Abstract
Rationale:
Tracheobronchial adenoid cystic carcinoma (TACC) is a rare malignancy. Surgical resection remains the standard treatment of choice. But it is frequently unresectable due to its local extension. The practicability and safety of hypofractionated radiotherapy (HRT) for TACC remains unknown since most of the TACCs are centrally located.
Patient concerns:
A 57-year-old female presented with paroxysmal cough, expectoration, and hemoptysis.
Diagnoses:
Computed tomography (CT) scan of the chest revealed a nodule originating from the wall of right primary bronchus, with 1.9∗1.2 cm in size. Bronchoscope confirmed the node on the medial wall of the right primary bronchus extending towards the carina, with a close distance of 0.5 cm. Biopsy from the node was considered as adenoid cystic carcinoma (ACC). The clinical stage of the patient was T3N0M0.
Interventions:
The patient underwent HRT with a total dose of 60Gy in twelve fractions.
Outcomes:
The patient experienced complete remission after HRT. No symptomatic radiation-induced toxicity (≥grade 2) was observed during the follow-up.
Lessons:
HRT may be a safe and effective modality for inoperable TACC.
Keywords: adenoid cystic carcinoma, bronchus, complete remission, hypofractionated radiotherapy
1. Introduction
Adenoid cystic carcinoma (ACC) of the central airway is a rare malignancy, representing about 10% of the histology. Tracheobronchial ACC (TACC) originates from the salivary glands in the mucosa and is considered as low malignant potential. However, it can be locally infiltrative and life-threatening when causing central airway obstruction. Surgery is the standard care for TACC. Unfortunately, it is frequently unresectable due to its local extension. Although ACC seems to possess radiosensitivity and most tumors respond to radiotherapy, the prognosis for locally advanced inoperable TACC is poor, with a mean survival time (MST) of 41 months and a 5-year overall survival (OS) of about 33.3%.[1] The majority of these cases treated with conventional definitive radiotherapy died later from local recurrence.
Hypofractionated radiotherapy (HRT) is now more widely prescribed due to improved techniques (intensity modulated radiotherapy, image-guided radiotherapy, and stereotactic radiotherapy), which brings an opportunity for improved outcomes of inoperable TACC. The practicability and safety of HRT for TACC remain unknown since most of the TACCs are centrally located.
Here we presented a case of unresectable TACC treated by HRT in our institution. To the best of our knowledge, this is the first reported case to elucidate the role of HRT for treating this rare disease.
2. Case report
A 57-year-old lady presented in December 2016 with history of paroxysmal cough and expectoration for 1 month. She also had blood in phlegm. There was no history of dyspnea, chest pain or fever. She had a medical history of chronic bronchitis and hypertension for more than ten years. No abnormalities were observed after general physical examination. Computed tomography (CT) scan of the chest revealed a nodule originating from the wall of right primary bronchus, with 1.9∗1.2 cm in size (Fig. 1 A–B). Bronchoscope confirmed the node on the medial wall of the right primary bronchus extending towards the carina, with a close distance of 0.5 cm (Fig. 2 A–B). Biopsy from the node was considered as ACC. The expression of p40, p63, CK7 were positively assessed by immunohistochemical analysis, while napsinA, TTF-1 were negative. CT of abdominal cavity, ultrasound of neck and scan of bone were also conducted, but none of them revealed abnormality. The clinical stage of the patient was T3N0M0 (UICC/AJCC 7th).
Figure 1.
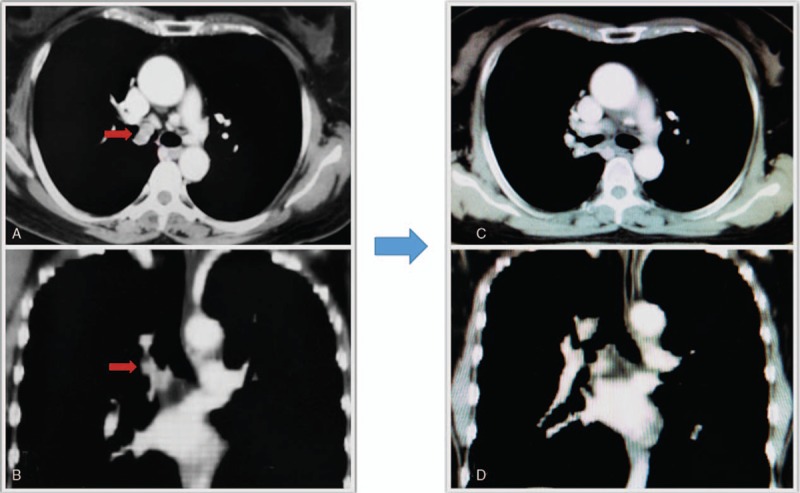
The images of the lesion examined by computer tomography (A–B before treatment, C–D 6 months after the treatment). Patient achieved complete remission after the treatment.
Figure 2.
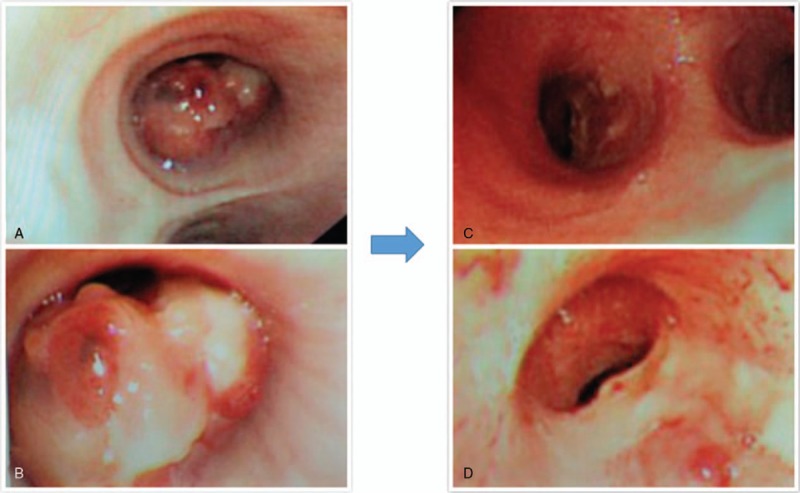
The bronchoscopic picture of the lesion (A–B before treatment, C–D 6 months after the treatment). The lesion was completely involuted after the treatment.
Surgical resection was contraindicated in view of its extensive local invasion from almost the carina down to the right main bronchus. Additionally, the patient has a history of chronic bronchitis for 10 years and poor pulmonary function, which would put the patient at high risk of postoperative complications. Thus the patient received HRT.
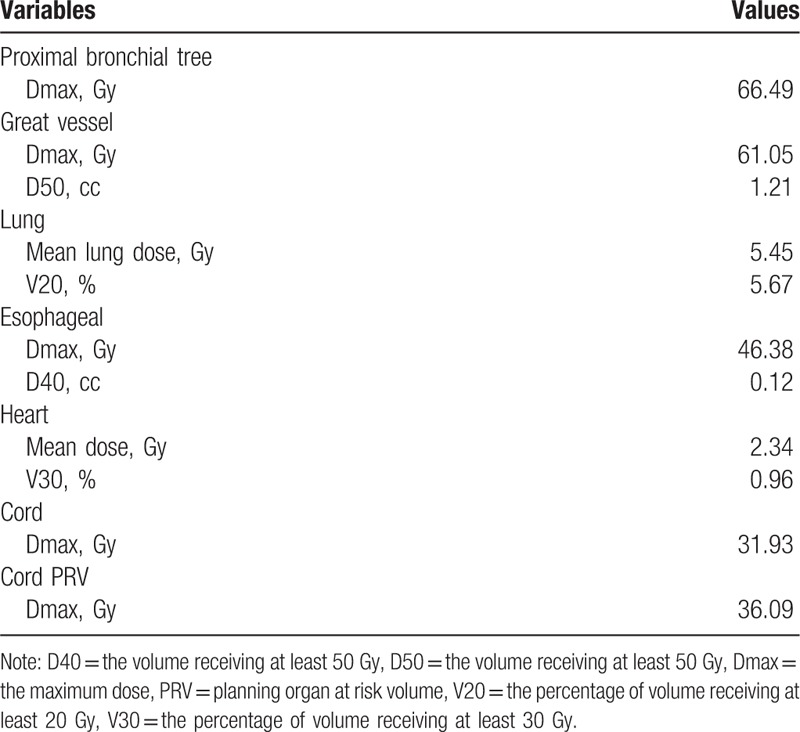
The patient was immobilized and a planning contrast-enhanced CT was conducted in a proper position. Target movement caused by breathing was detected by 4D mode. Internal gross tumor volume (IGTV) was contoured on CT images. Planning target volume (PTV) was expanded 1 cm in longitudinal and 0.5 cm in axial from primary tumor. Pinnacle System was used to design the plan and 6MV-X linear accelerator based IMRT to present radiotherapy. A total dose of 60Gy was conducted in twelve fractions, with 5Gy per fraction. The dose distribution in the radiation field and dose volume histogram were shown in Figure 3 and Figure 4. Dose to the organs at risk was shown in Table 1. The patient completed the whole treatment without interruption.
Figure 3.
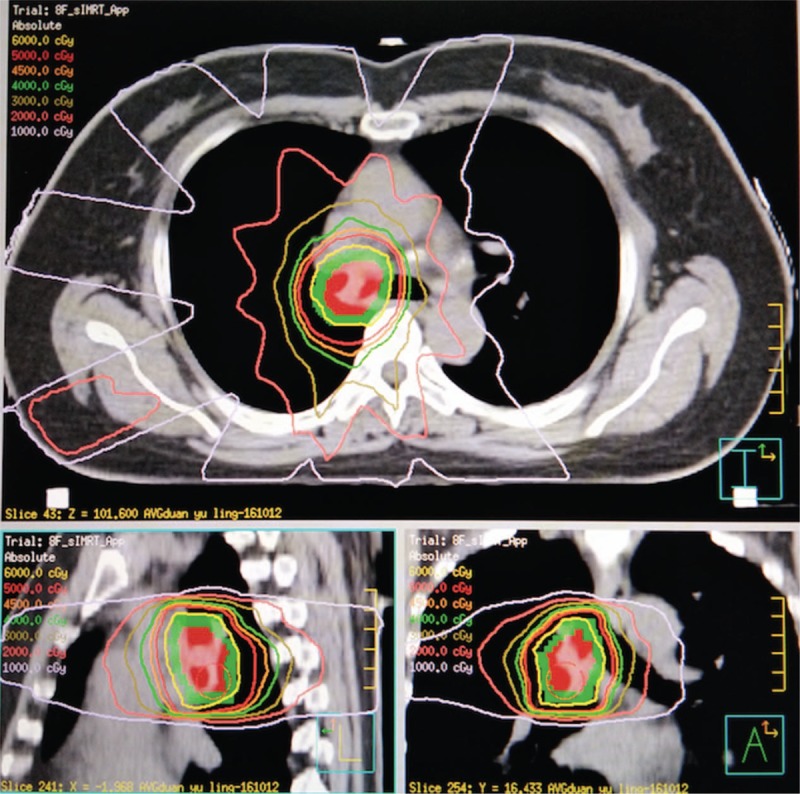
Dose distributions of HRT plan. Good conformability was observed for the target area. HRT = hypofractionated radiotherapy.
Figure 4.
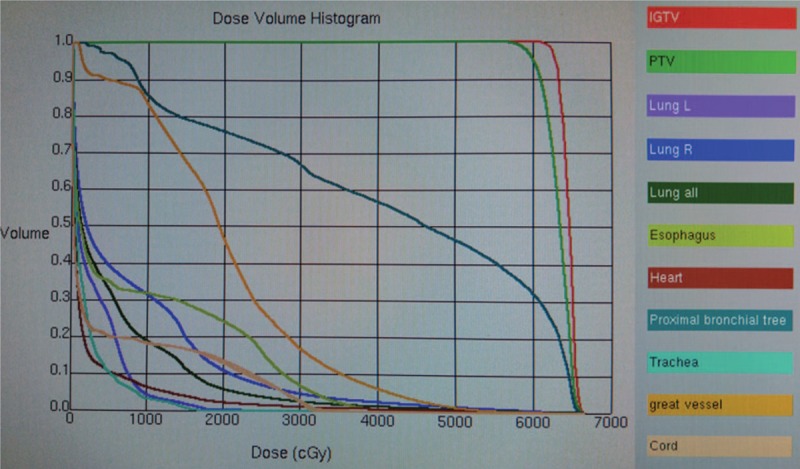
Dose volume histogram of the plan. 95% of the PTV received sufficient prescribed dose (60Gy). PTV = planning target volume.
Table 1.
Dose to the organs at risk.
The therapeutic outcomes were evaluated using CT (Fig. 1 C–D) and Bronchoscope (Fig. 2 C–D). Tumor shrinkage was observed at the end of the treatment, and complete response was achieved 6 months later. The date for last follow-up was May 1, 2018 and no sign of disease progression was observed.
3. Discussion
ACC of airway mostly originates from trachea and bronchus. Progressive airway obstruction is caused by the growth of tumor. The symptoms include cough, expectoration, wheeze and dyspnea, which are nonspecific. ACC always spread by direct extension and only 10% of patients have regional lymph-node metastasis or hematogenous metastasis.[2] Surgical resection remains the standard treatment for ACC if possible, with 5-year OS ranged from 70% to 100%.[3] Although the success rate of complete resection for ACC of airway is low, the addition of postoperative radiotherapy reduces risk of local recurrence, leading to a favourable outcome.
There might be a dose-response relationship between RT dose and treatment outcomes of ACC. Several studies with small size of examples have explored the efficiency of radiotherapy with conventional fractions in unresectable or medically inoperable patients.[3–5] In the study conducted by Je,[3] 9 patients with ACC of trachea were treated by radiotherapy with total dose of 66 to 80Gy. The radiotherapy was delivered at 1.8 to 2.2Gy per fraction. The patients were assigned into high dose group (≥70.8Gy) and low dose group (≤70.2Gy) according to total dose. As a result, patients with high dose achieve superior 5-year local-recurrence free survival (LRFS) (100% vs 0) and OS (83.3% vs 33.3%) than those with low dose.
HRT can confer great dose of radiation to the tumor target with highly conformal techniques, which is suitable for unresectable ACC. HRT is considered to be more applicable in malignant tumors with low value of α/β, such as prostate cancer.[6] Since ACC is a low-grade carcinoma, HRT is theoretically possible for the disease.
Compared with conventional radiotherapy, HRT has great advantage in raising local control rates with increased biological effective dose (BED), which has been widely used in peripherally located early stage non-small-cell lung cancer. Besides, various studies explored its role in centrally located tumors, the results remained controversial. Excessive toxicities of critical structures such as bronchial tree, major vessels, esophagus, heart, and brachial plexus limited the practice of HRT in patients with centrally located lesions.[7,8] Li[9] explored the efficacy of stereotactic ablative radiotherapy (SABR) using 70Gy in 10 fractions for 82 patients, 89% of which had T1-2N0M0, and 52% had centrally located lesions. The results showed that 2-year OS and local control rates were 66.9% and 96.2%. Only 2.4% suffered radiation pneumonitis with grade 3 and no patients developed brachial plexopathy, which was well tolerated. According to the guideline determined by American Society for Radiation Oncology, delivery of HRT in 6 to 15 fractions is recommended for central tumors in close proximity to the proximal bronchial tree, which may reduce the risks of severe toxicity.[10] Therefore, 12 fractions with total dose of 60Gy were applied in our case. Desipite maximum dose of bronchial tree exceed 60Gy, symptomatic bronchial injury was not observed. The patient did not suffered symptomatic radiation pneumonitis or esophagitis. We failed to observe long-term outcome with short follow-up time, but the result was encouraging since the patient achieved complete response with accepted toxicity. More practices are needed to explore ideal fractions and dose to warrant long-term outcome.
Author contributions
DQW participated in the management of the patient, the collection, analysis, and interpretation of data, and drafting of the manuscript. NB participated in the radiation management of the patient and revised the article. DFC and LHW contributed the radiotherapy components of the manuscript. All authors read and approved the final manuscript.
Data curation: Daquan Wang, Nan Bi, Luhua Wang.
Formal analysis: Daquan Wang, Nan Bi.
Investigation: Dongfu Chen.
Writing – original draft: Daquan Wang.
Writing – review & editing: Nan Bi, Dongfu Chen, Luhua Wang.
Footnotes
Abbreviations: ACC = adenoid cystic carcinoma, CT = computed tomography, HRT = hypofractionated radiotherapy, OS = overall survival, TACC = tracheobronchial adenoid cystic carcinoma.
Ethics approval is not necessary, because the paper is a case report.
Written informed consent was obtained from patient's legal guardian for publication of this case report and any accompanying images.
The authors have no conflicts of interest to disclose.
References
- [1].Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 2004;78:1889–96. [DOI] [PubMed] [Google Scholar]
- [2].Haresh KP, Prabhakar R, Rath GK, et al. Adenoid cystic carcinoma of the trachea treated with PET-CT based intensity modulated radiotherapy. J Thorac Oncol 2008;3:793–5. [DOI] [PubMed] [Google Scholar]
- [3].Je HU, Song SY, Kim DK, et al. A 10-year clinical outcome of radiotherapy as an adjuvant or definitive treatment for primary tracheal adenoid cystic carcinoma. Radiat Oncol 2017;12:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee JH, Jung EJ, Jeon K, et al. Treatment outcomes of patients with adenoid cystic carcinoma of the airway. Lung Cancer 2011;72:244–9. [DOI] [PubMed] [Google Scholar]
- [5].Maziak DE, Todd TR, Keshavjee SH, et al. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg 1996;112:1522–31. [DOI] [PubMed] [Google Scholar]
- [6].Nahum AE. The radiobiology of hypofractionation. Clin Oncol 2015;27:260–9. [DOI] [PubMed] [Google Scholar]
- [7].Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036–43. [DOI] [PubMed] [Google Scholar]
- [8].Senthi S, Haasbeek CJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013;106:276–82. [DOI] [PubMed] [Google Scholar]
- [9].Li Q, Swanick CW, Allen PK, et al. Stereotactic ablative radiotherapy (SABR) using 70 Gy in 10 fractions for non-small cell lung cancer: exploration of clinical indications. Radiother Oncol 2014;112:256–61. [DOI] [PubMed] [Google Scholar]
- [10].Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic body radiotherapy for early-stage non-small-cell lung cancer: american society of clinical oncology endorsement of the american society for radiation oncology evidence-based guideline. J Clin Oncol 2018;36:710–9. [DOI] [PubMed] [Google Scholar]


