Supplemental Digital Content is available in the text
Keywords: aggressive pancreas neoplasm, pancreas neoplasm, solid pseudopapillary neoplasm
Abstract
Background:
Solid pseudopapillary neoplasms (SPNs) of the pancreas are rare tumors considered to be benign although 10% to 15% of SPNs have been reported to be aggressive. Due to its rarity, there have only been a few cases reported regarding the clinical course of patients with aggressive SPNs. The goal of this study is to describe the clinical course of patients diagnosed with aggressive SPNs.
Methods:
A PubMed search was done looking for articles describing the clinical course of patients diagnosed with SPN that locally invaded, recurred, or metastasized. Institutional experience was also added to the pooled data. Patient information was extracted from the articles. Survival and recurrence curves were plotted and factors associated with survival and recurrences were analyzed.
Results:
A total of 59 patients were identified to have aggressive SPN. Seven patients were males and 52 were females and the mean age was 37.44 ± 2.21 years. Systemic metastasis constituted 81.4% while recurrence and deep tissue invasion were found in 11.9% and 6.8% of the patients, respectively. Disease-free survival was 45 ± 6.28 months and disease-specific survival was 152.67 ± 12.8 months. In survival analysis, age, gender, tumor size, tumor location, combined resection, type of recurrence, and stage IV on diagnosis were not significant factors in predicting survival. However, an unresectable tumor (hazards ratio [HR] = 4.871, 95% confidence interval [CI] 1.480–16.03, P = .009), and metastasis within 36 months (HR = 6.399, 95% CI: 1.390–29.452, P = .017) were identified as independent variables in predicting survival.
Conclusion:
SPNs of the pancreas carry a favorable course. Despite having aggressive properties, patients can still survive for more than 10 years as long as the tumor can be resected completely.
1. Introduction
Pancreatic solid pseudopapillary neoplasm (SPN), also referred to as Frantz tumor, is considered a rare tumor type representing 1% to 2% of all pancreatic tumors.[1–3] The tumor grows to be approximately 8–10 cm in diameter[4] and is usually identified using computed tomography (CT).[5–9] SPN usually affects young women between their 3rd and 4th decade of life and is associated with a good prognosis for patients, with a 5-year survival rate of up to 97%.[3,10–12]
Pancreatic SPNs are often considered to be benign conditions, but up to 10% to 15% of cases are reportedly aggressive.[13] Some SPNs are found to be locally aggressive and invade adjacent tissues,[14–17] while others are malignant and metastasize to the liver and peritoneum[1,13,18–20] or to more distant organs such as the lungs.[21] However, despite their malignant potential, curative R0, and en bloc resections have been testified to improve the overall survival and disease-free survival.[15,22–25] On the contrary, studies involving other pancreatic tumor types have shown that metastatic pancreatic cancers only carry a 5-year overall survival rate of 2%.[26–28]
However, clinical cases of aggressive SPNs are so rare that it is difficult to generalize and elucidate the natural course of aggressive SPN in clinical circumstances, thereby making it challenging for clinicians or surgeons to provide information to patients and their families regarding the prognosis of aggressive pancreatic SPNs.
In this review, we summarize and describe the long-term survival outcomes of patients with aggressive SPNs that have metastasized, recurred, or invaded adjacent organs, as well as determine the factors contributing to a decrease in the overall survival rate.
2. Materials and methods
2.1. Data collection
The PubMed database was searched using the search terms “aggressive,” “metastatic,” “recurrence,” “deep tissue invasion,” “peritoneal seeding,” “pseudopapillary,” and “pancreas.” Relevant articles in the English literature were reviewed, and references were cross-checked up to the 1st degree, in search of patients that have pathologically confirmed SPN exhibiting aggressive behavior (i.e., metastasis, recurrence, and deep tissue invasion). Patient data, such as age, sex, tumor size, tumor location, surgery, survival, recurrence, and time to recurrence, were extracted from those articles. Researches that grouped their patient characteristics as means had to be excluded from the study as they had heterogeneous endpoints. Local patients were also added to the data set. The complete list of journals from which articles were reviewed can be found in Appendix 1, whereas Figure 1 demonstrates the data collection flowchart. The institutional review board of Severance Hospital has approved this study.
Figure 1.
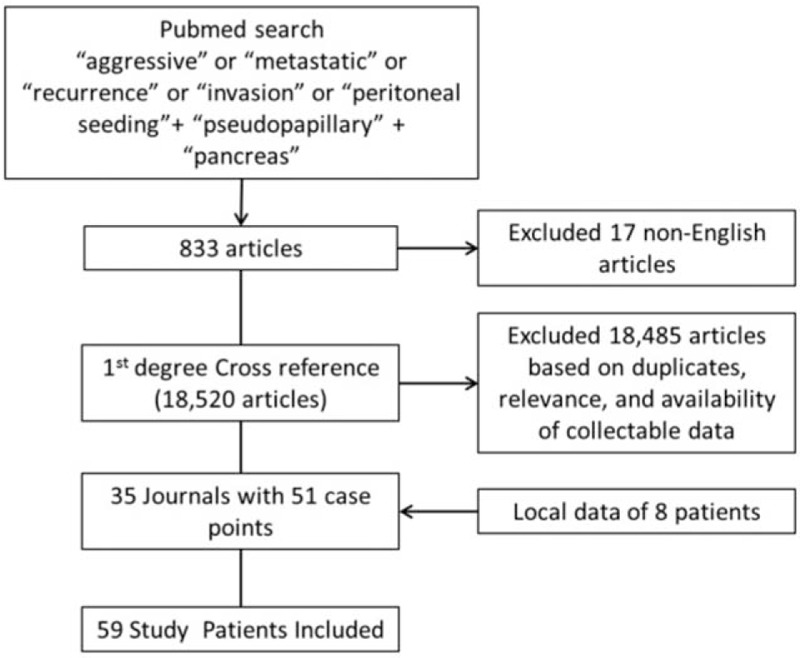
Flowchart of data collection.
2.2. Statistical analysis
The data collected were encoded into the statistical analysis software, SPSS v23.0. Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as frequency (percentage). Journals that had incomplete information were plotted as having missing variables. A Kaplan–Meier survival plot analysis was employed to determine overall survival, disease-free survival, and recurrence rate. A log-rank test was used to determine the significance of each parameter with regard to survival and recurrence. Characteristics that demonstrated a univariate association with survival at a significance level of P < .05 were entered as covariates into a multivariate proportional hazards regression model. All P-values <.05 were considered statistically significant.
3. Results
3.1. Search results and general characteristics of the patients with aggressive SPN
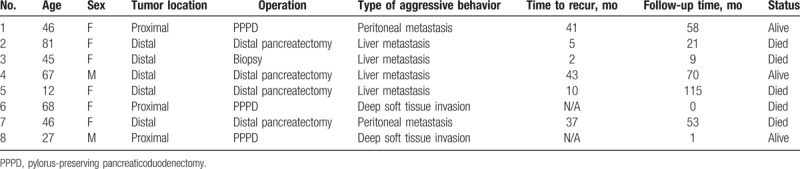
A systematic review of literature was performed to search for relevant articles. A total of 833 potential journals were identified. Seventeen journals that were not in English were excluded from the analysis. The remaining 816 journals were cross referenced and we were able to review 18,485 articles, of which, those that contained duplicate data sets, and did not contain patient details were also excluded from the analysis (Fig. 1). After the exclusion criteria were applied, we were able to collect 51 patients with aggressive SPN out of 462 (11.0%) total patients described with SPN from 31 journals (Table 1) and we added 8 more patients from our local data (Table 2).
Table 1.
Summary of journals reviewed.
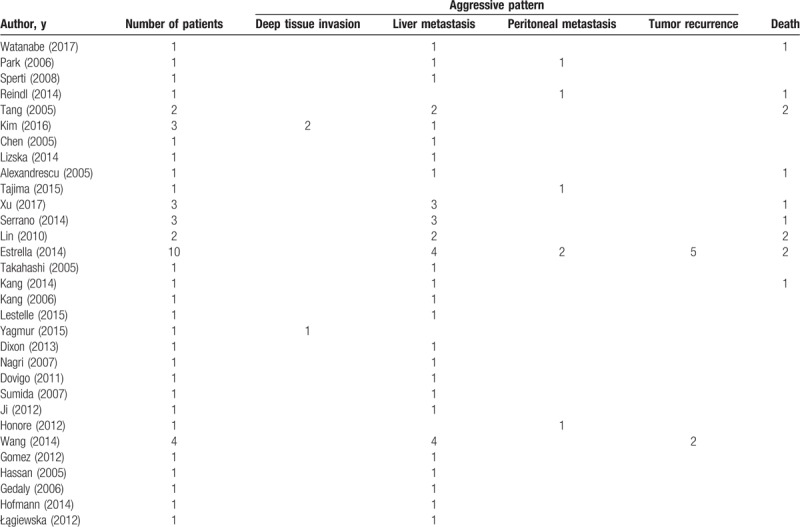
Table 2.
Summary of local patients.
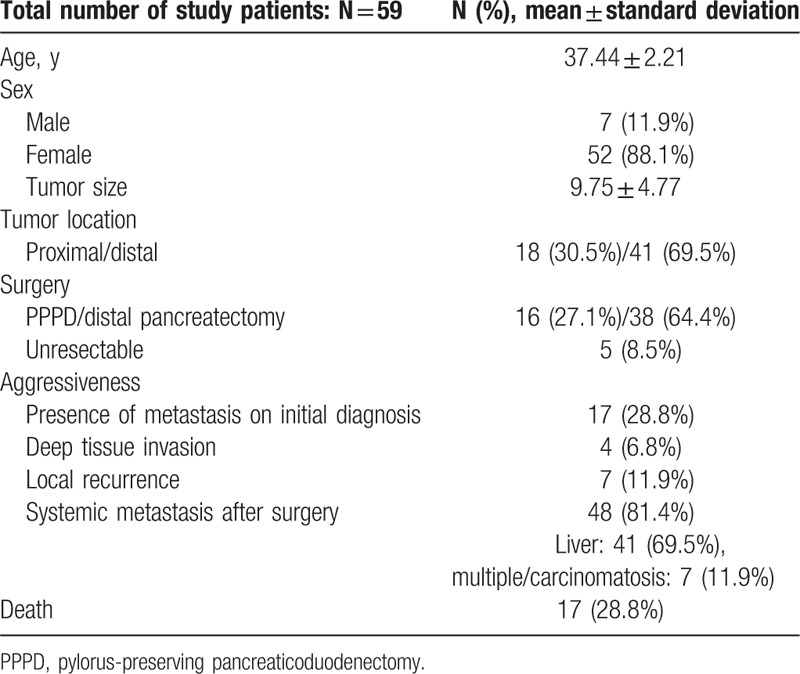
A total of 59 patients were included in the study. The number of women included in this meta-analysis was >7 times compared with the number of men (female to male ratio of 7.43:1). The mean age of the patients was 37.44 years. The most common type of aggressiveness observed was systemic metastasis, which constituted 81.4% of the adverse events. Local recurrence and deep tissue invasion were diagnosed in 11.9% and 6.8% of the patients, respectively. The rate of curative resection was 91.5%, whereas the tumor was deemed unresectable in 8.5% of the patients, and thus, only a biopsy was performed (Table 3).
Table 3.
General characteristics.
3.2. Survival analysis of aggressive SPN of the pancreas
Disease-free survival was noted to be 45 ± 6.28 months (95% confidence interval), and 5-year disease-free survival rate was 26.8% (Fig. 2A). Disease-specific survival for patients with aggressive SPNs was 152.67 ± 12.8 months (approximately 13 years) and the 5- and 10-year survival rates were 71.1% and 65.5%, respectively (Fig. 2A).
Figure 2.
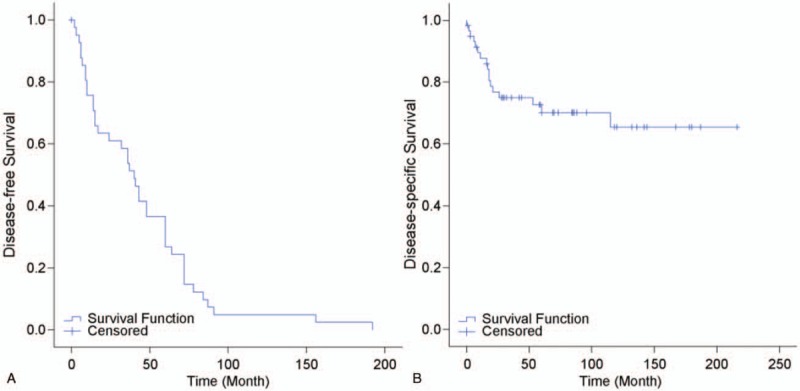
(A) Survival plot for patients with aggressive solid pseudopapillary neoplasm (SPN). (B) Recurrence plot for patients with aggressive SPN.
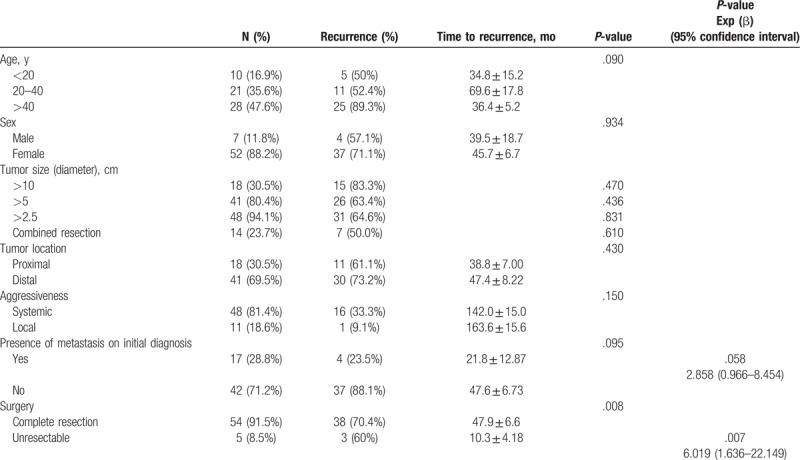
3.3. Factors affecting recurrence of aggressive SPNs
Among the factors measured, only tumors that were determined to be unresectable on initial evaluation were noted to have a significant effect (P = .008) on recurrence. For these patients, because the primary tumor was not removed, recurrence exclusively referred to the development of systemic metastasis.
Using Cox regression survival analysis on these factors, it was identified that only unresectable SPNs was a significant factor in determining recurrence (HR = 6.019, 95% CI: 1.636–22.149, P = .007) (Table 4).
Table 4.
Clinical and pathologic factors affecting recurrence.
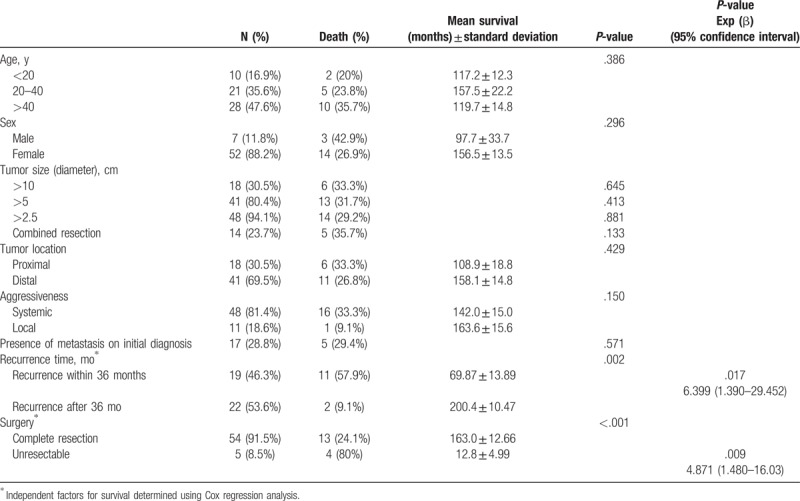
3.4. Prognostic factors affecting survival in aggressive SPN
It was noted that other factors were not significant in predicting survival, such as age, sex, tumor size, tumor location, combined resection (en bloc resection and/or metastasectomy), type of recurrence, and presence of metastasis on initial diagnosis (P < .05). However, unresectable tumors (P < .001) and tumors that metastasize/recur within 3 years (P = .002) had a statistically negative impact on survival. Using Cox regression survival analysis, it was analyzed that both unresectability (HR = 4.871, 95% CI: 1.480–16.03, P = .009) and recurrence within 36 months (HR = 6.399, 95% CI: 1.390–29.452, P = .017) are independent variables for determining survival period (Table 5).
Table 5.
Clinical and pathologic factors affecting long-term course.
4. Discussion
The natural course of SPNs remains to be an enigma. Due to the rarity of these tumors, only a few case reports and isolated institutional experiences describing aggressive SPNs have been published. Most of the selected journal articles were about the successful management of aggressive SPNs with various treatment strategies such as administration of multiple chemotherapeutic agents,[16,24,29] synchronous surgical excision of metastasis,[30,31] liver transplantation,[32–34] and even HIPEC.[35] This can create a form of selection bias in terms of reporting only successful outcomes. In addition, current staging systems investigate pathologic characteristics of the excised tumor which has an inconsistent correlation with the clinical course of the disease.[30] For example, Kim et al claim that the presence of angioinvasion, perineural invasion, and nuclear pleomorphism, pathologic features that are usually associated with malignant behavior, were not shown to be associated with aggressive SPN.[15] Watanabe et al and Reindl et al report that high proliferative index, as in Ki-67, is associated with aggressive course,[1,36] whereas Tang et al did not find a significant association between Ki-67 and aggressive SPN.[19]
The aim of this study was to get a better picture of the natural course of SPNs through compiling and analyzing published journal articles in the hope of increasing the number of comparable cases. To the best of our knowledge, this is the only report, to date, to have the largest pool of patients with aggressive SPNs. To overcome the inconsistent correlation between pathology and natural disease course, we focused on the clinical outcomes as the endpoint for determining aggressiveness.
Our meta-analysis reveals that despite having a 69.5% recurrence and metastasis rate, SPN still has a favorable survival period of 152.67 ± 12.8 months (approximately 13 years), with 5- and 10-year survival rates of 71.1% and 65.5%, respectively. We recognize that both unresectability and recurrence within 3 years are independent factors for decreasing the survival of patients with aggressive SPNs. We also note that unresectability and, to some degree, having metastasis on initial diagnosis, are factors that can lead to recurrence. Kang et al report similar findings in their previous multicenter analysis of 351 patients where having metastasis on diagnosis was significant prognostic factor for tumor recurrence.[37] Another study by Xu et al report that synchronous metastasis was also a significant predictor for recurrence.[17]
Our study is limited by the number of cases reported as well the heterogeneity of the published data. As previously mentioned, most of the information was collected from case reports, owing to which only basic information was found to be common among the published articles. Thus we were unable to analyze the effect of certain factors that have been thought to be associated with a more aggressive SPN. One of these is the Ki-67 index, a pathologic marker for proliferation. It is believed that an elevated Ki-67 index identifies a tumor that is more actively thriving and thus pertains to a more malignant tumor. Several studies have been made linking elevated Ki-67 with poorer disease-free and disease-specific survival.[1,38] Reindl et al reported that increased proliferative index, together with extensive necrosis and nuclear atypia, were uncommon pathologic findings in their patient leading to decreased survival,[36] whereas Kim et al noted the association of elevated Ki-67 with the development of distant metastasis.[39]
Aside from Ki-67 index, the preoperative positron emission tomography (PET)-CT image has also been identified as a potential predictor for aggressive pattern behavior for SPN. Park et al published their findings that a Ki-67 ≥ 3% was associated with increased maximum standard uptake value (SUVmax) on PET-CT.[40] This finding coincides with the research done by Kim et al, where they noted that the SUVmax was significantly higher in patients with T2 and T3 SPN compared with those with T1 tumors.[41]
Information derived from pathologic reports can usually give us a clue regarding the potentially aggressive behavior of most tumors. But in the case of SPNs, there has been some debate regarding the correlation of pathological aggressive characteristics with clinical aggressive behavior. Watanabe et al report that the presence of lymphovascular and capsular invasion, duct and vascular infarction, and tumor necrosis are associated with aggressive behavior of SPN.[1] A Chinese publication by Xu et al also reports that lymphovascular invasion and peripancreatic fat infiltration are associated with the development of distant metastasis.[17] On the contrary, the research of Tang et al claims that patients with either extrapancreatic extension, vascular or perineural invasion, or severe cellular pleomorphism did not demonstrate metastasis during their study period.[19] This claim is echoed by a study done in Korea on 31 patients with SPN. Kim et al report that angioinvasion, perineural invasion, and moderate nuclear pleomorphism were all not significantly correlated with clinically confirmed malignant behavior. Moreover, the absence of these pathological findings could not exclude aggressive behavior.[15]
The SPNs have a unique natural course, and we recommend further study into the possible causes for the aggressive behavior of SPNs. As mentioned, perioperative information such as Ki-67 index,[1,36] PET-CT scan quantitative reports,[18] and pathologic reports[1] have all been suggested to predict SPN behavior. These information can then be channeled for standardizing the reporting of other cases of aggressive SPNs to obtain clearer insights on this disease. Furthermore, we recommend that a surveillance program should be crafted for each patient, taking into account the possibility of delayed metastasis and the negative impact of early metastasis to overall survival outcomes.
Author contributions
Conceptualization: Chang Moo Kang.
Data curation: Emmanuel II Uy Hao.
Formal analysis: Emmanuel II Uy Hao, Chang Moo Kang, Ho Kyoung Hwang, Dong-Sub Yoon, Woo Jung Lee.
Investigation: Emmanuel II Uy Hao.
Methodology: Emmanuel II Uy Hao.
Software: Emmanuel II Uy Hao.
Supervision: Chang Moo Kang, Ho Kyoung Hwang, Dong-Sub Yoon, Woo Jung Lee.
Validation: Dong-Sub Yoon, Woo Jung Lee.
Writing – original draft: Emmanuel II Uy Hao.
Writing – review & editing: Chang Moo Kang, Ho Kyoung Hwang, Dong-Sub Yoon, Woo Jung Lee.
Supplementary Material
Footnotes
Abbreviations: CT = computed tomography, HIPEC = hyperthermic intraperitoneal chemotherapy, HR = hazards ratio, PET = positron-emission tomography, SPN = solid pseudopapillary neoplasm.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Watanabe Y, Okamoto K, Okada K, et al. A case of aggressive solid pseudopapillary neoplasm: comparison of clinical and pathologic features with non-aggressive cases. Pathol Int 2017;67:202–7. [DOI] [PubMed] [Google Scholar]
- [2].Martin RC, Klimstra DS, Brennan MF, et al. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol 2002;9:35–40. [DOI] [PubMed] [Google Scholar]
- [3].Bhutani N, Kajal P, Singla S, et al. Solid pseudopapillary tumor of the pancreas: experience at a tertiary care centre of Northern India. Int J Surg Case Rep 2017;39:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ahmed Z, Yaqoob N, Muzaffar S, et al. Solid cystic papillary epithelial neoplasm of pancreas: a series of three cases with review of literature. J Pak Med Assoc 2004;54:392–4. [PubMed] [Google Scholar]
- [5].Alexandrescu DT, O’Boyle K, Feliz A, et al. Metastatic solid-pseudopapillary tumour of the pancreas: clinico-biological correlates and management. Clin Oncol (R Coll Radiol) 2005;17:358–63. [DOI] [PubMed] [Google Scholar]
- [6].Zhong Y, Wang HY, Linghu EQ, et al. Clinical and MRI features of SPN of pancreas in male patients. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2017;39:471–6. [DOI] [PubMed] [Google Scholar]
- [7].Dong A, Wang Y, Dong H, et al. FDG PET/CT findings of solid pseudopapillary tumor of the pancreas with CT and MRI correlation. Clin Nucl Med 2013;38:e118–24. [DOI] [PubMed] [Google Scholar]
- [8].Chong A, Ha JM, Kwon SY. Multiple diagnostic imaging of a patient with solid pseudopapillary tumour of the pancreas: EUS, CT and FDG PET/CT. Nucl Med Mol Imaging 2014;48:82–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sunkara S, Williams TR, Myers DT, et al. Solid pseudopapillary tumours of the pancreas: spectrum of imaging findings with histopathological correlation. Br J Radiol 2012;85:e1140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg 2005;200:965–72. [DOI] [PubMed] [Google Scholar]
- [11].Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: a typically cystic carcinoma of low malignant potential. Semin Diagn Pathol 2000;17:66–80. [PubMed] [Google Scholar]
- [12].Luttges J. What's new? The 2010 WHO classification for tumours of the pancreas [in German]. Pathologe 2011;32:332–6. [DOI] [PubMed] [Google Scholar]
- [13].Kang CM, Kim KS, Choi JS, et al. Solid pseudopapillary tumor of the pancreas suggesting malignant potential. Pancreas 2006;32:276–80. [DOI] [PubMed] [Google Scholar]
- [14].Sperti C, Berselli M, Pasquali C, et al. Aggressive behaviour of solid-pseudopapillary tumor of the pancreas in adults: a case report and review of the literature. World J Gastroenterol 2008;14:960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim JH, Lee JM. Clinicopathologic review of 31 cases of solid pseudopapillary pancreatic tumors: can we use the scoring system of microscopic features for suggesting clinically malignant potential? Am Surg 2016;82:308–13. [PubMed] [Google Scholar]
- [16].Tajima H, Takamura H, Kitagawa H, et al. Multiple liver metastases of pancreatic solid pseudopapillary tumor treated with resection following chemotherapy and transcatheter arterial embolization: a case report. Oncol Lett 2015;9:1733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu Y, Zhao G, Pu N, et al. One hundred twenty-one resected solid pseudopapillary tumors of the pancreas: an 8-year single-institution experience at Zhongshan Hospital, Shanghai. China Pancreas 2017;46:1023–8. [DOI] [PubMed] [Google Scholar]
- [18].Kang CM, Cho A, Kim H, et al. Clinical correlations with (18)FDG PET scan patterns in solid pseudopapillary tumors of the pancreas: still a surgical enigma? Pancreatology 2014;14:515–23. [DOI] [PubMed] [Google Scholar]
- [19].Tang LH, Aydin H, Brennan MF, et al. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol 2005;29:512–9. [DOI] [PubMed] [Google Scholar]
- [20].Serrano PE, Serra S, Al-Ali H, et al. Risk factors associated with recurrence in patients with solid pseudopapillary tumors of the pancreas. JOP 2014;15:561–8. [DOI] [PubMed] [Google Scholar]
- [21].Takahashi Y, Fukusato T, Aita K, et al. Solid pseudopapillary tumor of the pancreas with metastases to the lung and liver. Pathol Int 2005;55:792–6. [DOI] [PubMed] [Google Scholar]
- [22].Law JK, Ahmed A, Singh VK, et al. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas 2014;43:331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nakagohri T, Kinoshita T, Konishi M, et al. Surgical outcome of solid pseudopapillary tumor of the pancreas. J Hepatobiliary Pancreat Surg 2008;15:318–21. [DOI] [PubMed] [Google Scholar]
- [24].Estrella JS, Li L, Rashid A, et al. Solid pseudopapillary neoplasm of the pancreas: clinicopathologic and survival analyses of 64 cases from a single institution. Am J Surg Pathol 2014;38:147–57. [DOI] [PubMed] [Google Scholar]
- [25].Lubezky N, Papoulas M, Lessing Y, et al. Solid pseudopapillary neoplasm of the pancreas: management and long-term outcome. Eur J Surg Oncol 2017;43:1056–60. [DOI] [PubMed] [Google Scholar]
- [26].Tao L, Yuan C, Ma Z, et al. Surgical resection of a primary tumor improves survival of metastatic pancreatic cancer: a population-based study. Cancer Manag Res 2017;9:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Malvezzi M, Bertuccio P, Levi F, et al. European cancer mortality predictions for the year 2013. Ann Oncol 2013;24:792–800. [DOI] [PubMed] [Google Scholar]
- [28].Sohal DP, Mangu PB, Khorana AA, et al. Metastatic pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2784–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gomez P, Yorke R, Ayala AG, et al. Solid-pseudopapillary neoplasm of pancreas with long delayed liver metastasis. Ann Diagn Pathol 2012;16:380–4. [DOI] [PubMed] [Google Scholar]
- [30].Liszka L, Mrowiec S, Pajak J, et al. Limited usefulness of histopathological features in identification of a clinically aggressive solid-pseudopapillary neoplasm of the pancreas. Pol J Pathol 2014;65:182–93. [DOI] [PubMed] [Google Scholar]
- [31].Wang WB, Zhang TP, Sun MQ, et al. Solid pseudopapillary tumor of the pancreas with liver metastasis: clinical features and management. Eur J Surg Oncol 2014;40:1572–7. [DOI] [PubMed] [Google Scholar]
- [32].Dovigo AG, Diaz MB, Gutierrez MG, et al. Liver transplantation as treatment in a massive metastasis from Gruber-Frantz pancreatic tumor: a case report. Transplant Proc 2011;43:2272–3. [DOI] [PubMed] [Google Scholar]
- [33].Sumida W, Kaneko K, Tainaka T, et al. Liver transplantation for multiple liver metastases from solid pseudopapillary tumor of the pancreas. J Pediatr Surg 2007;42:e27–31. [DOI] [PubMed] [Google Scholar]
- [34].Lagiewska B, Pacholczyk M, Lisik W, et al. Liver transplantation for nonresectable metastatic solid pseudopapillary pancreatic cancer. Ann Transplant 2013;18:651–3. [DOI] [PubMed] [Google Scholar]
- [35].Honore C, Goere D, Dartigues P, et al. Peritoneal carcinomatosis from solid pseudopapillary neoplasm (Frantz's tumour) of the pancreas treated with HIPEC. Anticancer Res 2012;32:1069–73. [PubMed] [Google Scholar]
- [36].Reindl BA, Lynch DW, Jassim AD. Aggressive variant of a solid pseudopapillary neoplasm: a case report and literature review. Arch Pathol Lab Med 2014;138:974–8. [DOI] [PubMed] [Google Scholar]
- [37].Kang CM, Choi SH, Kim SC, et al. Predicting recurrence of pancreatic solid pseudopapillary tumors after surgical resection: a multicenter analysis in Korea. Ann Surg 2014;260:348–55. [DOI] [PubMed] [Google Scholar]
- [38].Yang F, Yu X, Bao Y, et al. Prognostic value of Ki-67 in solid pseudopapillary tumor of the pancreas: Huashan experience and systematic review of the literature. Surgery 2016;159:1023–31. [DOI] [PubMed] [Google Scholar]
- [39].Kim EK, Jang M, Park M, et al. LEF1, TFE3, and AR are putative diagnostic markers of solid pseudopapillary neoplasms. Oncotarget 2017;8:93404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Park M, Hwang HK, Yun M, et al. Metabolic characteristics of solid pseudopapillary neoplasms of the pancreas: their relationships with high intensity (18)F-FDG PET images. Oncotarget 2018;9:12009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim YI, Kim SK, Paeng JC, et al. Comparison of F-18-FDG PET/CT findings between pancreatic solid pseudopapillary tumor and pancreatic ductal adenocarcinoma. Eur J Radiol 2014;83:231–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


