Supplemental Digital Content is available in the text
Keywords: Helicobacter pylori, meta-analysis, sicca syndrome, Sjögren syndrome
Abstract
Background:
Helicobacter pylori has been proved as a risk factor of many diseases. There are some researches trying to find connection between H. pylori and Sjögren syndrome (SS). However, the conclusions of these studies are controversial. We conducted this meta-analysis to evaluate the association between H. pylori and SS.
Methods:
We searched PubMed and Embase databases for researches which include the data of H. pylori infection rate in SS and control groups. A fixed-effects model was used to analyze the risk odds ratio (OR) with 95% confidence intervals (CIs) according to the heterogeneity across the selected studies.
Results:
Nine studies with 1958 participants including 619 patients with SS met the inclusion criteria. The total infection rate of H. pylori was 53.83% (1054/1958). We found that the patients with SS had a significantly higher H. pylori infection rate than control groups (OR = 1.19, 95% CI: 1.01–1.41, P = .033). Subgroup analysis demonstrated a significantly higher H. pylori infection rate in patients with primary SS than controls (OR = 1.24, 95% CI: 1.03–1.50, P = .026).
Conclusion:
This meta-analysis is the 1st meta-analysis about the association between H. pylori and SS. The pooled data suggested a significantly higher H. pylori infection rate in patients with SS. More prospective or multicenter retrospective researches could be conducted in the future.
1. Introduction
Helicobacter pylori is a widely prevalent bacterium, and its infection rate in population ranges from 28.3% to 98.6% in different areas over the world, which is especially higher in less developed countries.[1]Helicobacter pylori has been proved to be a risk factor of many diseases, including not only some gastrointestinal-related diseases like chronic atrophic gastritis,[2] gastric mucosa-associated lymphoid tissue (MALT) lymphoma,[3] peptic ulcer disease,[4] but also other systemic diseases like immune thrombocytopenic purpura,[5] and diabetes mellitus.[6] As a systemic autoimmune disease, Sjögren syndrome (SS) was reported to have a link with H. pylori infection by some researchers,[7–10] which characterized by lymphocytic infiltration and destruction of exocrine glands.[11] This phenomenon may result from bacterial induced autoimmune response, and H. pylori is one of commonly known infectious factors that trigger the autoimmune reactions. However, some studies found there were no significant differences of H. pylori infection rate between SS and control group.[12–16] Therefore, whether H. pylori infection is a risk factor of SS remains controversial.
We performed this meta-analysis to have a better understanding of whether the relation between H. pylori and SS existed. The association between the 2 diseases may be of great value for patients with SS with gastrointestinal diseases to receive more reasonable treatment.
2. Methods
2.1. Inclusion criteria
-
1.
Study design is a case–control study.
-
2.
Provision of raw data on H. pylori infection in SS group and control group.
-
3.
H. pylori infection was confirmed with at least 1 positive result by either mucosal biopsy, enzyme-linked immunosorbent assay (ELISA) or 13C-urea breathe test (13C-UBT).
-
4.
Studies written in English language.
2.2. Exclusion criteria
-
1.
Case report or observational researches without control group.
-
2.
The H. pylori infection raw data in SS group or in control group cannot be fully available.
-
3.
Papers written by the same authors.
-
4.
Animal studies only.
-
5.
The participants in studies received H. pylori eradication treatment including H2 blockers, proton pump inhibitors, or antibiotic drugs within 4 weeks.
2.3. Literature search
This meta-analysis was made by following the PRISMA guidelines. Two investigators (CQQ and XYZ) searched the PubMed and Embase databases from database inception to May 27, 2018 with a systemic literature search strategy. The keywords we used were included in supplementary data file. The 2 authors performed the search and repeated several times in different medical science information centers affiliated to Nanjing Medical University at different times independently. The full texts of the relevant papers in English were reviewed by the 2 investigators. The reference lists of the relevant papers or systemic reviews previously published were also taken into consideration for additional search.
2.4. Data extraction
Data extraction was conducted by the 2 authors (CQQ and XYZ) independently. We reviewed and collected the information including the 1st author(s), year of publication, country or area of study, study design type, way of diagnosis, amount of study subjects, population of H. pylori infection in SS and control group from the selected studies. We only included 1 study if the same raw data were published in different studies. The raw data of ELISA would be collected if the study contained more than 1 detection method.
2.5. Statistical analysis
We used Chi-squared test and I2 test to measure the heterogeneity among studies. There was no significant heterogeneity in this study when P > .1 and I2 < 50%. The Mantel–Haenszel fixed-effects model was applied to evaluate odds ratio (OR) and 95% confidence interval (CI) in this meta-analysis if heterogeneity was no significant, or a random-effect model was applied.[17] Publication bias was assessed by using funnel plots as a qualitative analysis, Egger linear regression test (Egger test) and Begg rank correlation test (Begg test) as quantitative analysis.[18,19]P-values <.05 of all tests in this article were statistically significant. We conducted statistical analysis with the STATA 12.0 (2000; STATA Corp, College Station, TX). No approval of ethics was needed because all studies included in this meta-analysis were published previously.
3. Results
3.1. Characteristics of the studies
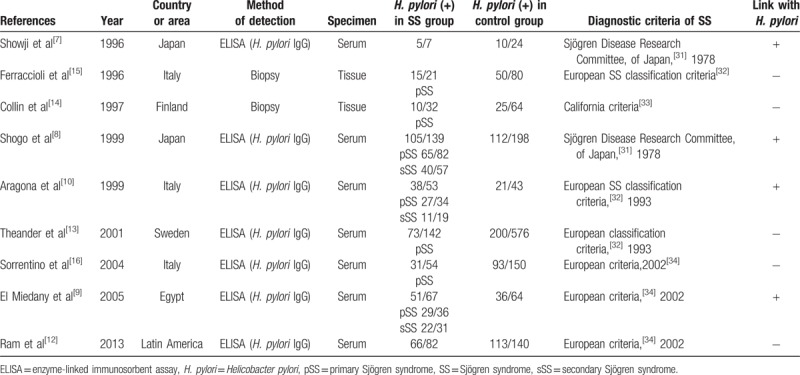
We totally included 9 studies which met the criteria in this meta-analysis. Table 1 showed the characteristics of the studies we included. The process of literature search and identification are shown in PRISMA Flow Diagram. There are 1958 participants including 619 patients with SS and 1339 control patients in these selected studies. The total prevalence rate of H. pylori was 53.83% (1054/1958), of which the SS group was 63.65% (394/614) and the control group was 49.29% (660/1339). About 68.89% (423/614) primary SS (pSS) and 17.43% (107/614) patients were reported in 7 studies. Two of the selected studies did not mention the type of patients with SS. The publication year of these studies ranged from 1996 to 2013. Participants from different countries or areas (5 from Europe, 2 from Japan, 1 from Latin America, and 1 from Egypt) were involved in the selected studies. Of the nine studies, 7 studies used ELISA as the confirmation way of H. pylori infection and the others used tissue biopsy. The positive correlation between H. pylori and SS was claimed to be found in 5 selected studies while no positive correlation was found in the other 4.
Table 1.
Main characteristics of the studies included in this meta-analysis on H. pylori in SS and non-SS controls.
3.2. Subgroup analysis
We found a small but significantly higher H. pylori infection rate in patients with SS than that in controls from the forest plot (Fig. 1) of overall meta-analysis (OR 1.19, 95% CI 1.01–1.41, P = .033 < .05). Though the heterogeneity was low (I2 = 0), subgroup analysis was made since there are 2 different types of SS (pSS and secondary SS). We excluded 2 literatures where the type of SS was unclear. The association still existed in patients with pSS according to the forest plot, in which OR was 1.24, 95% CI 1.03–1.50, P = .026 < .05 (Fig. 2). The patients with pSS had a more likely higher H. pylori infection rate than controls. However, no significant difference was found when comparing secondary SS (sSS) with controls after analysis of 3 literatures with mentioned sSS population (OR 1.24, 95% CI 0.87–1.76, P = .238) (Fig. 3) We also conducted a subgroup analysis according to the different detection ways of H. pylori infection. The patients with SS had a higher H. pylori infection rate than controls by using ELISA to confirm infection (OR 1.22, 95% CI 1.03–1.44, P = .024) (Fig. 4). The other 2 studies did not show significantly difference between SS and controls with tissues biopsy conformation.
Figure 1.
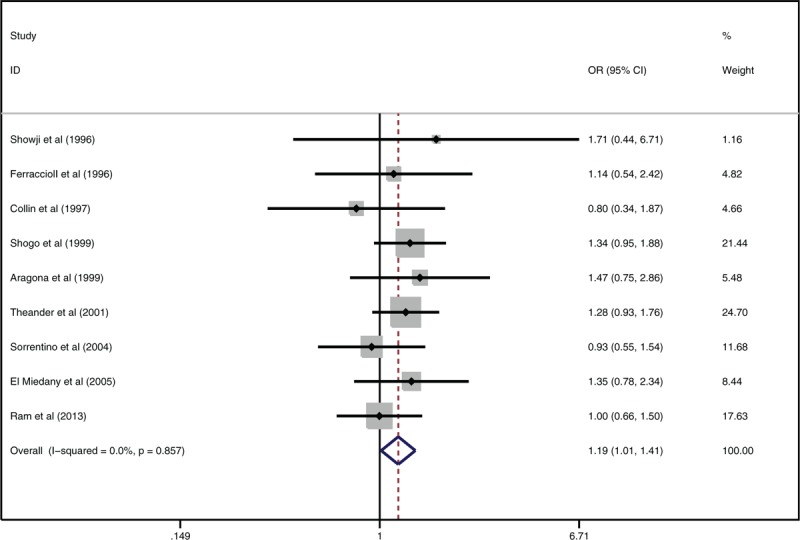
Forest plot about association between Helicobacter pylori infection and Sjögren syndrome.
Figure 2.

Meta-analysis of primary Sjögren syndrome groups of the selected studies.
Figure 3.

Meta-analysis of secondary Sjögren syndrome groups of the selected studies.
Figure 4.

subgroup analysis according to the different detection ways of Helicobacter pylori infection.
3.3. Evaluation of publication bias
From the funnel plot, we found no publication bias (Fig. 5). Egger test and Begg test did not show any publication bias (Egger test: P = .76 > .05, 95% CI: −1.79 to −1.38 [Fig. 6]; Begg test: z = 0.10 <1.96, P = .917 > .05 continuity corrected [Fig. 7]).
Figure 5.

Funnel plot of publication bias.
Figure 6.
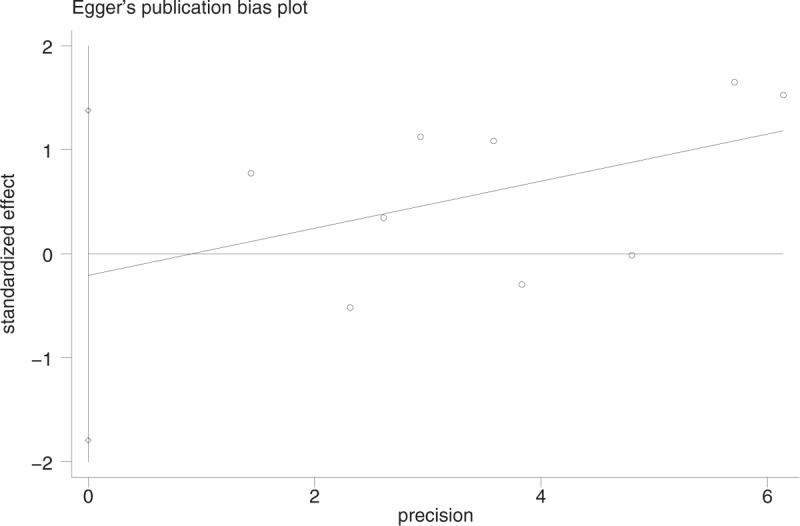
Publication bias with Egger test.
Figure 7.
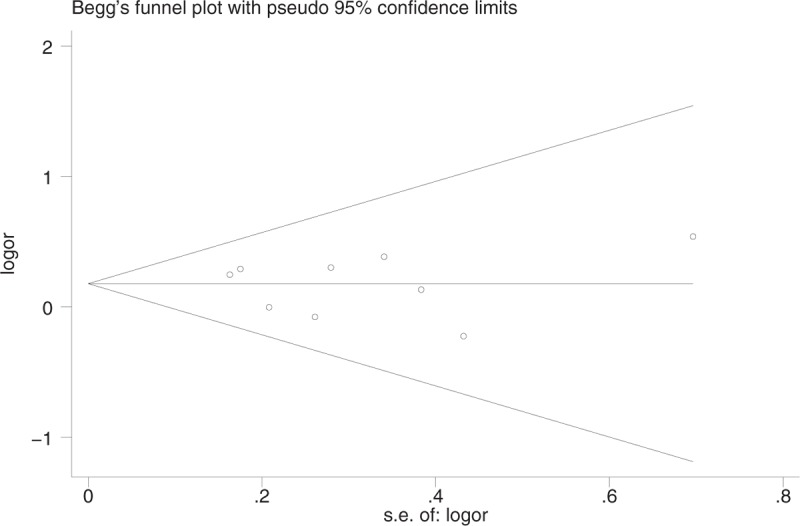
Publication bias with Begg test.
4. Discussion
We found a small but significant higher H. pylori infection in SS than controls from this meta-analysis. As we know, it was the 1st meta-analysis about the association between H. pylori and SS. Some assumptions were proposed to explain this association. Firstly, H. pylori infection has been proved to play an important role in the pathogenesis of many autoimmunity diseases, and similar mechanisms may also exist in SS. As antigens, H. pylori or other microbes induce immune response in human body. Some investigators found a heat shock protein of 60 kDa (HSP60) produced by H. pylori induced the activation of human lymphocytes by molecular mimicry as a component of H. pylori antigens, which could damage the immunologic tolerance of human body because of the protein's homology between human and microbes.[10,20] The significant higher prevalence rate of anti-HSP60 in patients with SS reported by Aragona et al[10] might demonstrate the role of HSP60 in the pathogenesis of SS. The correlation between H. pylori and SS may provide a new explanation of many interesting phenomena occurring in patients with SS clinically and histologically. It was reported that patients with SS have more possibilities of chronic gastritis,[21] which H. pylori infection has been widely considered as a main causative factor.[2] Moreover, the histologic manifestation of lymphocytic infiltration in gastric mucosa is similar to salivary glands in patients with SS.[22] Researchers tried most to explain the fact that patients with SS had a higher risk of development to lymphoma, and most of lymphoma following SS are MALT lymphoma.[23] As is well known, gastric MALT lymphoma is strongly correlated with H. pylori infection,[24] and eradication therapy will induce regression of the lymphoma.[25] Although the MALT lymphoma along with SS was mainly found in silvery glands[26] as a result of routine salivary glands biopsy for diagnosis according to the SS classification criteria,[27] regression of lymphoma was actually found after these patients with SS with gastric H. pylori infection received eradication therapy in some case reports.[28,29] Based on the results of our above study, it is likely that H. pylori infection would be a risk factor of SS-associated MALT lymphoma. H. pylori eradication might be effective as a prevention of lymphoma in patients with SS. More studies are needed to be conducted in this area.
4.1. Type of SS
In this meta-analysis, we found a significant higher H. pylori infection rate in patients with pSS than controls but nonsignificant in patients with sSS. Due to the lack of data or obfuscation of SS type, only three selected studies with 104 patients were included in sSS group analysis, while seven studies with 423 patients in pSS group. The unneglected gap of population may affect the meta-analysis results of sSS between pSS and sSS. Also, the components difference of primary diseases per se was possible to contribute to the different results though the association between these primary diseases (mainly other autoimmune diseases) and H. pylori are inconclusive.[20]
4.2. Ways of H. pylori testing
There were significant differences between SS group and controls by serologic test but such difference did not exist by tissue biopsy in our subgroup analysis. As only 2 studies were included, it was reasonable to believe that the difference was a result of small number of samples. Although serologic test was considered highly accurate and specific in the recent guidelines,[30] the 13C-UBT was regarded as the best recommended noninvasive way to diagnose H. pylori infection in recent guidelines.[30] However, none of the studies included in this meta-analysis used 13C-UBT to confirm H. pylori infection. Therefore, it is necessary to conduct studies by 13C-UBT to compare results among different methods of detection.
4.3. Limitations of the study
Firstly, the number of selected studies or participants was relatively small. Secondly, the diagnostic criteria of SS varied in details because of unavoidable limitation of areas and times.[31–34] Thirdly, the control groups were selected differently across studies included. Fourthly, geographical and socioeconomic difference in H. pylori infection may have an impact on the results. Lastly, exclusion of non-English in the meta-analysis could lead to statistically bias.
5. Conclusion
This meta-analysis was the 1st meta-analysis about the association between H. pylori and SS. And the pooled data suggested a significantly higher H. pylori infection rate in patients with SS. More prospective or multicenter retrospective researches could be conducted in the future.
Acknowledgment
The authors thank Dr Xinmin Si for valuable advice and support in spirit during the preparation of this manuscript.
Author contributions
Data curation: Qianqian Chen, Xiaoying Zhou.
Formal analysis: Qianqian Chen, Wenfeng Tan.
Investigation: Qianqian Chen, Xiaoying Zhou.
Methodology: Wenfeng Tan, Miaojia Zhang.
Project administration: Miaojia Zhang.
Software: Xiaoying Zhou, Wenfeng Tan.
Supervision: Miaojia Zhang.
Writing – original draft: Qianqian Chen, Xiaoying Zhou.
Writing – review & editing: Qianqian Chen, Xiaoying Zhou, Miaojia Zhang.
Miaojia Zhang orcid: 0000-0002-1056-3152.
Supplementary Material
Footnotes
Abbreviations: 13C-UBT = 13C-urea breathe test, CIs = confidence intervals, ELISA = enzyme-linked immunosorbent assay, H. pylori = Helicobacter pylori, HSP60 = heat shock protein of 60 kDa, MALT = mucosa associated lymphoid tissue, OR = odds ratio, pSS = primary Sjögren syndrome, SS = Sjögren syndrome, sSS = secondary Sjögren syndrome.
QC and XZ contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori Infection. Helicobacter 2014;19:1–5. [DOI] [PubMed] [Google Scholar]
- [2].Kuipers EJ, Uyterlinde AM, Pena AS, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet (London, England) 1995;345:1525–8. [DOI] [PubMed] [Google Scholar]
- [3].Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin 2016;66:153–71. [DOI] [PubMed] [Google Scholar]
- [4].Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212–39. [DOI] [PubMed] [Google Scholar]
- [5].Jackson S, Beck PL, Pineo GF, et al. Helicobacter pylori eradication: novel therapy for immune thrombocytopenic purpura? A review of the literature. Am J Hematol 2005;78:142–50. [DOI] [PubMed] [Google Scholar]
- [6].Zhou X, Zhang C, Wu J, et al. Association between Helicobacter pylori infection and diabetes mellitus: a meta-analysis of observational studies. Diabetes Res Clin Pract 2013;99:200–8. [DOI] [PubMed] [Google Scholar]
- [7].Showji Y, Nozawa R, Sato K, et al. Seroprevalence of Helicobacter pylori infection in patients with connective tissue diseases. Microbiol Immunol 1996;40:499–503. [DOI] [PubMed] [Google Scholar]
- [8].Shogo B, Matsumoto Y, Sugiura Y, et al. Seroprevalence of Helicobacter pylori and association with atrophic gastritis in patients with Sjogren's syndrome. Jpn J Rheumatol 1999;9:353–63. [Google Scholar]
- [9].El Miedany YM, Baddour M, Ahmed I, et al. Sjogren's syndrome: concomitant H. pylori infection and possible correlation with clinical parameters. Joint Bone Spine 2005;72:135–41. [DOI] [PubMed] [Google Scholar]
- [10].Aragona P, Magazzu G, Macchia G, et al. Presence of antibodies against Helicobacter pylori and its heat-shock protein 60 in the serum of patients with Sjogren's syndrome. J Rheumatol 1999;26:1306–11. [PubMed] [Google Scholar]
- [11].Fisher BA, Brown RM, Bowman SJ, et al. A review of salivary gland histopathology in primary Sjogren's syndrome with a focus on its potential as a clinical trials biomarker. Ann Rheum Dis 2015;74:1645–50. [DOI] [PubMed] [Google Scholar]
- [12].Ram M, Barzilai O, Shapira Y, et al. Helicobacter pylori serology in autoimmune diseases - fact or fiction? Clin Chem Lab Med 2013;51:1075–82. [DOI] [PubMed] [Google Scholar]
- [13].Theander E, Nilsson I, Manthorpe R, et al. Seroprevalence of Helicobacter pylori in primary Sjogren's syndrome. Clin Exp Rheumatol 2001;19:633–8. [PubMed] [Google Scholar]
- [14].Collin P, Karvonen AL, Korpela M, et al. Gastritis classified in accordance with the Sydney system in patients with primary Sjogren's syndrome. Scand J Gastroenterol 1997;32:108–11. [DOI] [PubMed] [Google Scholar]
- [15].Ferraccioli GF, Sorrentino D, De Vita S, et al. B cell clonality in gastric lymphoid tissues of patients with Sjogren's syndrome. Ann Rheum Dis 1996;55:311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sorrentino D, Faller G, DeVita S, et al. Helicobacter pylori associated antigastric autoantibodies: role in Sjogren's syndrome gastritis. Helicobacter 2004;9:46–53. [DOI] [PubMed] [Google Scholar]
- [17].John Wiley & Sons, Ltd, Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Sharples K, Woodward G, Barclay S, et al. Fixed-Effect Model. Introduction to Meta-Analysis 2009;63–7. [Google Scholar]
- [18].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [20].Smyk DS, Koutsoumpas AL, Mytilinaiou MG, et al. Helicobacter pylori and autoimmune disease: cause or bystander. World J Gastroenterol 2014;20:613–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Popov Y, Salomon-Escoto K. Gastrointestinal and Hepatic Disease in Sjogren Syndrome. Rheum Dis Clin North Am 2018;44:143–51. [DOI] [PubMed] [Google Scholar]
- [22].Kilpi A, Bergroth V, Konttinen YT, et al. Lymphocyte infiltrations of the gastric mucosa in Sjogren's syndrome. An immunoperoxidase study using monoclonal antibodies in the avidin-biotin-peroxidase method. Arthritis Rheum 1983;26:1196–200. [DOI] [PubMed] [Google Scholar]
- [23].Giannouli S, Voulgarelis M. Predicting progression to lymphoma in Sjogren's syndrome patients. Expert Rev Clin Immunol 2014;10:501–12. [DOI] [PubMed] [Google Scholar]
- [24].Pereira MI, Medeiros JA. Role of Helicobacter pylori in gastric mucosa-associated lymphoid tissue lymphomas. World J Gastroenterol 2014;20:684–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ruskone-Fourmestraux A, Fischbach W, Aleman BM, et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut 2011;60:747–58. [DOI] [PubMed] [Google Scholar]
- [26].Schreuder MI, van den Brand M, Hebeda KM, et al. Novel developments in the pathogenesis and diagnosis of extranodal marginal zone lymphoma. J Hematopathol 2017;10:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- [28].Iwai H, Nakamichi N, Nakae K, et al. Parotid mucosa-associated lymphoid tissue lymphoma regression after Helicobacter pylori eradication. Laryngoscope 2009;119:1491–4. [DOI] [PubMed] [Google Scholar]
- [29].Nishimura M, Miyajima S, Okada N. Salivary gland MALT lymphoma associated with Helicobacter pylori infection in a patient with Sjogren's syndrome. J Dermatol 2000;27:450–2. [DOI] [PubMed] [Google Scholar]
- [30].Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/florence consensus report. Gut 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- [31].Ohfuji T. Review on Research Report. Annual Report of the Ministry of Health and Welfare: Sjogren's Disease Research Committee 1978. [Google Scholar]
- [32].Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjogren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum 1993;36:340–7. [DOI] [PubMed] [Google Scholar]
- [33].Fox RI, Robinson C, Curd J, et al. First international symposium on Sjogren's syndrome: suggested criteria for classification. Scand J Rheumatol Suppl 1986;61:28–30. [PubMed] [Google Scholar]
- [34].Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


