Abstract
Background:
Total hip replacement (THR) is often accompanied by severe postoperative pain. We aimed to study whether oxycodone can be an effective alternative for fentanyl in the management of early postoperative pain after total hip replacement.
Methods:
We conducted a randomized controlled trial on 46 patients scheduled to undergo THR. We followed a standard general anesthetic technique, with endotracheal intubation. Twenty minutes before the end of surgery, single bolus injection of fentanyl, 50 μg (fentanyl group [group F], n = 23) or oxycodone, 4 mg (oxycodone group [group O], n = 23) was administered intravenously. Numeric rating scale (NRS) was used to assess pain in the post-anesthesia care unit (PACU). All patients had intravenous patient-controlled analgesia (PCA) with 10 μg/kg of fentanyl for 48 hours. Intravenous boluses of 50 μg were administered for breakthrough pain. The cumulative opioid dose administered at 6, 12, 24, and 48 h after surgery were recorded. A P value of less than .05 was considered statistically significant.
Results:
The NRS of group O in the PACU was significantly lower (P <.05); fewer patients in group O required additional fentanyl boluses in the PACU (P <.05). The cumulative opioid requirement was significantly less in group O at 6, 12, 24, and 48 hours after surgery. (P <.05)
Conclusions:
A single bolus injection of oxycodone is more effective than that of fentanyl in the acute phase of postoperative pain after THR. It may be used as an alternative drug for fentanyl in pain control after orthopedic surgery.
Keywords: fentanyl, oxycodone, postoperative pain, total hip replacement
1. Introduction
Adequate pain control is crucial in improving patient comfort and satisfaction in the postoperative period. Suboptimal pain control often delays rehabilitation and prolongs hospital stay.[1] Furthermore, unrelieved postoperative pain could lead to chronic post-surgical pain syndromes; hence, it is important to provide effective pain relief in the post-anesthesia care unit (PACU) soon after surgery. Especially, severe pain has been reported in the PACU in patients undergoing THR,[2] so adequate postoperative pain control is more important.
In several studies, oxycodone showed similar or superior analgesic effects compared to fentanyl[3,4] and was suggested as a substitute for fentanyl in postoperative pain control. However, it has been mainly used to control visceral pain, based on previous studies that show analgesic effect mediated by the kappa receptors.[5] To our knowledge, few studies have compared oxycodone with fentanyl for postoperative pain relief in patients undergoing orthopedic surgery.
Opioids are predominantly used in postoperative pain control, despite potential side effects. Morphine, oxycodone, tramadol, and fentanyl are the commonly used opioids.[6] Fentanyl has a rapid onset of action (5–7 min) and is effective when a rapid analgesic effect is required in the recovery room or intensive care unit. Compared with morphine, fentanyl showed less demand for additional analgesics, faster bowel recovery and shortened hospital stay after surgery.[7] Oxycodone is a potent agonist at the mu receptor; at higher doses, it binds to other opioid receptors, including kappa, and delta receptors in the central, peripheral, and autonomic nervous systems.[8] It has an onset of action of 10 to 15 minutes;[9] the duration of action is longer than that of morphine; however, it does not lead to histamine release.[10] It is used in both acute and chronic pain of moderate-to-severe intensity, with no ceiling effect, and oxycodone is more potent than morphine when used for postoperative pain relief.[11]
In several studies, oxycodone showed similar or superior analgesic effects compared to fentanyl[3,4] and was suggested as a substitute for fentanyl in postoperative pain control. However, it has been mainly used to control visceral pain, based on previous studies that show analgesic effect mediated by the kappa receptors.[5] To our knowledge, few studies have compared oxycodone with fentanyl for postoperative pain relief in patients undergoing orthopedic surgery.
In this randomized controlled trial, we aimed to compare the efficacy of oxycodone with that of fentanyl for postoperative pain relief in patients undergoing total hip replacement (THR).
2. Methods
We conduct a randomized controlled trial, double-blind, single center study. This study was approved by the University's Institutional Review Board (IRB #2016-09-047) and the trial was registered before patient enrollment at clinicaltrials.gov (NCT03019562). Written informed consent was obtained from all subjects participating in the trial on the day before surgery. We enrolled 46 patients aged 19 to 65 years old, with American Society of Anesthesiologists (ASA) physical status classification 1 to 2, scheduled for elective THR at Kyung Hee University Hospital. Exclusion criteria were:
-
(1)
history of allergy to oxycodone or fentanyl,
-
(2)
renal or hepatic dysfunction,
-
(3)
history of drug or alcohol abuse,
-
(4)
psychiatric disease, and
-
(5)
pregnancy.
We used a standardized anesthetic technique in all cases; no premedication was administered. In the operating room, routine monitoring was commenced including electrocardiography, blood pressure, pulse oximetry, end-tidal CO2, and bispectral index (BIS). Anesthesia was induced with 1 to 2 mg/kg of propofol and 0.8 mg/kg of rocuronium intravenously. Desflurane (5–7 vol%) was administered in a mixture of 50% oxygen and air to maintain anesthesia. Remifentanil was infused at a rate of 0.025 to 0.1 μg/kg/min intravenously during surgery. The BIS values were kept between 40 and 60.
Fentanyl, 50 μg, (fentanyl group [group F]) or oxycodone, 4 mg (oxycodone group [group O]) was administered intravenously 20 minutes before the end of surgery, in a double-blind manner. At the end of surgery, desflurane and remifentanil were discontinued. When the train-of-4 count returned to 2/4, sugammadex, 2 mg/kg, was administered intravenously to reverse muscle relaxant effect. Extubation was carried out after confirming sufficient recovery of consciousness and presence of adequate spontaneous respiration. Fentanyl 10 μg/kg and ramosetron 0.6 mg were mixed in 100 mL normal saline and administered intravenously through a patient-controlled analgesia (PCA) device (Ambix Anapa; IWha-Fresenius Kabi, Seoul, Korea) at the end of surgery. A continuous infusion of this mixture was set to run at 2 mL/h (approximately 0.21 μg/kg/h). Patients were transferred to the PACU and observed for at least 30 min before being transferred to the ward. Numeric rating scale (NRS; 0 = no pain, and 10 = worst possible pain) was used for assessing the severity of pain. In the PACU, if the NRS was 5 or more, a bolus dose of 50 μg of fentanyl was administered in both groups. Similarly, in the wards, an intravenous dose of 50 μg intravenous fentanyl was administered for breakthrough pain, instead of a PCA bolus. The time of administration and the cumulative fentanyl requirement at 6, 12, 24, and 48 hours postoperatively were recorded. We recorded any adverse effects including postoperative nausea and vomiting (PONV), dizziness, pruritus, headache, dizziness, sedation (alert, drowsy, and asleep), and respiratory depression, and. In case of PONV, an intravenous dose of 10 mg of metoclopramide was administered.
The primary endpoint is difference of NRS between oxycodone and group F in PACU, and the second endpoint is difference of cumulative opioid consumption at 6, 12, 24, and 48 hours postoperatively.
3. Statistical analysis
Based on a pilot study, the mean difference between group O and group F in NRS was 1.56. The sample size was calculated using G∗power 3.1 program (Erdfelder, Faul, & Buchner).[12] The minimum sample size required in each group at a significance level (α) of 0.05 and a statistical power of 0.8 was 20. Forty-six patients were included in the study allowing a 10% dropout due to incomplete data collection and exclusion. Patients were randomly allocated to 1:1 ratio either the group F (n = 23) or the group O (n = 23) using Microsoft Office Exel program. All statistical analyses were carried out using SPSS (software version 21.0, IBM Corporation, New York, NY). Levene test for equality of variances was performed. Variability of scores for each of the groups was similar (P >.05). Comparisons between groups were analyzed using the unpaired t-test for continuous variables and chi-square test for categorical data. A P value less than .05 was considered statistically significant. Data are presented as the mean ± standard deviation (SD) unless otherwise noted. This study was evaluated by a statistical expert.
4. Results
The study involved 46 patients, randomly assigned to the O (n = 23) or F (n = 23) groups, and were followed for 48 hours postoperatively (for a flow chart of participants’ selection see Fig. 1). Demographic and clinical characteristics of patients are shown in Table 1. There were no statistically significant differences in age, sex, ASA classification, weight, and height between groups (P >.05). The duration of surgery, the intraoperative dose of remifentanil, and the time to recovery from anesthesia were also not different.
Figure 1.
Flow Chart indicating participants.
Table 1.
Demographic data and anesthesia characteristics.
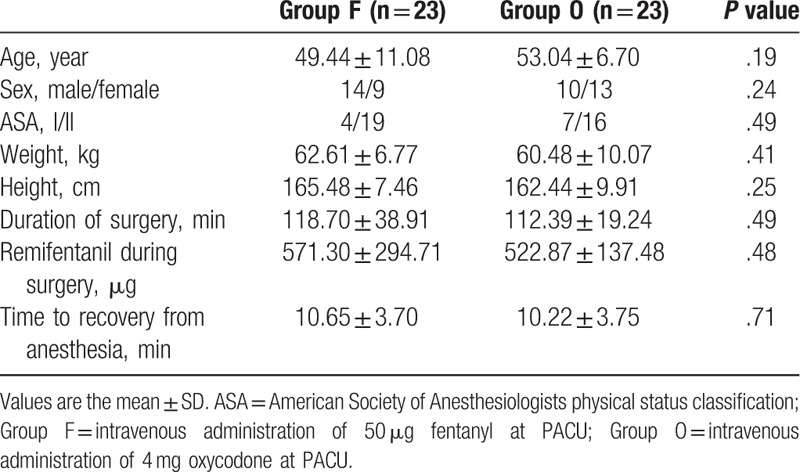
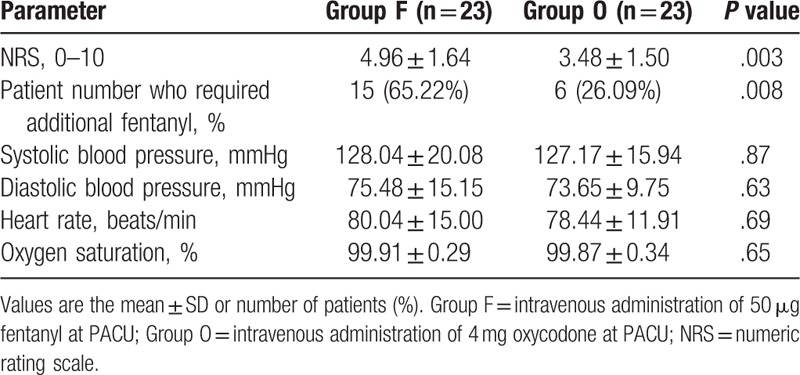
The NRS measured on arrival to the PACU was 3.48 ± 1.50 in group O and 4.96 ± 1.64 in group F (P = .003). In the PACU, additional doses of fentanyl were required, for an NRS of 5 or more, for 6 patients in group O and 15 in group F (P = .008). Vital signs were not significantly different between groups (Table 2).
Table 2.
Postoperative pain-related factors and vital signs in the post-anesthesia care unit (PACU).
The cumulative dose of fentanyl in group O was 93.86 ± 26.55 μg at 6 hours, 174.67 ± 37.87 μg at 12 hours, 336.30 ± 62.93 μg at 24 hours, and 659.13 ± 119.31 μg at 48 hours after surgery; In group F, the cumulative dose of fentanyl was 123.15 ± 34.36 μg at 6 hours, 212.39 ± 46.58 μg at 12 hours, 390.00 ± 75.65 μg at 24 hours, and 744.35 ± 142.25 μg at 48 hours after surgery was calculated. In group O, less fentanyl was administered compared to group F (P <.05) at each postoperative time periods (Fig. 2).
Figure 2.
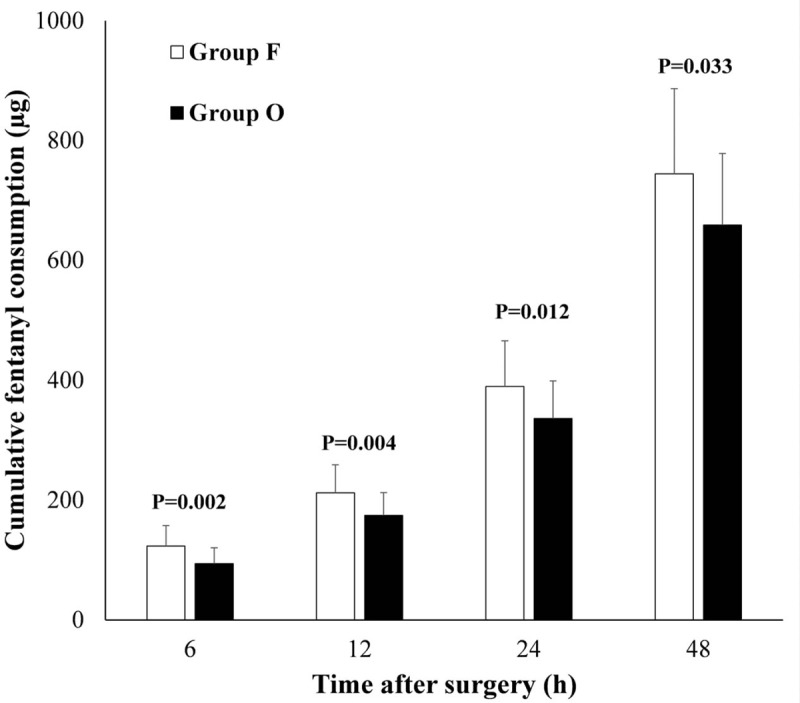
Cumulative fentanyl consumption (μg) at 6, 12, 24, and 48 hours postoperatively. P value <.05 was statistically significant.
Adverse events were observed for 48 hours postoperatively (Table 3). Three patients in group O and 1 in group F experienced PONV; all were successfully treated with anti-emetic agents (palonosetron or metoclopramide). One patient in group O had dizziness/headache in the PACU, and 1 patient in group F complained of pruritus. Excessive sedation was not noted in any patient.
Table 3.
Incidence rate (%) of adverse events during 48 hours.
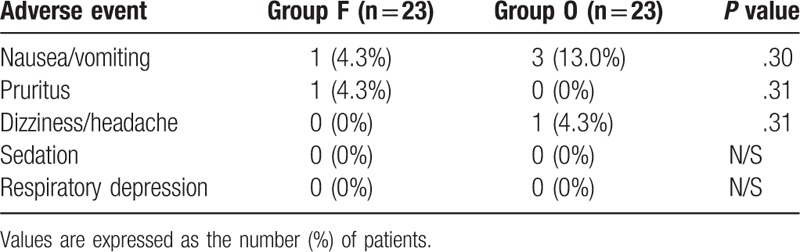
Adverse effects were not significantly different between the 2 groups. (P >.05).
5. Discussion
The purpose of this study was to compare the pain scores in the PACU and additional opioid requirement between single bolus injection of oxycodone and fentanyl during the postoperative period. There are several reports comparing the analgesic effects between oxycodone and fentanyl using intravenous PCA.[3,13,14] They reported that continuous injection of oxycodone provided superior analgesic effects than continuous injection of fentanyl but has more side effects. Considering that continuous injection of oxycodone via PCA increases the incidence of side effects, our study planned to administer single bolus oxycodone before surgery. Oxycodone has a longer duration of action than fentanyl and hence, may have a more sustained analgesic effect after a single bolus injection than fentanyl at the end of the surgery. In our study, the group O had lower NRS, fentanyl requirement in the PACU and cumulative fentanyl requirement during the 48 hours postoperative period, and showed similar incidence of side effects compared to the group F.
In our study, the group O had lower NRS in the PACU and less cumulative fentanyl requirements in the 48 hours study period. The half-life of oxycodone is known to be 3 to 6 hours, but longer duration of action than half-life of oxycodone was reported similar to our study.[15] It has been reported that sensitization of peripheral and central nervous system may lead to the development of acute pain that can potentially become chronic.[1] The sensitization of the pain of the peripheral nervous system amplifies the signal to the spinal cord, which leads to hyperexcitation of the dorsal horn and sensitization of the central nervous system. This eventually lowers the threshold for pain and increases the response to pain. It is thought that having a longer duration of action time than the half-life is due to the reduced pain sensitivity in the peripheral and central nervous system through the effective analgesic effect of oxycodone.
To compare the analgesic effects of 2 drugs, equivalent doses are needed. In a previous report, oxycodone and fentanyl were compared in the PACU for a period of 2 hours following intravenous administration after laparoscopic cholecystectomy.[16] Intravenous fentanyl, 100 μg was compared to 10 mg of oxycodone with lower pain scores in the group O. However, there was a higher incidence of side effects including severe nausea and vomiting with oxycodone. This study was the earliest to determine equivalent doses of oxycodone and fentanyl; lower doses of oxycodone were studied subsequently, including in our previous report.[3,14] Intravenous oxycodone is known to have equivalent or 3:4 ratio to morphine.[17] In addition, Herrick et al[18] compared intravenous fentanyl PCA with morphine and concluded that the group F needed more PCA boluses but reported the same VAS score in a fentanyl to morphine ratio of 1:100. Hwang et al[14] suggested a potency ratio of fentanyl to oxycodone of about 1:75 and morphine to oxycodone of 3:4. The previous studies show that the equivalent ratio of fentanyl to oxycodone is 1: 75 to 100, but since 1:100 is known to have more side effects in early studies of oxycodone,[16] a ratio of 1:80 was used in this study. A dose of 50 μg of fentanyl and 4 mg of oxycodone were administered 20 minutes before completion of surgery, based on these potency ratios. In addition, there was no difference in the patient's vital signs between the 2 groups in the PACU. If the administered oxycodone and fentanyl were not equipotent, the inhibition of cardiovascular system would have caused a difference in blood pressure or pulse rate.
Adverse effects associated with oxycodone use include constipation (25%–30%) nausea (25%), vomiting (10%–15%), drowsiness (25%), dizziness (15%), and pruritus (10%–15%).[9] A higher incidence of nausea and vomiting was reported with oxycodone compared to fentanyl in previous studies.[3,4] The precise mechanism of opioid-induced nausea and vomiting is unknown; multiple opioid effects may be involved, including enhanced vestibular sensitivity, direct effects on the chemoreceptor trigger zone, and delayed gastric emptying. Vestibular sensitivity may lead to dizziness, which may be caused by opioids activating on the mu-opioid receptors in the vestibular epithelium. Although previous studies have shown more nausea with oxycodone compared to other opioids, we did not find a significant difference in the incidence of PONV between the fentanyl and groups O. Most cases of nausea occurred within 12 hours of surgery and showed a tendency to decrease over time in both groups. One patient in group O complained of transient headache; there was no incidence of excessive sedation or respiratory depression in either group. Opioids can affect the recovery from anesthesia because they cause respiratory depression. The peak respiratory depressant effect of a single intravenous dose of fentanyl is noted 5 to 15 minutes following injection (product data sheet), and oxycodone takes effect 10 to 15 minutes after administration. There was no difference in the effect of respiratory depression on the recovery time from anesthesia in both groups. At PACU, patients of both groups did not show difficulty in breathing.
The hyperalgesia caused by remifentanil used during surgery should be considered because that could influence the outcome of this study. Remifentanil is a very potent and ultra-short-acting opioid drug widely used for general anesthesia. Nevertheless, it is known that opioid-induced hyperalgesia by remifentanil is developed more frequent when the drug is used for long periods and at a high dose. The dose of remifentanil (0.025–0.1 μg/kg/min) in this study is much lower than the concentration known to cause hyperalgesia (0.25 μg/kg/min).[19] There were no statistically significant differences in total usage and duration of surgery between the 2 groups. Therefore, the effect of remifentanil in postoperative pain is considered to be small.
We controlled the pain through PCA after surgery. In order to compare the effect of oxycodone bolus with fentanyl bolus, only an additional amount of fentanyl should be measured without PCA. However, since the severe pain is accompanied by THR, intravenous PCA is combined with additional fentanyl during the 48 hours postoperative period. Infusion rates of fentanyl between 0.12 and 0.67 μg/kg/h are usually used without any serious side effects for intravenous PCA.[20] We injected fentanyl at a low rate of 0.21 μg/kg/h to reduce the bias of the oxycodone and fentanyl used in the study.
There are several limitations to our study. First, the patients’ pain thresholds were not tested before conducting the study. Second, a sample size of the study is relatively small despite having calculated through a pilot study. Generalization through large-scale research is necessary. The other is that the patients in our study did not have long-term follow-up to determine the effect of oxycodone on chronic pain. Further research is needed to determine if oxycodone can reduce the prevalence of chronic pain syndrome.
In conclusion, single bolus injection of oxycodone at the end of surgery provided more effective postoperative pain relief compared to that of fentanyl when used with a conversion ratio of 1:80 in THR, with comparable adverse effects. Oxycodone has a longer duration of action compared to fentanyl, but because of the slow onset time, it is administered 20 minutes before the end of the surgery, so that early postoperative pain control can be achieved while reducing the need for additional analgesics after surgery.
Author contributions
Conceptualization: Hee Yong Kang.
Data curation: Sang Eun Ahn, Hee Yong Kang.
Formal analysis: Jeong-Hyun Choi.
Investigation: Hee Yong Kang.
Methodology: Eunsil Shin.
Project administration: Hee Yong Kang.
Supervision: Sung Wook Park.
Writing – original draft: Mi Kyeong Kim.
Writing – review & editing: Hee Yong Kang.
Hee Yong Kang orcid: 0000-0001-7506-1375.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BIS = bispectral index, group F = fentanyl group, group O = oxycodone group, NRS = numeric rating scale, PACU = post anesthesia care unit, PCA = patient-controlled analgesia, PONV = postoperative nausea and vomiting, SD = standard deviation, THR = total hip replacement.
The authors report no conflicts of interest.
References
- [1].Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North Am 2005;23:21–36. [DOI] [PubMed] [Google Scholar]
- [2].Minville V, Lubrano V, Bounes V, et al. Postoperative analgesia after total hip arthroplasty: patient-controlled analgesia versus transdermal fentanyl patch. J Clin Anesth 2008;20:280–3. [DOI] [PubMed] [Google Scholar]
- [3].Kim NS, Lee JS, Park SY, et al. Oxycodone versus fentanyl for intravenous patient-controlled analgesia after laparoscopic supracervical hysterectomy: a prospective, randomized, double-blind study. Medicine 2017;96:e6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ding Z, Wang K, Wang B, et al. Efficacy and tolerability of oxycodone versus fentanyl for intravenous patient-controlled analgesia after gastrointestinal laparotomy: a prospective, randomized, double-blind study. Medicine 2016;95:e4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Eisenach JC, Carpenter R, Curry R. Analgesia from a peripherally active kappa-opioid receptor agonist in patients with chronic pancreatitis. Pain 2003;101:89–95. [DOI] [PubMed] [Google Scholar]
- [6].Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am 2015;95:301–18. [DOI] [PubMed] [Google Scholar]
- [7].Russo A, Grieco DL, Bevilacqua F, et al. Continuous intravenous analgesia with fentanyl or morphine after gynecological surgery: a cohort study. J Anesth 2017;31:51–7. [DOI] [PubMed] [Google Scholar]
- [8].Kalso E. Oxycodone. J Pain Symp Manag 2005;29suppl 5:S47–56. [DOI] [PubMed] [Google Scholar]
- [9].Ordonez Gallego A, Gonzalez Baron M, Espinosa Arranz E. Oxycodone: a pharmacological and clinical review. Clin Transl Oncol 2007;9:298–307. [DOI] [PubMed] [Google Scholar]
- [10].Ennis M, Schneider C, Nehring E, et al. Histamine release induced by opioid analgesics: a comparative study using porcine mast cells. Agents Actions 1991;33:20–2. [DOI] [PubMed] [Google Scholar]
- [11].Kalso E, Poyhia R, Onnela P, et al. Intravenous morphine and oxycodone for pain after abdominal surgery. Acta Anaesthesiol Scand 1991;35:642–6. [DOI] [PubMed] [Google Scholar]
- [12].Kang H, Yeon K, Han ST. A review on the use of effect size in nursing research. J Korean Acad Nurs 2015;45:641–9. [DOI] [PubMed] [Google Scholar]
- [13].Kim NS, Kang KS, Yoo SH, et al. A comparison of oxycodone and fentanyl in intravenous patient-controlled analgesia after laparoscopic hysterectomy. Korean J Anesthesiol 2015;68:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hwang BY, Kwon JY, Kim E, et al. Oxycodone vs. fentanyl patient-controlled analgesia after laparoscopic cholecystectomy. Int J Med Sci 2014;11:658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang N, Wang Y, Pang L, et al. Effect of preemptive analgesia with intravenous oxycodone in the patients undergoing laparoscopic resection of ovarian tumor. Pakistan J Med Sci 2015;31:300–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koch S, Ahlburg P, Spangsberg N, et al. Oxycodone vs. fentanyl in the treatment of early post-operative pain after laparoscopic cholecystectomy: a randomised double-blind study. Acta Anaesthesiol Scand 2008;52:845–50. [DOI] [PubMed] [Google Scholar]
- [17].Leow KP, Cramond T, Smith MT. Pharmacokinetics and pharmacodynamics of oxycodone when given intravenously and rectally to adult patients with cancer pain. Anesth Analg 1995;80:296–302. [DOI] [PubMed] [Google Scholar]
- [18].Herrick IA, Ganapathy S, Komar W, et al. Postoperative cognitive impairment in the elderly. Choice of patient-controlled analgesia opioid. Anaesthesia 1996;51:356–60. [DOI] [PubMed] [Google Scholar]
- [19].Yu EH, Tran DH, Lam SW, et al. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain. Anaesthesia 2016;71:1347–62. [DOI] [PubMed] [Google Scholar]
- [20].Shin S, Min KT, Shin YS, et al. Finding the ‘ideal’ regimen for fentanyl-based intravenous patient-controlled analgesia: how to give and what to mix. Yonsei Med J 2014;55:800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


