Abstract
The average age of hyperuricemia patients has gradually decreased, but young patients with primary hyperuricemia often do not exhibit clinical symptoms and have not received sufficient attention. However, a lack of symptoms with primary hyperuricemia does not mean that high serum uric acid (UA) levels cannot lead to pathological effects, such as oxidative stress and inflammation, and the specific damage is still unclear. We aimed to determine the relationship between oxidative stress and inflammation to explore the possible role of pathological damage in asymptomatic young patients with primary hyperuricemia.
A total of 333 participants were enrolled in our study: 158 asymptomatic young patients with primary hyperuricemia and 175 healthy persons from a health check-up population. Malondialdehyde (MDA), superoxide dismutase (SOD), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and general biochemical markers were measured.
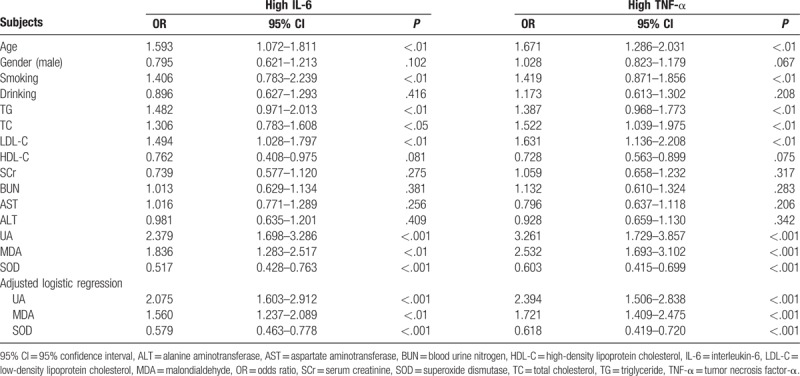
We found no differences in biochemical markers (fasting glucose, TG, TC, LDL-C, HDL-C, SCr, BUN, AST, and ALT) between the patients and healthy persons. Subsequent analyses of oxidative stress and inflammation revealed that the serum levels of MDA, IL-6, and TNF-α in the patients were significantly higher than those in the healthy control group (P < .001), and the SOD activity was significantly lower (P < .001). As the UA levels increased, MDA increased significantly and SOD decreased significantly; likewise, IL-6 and TNF-α increased significantly as the UA level increased. MDA showed a significant positive correlation with IL-6 (r = 0.367, P < .001) and TNF-α (r = 0.319, P < .001), and SOD was negatively correlated with IL-6 (r = −0.241, P < .01) and TNF-α (r = −0.308, P < .001). Multivariable logistic regression analysis showed that UA (OR: 2.379, 95% CI: 1.698–3.286, P < .001; OR: 3.261, 95% CI: 1.729–3.857, P < .001; for IL-6 and TNF-α, respectively) and MDA (OR: 1.836, 95% CI: 1.283–2.517, P < .01; OR: 2.532, 95% CI: 1.693–3.102, P < .001; for IL-6 and TNF-α, respectively) were risk factors for high IL-6 and TNF-α and that SOD (OR: 0.517, 95% CI: 0.428–0.763, P < .01; OR: 0.603, 95% CI: 0.415–0.699, P < .001; for IL-6 and TNF-α, respectively) was a protective factor.
In our study, some abnormal pathological effects were found in asymptomatic young patients with hyperuricemia, suggesting that in young hyperuricemia patients, oxidative stress, inflammation and the inflammatory response may be related to the oxidative stress induced by UA. Therefore, we should pay more attention to the pathological damage caused by these alterations.
Keywords: inflammation, oxidative stress, primary hyperuricemia
1. Introduction
Serum uric acid (UA) is the final product of purine catabolic metabolism in the human body. In cases with an in vivo loss of uricase, which is a biological enzyme that can specifically break down UA, or an impairment in renal excretion of UA, UA levels will increase, leading to hyperuricemia. The incidence of hyperuricemia is increasing gradually worldwide with the improvement in human living standard and dietary structure changes, as well as genetic and environmental factors.[1,2] Epidemiological studies[3–5] at present showed that hyperuricemia, an independent risk factor for the morbidity and mortality of chronic diseases, may be related to obesity, hypertension, diabetes, and metabolic syndrome. Ogura et al[6] studied the relationship between obesity and serum UA and demonstrated a 30% increase in fat as serum UA levels increased 59.5 mol/L. A prospective study[7] among 85,286 patients with hyperuricemia reported that serum UA was positively correlated with systolic and diastolic blood pressure (SBP and DBP, respectively).
Elevated UA can promote the expression of inflammatory proteins by triggering complex proinflammatory cascades that damage cells and tissues.[8] Oxidative stress is considered to play a crucial role in this process. Many studies[9,10] have proved that the oxidative stress induced by UA is similar to a second messenger for inflammation. In previous studies, UA was considered an antioxidant that helps protect the body from damage caused by oxidative stress[11,12]; however, further studies have found that hyperuricemia causes much greater damage to vascular endothelial cells than antioxidants.[13,14]
A pathological consequence of the tendency of excess UA to form crystals is the characteristic alteration resulting in gouty arthritis or UA stones. Doctors and patients typically are concerned only by hyperuricemia with distinct clinical symptoms or complications and usually do not worry about hyperuricemia without clinical signs. However, more recent evidence[15,16] suggested that asymptomatic patients may exhibit inflammation, which can slowly cause pathological harm locally to tissues or systemically. This study focused on young patients who suffer from primary hyperuricemia and have been diagnosed with a health check-up, sought to determine the relationship between oxidative stress and inflammation, and attempted to elucidate a possible pathological mechanism in asymptomatic patients with primary hyperuricemia.
2. Patients and methods
2.1. Patients
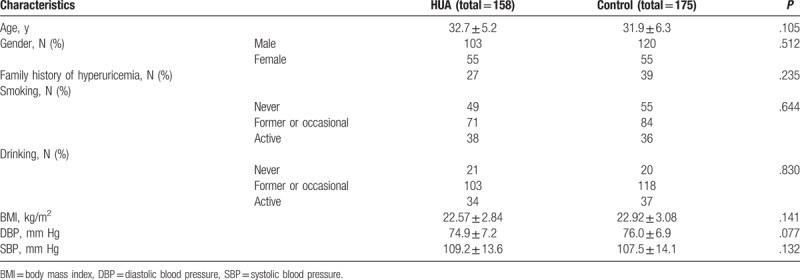
Of 2568 Chinese people aged 18 and 44 years who underwent a routine health examination between January 2015 and December 2016, 196 individuals were diagnosed at Suining Central Hospital, a district hospital covering a region with 5 million people in western China. In this study, a total of 158 participants (HUA group) met the requirement after screening. The inclusion criteria included age 18 to 44 years, hyperuricemia consistent with the diagnostic criteria of serum UA concentrations above 416 and 357 μmol/L for men and women, respectively, and no clinical signs of hyperuricemia, such as gouty arthritis or UA stones. Exclusion criteria included the following: failure to meet the hyperuricemia diagnostic criteria (serum UA concentrations were measured in all participants, with a 1-week interval between every 2 measurements); use of medications that can affect UA concentrations or oxidative stress levels and inflammation in the past 3 months; diagnosis of diabetes, hypertension, liver disease, renal failure, or obesity before this routine health examination; and presence of autoimmune diseases, cancer, or acute infection or trauma that can cause stress responses. In addition, we also excluded individuals who had not undergone a health check-up in the past 3 years to ensure that the physiological indexes of participants had not been affected by a long period of high blood UA. Additionally, 175 healthy persons who received a physical examination at this period time were used as the controls. The HUA group and control group were strictly matched by age. A comparison of the age, gender, weight, smoking, drinking and other data between the 2 groups is shown in Table 1. This study was registered and approved by the Ethics Committee of the Suining Central Hospital, Sichuan province, China. The purpose, procedures and importance of the research were explained to all participants, and written informed consent was obtained before enrollment in this study.
Table 1.
The characteristics of participants.
2.2. Measurement of laboratory parameters
All participants completed questionnaires about smoking, drinking, and family medical history of hyperuricemia to assess general conditions; because these questionnaires involved private features of individuals, the questionnaires should be anonymous. Each participant was required to complete 2 questionnaires by his or herself, and the resulting estimates were the only data about general conditions available to the researchers.
Venous blood (5 mL) was drawn from participants after an overnight fast for more than 12 hours. Then, the blood samples were centrifuged and cryopreserved until measurements were performed. Biochemical indicators were measured using a Hitachi 7600 automatic biochemical analyzer with a turbidimetric immunoassay, enzyme-coupled spectrophotometric assay or oxidase method; the indicators included UA, fasting plasma glucose, TG, LDL-C, HDL-C, SCr, BUN, AST, and ALT. Serum malondialdehyde (MDA) and superoxide dismutase (SOD) activity were measured by the thiobarbituric acid method and the xanthine oxidase method, respectively. The commercial kits were purchased from Biyuntian Biotech Corp (Shang Hai, China). The serum IL-6 and TNF-α levels were measured by ELISA with a kit purchased from Biyuntian Biotech Corp.
All of the biochemical variables were measured in the clinical laboratory department of Suining Central Hospital, the temperature and humidity of the laboratory conformed to the requirements of kit, and all procedures were performed in strict accordance with the kit instructions.
2.3. Statistical analysis
The statistical software Statistical Program for Social Sciences (version 21.0) was used for all statistical analyses, and all data were first analyzed for normality. For patients and healthy persons, the data with a normal distribution are presented as the mean ± standard deviation (SD) and were analyzed with independent Student t test. Analogous data with a nonnormal distribution are presented at the median (25th percentile, 75th percentile) and were analyzed with the Wilcoxon rank-sum test. The data of characteristics are presented as numbers and were analyzed with the Chi-square test.
The statistical analyses for patients with high, moderate, and low UA levels and the data with normal distributions were analyzed with ANOVA, and the data with nonnormal distributions were analyzed with the Kruskal–Wallis test. The relationship between markers of oxidative stress and inflammation was analyzed with Pearson correlation analysis. Risk factors for high IL-6 and high TNF-α levels were further analyzed using multivariable logistic regression analysis and were adjusted for age, smoking, TG, TC, and LDL-C.
A P-value < .05 was considered statistically significant for all statistical results.
3. Results
3.1. Participant characteristics
There were 158 participants in the HUA group and 175 participants in the healthy control group, and we analyzed the characteristics of participants to assess general conditions. There were no significant differences regarding age, gender, family history of hyperuricemia, smoking, drinking, BMI, DBP, and SBP between the 2 groups, as presented in Table 1. We continued to compare the general biochemical parameters between the 2 groups; these parameters can estimate glucose metabolism, lipid metabolism, liver and renal metabolism in participants, as presented in Table 2. The UA levels were 502.4 ± 39.7 μmol/L and 286.9 ± 31.5 μmol/L in the HUA group and the healthy control group, respectively. The parameters other than UA, including fasting glucose, TG, TC, LDL-C, HDL-C, SCr, BUN, AST, and ALT had no significant differences between the 2 groups.
Table 2.
The general biochemical parameters of participants.

3.2. The oxidative stress and inflammation of patients with primary hyperuricemia were higher than those in the healthy control group
MDA is the product of the oxygen radical chain reaction in organisms and can indirectly represent the formation and scavenging of oxygen free radicals in organisms.[17] SOD is an important antioxidant substance in organism and can increase the activity of antioxidant enzymes and eliminate oxygen radicals produced by internal metabolism[18] to protect organisms from oxidative stress damage. In our study, we aimed to explore the oxidative stress and inflammation in patients with hyperuricemia. The MDA levels of the HUA group and healthy control group were 7.55 mmol/mL (5.12–10.87) and 4.16 mmol/mL (1.71–5.52), respectively, with higher values in the HUA group; however, the SOD activity of the healthy control group was significantly higher than that of the HUA group, with values of 926.3 U/L (695.3–1382.7) and 725.4 U/L (565.9–1058.4) respectively. In the present study, IL-6 and TNF-α were used as the marker of inflammation, and we found that the IL-6 and TNF-α levels of the HUA group were significantly higher than those of the healthy control group, with values of 32.78 pg/mL (18.53–39.20) versus 18.63 pg/mL (11.75–26.18) and 41.32 pg/mL (20.65–51.43) versus 25.52 pg/mL (14.93–31.68), respectively (Fig. 1).
Figure 1.
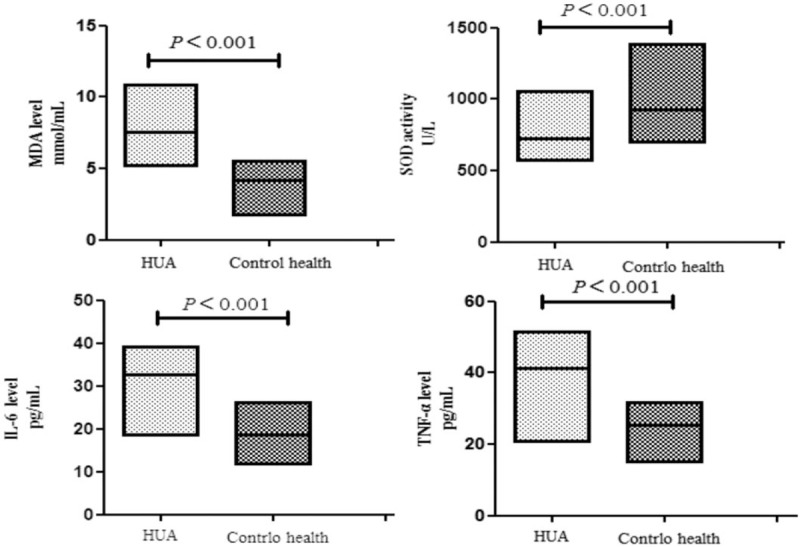
The MAD, SOD, IL-6, and TNF-α levels of hyperuricemia group and health control group, presented at the median (25th percentile, 75th percentile).
3.3. For patients with primary hyperuricemia, increases in UA levels were associated with higher oxidative stress and inflammation
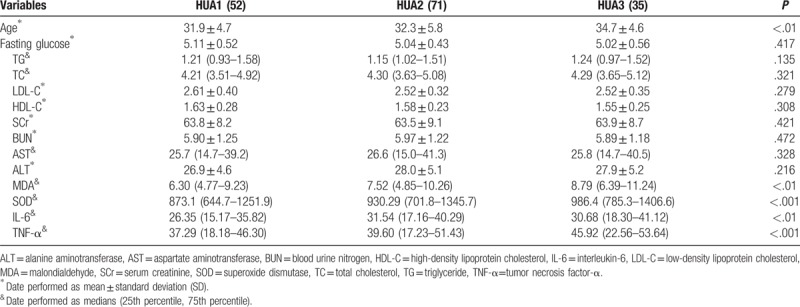
We classified the patients into 3 groups based on their UA levels, HUA1 group (low UA level: 357–460 μmol/L), HUA2 group (moderate UA level: 461–550 μmol/L) and HUA3 group (high UA level: above 551 μmol/L), to investigate the difference in biochemical variables in patients with high, moderate and low UA levels. This study found no significant differences in the general biochemical parameters, including fasting glucose, TG, TC, LDL-C, HDL-C, SCr, BUN, AST, and ALT. Surprisingly, as UA levels increased, MDA increased significantly and SOD decreased significantly; likewise, the IL-6 and TNF-α levels increased significantly as UA levels increased. In addition, the patient age tended to increase with the UA level, and the patient age in the subgroup with high UA levels was significantly higher than those in the subgroups with moderate and low UA levels. All data are shown in Table 3.
Table 3.
The biochemical variables in patients with difference levels uric acid.
3.4. The relationship between oxidative stress and inflammation in patients with primary hyperuricemia
In this study, the relationship between oxidative stress and inflammation was assessed by Pearson correlation analyses, and the results showed a significant positive correlation of MDA with IL-6 (r = 0.367, P < .001) and TNF-α (r = 0.319, P < .001). Furthermore, SOD was significantly negatively correlated with IL-6 (r = −0.241, P < .01) and TNF-α (r = −0.308, P < .001), as presented in Fig. 2.
Figure 2.
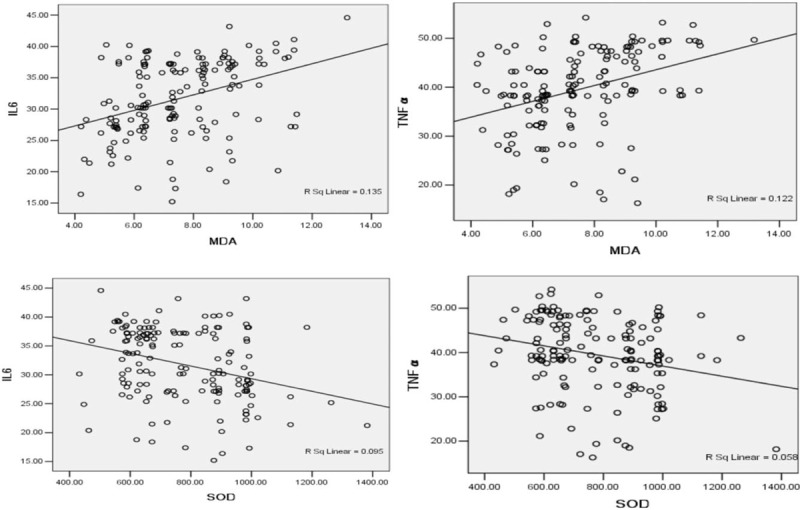
The relationship between MDA and IL-6, relationship between MDA and TNF-α, relationship between SOD and IL-6, relationship between SOD and TNF-α.
3.5. Logistic regression analysis for high IL-6 and high TNF-α
According to the median values of IL-6 and TNF-α, we defined the following groups as dependent variables: high IL-6 (above 32.78 pg/mL) and high TNF-α (above 41.32 pg/mL). We used multivariable logistic regression to analyze the risk factors for high IL-6 and high TNF-α in patients with hyperuricemia. The multivariable logistic regression analysis showed that UA and MDA were risk factors for high IL-6 and TNF-α; in contrast, SOD was a protective factor against high IL-6 and TNF-α. Additionally, age, smoking, TG, TC, and LDL-C were also shown to be risk factors for high IL-6 and TNF-α. To avoid obstruction from other potential confounding factors, UA, MDA, and SOD were used for multivariable logistic regression analysis with a model adjusted for age, smoking, TG, TC, and LDL-C. Importantly, UA and MDA were still risk factors for IL-6 and TNF-α, and SOD was still a protective factor. Thus, the regression analysis results demonstrate that UA and UA-induced oxidative stress may influence elements of inflammation in patients with primary hyperuricemia, as presented in Table 4.
Table 4.
The multivariable logistic regression analysis for IL-6 and TNF-α.
4. Discussion
In our present study, we found that asymptomatic young patients with primary hyperuricemia did not differ from healthy persons in terms of general biochemical parameters; however, the patients had significantly higher oxidative stress and inflammation than healthy persons. Moreover, patients with elevated UA levels had higher oxidative stress and inflammation, and a relationship between oxidative stress and inflammation was observed. Our further analysis showed that UA and UA-induced oxidative stress may be risk factors for inflammation in patients with primary hyperuricemia.
We generally consider that young persons constitute one of multiple populations at risk for primary hyperuricemia[19] and a recent report by Reschke et al[20] indicated an increased incidence of hyperuricemia among adults aged 3 to 19 years. The most common clinical symptom of hyperuricemia is gouty arthritis, so the majority of patients are treated; furthermore, hyperuricemia may also cause cardiovascular disease, cerebrovascular disease, and metabolic syndrome. Young patients usually have a shorter duration of illness, and young patients have a strong resistance to the slight pathological damage induced by UA.[21] Although primary hyperuricemia is generally not a concern for young persons. However, numerous findings from related studies have suggested that asymptomatic patients may exhibit inflammation induced by UA, which can slowly cause pathological harm locally (tissues) or systemically.[15,16,22] Previous studies focused on clinical onset patients with hyperuricemia or a combination of other complications.[3–5,23–25] Nevertheless, our research attempts to elucidate possible pathological alterations in asymptomatic young patients with primary hyperuricemia, with an emphasis on oxidative stress and inflammation. Hyperuricemia is a metabolic disease related to habits, customs, age, gender, and inherited factors, and hyperuricemia is also correlated with obesity, hypertension, diabetes, renal failure, dyslipidemia, and liver diseases.[3,4,23] The healthy persons enrolled in this study were not significantly different from patients in terms of their characteristics (Supplementary Table 1) with patients, so we further analyzed general biochemical parameters (Supplementary Table 2). The results revealed no significant differences in these biochemical parameters between patients and healthy control persons, suggesting that young hyperuricemia patients or patients with a shorter duration of hyperuricemia have no significant alterations in general biochemical markers.
UA contributes to the initiation an inflammatory response in vascular endothelial cells via complex inflammatory signaling pathways, which have been verified to be basic pathological changes in hyperuricemia-related diseases.[8,10,26–28] Elevated UA in peripheral blood can give rise to disordered structure and function of vascular endothelial cells by causing leukocyte adhesion and hormesis, causing further efforts to produce proinflammatory cytokines to set off an inflammation chain reaction, leading to a vicious cycle of endothelial dysfunction.[28,29] In our present study, we measured the levels of IL-6 and TNF-α in asymptomatic young patients with primary hyperuricemia and healthy persons and found that the levels of IL-6 and TNF-α in patients were significantly higher than those in healthy persons, which proved that asymptomatic young patients with primary hyperuricemia may have inflammation.
Oxidative stress is considered to play a crucial role in the inflammatory response induced by various damaging substances. Additionally, previous literature[9,10,30] suggested that oxidative stress is similar to a second messenger for the inflammatory signaling pathway induced by UA. Massive amounts of active oxygen species (ROS) are produced in vivo, and the ability to remove ROS is inhibited, leading to an imbalance in the oxidative response and antioxidation response and the subsequent occurrence of oxidative stress in multiple systems.[31] Elevated UA can induce oxidative stress and ROS production in vascular endothelial cells, and massive ROS can upregulate IL-6 and TNF-α expression. We explored the oxidative stress of young patients with primary hyperuricemia and healthy persons and found that patients showed higher MDA and lower SOD levels than healthy persons. MDA and SOD can be used as markers of oxidative stress; the former can indirectly represent the formation and scavenging of oxygen free radicals, and the latter can protect organisms from oxidative stress damage.[17,18] In addition, our study found oxidative stress and inflammation in asymptomatic young patients with primary hyperuricemia.
Primary hyperuricemia, a disease with stepwise progression, aggravates the damage in organisms with a moderately increased UA level. In our research, we discovered that IL-6 and TNF-α were significantly higher with increased UA levels and that MDA was enhanced and SOD was decreased, which might indicate that higher UA levels was accompanied by more serious inflammation and oxidative stress. Our findings agreed with previous studies.
Earlier studies[12,32] had considered that UA acts as an antioxidant to protect the body from pathological damage. Kuzkaya et al[33] verified in vitro experimental research that UA can prevent the uncoupling of nitric oxide synthase through purging peroxynitrite anions to increase NO synthesis for antioxidative purposes. However, an increasing number of studies[34,35] have proven that a high level of serum UA is the major determinant of the oxidative response in UA-related metabolic diseases. Xie et al[36] found that higher UA not only increased the expression of inflammatory cytokines, such as IL-6, intercellular adhesion molecule-1, TNF-α and monocyte chemoattractant protein, but also increased the formation of oxidants in human umbilical vein endothelial cells. Recent research[37] on the treatment of severe gouty arthritis by allopurinol and febuxostat reported that MDA and NADPH oxidase (NOX) levels decline when UA is reduced, suggesting that oxidative stress may be involved in the inflammation induced by UA. The relationship between inflammation and oxidative stress was analyzed in our present study. There were significant positive correlations between MDA and IL-6 (r = 0.367, P < .001) and TNF-α (r = 0.319, P < .001); in contrast, SOD was negatively correlated with IL-6 (r = −0.241, P < .01) and TNF-α (r = −0.308, P < .001). Therefore, we speculate that the oxidative stress triggered by UA was involved in the inflammatory process of patients with hyperuricemia. The downstream inflammatory signaling pathway is activated by excessive ROS via the regulation of phosphorylation of extracellular signal-regulated kinase, phosphatidylinositol 3-hydroxy kinase and p38 mitogen-activated protein kinase to impact the expression of terminal gene transcription, protein translation, cell proliferation and apoptosis, and endothelium-dependent vasodilation.[38–40] In addition, oxidative stress directly interacts with nuclear transcription factors to promote the expression of inflammatory proteins.[41]
There is evidence that hyperuricemia is an independent risk factor for hypertension,[42] myocardial ischemia,[43] stroke,[44] and atherosclerosis,[45] and the vascular inflammation that results from high UA is regarded as a fundamental pathological mechanism of those diseases. Multivariable logistic regression analysis indicated that UA and MDA were risk factors for high IL-6 and TNF-α; conversely, SOD was a protective factor. We believe that high UA in peripheral blood induces vascular inflammation by triggering the production of oxidants and inhibiting antioxidative enzymes. However, many physiological or pathological factors result in chronic inflammation in vascular endothelial tissue. In fact, our regression analysis also showed that age, smoking, TG, TC, and LDL-C were risk factors for high IL-6 and TNF-α. Further multivariable logistic regression analysis adjusted for age, smoking, TG, TC, and LDL-C still reached the same conclusions as the previous (unadjusted) analysis, providing persuasive evidence that oxidative stress is involved in inflammation in young patients with primary hyperuricemia.
The main strength of this study is that we recruited asymptomatic young patients with hyperuricemia who served as the subject of our study to investigate the pathological mechanism in these patients, increasing the attention paid to young patients with asymptomatic hyperuricemia and to the need for early intervention to prevent the development of hyperuricemia. The other advantage is that we excluded patients who had no health check-up in the past 3 years to ensure that the physiological indexes had not been affected by long periods of high blood UA, so our study suggests that even a short period of hyperuricemia can lead to pathological damage. However, there are several limitations. Although this hospital is a regional medical center that can cover five million people, collaborative research in a multicenter setting would improve our study.
In conclusion, we found oxidative stress and microinflammatory responses in asymptomatic young patients with primary hyperuricemia and found that the inflammatory response may be related to oxidative stress induced by high UA.
Author contributions
Conceptualization: You Zhou, Mingcai Zhao.
Data curation: Zheyan Pu, Guoqiang Xu, Xiangkun Li.
Formal analysis: You Zhou.
Investigation: Mingcai Zhao.
Methodology: You Zhou, Mingcai Zhao.
Project administration: You Zhou.
Writing – original draft: You Zhou.
Writing – review & editing: You Zhou.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, BUN = blood urine nitrogen, DBP = diastolic blood pressure, HDL-C = high-density lipoprotein cholesterol, HUA = hyperuricemia, IL-6 = interleukin-6, LDL-C = low-density lipoprotein cholesterol, MDA = malondialdehyde, NOX = NADPH oxidase, OR = odds ratio, ROS = active oxygen species, SBP = systolic blood pressure, SCr = serum creatinine, SOD = superoxide dismutase, TC = total cholesterol, TG = triglyceride, TNF-α = tumor necrosis factor-α.
Funding: This work was supported by the research project of the Sichuan Provincial Health Planning Commission, and the reference number is 17PJ067.
The authors have no conflicts of interest to disclose.
References
- [1].Von Lueder TG, Girerd N. The prognostic role of serum uric acid (SUA) in coronary artery disease: perSUAsive data plea for a large morbidity-mortality trial. Cardiology 2016;134:357–9. [DOI] [PubMed] [Google Scholar]
- [2].Chatzipavlou M, Magiorkinis G, Koutsogeorgopoulou L, et al. Mediterranean diet intervention for patients with hyperuricemia: a pilot study. Rheumatol Int 2014;34:759–62. [DOI] [PubMed] [Google Scholar]
- [3].Zhang N, Chang Y, Guo X, et al. A Body Shape Index and Body Roundness Index: two new body indices for detecting association between obesity and hyperuricemia in rural area of China. Eur J Intern Med 2016;29:32–6. [DOI] [PubMed] [Google Scholar]
- [4].Cho J, Kim C, Kang DR, et al. Hyperuricemia and uncontrolled hypertension in treated hypertensive patients: K-MetS Study. Medicine (Baltimore) 2016;95:e4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shah P, Bjornstad P, Johnson RJ. Hyperuricemia as a potential risk factor for type 2 diabetes and diabetic nephropathy. J Bras Nefrol 2016;38:386–7. [DOI] [PubMed] [Google Scholar]
- [6].Ogura T, Matsuura K, Matsumoto Y, et al. Recent trends of hyperuricemia and obesity in Japanese male adolescents, 1991 through 2002. Metabolism 2004;53:448–53. [DOI] [PubMed] [Google Scholar]
- [7].Yokokawa H, Fukuda H, Suzuki A, et al. Association between serum uric acid levels/hyperuricemia and hypertension among 85,286 Japanese workers. J Clin Hypertens (Greenwich) 2016;18:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen L, Lan Z. Polydatin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation by inhibiting NF-κB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food Funct 2017;8:1785–92. [DOI] [PubMed] [Google Scholar]
- [9].Kaneko C, Ogura J, Sasaki S, et al. Fructose suppresses uric acid excretion to the intestinal lumen as a result of the induction of oxidative stress by NADPH oxidase activation. Biochim Biophys Acta 2017;1861:559–66. [DOI] [PubMed] [Google Scholar]
- [10].Wang M, Zhao J, Zhang N, et al. Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomed Pharmacother 2016;83:975–88. [DOI] [PubMed] [Google Scholar]
- [11].Merriman TR. An update on the genetic architecture of hyperuricemia and gout. Arthritis Res Ther 2015;17:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alonso A, Sovell KA. Gout, hyperuricemia, and Parkinson's disease: a protective effect? Curr Rheumatol Rep 2010;12:149–55. [DOI] [PubMed] [Google Scholar]
- [13].Desideri G, Gentile R, Antonosante A, et al. Uric acid amplifies Aβ amyloid effects involved in the cognitive dysfunction/dementia: evidences from an experimental model in vitro. J Cell Physiol 2017;232:1069–78. [DOI] [PubMed] [Google Scholar]
- [14].Cristóbal-García M, García-Arroyo FE, Tapia E, et al. Renal oxidative stress induced by long-term hyperuricemia alters mitochondrial function and maintains systemic hypertension. Oxid Med Cell Longev 2015;2015:535686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eleftheriadis T, Golphinopoulos S, Pissas G, et al. Asymptomatic hyperuricemia and chronic kidney disease: narrative review of a treatment controversial. J Adv Res 2017;8:555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Choi HY, Kim SH, Choi AR, et al. Hyperuricemia and risk of increased arterial stiffness in healthy women based on health screening in Korean population. PLoS ONE 2017;12:e0180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gol M, Ghorbanian D, Soltanpour N, et al. Protective effect of raisin (currant) against spatial memory impairment and oxidative stress in Alzheimer disease model. Nutr Neurosci 2017;16:1–9. [DOI] [PubMed] [Google Scholar]
- [18].Huang C, Lai C, Xu P, et al. Lead-induced oxidative stress and antioxidant response provide insight into the tolerance of Phanerochaete chrysosporium to lead exposure. Chemosphere 2017;187:70–7. [DOI] [PubMed] [Google Scholar]
- [19].Saladini F, Mos L, Fania C, et al. Regular physical activity prevents development of hypertension in young people with hyperuricemia. J Hypertens 2017;35:994–1001. [DOI] [PubMed] [Google Scholar]
- [20].Reschke LD, Miller ER, III, Fadrowski JJ, et al. Elevated uric acid and obesity-related cardiovascular disease risk factors among hypertensive youth. Pediatr Nephrol 2015;30:2169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang J, Liu Z, Zhang C, et al. The prevalence of hyperuricemia and its correlates in an inland Chinese adult population, urban and rural of Jinan. Rheumatol Int 2013;33:1511–7. [DOI] [PubMed] [Google Scholar]
- [22].Wasilewska A, Tenderenda E, Taranta-Janusz K, et al. Markers of systemic inflammation in children with hyperuricemia. Acta Paediatr 2012;101:497–500. [DOI] [PubMed] [Google Scholar]
- [23].Sattui SE, Gaffo AL. Treatment of hyperuricemia in gout: current therapeutic options, latest developments and clinical implications. Ther Adv Musculoskelet Dis 2016;8:145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Murray K, Burkard T. Hyperuricemia, gout and cardiovascular diseases. Ther Umsch 2016;73:141–6. [DOI] [PubMed] [Google Scholar]
- [25].Sheikh M, Movassaghi S, Khaledi M, et al. Hyperuricemia in systemic lupus erythematosus: is it associated with the neuropsychiatric manifestations of the disease? Rev Bras Reumatol Engl Ed 2016;56:471–7. [DOI] [PubMed] [Google Scholar]
- [26].Karbowska A, Boratynska M, Kusztal M, et al. Hyperuricemia is a mediator of endothelial dysfunction and inflammation in renal allograft recipients. Transplant Proc 2009;41:3052–5. [DOI] [PubMed] [Google Scholar]
- [27].Huang Z, Hong Q, Zhang X, et al. Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Commun Signal 2017;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liang WY, Zhu XY, Zhang JW, et al. Uric acid promotes chemokine and adhesion molecule production in vascular endothelium via nuclear factor-kappa B signaling. Nutr Metab Cardiovasc Dis 2015;25:187–94. [DOI] [PubMed] [Google Scholar]
- [29].Cai W, Duan XM, Liu Y, et al. Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway. Biomed Res Int 2017;2017:4391920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hong Q, Yu S, Mei Y, et al. Smilacis Glabrae Rhizoma reduces oxidative stress caused by hyperuricemia via upregulation of catalase. Cell Physiol Biochem 2014;34:1675–85. [DOI] [PubMed] [Google Scholar]
- [31].Newsholme P, Cruzat VF, Keane KN, et al. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J 2016;473:4527–50. [DOI] [PubMed] [Google Scholar]
- [32].Menè P, Punzo G. Uric acid: bystander or culprit in hypertension and progressive renal disease? J Hypertens 2008;26:2085–92. [DOI] [PubMed] [Google Scholar]
- [33].Kuzkaya N, Weissmann N, Harrison DG, et al. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol 2005;70:343–54. [DOI] [PubMed] [Google Scholar]
- [34].Pingmuangkaew P, Tangvarasittichai O, Tangvarasittichai S. Association of elevated serum uric acid with the components of metabolic syndrome and oxidative stress in abdominal obesity subjects. Indian J Clin Biochem 2015;30:286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chida R, Hisauchi I, Toyoda S, et al. Impact of irbesartan, an angiotensin receptor blocker, on uric acid level and oxidative stress in high-risk hypertension patients. Hypertens Res 2015;38:765–9. [DOI] [PubMed] [Google Scholar]
- [36].Xie H, Sun J, Chen Y, et al. EGCG Attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the NOTCH pathway. Oxid Med Cell Longev 2015;2015:214836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tausche AK, Christoph M, Forkmann M, et al. As compared to allopurinol, urate-lowering therapy with febuxostat has superior effects on oxidative stress and pulse wave velocity in patients with severe chronic tophaceous gout. Rheumatol Int 2014;34:101–9. [DOI] [PubMed] [Google Scholar]
- [38].Kučera J, Netušilová J, Sladeček S, et al. Hypoxia downregulates MAPK/ERK but not STAT3 signaling in ROS-dependent and HIF-1-independent manners in mouse embryonic stem cells. Oxid Med Cell Longev 2017;2017:4386947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhu Y, Fan S, Wang N, et al. NADPH oxidase 2 inhibitor diphenyleneiodonium enhances ROS-independent bacterial phagocytosis in murine macrophages via activation of the calcium-mediated p38 MAPK signaling pathway. Am J Transl Res 2017;9:3422–32. [PMC free article] [PubMed] [Google Scholar]
- [40].Kim M, Chun J, Jung HA, et al. Capillarisin attenuates exercise-induced muscle damage through MAPK and NF-κB signaling. Phytomedicine 2017;32:30–6. [DOI] [PubMed] [Google Scholar]
- [41].Song XM, Yu Q, Dong X, et al. Aldose reductase inhibitors attenuate β-amyloid-induced TNF-α production in microlgia via ROS-PKC-mediated NF-κB and MAPK pathways. Int Immunopharmacol 2017;50:30–7. [DOI] [PubMed] [Google Scholar]
- [42].Perticone M, Tripepi G, Maio R, et al. Risk reclassification ability of uric acid for cardiovascular outcomes in essential hypertension. Int J Cardiol 2017;243:473–8. [DOI] [PubMed] [Google Scholar]
- [43].Lai YC, Yew YW. Psoriasis as an independent risk factor for cardiovascular disease: an epidemiologic analysis using a national database. J Cutan Med Surg 2016;20:327–33. [DOI] [PubMed] [Google Scholar]
- [44].Kumral E, Karaman B, Orman M, et al. Association of uric acid and carotid artery disease in patients with ischemic stroke. Acta Neurol Scand 2014;130:11–7. [DOI] [PubMed] [Google Scholar]
- [45].Okazaki H, Shirakabe A, Kobayashi N, et al. Are atherosclerotic risk factors associated with a poor prognosis in patients with hyperuricemic acute heart failure? The evaluation of the causal dependence of acute heart failure and hyperuricemia. Heart Vessels 2017;32:436–45. [DOI] [PubMed] [Google Scholar]


