), I2 was 65.77%; specificity was 0.96 (95%CI, 0.88–0.99), I2 was 31.46%, results were showed in Figure 3B; ROC AUC was 0.97 (CI, 0.95–0.98) showed in Figure 4B. There is nearly no difference in DILI and APAP-ILI both in sensitivity and specificity, which means the diagnostic role of miR-122 in DILI may not related with drug species.
Figure 3.
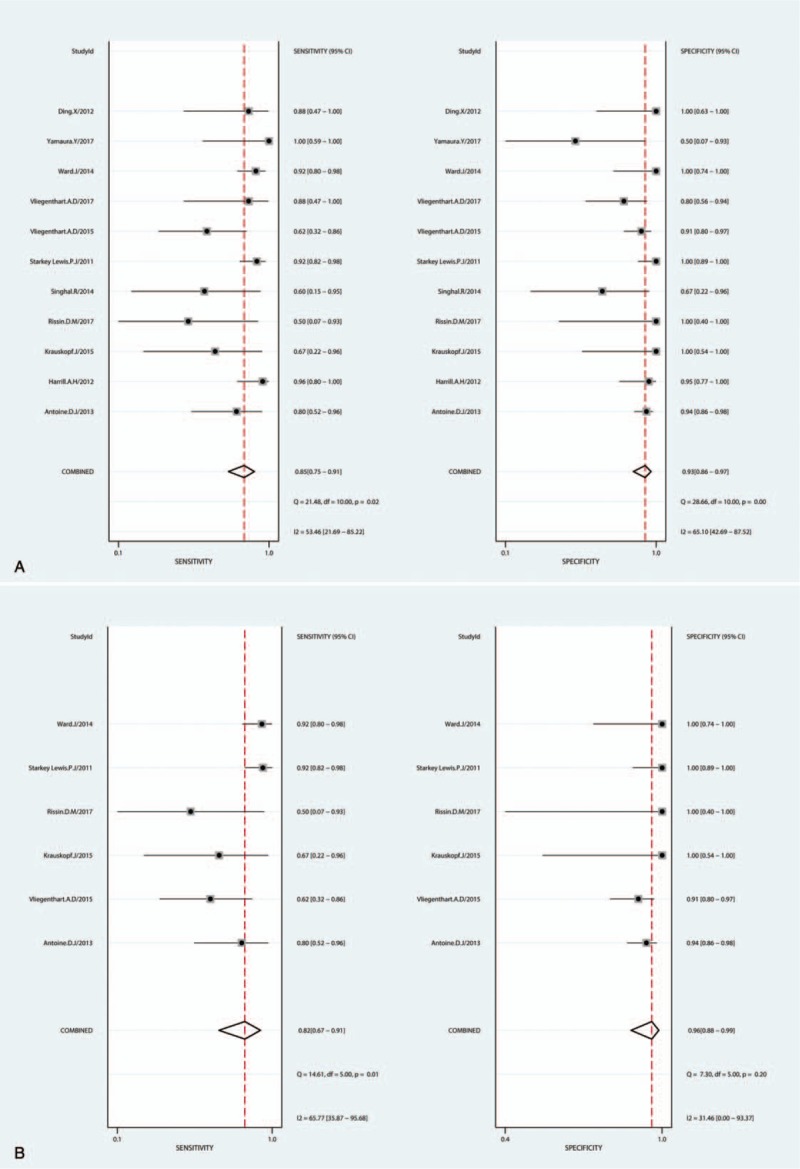
Sensitivity and specificity forest plots of miR-122 in diagnosing DILI (A) and APAP-ILI (B). APAP = acetaminophen, DILI = drug-induced liver injury.
Figure 4.
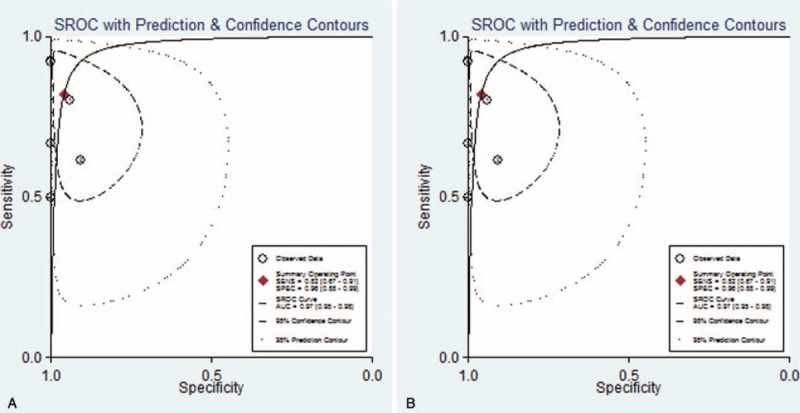
Summary ROC curve showing performance of miR-122 in diagnosing DILI (A) and APAP-ILI (B). ROC curve = paired receiver operating characteristic curve.
3.3. Heterogeneity investigation and publication bias
Q test and I2 test were used to estimate heterogeneity, results were: sensitivity (Q = 21.48, P = .02), specificity (Q = 28.66, P < .001), I2 of sensitivity and specificity were 53.49% and 65.10%, suggested that there exist moderate heterogeneity. Threshold analysis: Spearman correlation coefficient = 0.391, P = .235 showed that were no threshold effect in these studies. Meta-regression analysis were used to investigate heterogeneity: using region, study type, drug, control group, reference standard and number of patients as covariate, but no heterogeneity were found in those sources. A sensitivity analysis, in which studies were removed one-by-one confirmed the stability of our results (Table 1).
Visual inspection of the Deeks funnel plot suggests possible publication bias as in Figure 5A. And a Deeks funnel plot of studies of APAP-ILI was shown in Figure 5B.
Figure 5.
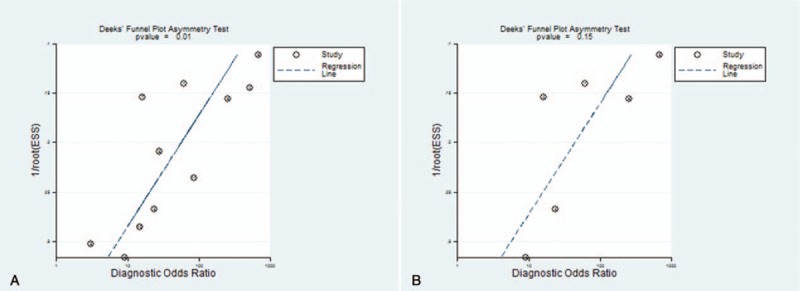
The publication bias of all selected DILI studies showed as Deeks funnel plot (A) and APAP-ILI studies (B). APAP = acetaminophen, DILI = drug-induced liver injury.
4. Discussion
Current diagnosis of DILI depends on expert opinion that is based on patient data and the typical ‘signatures’ associated with certain drugs.[28] A major component of diagnosis relies on a liver function test, elevations of alanine aminotransferase (ALT)/aspartate aminotransferase (AST) in the serum correlate with hepatocyte necrosis, alkaline phosphatase (ALP) related to the biliary epithelial cells or canalicular membrane damage and total bilirubin (TBIL) is indicative of whole liver function.[29] Also, the Food and Drug Administration (FDA) endorses the use of Hy's law: a drug which causes elevation of ALT/AST > × 3 and TBIL > × 2 upper limit of normal (ULN) in the absence of other cholestatic/hepatic co-morbidities is likely to cause hepatotoxicity.[30,31] But the Positive Predictive Value (PPV) was only 2% given the low incidence rate of drug-induced ALF at 1.6/1,000,000 person-year using Hy's Law to identify ALF in DILI patients[32]; ALT and AST are present in skeletal muscle and have shown elevation in patients under polymyositis or extreme exercise[33]; ALP is present in bone tissue and increased to osteoblast activity; TBIL can also elevated by the processing of erythrocytes and subsequent degradation of haemoglobin or by alteration of bilirubin transporters.[34] DILI diagnose remains a critical clinical problem for doctors.[10,35]
When patients are combined with a underlying liver disease, it requires strategies to assess whether test changes are due to DILI or due to flares of the underlying liver disease.[36] Lacking a valid diagnostic biomarker, DILI is basically a diagnosis of exclusion and typically requires causality assessment such as RUCAM to establish an individual causality grading of the suspected drug, but some studies showed it still has significant inter-rater variability.[37] The United States DILI network (DILIN) prospective study attempted to structure expert opinion process to minimise variability and bias, and the structured DILIN expert opinion process produced higher agreement rates and likelihood scores than RUCAM in assessing causality, but there still has considerable interobserver variability.[38] One systematic review and meta-analysis of algorithms used to identify DILI in health record databases shows that using diagnosis scales (including RUCAM, Maria, Victorino and DDW-J) as a search criteria for inclusion in the algorithm does not add significant additional value to the PPV for DILI.[39] Efforts to obtain reliable information about it is diagnosis and treatment are being made worldwide, especially to find a easily detected biomarker for early diagnosising DILI.
For a biomarker to be in clinical use, it must have both biological and bioanalytical sensitivity, tissue/organ specificity, easy accessibility and it must display a change early enough for a therapeutic intervention to be effective.[40] Although the identities, expression and functional roles of miRNAs have been extensively studied, little is known about the fate of extracellular miRNAs after exposure to an organ-specific toxicant and their usage as biomarkers for tissue injury.[41] Robles et al reviewed biomarkers in DILI found that miRNAs have received much attention lately as potential non-invasive DILI biomarker candidates, in particular miR-122,[42] but Teschke et al in 2017 found that biomarkers for DILI are not yet available.[43] The polymerase chain reaction (PCR) and next generation sequencing (NGS) technologies has been widely used to detect RNAs and scientists also found that the electrochemical sensors have received significant attention owing to their quick response, low cost, simple preparation and extensive applications in different fields.[44] Therefore, using PCR technique to detect miRNA-122 is very convenient in clinic, the cost is not high and if we can test it in suspected DILI patients, we can avoid deterioration of liver damage and targeted treatment in time, rather than blindly exclude all other liver diseases which may take more manpower material resources.
Here, for the 1st time, we made systematic review and meta-analysis to report the utility of miR-122 as a possible biomarker for DILI, and its assimilation with traditional methods for evaluating hepatic integrity to investigate the onset liver injury caused by drugs. Although miR-122 was not only evaluated in DILI but also in other liver damages such as hepatic virus infection, inflammation disease and hepatocellular carcinoma,[11,45,46] 1 study examined several mechanistic biomarkers of DILI in patients who had ingested a single toxic dose of APAP, demonstrated that initial miR-122 levels were able to predict maximum ALT activity and peak international normalized ratio score during the hospital stay of the patient, and it raised very early during liver injury,[18] another study found that miR-122 demonstrated the highest performance for prediction of APAP-induced ALF in a large cohort of overdose patients with normal ALT levels, confirmed the result of the earlier study.[47] And a prospective result was showed in our study.
This study findings should be interpreted in the following limitations. First, data are derived predominantly from APAP-nonAPAP comparisons which may lead to the doubt of miR-122 in diagnosing DILI or APAP-ILI. Second, while we used strict inclusion and exclusion criteria to ensure comparability across trials, there are few randomized controlled trial (RCT) which lead to a low reliability of this study and some significant studies cannot extract TP, FP, FN,TN data have to be missed. Third, research analysis found moderate heterogeneity, but no sources were found by meta-regression analysis using region, study type, drug, control group and reference standard as covariate, insufficient number of included studies may limit to find the source of heterogeneity. Moreover, we have only retrieved languages in English, and the related research of other languages will be omitted, which may result in the bias of language.
In conclusion, while many studies approved miR-122 maybe a advantageous diagnostic biomarker of liver damage, further research is still needed to evaluate the diagnostic value of miR-122 in DILI with only 194 patients included. Two clinical trials recorded in National Institutes of Health (NIH) named 1. Comparative Study of Circulating microRNA Changes in Patients With Liver Injury and 2. Healthy Subjects and Serum miR-122 as a Real-time Detection Biomarker of Drug-induced Liver Injury by Chemotherapy are both under investigation, thus in a worthy looking forward future, we may know more details about the diagnositic role of miR-122 in DILI.
Author contributions
Yiqi Liu and Ping Li carried out the idea, study design and the meta-analysis experimental work, Yiqi Liu and Liang Liu did the data collection and interpretation, Yilian Zhang participated in the design and coordination of the meta-analysis work, Yiqi Liu drafted the manuscript, Ping Li put forward constructive revisions to the paper. All authors have read and approved the final manuscript.
Conceptualization: Yiqi Liu, Ping Li.
Data curation: Yiqi Liu, Liang Liu, Yilian Zhang.
Formal analysis: Ping Li.
Project administration: Ping Li.
Resources: Yiqi Liu, Liang Liu, Yilian Zhang.
Software: Yiqi Liu, Liang Liu, Yilian Zhang.
Supervision: Ping Li.
Writing – original draft: Yiqi Liu.
Writing – review & editing: Ping Li.
Footnotes
Abbreviations: APAP = acetaminophen, AUROC = area under the ROC curve, DILI = drug-induced liver injury, PRISMA = preferred reporting items for systematic reviews and Meta-analyses, QUADAS-2 = quality assessment of diagnostic accuracy studies, ROC curve = paired receiver operating characteristic curve, RUCAM = Roussel-Uclaf Causality Assessment Method.
YL and PL contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Lee WM. Drug-induced acute liver failure. Clin Liver Dis 2013;17:575–86. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fontana RJ, Hayashi PH, Gu J, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterol 2014;147:96–108. e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002;137:947–54. [DOI] [PubMed] [Google Scholar]
- [4].Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatol 2002;36:451–5. [DOI] [PubMed] [Google Scholar]
- [5].Bjornsson ES, Bergmann OM, Bjornsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterol 2013;144:1419–25. 1425.e1411-1413; quiz e1419-1420. [DOI] [PubMed] [Google Scholar]
- [6].Zhang YM, Sun WJ, Wen LZ, et al. Clinical features of patients with drug-induced liver injury in China in the last five years. J Clin Hepatol 2018;34:562–6. [Google Scholar]
- [7].Alfirevic A, Pirmohamed M. Genomics of adverse drug reactions. Trends Pharmacol Sci 2017;38:100–9. [DOI] [PubMed] [Google Scholar]
- [8].Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci 2015;17(1.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Garcia-Cortes M, Stephens C, Lucena MI, et al. Causality assessment methods in drug induced liver injury: strengths and weaknesses. J Hepatol 2011;55:683–91. [DOI] [PubMed] [Google Scholar]
- [10].Kullak-Ublick GA, Andrade RJ, Merz M, et al. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut 2017;66:1154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bala S, Petrasek J, Mundkur S, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatol 2012;56:1946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kia R, Kelly L, Sison-Young RL, et al. MicroRNA-122: a novel hepatocyte-enriched in vitro marker of drug-induced cellular toxicity. Toxicol Sci 2015;144:173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vliegenthart AD, Shaffer JM, Clarke JI, et al. Comprehensive microRNA profiling in acetaminophen toxicity identifies novel circulating biomarkers for human liver and kidney injury. Sci Rep 2015;5:15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krauskopf J, de Kok TM, Schomaker SJ, et al. Serum microRNA signatures as “liquid biopsies” for interrogating hepatotoxic mechanisms and liver pathogenesis in human. PLoS One 2017;12:e0177928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J (Clin Res Ed) 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [17].Murad MH, Montori VM, Ioannidis JP, et al. How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA 2014;312:171–9. [DOI] [PubMed] [Google Scholar]
- [18].Antoine DJ, Dear JW, Lewis PS, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatol 2013;58:777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Krauskopf J, Caiment F, Claessen SM, et al. Application of high-throughput sequencing to circulating microRNAs reveals novel biomarkers for drug-induced liver injury. Toxicol Sci 2015;143:268–76. [DOI] [PubMed] [Google Scholar]
- [20].Rissin DM, Lopez-Longarela B, Pernagallo S, et al. Polymerase-free measurement of microRNA-122 with single base specificity using single molecule arrays: detection of drug-induced liver injury. PLoS One 2017;12:e0179669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Starkey Lewis PJ, Dear J, Platt V, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatol 2011;54:1767–76. [DOI] [PubMed] [Google Scholar]
- [22].Ward J, Kanchagar C, Veksler-Lublinsky I, et al. Circulating microRNA profiles in human patients with acetaminophen hepatotoxicity or ischemic hepatitis. Proc Natl Acad Sci U S A 2014;111:12169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harrill AH, Roach J, Fier I, et al. The effects of heparins on the liver: application of mechanistic serum biomarkers in a randomized study in healthy volunteers. Clin Pharmacol Ther 2012;92:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Singhal R, Harrill AH, Menguy-Vacheron F, et al. Benign elevations in serum aminotransferases and biomarkers of hepatotoxicity in healthy volunteers treated with cholestyramine. BMC Pharmacol Toxicol 2014;15:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ding X, Ding J, Ning J, et al. Circulating microRNA-122 as a potential biomarker for liver injury. Mol Med Rep 2012;5:1428–32. [DOI] [PubMed] [Google Scholar]
- [26].Vliegenthart ADB, Berends C, Potter CMJ, et al. MicroRNA-122 can be measured in capillary blood which facilitates point-of-care testing for drug-induced liver injury. Br J Clin Pharmacol 2017;83:2027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yamaura Y, Tatsumi N, Takagi S, et al. Serum microRNA profiles in patients with chronic hepatitis B, chronic hepatitis C, primary biliary cirrhosis, autoimmune hepatitis, nonalcoholic steatohepatitis, or drug-induced liver injury. Clin Biochem 2017. [DOI] [PubMed] [Google Scholar]
- [28].Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950–66. quiz 967. [DOI] [PubMed] [Google Scholar]
- [29].Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatol 2011;53:1377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Regev A, Bjornsson ES. Drug-induced liver injury: morbidity, mortality, and Hy's law. Gastroenterol 2014;147:20–4. [DOI] [PubMed] [Google Scholar]
- [31].Chalasani N, Bonkovsky HL, Fontana R, et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterol 2015;148:1340–52. e1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lo Re V, 3rd, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy's law and a new prognostic model. Clin Gastroenterol Hepatol 2015;13:2360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nathwani RA, Pais S, Reynolds TB, et al. Serum alanine aminotransferase in skeletal muscle diseases. Hepatol 2005;41:380–2. [DOI] [PubMed] [Google Scholar]
- [34].Church RJ, Watkins PB. The transformation in biomarker detection and management of drug-induced liver injury. Liver Int 2017;37:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hayashi PH, Rockey DC, Fontana RJ, et al. Death and liver transplantation within 2 years of onset of drug-induced liver injury. Hepatol 2017;66:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Teschke R, Danan G. Diagnosis and management of drug-induced liver injury (DILI) in patients with pre-existing liver disease. Drug Saf 2016;39:729–44. [DOI] [PubMed] [Google Scholar]
- [37].Rochon J, Protiva P, Seeff LB, et al. Reliability of the Roussel Uclaf causality assessment method for assessing causality in drug-induced liver injury. Hepatol 2008;48:1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatol 2010;51:2117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tan EH, Low EXS, Dan YY, et al. Systematic review and meta-analysis of algorithms used to identify drug-induced liver injury (DILI) in health record databases. Liver Int 2017. [DOI] [PubMed] [Google Scholar]
- [40].Hornby RJ, Starkey Lewis P, Dear J, et al. MicroRNAs as potential circulating biomarkers of drug-induced liver injury: key current and future issues for translation to humans. Expert Rev Clin Pharmacol 2014;7:349–62. [DOI] [PubMed] [Google Scholar]
- [41].Cho YE, Kim SH, Lee BH, et al. Circulating plasma and exosomal microRNAs as indicators of drug-induced organ injury in rodent models. Biomol Ther (Seoul) 2017;25:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Robles-Diaz M, Medina-Caliz I, Stephens C, et al. Biomarkers in DILI: one more step forward. Front Pharmacol 2016;7:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Teschke R, Schulze J, Eickhoff A, et al. Drug induced liver injury: can biomarkers assist RUCAM in causality assessment? Int J Mol Sci 2017;18(4.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Su S, Cao W, Liu W, et al. Dual-mode electrochemical analysis of microRNA-21 using gold nanoparticle-decorated MoS2 nanosheet. Biosens Bioelectron 2017;94:552–9. [DOI] [PubMed] [Google Scholar]
- [45].El-Abd NE, Fawzy NA, El-Sheikh SM, et al. Circulating miRNA-122, miRNA-199a, and miRNA-16 as biomarkers for early detection of hepatocellular carcinoma in Egyptian patients with chronic Hepatitis C virus infection. Mol Diagn Ther 2015;19:213–20. [DOI] [PubMed] [Google Scholar]
- [46].Park HK, Jo W, Choi HJ, et al. Time-course changes in the expression levels of miR-122, -155, and -21 as markers of liver cell damage, inflammation, and regeneration in acetaminophen-induced liver injury in rats. J Vet Sci 2016;17:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dear JW, Clarke JI, Francis B, et al. Risk stratification after paracetamol overdose using mechanistic biomarkers: results from two prospective cohort studies. Lancet Gastroenterol Hepatol 2018;3:104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


