Supplemental Digital Content is available in the text
Keywords: D2-40, ductal adenocarcinoma, lymphatic metastasis, pancreas, pancreaticoduodenectomy, prognosis, survival analysis
Abstract
Little is known concerning the prognostic significance of the degree of lymphatic vessel invasion in pancreatic head cancer. To address this gap in knowledge, we retrospectively examined 60 patients with locally advanced, surgically resectable pancreatic head cancer who underwent pancreaticoduodenectomy and lymph node (LN) dissection.
All cases were histopathologically diagnosed as ductal adenocarcinoma, stage II (25 pT3N0 cases, 35 pT3N1 cases). The following variables were investigated: age; sex; neoadjuvant therapy; adjuvant therapy; tumor size; tumor grade; invasion into the serosa, retropancreatic tissue, duodenum, bile duct, portal venous system and perineural area; cut margins; LN metastasis; and the number of invaded lymphatic vessels (LVI-score).
Univariate analysis demonstrated that LN metastasis and an LVI-score ≥5 were significantly associated with poor disease-free survival. Multivariate Cox regression analysis confirmed that LN metastasis and an LVI-score ≥7 were significantly associated with poor disease-free survival. Additionally, LVI-scores ≥9 and ≥10 were comparable to or surpassed the significance of LN metastasis based on the hazard ratio. Univariate analysis demonstrated that tumor size >30 mm, duodenal invasion, LN metastasis and an LVI-score ≥2 were significantly associated with poor overall survival. Multivariate Cox regression analysis confirmed that LN metastasis and LVI-scores ≥9 and ≥10 were significantly associated with poor overall survival, and an LVI-score ≥10 was comparable to or surpassed the significance of LN metastasis based on the hazard ratio.
Our study strongly suggests that a high degree of lymphatic vessel invasion is associated with a poor prognosis in patients with locally advanced, surgically resectable pancreatic head cancer.
1. Introduction
Pancreatic cancer remains a serious disease, with an estimated 330,400 deaths (173,800 men and 156,600 women) in 2012 worldwide.[1] Despite exhaustive efforts to detect early-stage pancreatic ductal adenocarcinoma, only 10% to 20% or fewer of cases are surgically resectable at the time of diagnosis, resulting in low survival rates.[2] One study reported that the 5-year overall survival rate for localized cases (T1/2 N0 M0) is 41.5% to 43.7%, the 5-year overall survival rate for regional cases (T3/4 N0 M0 or any T N+ M0) is 14.4% to 16.7%, and the 5-year overall survival rate for distant cases (any T any N M+) is 3.7% to 4.3% in the United States and Germany.[3]
Pancreatic ductal adenocarcinoma metastasizes to lymph nodes (LNs) in greater than one-half to three-quarters of surgically resectable cases, and the presence of LN metastases is one of the most powerful predictors of postoperative survival.[4–7] Moreover, several studies have demonstrated that the metastatic LN ratio and the number of positive nodes are significant factors associated with overall survival in patients with resectable pancreatic ductal adenocarcinoma.[8–10] However, the number of retrieved and evaluated regional LNs is influenced by the extent of LN dissection and the accuracy of pathological examination. Warschkow et al revealed that a higher number of retrieved regional LNs in pancreatic cancer decreases the rate of stage migration and is associated with an improved oncological outcome in node-negative and node-positive pancreatic cancer.[11] Thus, further elucidation of lymphatic pathology may help stratify pancreatic cancer patients to provide therapeutic strategies.
Given that lymphatic vessel invasion is the first step toward establishing lymphogenous metastasis of cancer, it is important to determine the pathological status of lymphatic vessel invasion in the primary lesion.[12] However, only a few studies have focused on this topic. Several studies reported that the presence of lymphatic vessel invasion was associated with survival in patients with resectable pancreatic cancer.[13,14] However, these studies did not possess robust, reproducible methodology for the assessment of lymphatic vessel invasion. Thus, our central question is the following: to what extent is lymphatic vessel invasion actually involved in the prognosis when lymphatic vessel invasion is assessed by a constant measurement criterion?
The aim of this study was to elucidate the prognostic significance of the degree of lymphatic vessel invasion based on a constant measurement criterion and sequential analysis using immunohistochemistry and a continuous natural number method in patients with locally advanced, surgically resectable pancreatic head cancer.
2. Materials and methods
2.1. Case collection and clinical evaluation
This retrospective study was approved by our institutional review board (No. A16-052). The inclusion criteria consisted of pancreaticoduodenectomy for pancreatic head cancer at Jichi Medical University Hospital. The exclusion criteria included stage I, III, and IV disease and a lack of available pathological samples and follow-up information. The medical records of potentially eligible patients were retrospectively and consecutively retrieved from our computerized database between January 2002 and December 2015. Board-certified surgeons reviewed the patients’ medical charts and evaluated information regarding patient age, clinical history, surgical procedures, and administration of chemotherapy for pancreatic cancer.
2.2. Pathological evaluation
Materials obtained from pancreaticoduodenectomy and LN dissection were fixed in 15% formalin. Pancreatic and duodenal tissues were sliced at a nearly horizontal sectional view at approximately 5 mm intervals. Paraffin-embedded tissue blocks were generated from the slices by total segmentation. Routine histological examination was performed by board-certified anatomic pathologists using hematoxylin and eosin staining of these tissue samples. Diagnosis was based on the UICC TNM classification system[15] and the World Health Organization classification of tumors of the digestive system.[2]
Immunohistochemistry was performed on whole mount tissue sections that completely covered the maximum division surface of the tumor in each case using an avidin-biotin-peroxidase complex method, an automated staining system (Ventana Benchmark ULTRA; Roche Diagnostics; Tokyo, Japan), and an antibody against podoplanin (D2-40; Nichirei Bioscience; Tokyo, Japan) along with positive and negative controls according to the manufacturer's instructions. In addition, a serial section slide stained with hematoxylin and eosin was made corresponding to each D2-40 immunohistochemical slide. Cancer cells surrounded by endothelium that were strongly and continuously positive for D2-40 were considered to represent lymphatic vessel invasion, whereas cancer cells adjacent to stromal cells that were weakly or intermittently positive for D2-40 and histologically interpreted to be fibrous components were not regarded as lymphatic vessel invasion. In addition, cancer cells with squamous differentiation that reacted with D2-40 were not considered to represent lymphatic vessel invasion. The degree of lymphatic vessel invasion was assessed using the lymphatic vessel invasion score (LVI-score), which was defined as the total number of invaded lymphatic vessels observed in the whole mounted section slides that covered the maximum division surface of the tumor. Lymphatic vessel invasion was evaluated twice, with an interval longer than one month, using these slides in each case, by a board-certified pathologist who was blinded to the prognostic data, and the final LVI-score was confirmed by reviewing these measurement results.
We also analyzed the number of invaded lymphatic vessels according to the following four anatomical locations: right, left, anterior, and posterior regions (Fig. 1).
Figure 1.
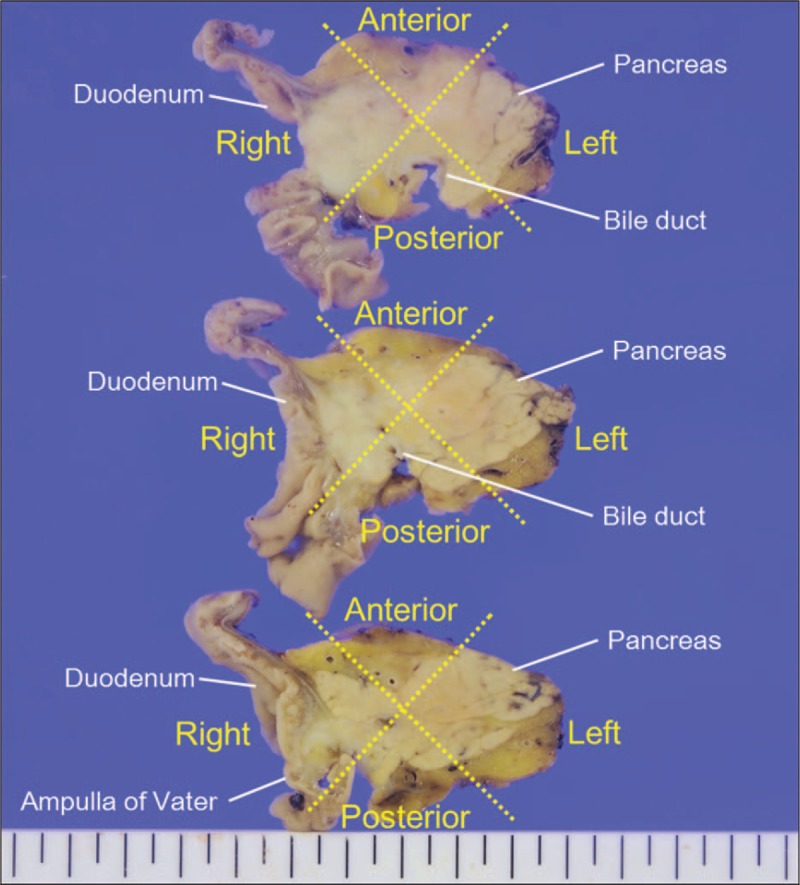
Anatomical classification of the pancreatic head portion (horizontal view).
2.3. Clinicopathological parameters and endpoints
The following variables were investigated: age, sex, neoadjuvant therapy, adjuvant therapy, tumor size as measured histopathologically by the greatest dimension, histological tumor grade, serosal invasion, retropancreatic tissue invasion, duodenal invasion, bile duct invasion, portal venous system invasion, intrapancreatic perineural invasion, pancreatic cut margin, dissected peripancreatic tissue margin, LN metastasis, and the LVI-score. Disease-free survival and overall survival were chosen as endpoints. Disease-free survival was defined as the time from pancreaticoduodenectomy to the following events: locoregional recurrence, distant metastasis, second primary same or other cancer, death from same or other cancer, non-cancer-related death, or treatment-related death.[16] Overall survival was defined as the time from pancreaticoduodenectomy to death, irrespective of the cause.[16]
2.4. Statistical analysis
Fisher's exact test was used to investigate the relationship between lymphatic vessel invasion and clinicopathological findings. Pearson's correlation coefficient was used to examine correlations between the tumor size as measured histopathologically by the greatest dimension and the LVI-score. Friedman's test was used to investigate differences in the frequency of lymphatic vessel invasion according to the anatomical location. A receiver operating characteristic curve was used to analyze the diagnostic performance of the LVI-score to determine the cutoff point that yielded the highest combined sensitivity and specificity with respect to distinguishing patients with events described above from those without such events. The Kaplan–Meier method was used to estimate survival rates. All explanatory variables were dichotomized for survival analysis and compared using the log-rank test. The Cox proportional hazard model was used to identify independent risk factors for survival. A value of P < .05 was considered statistically significant (two-tailed test). These statistical analyses were performed with IBM SPSS Statistics 21 (IBM Japan; Tokyo, Japan) or EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).[17]
3. Results
3.1. Patient characteristics
A total of 63 patients who underwent pancreaticoduodenectomy and LN dissection for pancreatic head cancer were identified from our database during the study period, among which three cases were stage I and were thereby eliminated based on the exclusion criteria. The remaining 60 cases were all stage II and eligible for the study. The median age was 70 years (range 36–83 years), and the mean patient age was 67.8 years (S.E. = 1.3). The study included 29 males and 31 females. Nine patients underwent neoadjuvant therapy, including 7 patients who underwent chemoradiotherapy with gemcitabine or tegafur and 2 patients who underwent gemcitabine-based chemotherapy. Forty-seven patients underwent adjuvant chemotherapy with gemcitabine or tegafur or a combination of these drugs. The 60 cases were further classified as stage IIA: pT3N0M0 (25 cases) and stage IIB: pT3N1M0 (35 cases). All 60 cases were histopathologically diagnosed as ductal adenocarcinoma, including two cases of adenosquamous carcinoma and one case of undifferentiated carcinoma. The median tumor size was 30 mm (range 12–64 mm), and the mean tumor size was 30.7 mm (S.E. = 1.4). Three cases were classified as histological tumor grade 1, 26 cases were classified as grade 2, and 31 cases were classified as grade 3. The median number of dissected LNs was 16 (range 2–39), and the median number of metastatic LNs was 1 (range 0–12). Only one patient exhibited cancer invasion into the extrapancreatic nerve plexus, and only 1 patient was positive for the bile duct cut-end margin.
3.2. Characteristics of lymphatic vessel invasion
Forty-four patients exhibited lymphatic vessel invasions, as identified by the D2–40 immunohistochemical glass slides that completely covered the maximum division surface of the tumor (Fig. 2). The median LVI-score was 2 (range 0–112). The median number of D2-40 immunohistochemical glass slides used for evaluating lymphatic vessel invasion was 2 (range 1–4).
Figure 2.
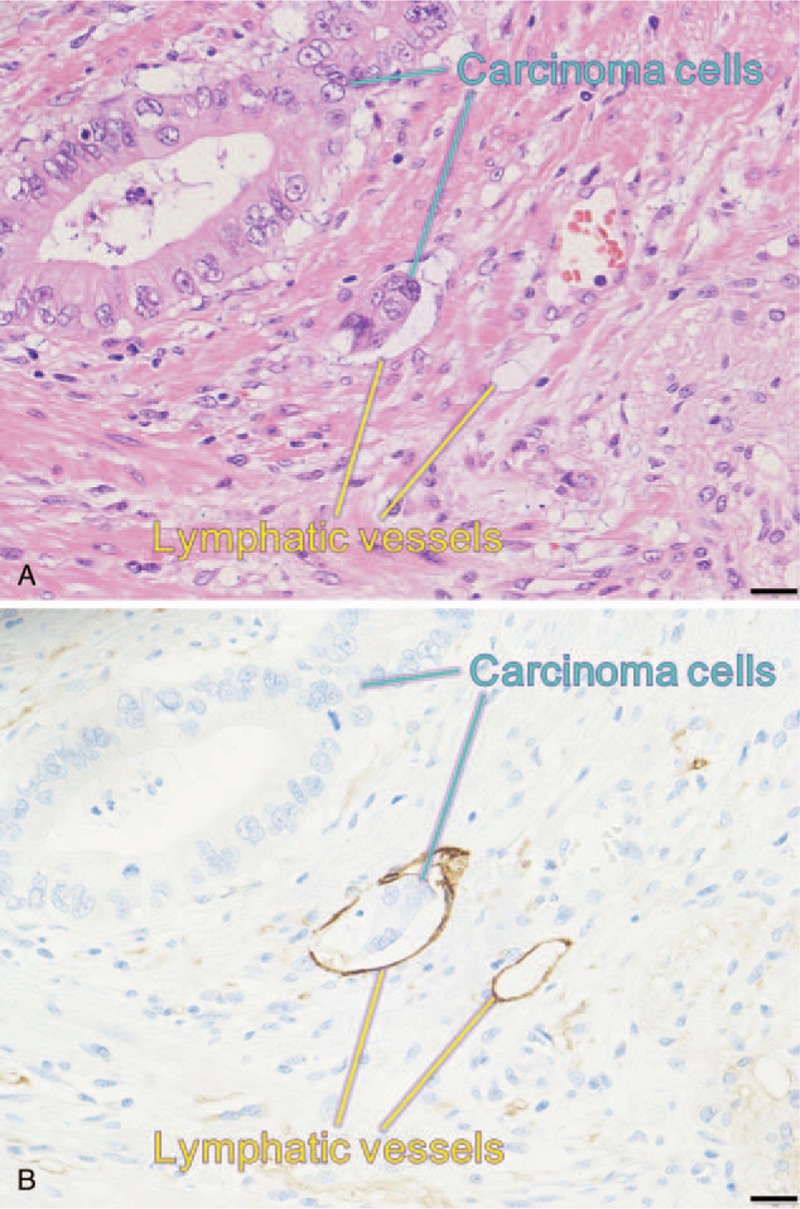
Representative histopathological findings of pancreatic head cancer. (A) Barely visible lymphatic vessel invasion of ductal adenocarcinoma cells shown by hematoxylin and eosin staining (the bar indicates 20 μm). (B) Clearly visible lymphatic vessel invasion of ductal adenocarcinoma cells shown by D2-40 immunohistochemistry (serial section of A, the bar indicates 20 μm).
Lymphatic vessel invasion was frequently observed in patients with LN metastasis (Fisher's exact test, P < .001) and in patients with bile duct invasion (P = .003) but was much less frequently observed in patients who received neoadjuvant therapy (P < .001) (Table 1). Pearson's correlation coefficient for the relationship between the tumor size and LVI-score was 0.220 (Pearson's correlation coefficient, P = .091). Lymphatic vessel invasion was more frequently observed in the right and posterior regions of the pancreaticoduodenectomy material than in the left and anterior regions (Friedman's test, P < .001) (Table 2).
Table 1.
Association between lymphatic vessel invasion and clinico-pathological factors in 60 patients with stage II pancreatic head cancer.
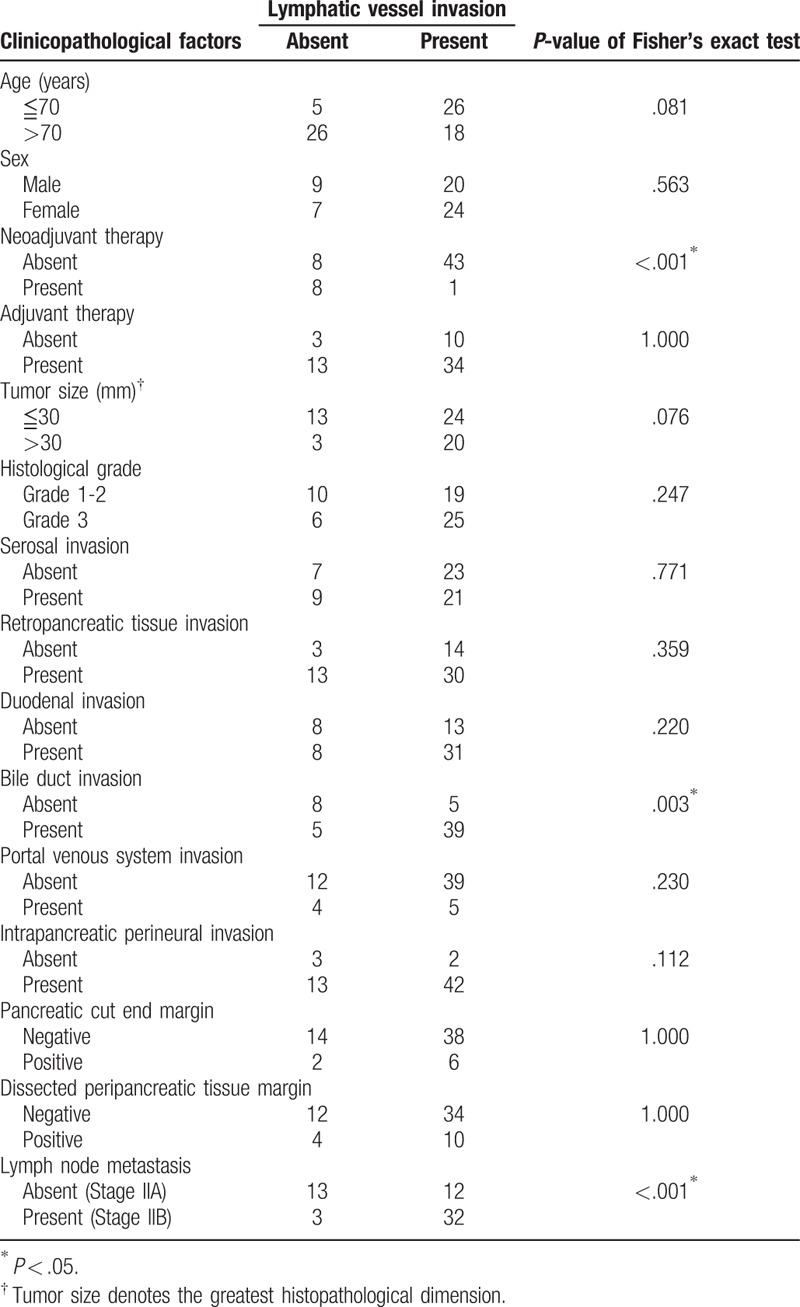
Table 2.
Regional differences of the number of invaded lymphatic vessels observed in 45 patients with stage II pancreatic head cancer.

3.3. Disease-free survival analysis
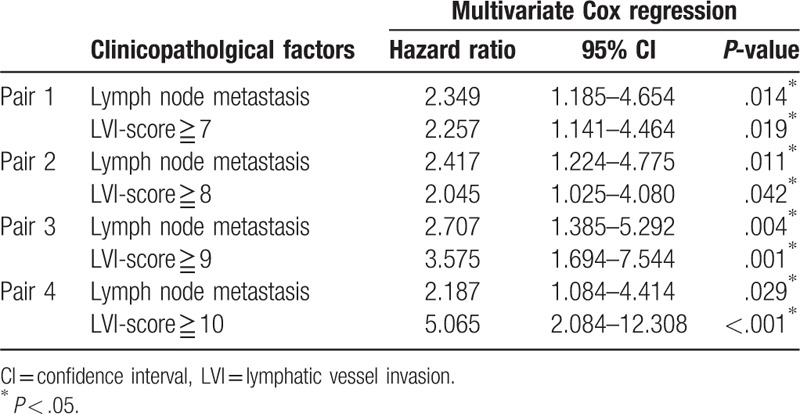
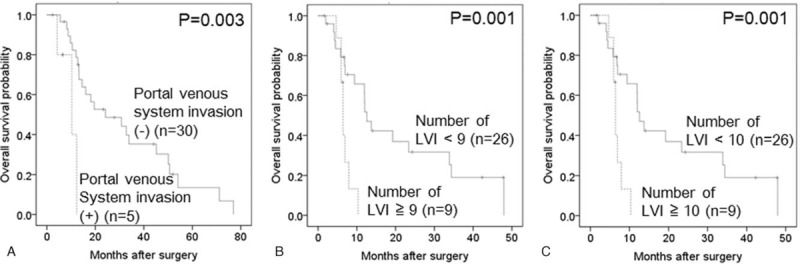
The median disease-free survival time was 12.1 months (95% confidence interval 9.0–15.1 months), and the 5-year disease-free survival rate was 20% in our study cohort of 60 patients. The area under the receiver operating characteristic curve used to analyze the diagnostic performance of the LVI-score for disease-free survival was 0.673 (95% confidence interval 0.530–0.817), and the LVI-score that yielded the maximal sensitivity and specificity was 3; at this threshold, the sensitivity was 0.533, and the specificity was 0.733 (Supplemental Digital Content 1). Kaplan–Meier curves and the log-rank test demonstrated that LN metastasis as well as LVI-scores of ≥5, ≥6, ≥7, ≥8, ≥9 and ≥10 were significantly associated with poor disease-free survival (Supplemental Digital Content 2, Fig. 3). Multivariate Cox regression analysis confirmed that LN metastasis and LVI-scores of ≥7, ≥8, ≥9 or ≥10 were significantly associated with poor disease-free survival (Table 3). Most notably, LVI-scores of ≥9 and ≥10 were as or more significant than LN metastasis based on hazard ratio analysis.
Figure 3.
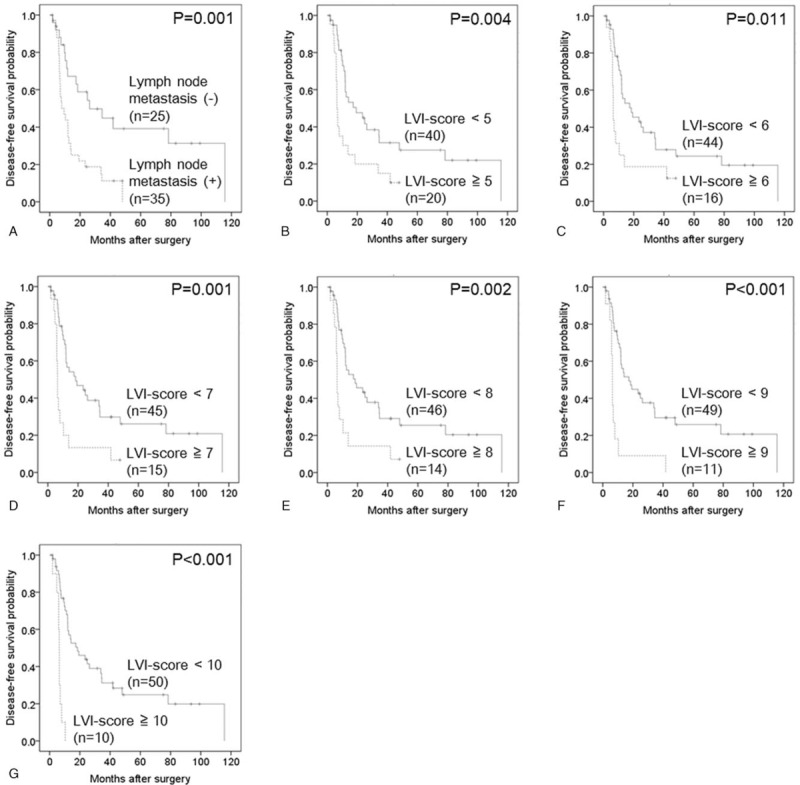
Kaplan–Meier curves showing the disease-free survival of 60 patients with pancreatic head cancer. (A) Lymph node metastasis (−) versus lymph node metastasis (+) (P = .001). (B) Lymphatic vessel invasion (LVI)-score <5 versus LVI-score ≥5 (P = .004). (C) LVI-score <6 versus LVI-score ≥6 (P = .011). (D) LVI-score <7 versus LVI-score ≥7 (P = .001). (E) LVI-score <8 versus LVI-score ≥8 (P = .002). (F) LVI-score <9 versus LVI-score ≥9 (P < .001). (G) LVI-score <10 versus LVI-score ≥10 (P < .001). LVI = lymphatic vessel invasion.
Table 3.
Multivariate Cox regression analysis of disease-free survival in 60 patients with stage II pancreatic head cancer.
In the subgroup analysis, an LVI-score ≥10 was significantly associated with poor disease-free survival in 25 cases of stage IIA disease (pT3N0) (Table 4, Fig. 4), whereas LVI-scores of ≥7, ≥9 and ≥10 were significantly associated with poor disease-free survival in 35 cases of stage IIB disease (pT3N1) (Table 5, Fig. 5). In this subgroup, the area under the receiver operating characteristic curve used to analyze the diagnostic performance of the LVI-score for disease-free survival was 0.66 (95% confidence interval 0.46–0.85), and the LVI-score that yielded the maximal sensitivity and specificity was 2; at this threshold, the sensitivity was 0.375, and the specificity was 0.889 (Supplemental Digital Content 3).
Table 4.
Disease-free survival analysis of 25 patients with stage IIA (pT3N0) pancreatic head cancer.
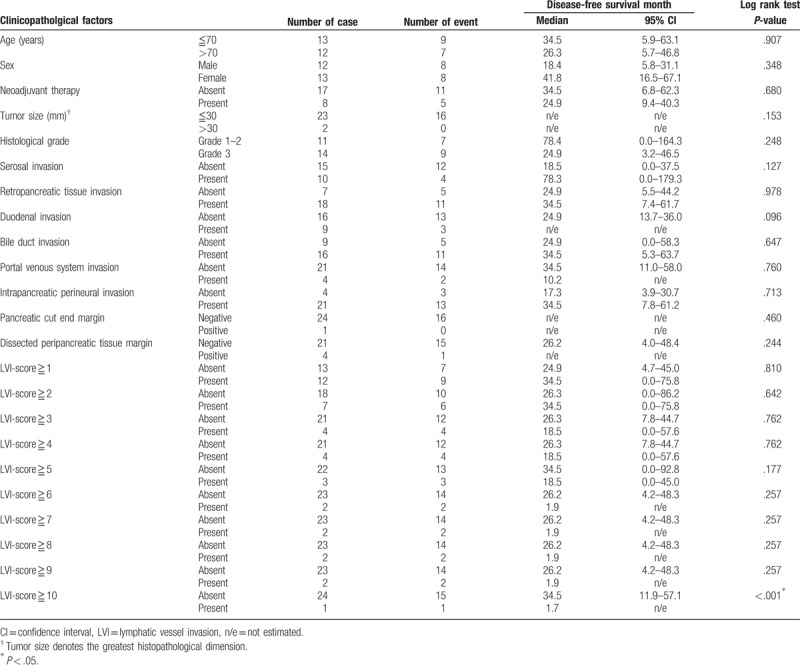
Figure 4.
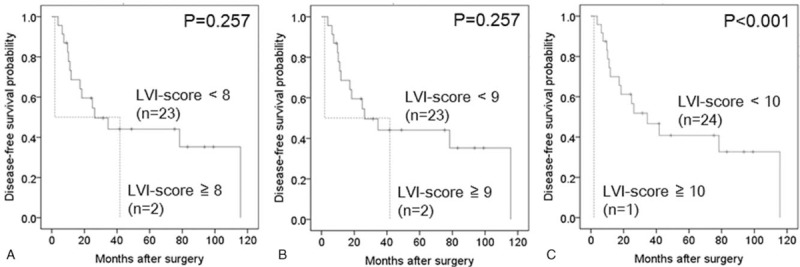
Kaplan–Meier curves depicting the disease-free survival of 25 patients with stage IIA pancreatic head cancer. (A) Lymphatic vessel invasion (LVI)-score <8 versus LVI-score ≥8 (P = .257). (B) LVI-score <9 versus LVI-score ≥9 (P = .257). (C) LVI-score <10 versus LVI-score ≥10 (P < .001). LVI = lymphatic vessel invasion.
Table 5.
Disease-free survival analysis of 35 patients with stage IIB (pT3pN1) pancreatic head cancer.
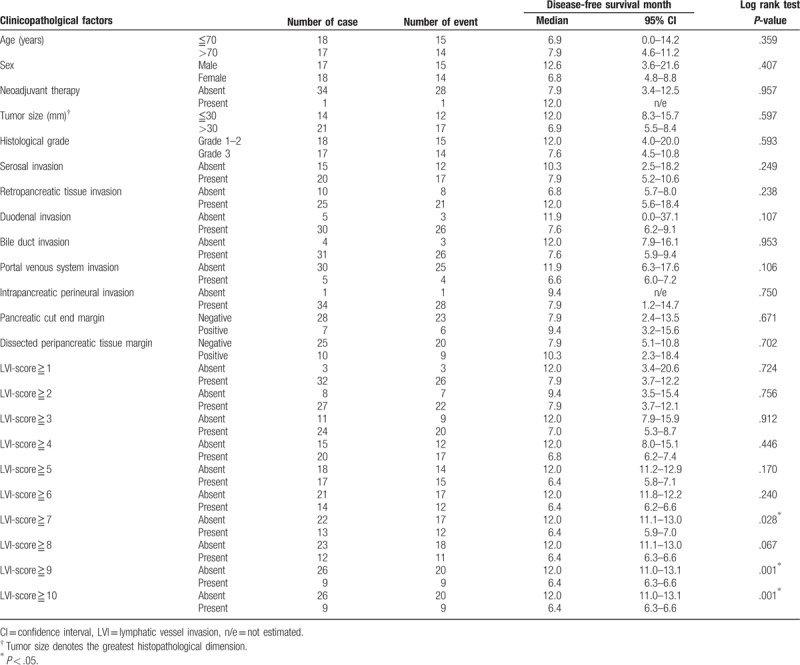
Figure 5.
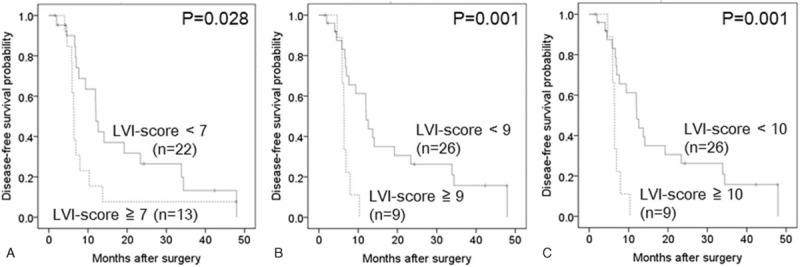
Kaplan–Meier curves depicting the disease-free survival of 35 patients with stage IIB pancreatic head cancer. (A) Lymphatic vessel invasion (LVI)-score <7 versus LVI-score ≥7 (P = .028). (B) LVI-score <9 versus LVI-score ≥9 (P = .001). (C) LVI-score <10 versus LVI-score ≥10 (P = .001). LVI = lymphatic vessel invasion.
3.4. Overall survival analysis
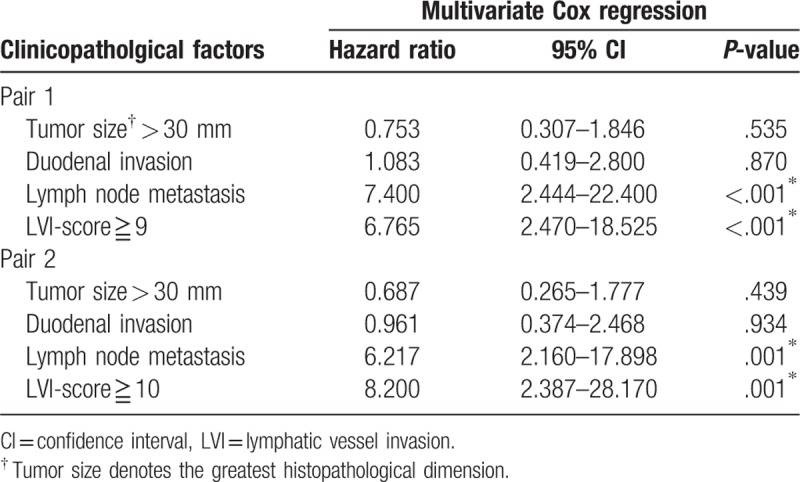
The median overall survival time was 50.1 months (95% confidence interval 28.9–71.3 months), and the 5-year overall survival rate was 34.7% in our study cohort consisting of 60 patients. The area under the receiver operating characteristic curve used to analyze the diagnostic performance of the LVI-score for predicting overall survival was 0.710 (95% confidence interval 0.580–0.842), and the LVI-score that yielded the maximal sensitivity and specificity was 3; at this threshold, the sensitivity was 0.656, and the specificity was 0.750 (Supplemental Digital Content 4). Kaplan–Meier curves and the log-rank test demonstrated that tumor size >30 mm; duodenal invasion; LN metastasis; and LVI-scores of ≥2, ≥3, ≥4, ≥5, ≥6, ≥7, ≥8, ≥9 and ≥10 were significantly associated with poor overall survival (Supplemental Digital Content 4, Fig. 6). Multivariate Cox regression analysis confirmed that LN metastasis and LVI-scores ≥9 or ≥10 were significantly associated with poor overall survival (Table 6). Most notably, an LVI-score ≥10 was as or more significant than LN metastasis based on hazard ratio analysis.
Figure 6.
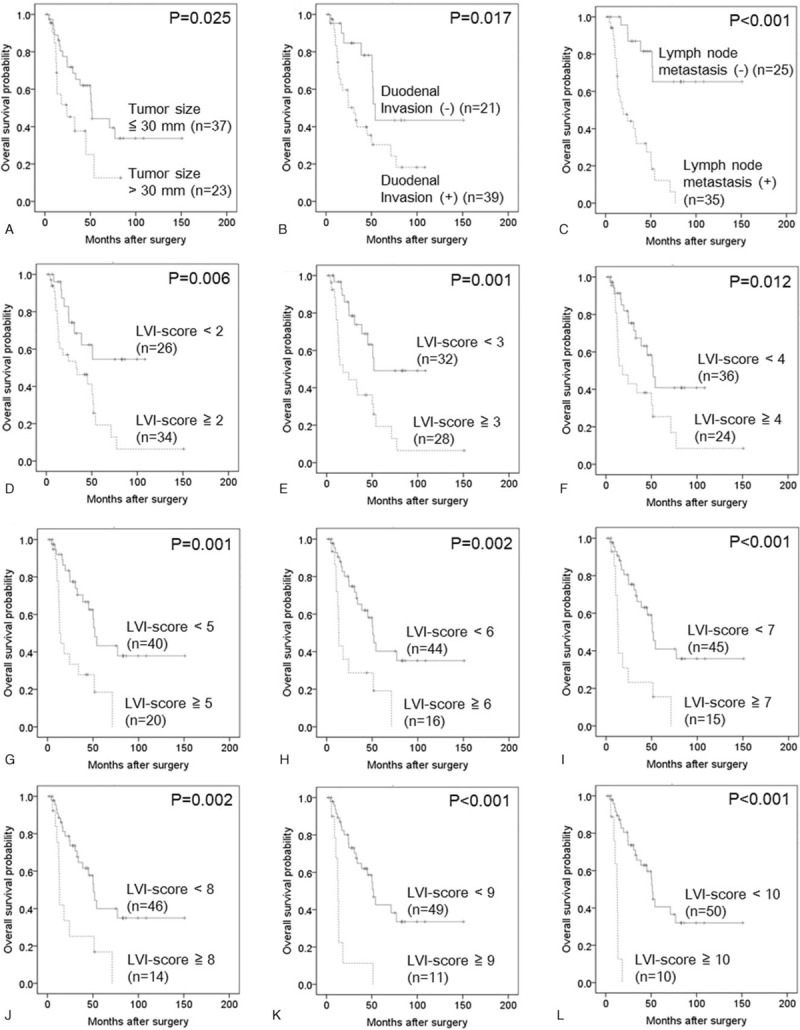
Kaplan–Meier curves depicting the overall survival of 60 patients with pancreatic head cancer. (A) Tumor size ≤30 mm versus tumor size >30 mm (P = .025). (B) Duodenal invasion (−) versus duodenal invasion (+) (P = .017). (C) Lymph node metastasis (−) versus lymph node metastasis (+) (P < .001). (D) Lymphatic vessel invasion (LVI)-score <2 versus LVI-score ≥2 (P = .006). (E) LVI-score <3 versus LVI-score ≥3 (P = .001). (F) LVI-score <4 versus LVI-score ≥4 (P = .012). (G) LVI-score <5 versus LVI-score ≥5 (P = .001). (H) LVI-score <6 versus LVI-score ≥6 (P = .002). (I) LVI-score <7 versus LVI-score ≥7 (P < .001). (J) LVI-score <8 versus LVI-score ≥8 (P = .002). (K) LVI-score <9 versus LVI-score ≥9 (P < .001). (L) LVI-score <10 versus LVI-score ≥10 (P < .001). LVI = lymphatic vessel invasion.
Table 6.
Multivariate Cox regression analysis of overall survival in 60 patients with stage II pancreatic head cancer.
In the subgroup analysis, no variable was significantly associated with overall survival in 25 cases of stage IIA disease (pT3N0) (Table 7), whereas portal venous system invasion and LVI-scores ≥9 and ≥10 were significantly associated with poor overall survival in 35 cases of stage IIB disease (pT3N1) (Table 8, Fig. 7). In this subgroup, the area under the receiver operating characteristic curve used to analyze the diagnostic performance of the LVI-score for overall survival was 0.526 (95% confidence interval 0.26–0.80), and the LVI-score that yielded the maximal sensitivity and specificity was 9; at this threshold, the sensitivity was 0.167, and the specificity was 0.947 (Supplemental Digital Content 6).
Table 7.
Overall survival analysis of 25 patients with stage IIA (pT3N0) pancreatic head cancer.
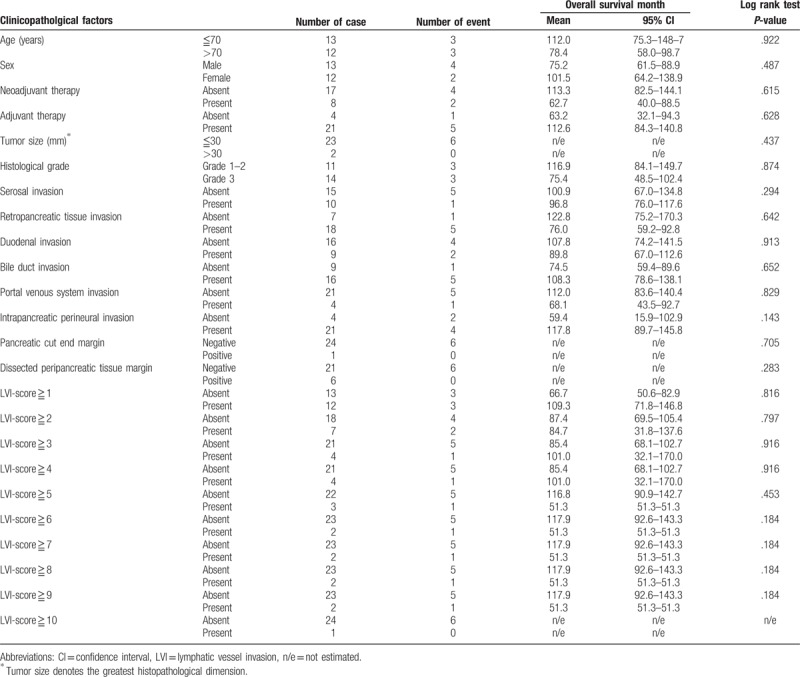
Table 8.
Overall survival analysis of 35 patients with stage IIB (pT3pN1) pancreatic head cancer.
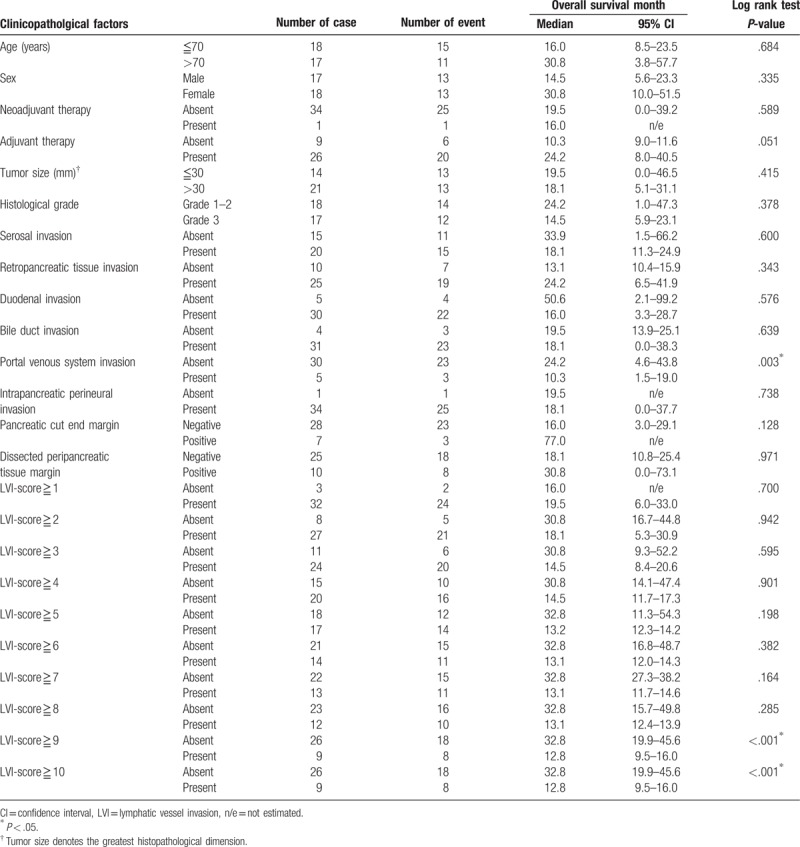
Figure 7.
Kaplan–Meier curves depicting the overall survival of 35 patients with stage IIB pancreatic head cancer. (A) Portal venous system invasion (−) versus portal venous system invasion (+) (P = 0.003). (B) Lymphatic vessel invasion (LVI)-score <9 versus LVI-score ≥9 (P = .001). (C) LVI-score <10 versus LVI-score ≥10 (P = .001). LVI = lymphatic vessel invasion.
4. Discussion
The main finding of this study was that a high degree of lymphatic vessel invasion is an independent prognostic factor for both disease-free and overall survival in patients with locally advanced, surgically resectable pancreatic head cancer. In addition, subgroup analysis based on the presence or absence of LN metastasis demonstrated that a high degree of lymphatic vessel invasion was significantly associated with shorter disease-free survival in patients without LN metastasis (LVI-score ≥10) and those with LN metastasis (LVI-score ≥7, ≥9 and ≥10), whereas a high degree of lymphatic vessel invasion was significantly associated with shorter overall survival exclusively in patients with LN metastasis (LVI-score ≥9 and ≥10).
In routine diagnostic practice, histopathological grading for lymphatic vessel invasion is recommended using a four-tier code described by the Japan Pancreatic Society: ly0 (no evidence of invasion), ly1 (slight invasion), ly2 (moderate invasion), and ly3 (marked invasion).[18] However, this definition is vague and can lead to inter-observer variability. Our approach to assess the degree of lymphatic vessel invasion is simple, objective and inexpensive, requiring the preparation of only a few immunohistochemical slides. Furthermore, the findings are predictive of the prognosis by counting at most 10 invaded lymphatic vessels in each case.
Given that the lymphatic system is a continuum including lymphatic capillaries, collecting lymphatic vessels, intervening LNs and the thoracic duct, LN metastasis itself represents only a part of the lymphogenous spread of cancer.[19,20] The intralymphatic cancer cells observed in the primary lesion can be interpreted as not only a predictor of LN metastasis but also as a result of downstream lymphostasis by lymphogenous spread of cancer outside the primary lesion.[19,20] Therefore, it is reasonable that a high degree of lymphatic vessel invasion is associated with a poor prognosis of pancreatic cancer.
It is interesting to note that lymphatic vessel invasion was more frequently observed in the right and posterior regions of the pancreaticoduodenectomy material than in the left and anterior regions. This finding may be due to the inherent anatomical distribution of the lymphatics. The right region contains duodenal lymphatics, which are well developed through the wall and drain into the downstream lymphatics around the upper para-aortic region.[21,22] The lymphatics in the posterior region are also well developed with a number of collecting lymphatics that drain into the downstream lymphatics around the upper para-aortic region.[21–23]
In the present study, decreased lymphatic vessel invasion was associated with neoadjuvant therapy. Seven of the 9 patients receiving neoadjuvant therapy exhibited no lymphatic vessel invasion. Of these, 4 patients underwent radiotherapy combined with gemcitabine chemotherapy, and 3 patients underwent radiotherapy combined with tegafur. Although the mechanism remains to be elucidated, we hypothesize that chemoradiotherapy plays a key role in damaging both cancer-associated, small-sized lymphatic vessels and intralymphatic cancer cells.[24–27]
The present study has several limitations. First, only the lymphatic vessels that were strongly and continuously positive for D2-40 by immunohistochemistry were assessed. However, D2-40 expression could be weakened in certain pathological conditions or in a large-sized collecting lymphatic vessel, and therefore we could have potentially overlooked some invaded lymphatic vessels. To ensure accuracy, use of other lymphatic endothelial markers, such as prox1, may be useful.[28] Second, only a single pathologist assessed the lymphatic vessel invasion, which could be a source of bias. However, the pathologist who assessed lymphatic vessel invasion was board-certified with 10 years of experience in tumor pathology; therefore, we believe that the degree of measurement error due to the rater is within acceptable levels. Third, this study was performed using a relatively small number of cases of stage II pancreatic head cancer in a single institution; therefore, it might be difficult to generalize our results. For example, we found that the difference in lymphatic vessel invasion frequency was significantly different between the neoadjuvant and non-neoadjuvant groups, but we could not demonstrate for a significant difference in the survival analysis between the groups, likely due to the small number of cases analyzed, which is inconsistent with the previously reported result.[29] Besides, in the Stage IIA (pT3N0) subgroup analyses using the area under the receiver operating characteristic curve, statistically significant associations could not been observed between the LVI-score and prognoses probably due to a low statistical power inherent to a small sample size, although the disease-free survival approached significance (the lower limit of 95% confidence interval was 0.46). However, to our knowledge, this study is the first to attempt to investigate the relationship between the degree of lymphatic vessel invasion and the prognosis of pancreatic cancer. Further comprehensive, large-scale surveys are needed to elucidate whether the LVI-score can be a robust prognostic predictor in pancreatic cancer patients without LN metastasis.
It can be assumed that some tumors may be larger than others; thus, the number of invaded lymphatic vessels should be stated per unit area. However, measurement of the number of invaded lymphatic vessels per unit area of the tumor is not feasible due to the following reasons:
-
(1)
demarcation of the tumor is often difficult in pancreatic cancer, which almost always contains a considerable degree of fibrous stromal elements, thereby leading to measurement uncertainty;
-
(2)
lymphatic vessel invasion is not exclusively observed in the tumor;
lymphatic vessel invasion was actually often found in the duodenum or other extrapancreatic tissues apart from the tumor in the present case series, thereby leading to the loss of potentially important information about lymphatic vessel invasion as a whole. Furthermore, the correlation between the tumor size and the number of invaded lymphatic vessels was not significant in the present study, indicating that tumor sizes did not necessarily influence the number of invaded lymphatic vessels. We suggest that our methodology used in the present study can be applied for day-to-day clinical practice, and this approach may provide further insights into the development of lymphogenous metastasis of pancreatic cancer.
In conclusion, the present study suggests that a high degree of lymphatic vessel invasion is associated with a poor prognosis in patients with locally advanced, surgically resectable pancreatic head cancer. Further elucidation of the lymphatic pathology will help to stratify pancreatic cancer patients and establish future therapeutic strategies.
Author contributions
Conceptualization: Kohei Morita and Hisashi Oshiro.
Data curation: Kohei Morita, Hisashi Oshiro, Kumiko Mito, Mio Tamba, Toshiro Niki, Atsushi Miki, Masaru Koizumi, Yasunaru Sakuma, Toshihide Komatsubara, Naohiro Sata, and Noriyoshi Fukushima.
Formal analysis: Kohei Morita, Hisashi Oshiro, Kumiko Mito, Makiko Naka Mieno, Mio Tamba, and Noriyoshi Fukushima.
Funding acquisition: Kohei Morita, Hisashi Oshiro, and Noriyoshi Fukushima.
Investigation: Kohei Morita, Hisashi Oshiro, Kumiko Mito, Makiko Naka Mieno, Mio Tamba, Atsushi Miki, Masaru Koizumi, Yasunaru Sakuma, Toshihide Komatsubara, Naohiro Sata, and Noriyoshi Fukushima.
Methodology: Kohei Morita, Hisashi Oshiro, Makiko Naka Mieno, and Noriyoshi Fukushima.
Project administration: Kohei Morita, Hisashi Oshiro, and Noriyoshi Fukushima.
Resources: Kohei Morita, Hisashi Oshiro, Kumiko Mito, Toshiro Niki, Masaru Koizumi, Toshihide Komatsubara, Naohiro Sata, and Noriyoshi Fukushima.
Software: Kohei Morita and Hisashi Oshiro.
Supervision: Kohei Morita, Hisashi Oshiro, Makiko Naka Mieno, Toshiro Niki, Masaru Koizumi, Yasunaru Sakuma, Toshihide Komatsubara, Naohiro Sata, and Noriyoshi Fukushima.
Validation: Kohei Morita, Hisashi Oshiro, Makiko Naka Mieno, Toshiro Niki, Atsushi Miki, Naohiro Sata, and Noriyoshi Fukushima.
Visualization: Kohei Morita and Hisashi Oshiro.
Writing – original draft: Kohei Morita and Hisashi Oshiro.
Writing – review & editing: Kohei Morita and Hisashi Oshiro.
Supplementary Material
Footnotes
Abbreviations: LN = lymph node, LVI-score = the lymphatic vessel invasion score.
KM and HO contributed equally to this work.
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 26460425 to N.F. and No. 16K11158 to H.O.).
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system. Lyon, France: World Health Organization; 2010. [Google Scholar]
- [3].Sirri E, Castro FA, Kieschke J, et al. Recent trends in survival of patients with pancreatic cancer in Germany and the United States. Pancreas 2016;45:908–14. [DOI] [PubMed] [Google Scholar]
- [4].Hirata K, Sato T, Mukaiya M, et al. Results of 1001 pancreatic resections for invasive ductal adenocarcinoma of the pancreas. Arch Surg 1997;132:771–6. discussion 777. [DOI] [PubMed] [Google Scholar]
- [5].House MG, Gonen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg 2007;11:1549–55. [DOI] [PubMed] [Google Scholar]
- [6].Kuhn Y, Koscielny A, Glowka T, et al. Postresection survival outcomes of pancreatic cancer according to demographic factors and socio-economic status. Eur J Surg Oncol 2010;36:496–500. [DOI] [PubMed] [Google Scholar]
- [7].Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 2006;10:1199–210. discussion 1210-1191. [DOI] [PubMed] [Google Scholar]
- [8].Elshaer M, Gravante G, Kosmin M, et al. A systematic review of the prognostic value of lymph node ratio, number of positive nodes and total nodes examined in pancreatic ductal adenocarcinoma. Ann R Coll Surg Engl 2017;99:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Basturk O, Saka B, Balci S, et al. Substaging of lymph node status in resected pancreatic ductal adenocarcinoma has strong prognostic correlations: proposal for a revised N classification for TNM staging. Anna Surg Oncol 2015;22:1187–95. [DOI] [PubMed] [Google Scholar]
- [10].Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional validation study of the american joint commission on cancer (8th Edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg 2017;265:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Warschkow R, Widmann B, Beutner U, et al. The more the better-lower rate of stage migration and better survival in patients with retrieval of 20 or more regional lymph nodes in pancreatic cancer: a population-based propensity score matched and trend SEER analysis. Pancreas 2017;46:648–57. [DOI] [PubMed] [Google Scholar]
- [12].Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest 2014;124:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Helm J, Centeno BA, Coppola D, et al. Histologic characteristics enhance predictive value of American Joint Committee on cancer staging in resectable pancreas cancer. Cancer 2009;115:4080–9. [DOI] [PubMed] [Google Scholar]
- [14].Shimada K, Nara S, Esaki M, et al. Intrapancreatic nerve invasion as a predictor for recurrence after pancreaticoduodenectomy in patients with invasive ductal carcinoma of the pancreas. Pancreas 2011;40:464–8. [DOI] [PubMed] [Google Scholar]
- [15].John Wiley & Sons, Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 2011. [Google Scholar]
- [16].Punt CJ, Buyse M, Kohne CH, et al. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst 2007. 99. [DOI] [PubMed] [Google Scholar]
- [17].Kanda Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone marrow transplantation 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Japan-Pancreas-Society Classification of pancreatic carcinoma, 4th English ed. Tokyo, Japan: Kanehara & Co., Ltd.; 2017. [Google Scholar]
- [19].Oshiro H, Osaka Y, Tachibana S, et al. Retrograde lymphatic spread of esophageal cancer: a case report. Medicine (Baltimore) 2015;94:e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nagai T, Oshiro H, Sagawa Y, et al. Pathological characterization of ovarian cancer patients who underwent debulking surgery in combination with diaphragmatic surgery: a cross-sectional study. Medicine (Baltimore) 2015;94:e2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sato T. Ohtani O, Kato S, Uchino S. Topographical anatomy of the lymphatic system. Abdomen, pelvis and lower extremities. Lymphatics: Nishimura-shoten. Japan: Niigata; 1997. 274–85. [Google Scholar]
- [22].Deki H, Sato T. Topographical anatomy required for pancreatic surgery. Shujutsu 1992;46:1337–47. [Google Scholar]
- [23].Jin G, Sugiyama M, Tuo H, et al. Distribution of lymphatic vessels in the neural plexuses surrounding the superior mesenteric artery. Pancreas 2006;32:62–6. [DOI] [PubMed] [Google Scholar]
- [24].Shi Y, Tong M, Wu Y, et al. VEGF-C ShRNA inhibits pancreatic cancer growth and lymphangiogenesis in an orthotopic fluorescent nude mouse model. Anticancer Res 2013;33:409–17. [PubMed] [Google Scholar]
- [25].Russell NS, Floot B, van Werkhoven E, et al. Blood and lymphatic microvessel damage in irradiated human skin: The role of TGF-beta, endoglin and macrophages. Radiother Oncol 2015;116:455–61. [DOI] [PubMed] [Google Scholar]
- [26].Leroi N, Lallemand F, Coucke P, et al. Impacts of Ionizing Radiation on the Different Compartments of the Tumor Microenvironment. Front Pharmacol 2016;7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhan HX, Xu JW, Wu D, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med 2017;6:1201–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Castro EC, Galambos C. Prox-1 and VEGFR3 antibodies are superior to D2-40 in identifying endothelial cells of lymphatic malformations–a proposal of a new immunohistochemical panel to differentiate lymphatic from other vascular malformations. Pediatr Dev Pathol 2009;12:187–94. [DOI] [PubMed] [Google Scholar]
- [29].Nipp RD, Zanconato A, Zheng H, et al. Predictors of early mortality after surgical resection of pancreatic adenocarcinoma in the era of neoadjuvant treatment. Pancreas 2017;46:183–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


