Abstract
Rationale:
Pancreatic ductal carcinoma is a hypovascular tumor, and characteristic findings are observed on imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI), in most cases.
Patient concerns:
Here we report a case of anaplastic carcinoma of the pancreas (ACP) with characteristics of hypervascular tumor diagnosed by endoscopic ultrasound guided fine needle aspiration (EUS-FNA). A 70-year-old woman was admitted to hospital because of exacerbation of diabetes. Contrast-enhanced CT revealed a hypervascular tumor at the head of the pancreas.
Diagnosis:
EUS-FNA was performed. Osteoclast-like giant cells and tumor cells with polymorphic nuclei were found on pathological examination and she was diagnosed with ACP.
Interventions:
Although it was a surgical indication at the time of diagnosis, the tumor rapidly worsened. Oral administration of TS-1 (tegafur/gimeracil/oteracil) was initiated. Chemotherapy was discontinued after the end of 2 courses because the tumor had increased prominently on CT.
Outcomes:
She died approximately a year since the onset of the illness.
Lessons:
ACP occasionally exhibits the characteristics of a hypervascular tumor and may require differentiation from other pancreatic tumors, such as neuroendocrine tumor. Therefore, pathological diagnosis by EUS-FNA at an early stage is important to determine treatment strategies.
Keywords: anaplastic carcinoma, endoscopic ultrasound guided fine needle aspiration, hypervascular tumor, pancreatic ductal carcinoma
1. Introduction
Generally, pancreatic ductal carcinoma is a hypovascular tumor.[1,2] In cases showing characteristic findings on imaging modalities, such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography, pancreatic ductal carcinoma is often diagnosed without pathological examination. On the other hand, hypervascular pancreatic tumor has a wide range of differentiation, such as neuroendocrine tumor and acinar cell carcinoma; therefore, pathological examination is often required. Anaplastic carcinoma of the pancreas (ACP) is a malignant tumor with very poor prognosis.[3] Because many cases of ACP are progressive at the time of diagnosis and only few can be resected, pathological examination is necessary for treatment decisions, such as surgical resection and chemotherapy. Herein, we report the case of a patient with ACP showing hypervascular tumor diagnosed by endoscopic ultrasound guided fine needle aspiration (EUS-FNA).
2. Case report
A 70-year-old woman was admitted to our hospital because of an exacerbation of diabetes. She had a history of appendicitis, diabetes, and hyperlipidemia. Fasting blood glucose (FBG) and hemoglobin A1c (HbA1c) were increased markedly compared with that from 6 months ago. Hepatobiliary and pancreatic enzymes were normal. Laboratory findings on admission showed FBG, 266 mg/dL; HbA1c, 10.9%; aspartate aminotransferase (AST), 30 U/L; alanine aminotransferase (ALT), 29 U/L; alkaline phosphatase (ALP), 258 U/L; γ-glutamyl transferase (γ-GTP), 41 U/L; and T-bilirubin, 1.19 mg/dL.
Abdominal contrast-enhanced CT revealed an enhanced mass (20 × 22 mm) in the pancreatic head (Fig. 1). The mass was rich in blood flow compared with normal pancreas. A neuroendocrine or metastatic pancreatic tumor was suspected. Compared with pancreatic parenchyma, the mass was well enhanced in the early phase and showed low absorption in the late phase. The pancreatic duct was 3-mm thick and slightly expanded. For pathological diagnosis, EUS-FNA was performed. On EUS, the tumor was an isoechoic circle with a clear boundary, and the inside of the mass was uniform (Fig. 2). Therefore, we performed EUS-FNA using a 19-gage needle (Boston EXPECT; Boston Scientific, Marlborough, MA). The tumor was punctured only once and stroked approximately 10 times. Pathological examination revealed proliferation of osteoclast-like giant cells (OCGCs) and tumor cells with polymorphic nuclei. Immunostaining revealed cytokeratin AE1/AE3 (–), EMA (+), CA19–9 (–), vimentin (+), and CD68 (+) (Fig. 3). Based on the above findings, she was diagnosed with ACP. There was no invasion into the surrounding blood vessel, and the classification was clinical stage IB (T2N0M0) according to the International Union Against Cancer (UICC) tumor, nodes, and metastasis staging system. Although we had suggested surgery, it took time to obtain approval from the patient and her family. Approximately 40 days after diagnosis, the tumor had increased to 40 mm in diameter on CT (Fig. 4). As the tumor invaded the surrounding blood vessels, unresectable pancreatic cancer was diagnosed. Therefore, surgery was avoided and chemotherapy or chemoradiotherapy were offered. She refused chemotherapy with infusion and radiotherapy; therefore, oral administration of TS-1 (tegafur/gimeracil/oteracil) was initiated. Chemotherapy was discontinued after the end of 2 courses (4-week administration and 2-week withdrawal). Chemotherapy was discontinued after the end of 2 courses (4-week administration and 2-week withdrawal), because the tumor had increased prominently on CT. At 11 months after diagnosis, the tumor was huge, with a diameter of 180 mm (Fig. 5), and the patient died approximately 12 months after the diagnosis of the tumor.
Figure 1.
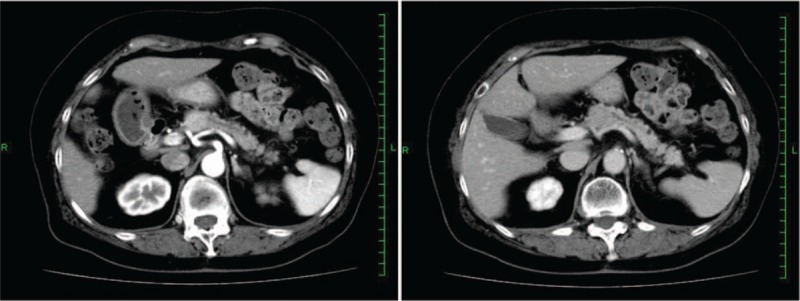
Contrast-enhanced abdominal computed tomography: a hypervascular tumor was found in the head of the pancreas.
Figure 2.
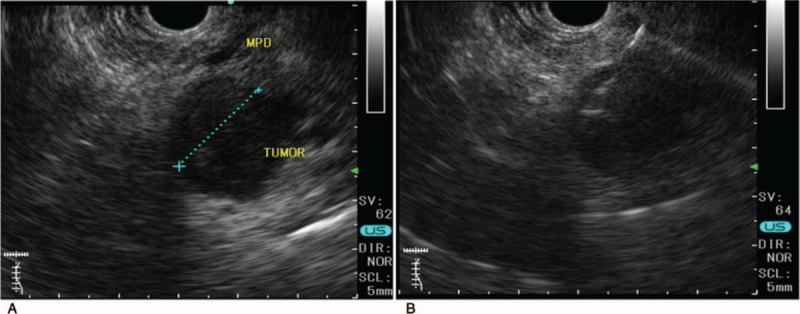
EUS images. (A) A circular tumor with a diameter of 20 mm was visible clearly in the pancreas. (B) The tumor was punctured with a 19-gage needle. EUS = endoscopic ultrasound.
Figure 3.

Pathology of the pancreatic tumor. Osteoclastic giant cells and tumor cells with polymorphic nuclei are proliferating. Vimentin is diffusely positive. CD68 is strongly positive for multinucleated giant cell. (A) Hematoxylin and eosin × 100. (B) Hematoxylin and eosin × 200. (C) Vimentin. (D) CD68.
Figure 4.

Contrast-enhanced abdominal computed tomography: 40 days after onset. The tumor had increased to 40 mm in diameter.
Figure 5.
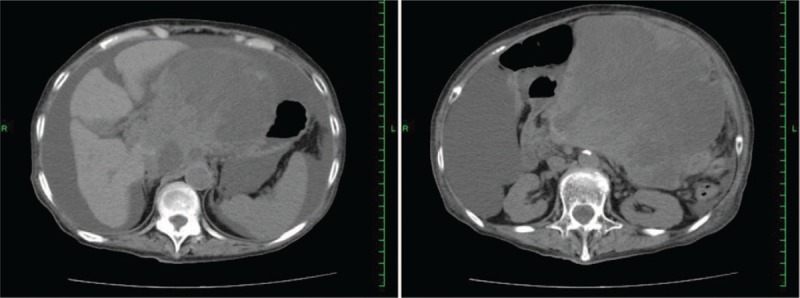
Contrast-enhanced abdominal computed tomography: 11 months after onset. The tumor was huge, with a diameter of 180 mm.
3. Discussion
A typical pancreatic ductal carcinoma is a hypovascular tumor, and imaging modalities often are important for diagnosis. The present case showed that, among pancreatic ductal carcinomas, ACPs can be hypervascular, making proper diagnosis difficult by image modalities alone. Therefore, pathological diagnosis by EUS-FNA immediately after tumor onset is important. ACP was reported by Sommer and Meissner[4] in 1954. ACP is a rare tumor accounting for 40 cases (0.31%) among the 13,029 normal pancreatic cancers reported between 1981 and 2004 in Japan. Because ACP has a poor prognosis compared with normal-type pancreatic cancer, it is important to decide therapy according to the pathologic diagnosis.
ACP may be a hypervascular pancreatic tumor. The most frequent type of hypervascular pancreatic tumor is neuroendocrine tumor (pancreatic neuroendocrine neoplasm; PNEN). In addition, differentiation of serous cystic neoplasm, pancreatic acinar cell carcinoma, solid pseudopapillary neoplasm, and metastatic pancreatic tumor (multiple blood tumor, such as kidney cancer) is necessary. CT is useful to diagnose pancreatic ductal carcinoma and its sensitivity is reported to be 90% to 99%.[5] Sensitivity increases with tumor growth.[6–8] The sensitivity is approximately 70% and almost 100% for tumors <20 and >20 mm in diameter, respectively.[9] Typical features of pancreatic ductal carcinoma are visualized as low density tumors by CT. Blood flow of pancreatic ductal carcinoma is poor; therefore, the findings are prominent on contrast CT. Another feature is the disruption of the pancreatic duct and expansion of the pancreatic duct/bile duct. Although this case showed slight dilatation of the pancreatic duct, it was an atypical hypervascular pancreatic ductal carcinoma. ACP has a contrast effect on the periphery of the tumor.[10] As it rapidly increases, it appears as a low density tumor at the necrosis of the central part. Because vascularity of ACP is higher than that of a normal pancreatic ductal carcinoma,[11] if it is found at an early stage, like in the present case, the tumor may be hypervascular. ACP must be considered as a differential diagnosis of hypervascular tumor.
For treatment decisions, pathological diagnosis by EUS-FNA immediately after onset of illness at an early stage is important. In 1992, Vilmann et al[12] reported on EUS-FNA, which is a standard diagnostic method for pancreatic tumor. The diagnostic ability of EUS-FNA is high. According to a recent meta-analysis, the sensitivity is 85% to 89% and specificity is 98% to 99%.[13,14] ACP has a poor prognosis compared with normal pancreatic ductal carcinoma. Clark et al[15] reported that the overall survival (OS) rate of ACP was significantly lower than that of normal pancreatic ductal carcinoma (hazard ratio [HR] = 1.9; 95% confidence interval [CI], 1.7–2.1; P < .001). Because of the rapid progression, surgical benefits cannot be obtained even when diagnosed in an operable state, and patients often do not survive for long. On the other hand, other reports have found no difference in OS between resectable ACP and normal-type pancreatic ductal carcinoma,[16] and long-term survival has been obtained postoperatively in cases of resectable ACP.[17] In recent years, prognosis has been reported to vary depending on tissue type among cases of ACP. The Japan Pancreas Society has classified ACP into the following 3 subtypes: pleomorphic type, spindle cell type, and ACP with OCGCs. The presence of OCGCs is a particularly rare histologic variant of ACP. Some investigators have reported improved OS in patients with the ACP variant with OCGCs.[18] Therefore, surgical resection should be performed in patients with resectable ACP with OCGCs. In the present case, we were able to diagnose resectable ACP with OCGC by EUS-FNA, so we could propose surgery to the patient. However, the consent for surgery could not be obtained, the tumor progressed rapidly, and chemotherapy was initiated. Pathological diagnosis by EUS-FNA is necessary at an early stage when a small hypervascular pancreatic tumor is observed.
ACP can be a hypervascular tumor. If a hypervascular tumor is recognized in the pancreas, it is necessary to rule out ACP in addition to PNEN and pancreatic tumor metastasis from other organ cancers. Histological diagnosis by EUS-FNA immediately after onset is important because ACP with OCGC may be a long-term prognosis by surgical treatment.
Author contributions
Conceptualization: Takehide Fujimoto, Osamu Inatomi.
Investigation: Ryo Mizuno, Shuhei Shintani, Atsushi Nishida.
Supervision: Osamu Inatomi, Akira Andoh.
Validation: Shigeki Bamba, Mitsushige Sugimoto.
Writing – original draft: Takehide Fujimoto.
Footnotes
Abbreviations: ACP = anaplastic carcinoma of the pancreas, CT = computed tomography, EUS-FNA = endoscopic ultrasound guided fine needle aspiration, FBG = fasting blood glucose, MRI = magnetic resonance imaging, OCGC = osteoclast-like giant cells, OS = overall survival.
Informed consent: The patient's family has given written informed consent for the case to be reported.
Financial support: There is no funding involved in the production of this manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol 2018;24:2047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ahn SS, Kim MJ, Choi JY, et al. Indicative findings of pancreatic cancer in prediagnostic CT. Eur Radiol 2009;19:2448–55. [DOI] [PubMed] [Google Scholar]
- [3].Strobel O, Hartwig W, Bergmann F, et al. Anaplastic pancreatic cancer: presentation, surgical management, and outcome. Surgery 2011;149:200–8. [DOI] [PubMed] [Google Scholar]
- [4].Sommers SC, Meissner WA. Unusual carcinomas of the pancreas. AMA Arch Pathol 1954;58:101–11. [PubMed] [Google Scholar]
- [5].Megibow AJ, Zhou XH, Rotterdam H, et al. Pancreatic adenocarcinoma: CT versus MR imaging in the evaluation of resectability--report of the Radiology Diagnostic Oncology Group. Radiology 1995;195:327–32. [DOI] [PubMed] [Google Scholar]
- [6].Horton KM, Fishman EK. Adenocarcinoma of the pancreas: CT imaging. Radiol Clin North Am 2002;40:1263–72. [DOI] [PubMed] [Google Scholar]
- [7].Gritzmann N, Macheiner P, Hollerweger A, et al. CT in the differentiation of pancreatic neoplasms--progress report. Dig Dis 2004;22:6–17. [DOI] [PubMed] [Google Scholar]
- [8].Scaglione M, Pinto A, Romano S, et al. Using multidetector row computed tomography to diagnose and stage pancreatic carcinoma: the problems and the possibilities. JOP 2005;6:1–5. [PubMed] [Google Scholar]
- [9].Bronstein YL, Loyer EM, Kaur H, et al. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol 2004;182:619–23. [DOI] [PubMed] [Google Scholar]
- [10].Okazaki M, Makino I, Kitagawa H, et al. A case report of anaplastic carcinoma of the pancreas with remarkable intraductal tumor growth into the main pancreatic duct. World J Gastroenterol 2014;20:852–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yamaguchi K, Nakamura K, Shimizu S, et al. Pleomorphic carcinoma of the pancreas: reappraisal of surgical resection. Am J Gastroenterol 1998;93:1151–5. [DOI] [PubMed] [Google Scholar]
- [12].Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc 1992;38:172–3. [DOI] [PubMed] [Google Scholar]
- [13].Hebert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology 2013;24:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology 2013;13:48–57. [DOI] [PubMed] [Google Scholar]
- [15].Clark CJ, Graham RP, Arun JS, et al. Clinical outcomes for anaplastic pancreatic cancer: a population-based study. J Am Coll Surg 2012;215:627–34. [DOI] [PubMed] [Google Scholar]
- [16].Paniccia A, Hosokawa PW, Schulick RD, et al. A matched-cohort analysis of 192 pancreatic anaplastic carcinomas and 960 pancreatic adenocarcinomas: a 13-year North American experience using the National Cancer Data Base (NCDB). Surgery 2016;160:281–92. [DOI] [PubMed] [Google Scholar]
- [17].Fujii K, Nitta T, Kawasaki H, et al. Anaplastic carcinoma of the pancreas arising in an intraductal papillary mucinous neoplasm: a case report. Mol Clin Oncol 2016;4:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hoshimoto S, Matsui J, Miyata R, et al. Anaplastic carcinoma of the pancreas: case report and literature review of reported cases in Japan. World J Gastroenterol 2016;22:8631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


