Abstract
Rationale:
Metastatic pancreatic cancer has a dismal prognosis. Many patients seek integrative care as an add-on to their conventional cancer treatment. Viscum album extracts (VAE)—widely used as an adjunct to cancer treatment—have cytotoxic, apoptogenic, and immune stimulatory properties. A statistically significant survival benefit has been demonstrated for VAE in advanced pancreatic cancer.
Patient concerns and diagnosis:
A 28-year old patient presented with painless jaundice and was subsequently diagnosed as pancreatic adenocarcinoma with liver metastases.
Interventions:
He was treated with FOLFIRINOX/Mitomycin, hyperthermia and fever-inducing VAE.
Outcomes:
Subsequently, the liver metastases regressed. Surgical intervention involved successful R0-resection of the primary tumor, as well as an atypical liver resection. A relapse was again treated with FOLFIRINOX/Mitomycin and hyperthermia. As of publication of this report, 49 months after initial diagnosis, the patient exhibits good condition, and is unrestricted in quality of life (till publication).
Lessons:
This case demonstrates the favorable outcome of a patient with metastatic pancreatic cancer following treatment with chemotherapy, integrative medicine, and surgical excision. As other positive outcomes in pancreatic cancer patients are related to inflammatory events, we presume the immunologic effects of VAE to have contributed to the favorable outcome here. Based on this case, and the other positive results of VAE use in pancreatic cancer, further investigations seem highly worthwhile.
Keywords: fever, integrative medicine, mistletoe, pancreatic cancer, Viscum album
1. Introduction
Pancreatic cancer (PC) is a common malignancy (incidence 12.4/100.000 in the US),[1] with poor prognosis (5-year survival of 2%),[2] especially in cases involving metastatic disease (a median survival of 3.7 months[3] and 6.4 months, despite chemotherapy).[4] In all cases, in which R0-resection seems feasible, surgery is recommended. Chemotherapy is recommended as a primary or adjuvant treatment. Type of chemotherapy regimen is chosen according to tumor stage and the patient's performance status. For metastatic disease, palliative FOLFIRINOX provides best results in terms of quality of life and survival; however, due to its high toxicity, it is restricted to younger patients with favorable overall condition.[5]
Viscum album extracts (VAE)—aqueous extracts made from European mistletoe (Viscum album L.)—possess a variety of antineoplastic properties including cytotoxic effects, apoptosis induction, immune stimulation, downregulation of cancer genes (e.g., transforming growth factor β and matrix-metalloproteinases), reduction of cell migration, and interference with tumor angiogenesis.[6–8] Pharmacologically active compounds include mistletoe lectins (ML), viscotoxins, oligo- and polysaccharids, flavonoids, and triterpene acids.[8] Different injectable forms of VAE preparations (typically for subcutaneous use, or in some cases, for intralesional or intravenous application) are commercially available, and are used as supportive therapy in patients with cancer.[9] Besides a more common range of side effects such as erythema at the injection site, fever, flu-like symptoms, and more rarely, pseudo allergic reactions, VAE are safe, even when used in higher dosages.[9] In a phase III randomized trial with 220 patients, a survival benefit of 2.1 months (hazard ratio = 0.49; P < .0001) was documented in cases of inoperable advanced pancreatic cancer treated with VAE. Compared to the control group, which received best supportive care only, the VAE group demonstrated better outcomes for several quality of life parameters, and exhibited positive weight gain effects.[10,11] This result is in line with previous investigations, though these are of lower methodological quality.[12]
We present a case of a young patient with advanced PC, treated with standard treatment and added hyperthermia and VAE leading to response to treatment and long-term survival. The case is presented in accordance to the CARE guideline.[13]
2. Case presentation
A lean, nonsmoking, 28-year old Caucasian marketing manager, with no family history of cancer, presented with painless jaundice. He was initially diagnosed with obstruction of the bile duct, most likely resulting from ampullary adenoma without elevation of tumor markers. Six months later, the patient underwent explorative laparotomy, which surprisingly revealed an adenocarcinoma of the pancreatic head (56 × 40 mm), and liver metastases (26 × 20 mm in segment II/III, 18 × 12 mm in segment V, and 5 mm diameter in segment VIII) (cT4, cN1, cM1: UICC stage IV). Histologic investigation of the liver metastasis biopsy showed a moderately differentiated ductal adenocarcinoma, displaying moderate nuclear pleomorphism, multiple atypical mitoses, and a strong expression of cytokeratin-7 and S100-protein, but no cytokeratin-20 staining (see Fig. 1). Carbohydrate antigen 19-9 was elevated to 895.5 U/mL, whereas carcinoembryonic antigen was in the normal range. As part of the palliative treatment plan, the patient was commenced on a first chemotherapy cycle (2 treatments with an interval of 7 days) of FOLFOX/Mitomycin (administered as an intravenous bolus of mitomycin C 5 mg, followed by a 30-minutes infusion of oxaliplatin 50 mg, a 1-hour infusion of leucovorin 400 mg, a bolus of fluorouracil 500 mg, and a subsequent 24-hour continuous infusion of 1000 mg). This was supported with additional integrative medical treatments such as hyperthermia (locoregional deep hyperthermia in the upper abdomen with Oncotherm-EHY2010/3010 and whole body hyperthermia) and phytotherapeutic and supplemental agents. These included extracts from milk thistle, artichoke leaf extract, zinc, selenium, vitamin D, and pancreatic enzymes. After a second chemotherapy cycle (2 treatments with an interval of 7 days), which was carried out per a FOLFIRINOX/Mitomycin protocol (intravenous bolus of mitomycin C 5 mg, followed by a 30-minutes infusion of oxaliplatin 50 mg, a 20-minutes infusion of irinotecan 80 mg, a 2-hour infusion of leucovorin 400 mg, a bolus of fluorouracil 500 mg, and a subsequent 24-hour continuous infusion of 1500 mg), a CT scan showed regression of 2 of the liver metastases (from 26×20 to 13 mm in diameter, and from 18×12 mm to 12×10 mm). A further metastasis remained, however, largely unchanged (from 5 to 6 mm). The patient was then administered VAE both subcutaneously (AbnobaViscum Fraxini 40 mg, containing 700 ng/mg ML and 4.75 μg/mg viscotoxin), as well as intralesionally, via endosonography directly into the primary tumor (AbnobaViscum Fraxini 80 mg). The patient subsequently developed fever up to 39.9°C which was well tolerated with fluid replacement. At the subcutaneous injection site, a self-limiting local skin reaction developed (erythema, swelling, induration and itching). Subcutaneous VAE therapy was continued with weekly high-dose VAE (Iscucin salicis Stärke H, 5 mg VAE/mL, ML: 5900 ng/mL)[14] and weekly highly diluted VAE (Iscucin salicis Stärke A, high dilution 1:1, 024×10).[13] After 2 weeks, the patient stopped the VAE treatment due to high-fever reactions following the high-dose injections; after treatment with highly diluted VAE, the patient also described a temperature elevation of approximately 1°C. FOLFIRINOX was continued for another 2 cycles. At the following CT scan, no liver metastases could be detected.
Figure 1.
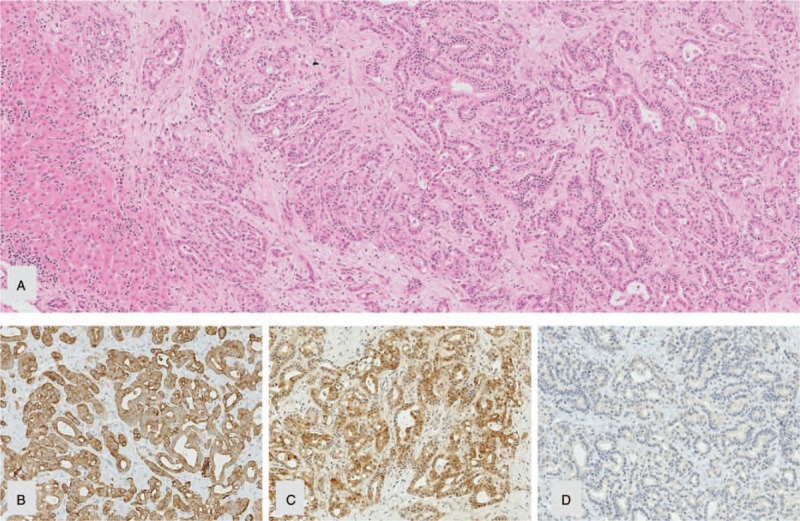
(A) Liver metastasis of an adenocarcinoma with immunophenotype ([B] CK 7 pos.; [C] S100P pos.; [D] CK 20 neg.) of a primary in the upper GI-tract (in particular ductal pancreatic adenocarcinoma) before treatment (obj.-magn. 10×).
Due to this favorable treatment response, a multidisciplinary tumor board panel decided to perform a resection of the primary tumor and the liver lesions. A pylorus-preserving pancreaticoduodenectomy with atypical liver resection was performed (Fig. 2 for the resected tumor and Fig. 3 for the situs), along with a histopathological R0 resection. At the site of liver metastases, the tissue showed necrotic lesions, with no viable tumor cells (see Fig. 4). The primary tumor was classified as a ductal adenocarcinoma of the pancreatic head, with lymph node metastasis and perineural invasion (ypT1, ypN1 (mi) (1/29), L0, V0, Pn1, R0, Gx). The histopathological tumor regression was classified as a “major response.”[15] During the postoperative course, the patient underwent further repeat laparotomies because of a complicated pancreatic fistula, but recovered and was discharged after a prolonged hospital stay. Eight months after surgery the patient remained well and displayed no limitations to quality of life. He maintained full capacity and continued working in his job. After a postoperative follow-up of 27 months (33 months after initial diagnose), the patient showed elevated tumor markers (CA19–9: 291.4 U/mL) and tumor cells were found in biopsies of the pancreatic tail. The patient was treated with 2 cycles of FOLFIRINOX/Mitomycin with hyperthermia and recovered after this treatment. To prevent further relapses, complete extirpation of the remaining pancreas plus spleen was performed. Pathologic investigation showed multifocal tumor invasions within the pancreatic tissue and 2 lymph node metastases (of 23 nodes); minimal resection border was 1 mm (positive circumferential resection margin). The patient exhibits good condition, is unrestricted in quality of life, and working full-time in his job, 49 months after initial diagnosis (till publication; for details of the course see Fig. 5).
Figure 2.
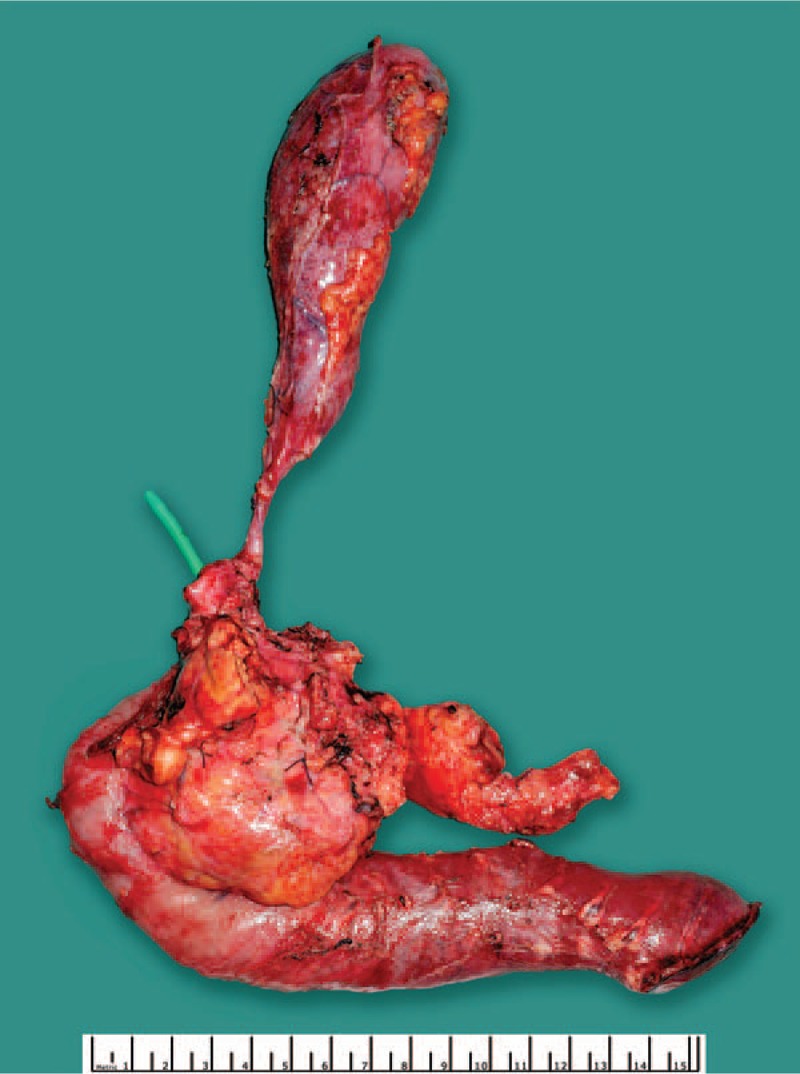
Tumor of the patient after surgical excision.
Figure 3.
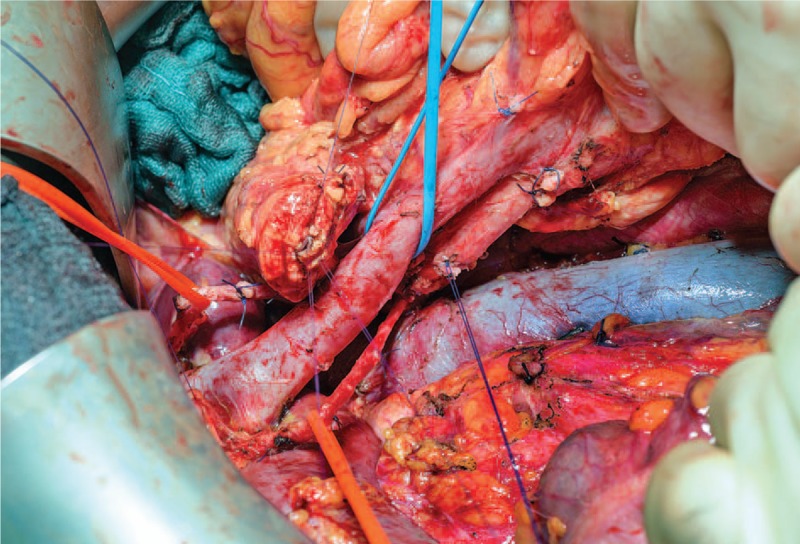
Surgical situs of the patient after removal of the patients’ tumor.
Figure 4.
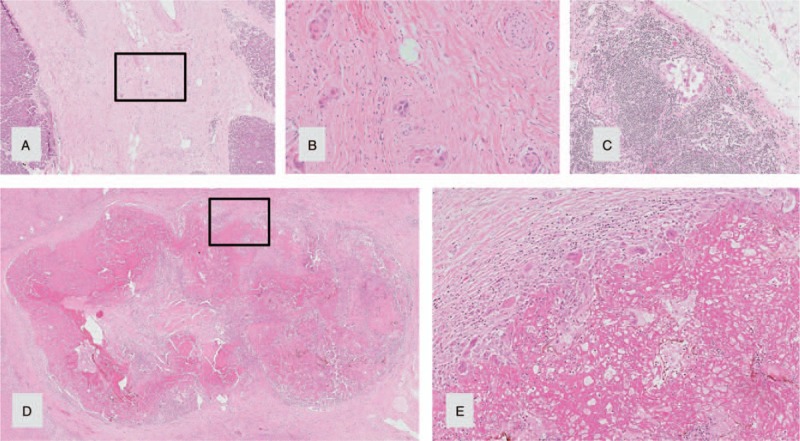
Pylorus-preserving pancreaticoduodenectomy specimen and liver metastasis after neoadjuvant treatment showing major histopathological response (14): (A) pancreatic tissue with dense fibrosis and single groups of residual tumor/carcinoma cells (HE, obj.-magn. 20×). (B) Residual tumor cells, higher magnification of A (obj. magn. 40×). (C) Micrometastasis to a regional peripancreatic lymph node (Obj.-magn. 10×). (D) Complete tumor regression of a liver metastasis with necrosis, fibrosis and no residual cancer cells (HE, obj.-magn, 1×). (E) Resorptive inflammatory reaction with histiocytes, multinucleated histiocytic giant cells and lymphocytes, higher magnification of D (HE, obj.-magn, 10×).
Figure 5.
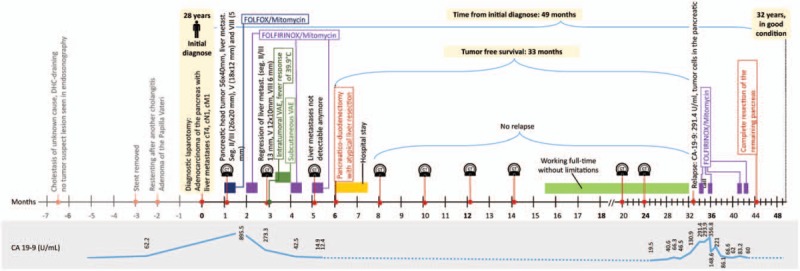
Timeline of the patient with metastatic pancreatic cancer.
3. Discussion
The current case report details the ongoing, long-time survival (49 months) of a patient with an initially inoperable metastatic pancreatic cancer, which, following treatment, underwent complete regression of liver metastases. Successful R0-resection was performed. Therapeutic measures included 1 cycle of FOLFOX/Mitomycin, 3 cycles of FOLFIRINOX/Mitomycin, 4 cycles of FOLFIRINOX/Mitomycin after relapse, surgical treatment, and integrative medical interventions such as local and systemic VAE administration, resulting in strong immunological reactions (high fever, erythema, swelling, induration and itching at the injection site).
In the setting of PC, neoadjuvant treatment with FOLFIRINOX is typically only considered in patients with borderline tumors, but is not usually considered in patients with metastatic disease.
Complete response following treatment with FOLFIRINOX is rare. For example, in the PRODIGE 4 ACCORD 11 trial, of the 171 metastatic PC patients who received a median of 10 cycles of FOLFIRINOX, there was only one complete remission.[16] Furthermore, in a study by Hackert et al,[17] of the 125 patients diagnosed with locally advanced, unresectable PC, and who were treated with neoadjuvant FOLFIRINIOX, and—where possible—underwent surgery, only 2 patients reached an UICC stage 0. Two other complete remissions of metastatic PC, and another case of remission of borderline resectable PC, were described as occurring after 8 and 13 cycles of FOLFIRINOX.[18,19] The rare event of spontaneous regression in PC is often associated with fever. In a metastatic PC patient undergoing complete remission of liver metastases after FOLFIRINOX treatment a strong inflammatory event (pneumonia) during chemotherapy was reported.[20] Furthermore, inflammatory events (e.g., peritonitis, cholangitis) were also observed in patients with advanced inoperable PC, who then demonstrated unexpectedly long survival.[21]
Remarkable in our case report is the long tumor-free survival period of 49 months to date. Hackert et al[17] reported 29 patients with metastatic PC, which, after neo-adjuvant treatment with FOLFIRINOX, became resectable. However, median survival in this group of patients was only 13 months (tumor-free survival was not reported). In a recent case report a long-time survival period of 30 and 36 months were reported for 2 patients receiving FOLFIRINOX in an integrative medicine setting including hyperthermia and VAE.[22]
High-dose fever-inducing VAE treatment has been described in small trials on hepatic and breast cancer,[23,24] as well as in case reports.[25] All reports describe relevant, long-lasting tumor regressions. Historically, similar courses of tumor regressions were seen following treatment with fever-inducing bacterial vaccines prepared from Streptococcus pyogenes, and Serratia marcescens (“Coley's toxins”), and spontaneous regressions have been reported in the course of Erysipelas.[26] Therefore, we presume that VAE treatment with high fever responses—despite the relatively short treatment period—may have contributed to the favorable outcome in this case.
Strong immunological reactions to VAE are explainable based on its immunological properties. These include induction of pro-inflammatory cytokines (interleukin-6, tumor necrosis factor alpha, interleukin-1), the stimulation of both proliferation and antigen presentation of dendritic cells and CD4 + T cells, the increase of natural killer cell-mediated cytotoxicity, and various other pathways.[8] Besides the immunological effects however, direct cytotoxic and synergistic effects of VAE (as adjunct to chemotherapy) may have contributed to the positive outcome in this case. Similar cytotoxic effects have also been documented in PC cell lines and animal studies (PA-TU-8902, PAXF 736, PAXF 546).[27–29] Furthermore, a pilot study of intratumoral VAE treatment of inoperable advanced pancreatic cancer patients showed a response in 57% of patients, and progression to stable disease in 36%.[30] Synergistic antineoplastic effects of VAE administered along with gemcitabine were also seen in PC cell lines.[27]
Overall survival in patients with PC stage IV is poor, and further treatment options are therefore needed and being sought. As VAE shows positive effects on PC, further research should be carried out regarding its efficacy, as well as specifications for application forms and dosages.
3.1. Informed consent
Informed consent was received from the patient for the publication of the report and accompanying images. The patient read the submission version of the report and confirmed its content.
Acknowledgments
We are thankful to the Klinik St. Georg, Bad Aibling for providing detailed data about the patient's course of disease, to Dr Helmut Kiene (IFAEMM), Dr Arnoud Templeton (Department of Onkology, St. Claraspital Basel, Switzerland) and Dr Reiner Penter (Department of Onkology, Klinik Arlesheim, Arlesheim, Switzerland) for revision of the manuscript, to Prof. Dale O’Brien, MD, MPH, Executive Director of Cancer Patients Alliance, for his help in the literature search and to the “Stiftung Integrative Medizin” for financial support.
Author contributions
PGW, PI and GSK contributed to the case report design. PI was the physician in charge who provided the patient's information. PGW and PI collected and provided the data. TW and RG were involved in the perioperative and operative treatment of the patient, provided the respective data, and helped to draft the manuscript. RG provided the surgical pictures. AKS provided the histologic pictures and revised the diagnosis. MD and MGS have special experience with intratumoral Viscum album extract therapy, carried out the intratumoral treatment of the patient and provided the respective data. PGW was the principle author of the paper, had full access to all data and is the guarantor. GSK supervised the report and the publication process.
Conceptualization: Paul Georg Werthmann, Gunver Sophia Kienle.
Data curation: Paul Georg Werthmann, Pia Inter, Thilo Welsch, Anne-Kathrin Sturm.
Investigation: Paul Georg Werthmann, Thilo Welsch, Anne-Kathrin Sturm, Robert Grützmann, Markus Debus, Martin-Günther Sterner, Gunver Sophia Kienle.
Methodology: Paul Georg Werthmann, Gunver Sophia Kienle.
Supervision: Gunver Sophia Kienle.
Validation: Paul Georg Werthmann, Pia Inter, Anne-Kathrin Sturm, Gunver Sophia Kienle.
Visualization: Paul Georg Werthmann.
Writing – original draft: Paul Georg Werthmann.
Writing – review & editing: Paul Georg Werthmann, Pia Inter, Thilo Welsch, Anne-Kathrin Sturm, Robert Grützmann, Markus Debus, Martin-Günther Sterner, Gunver Sophia Kienle.
Paul Georg Werthmann orcid: 0000-0002-1808-7787.
Footnotes
Abbreviations: CT = computed tomography, FOLFIRINOX = folinic acid, fluorouracil, irinotecan, oxaliplatin, FOLFOX = folinic acid, fluorouracil, oxaliplatin, ML = mistletoe lectin, PC = pancreatic cancer, UICC = Union for International Cancer Control, VAE = viscum album axtracts.
The authors have no conflicts of interest to disclose.
References
- [1].National Cancer Institute. SEER Cancer Stat Facts: Pancreas Cancer. Available at: https://seer.cancer.gov/statfacts/html/pancreas.html. Published July 2018 Accessed July 16, 2018. [Google Scholar]
- [2].Tabernero J, Chiorean EG, Infante JR, et al. Prognostic Factors of Survival in a Randomized Phase III Trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist 2015;20:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wade TP, Kraybill WG, Virgo KS, et al. Pancreatic cancer treatment in the US veteran from 1987 to 1991: effect of tumor stage on survival. J Surg Oncol 1995;58:104–11. [DOI] [PubMed] [Google Scholar]
- [4].Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013;63:318–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26suppl 5:v56–68. [DOI] [PubMed] [Google Scholar]
- [6].Beztsinna N, de Matos MBC, Walther J, et al. Quantitative analysis of receptor-mediated uptake and pro-apoptotic activity of mistletoe lectin-1 by high content imaging. Sci Rep 2018;8:2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Podlech O, Harter PN, Mittelbronn M, et al. Fermented mistletoe extract as a multimodal antitumoral agent in gliomas. Evid Based Complement Alternat Med 2012;2012:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Singh BN, Saha C, Galun D, et al. European Viscum album: a potent phytotherapeutic agent with multifarious phytochemicals, pharmacological properties and clinical evidence. RSC Adv 2016;6:23837–57. [Google Scholar]
- [9].Kienle GS, Grugel R, Kiene H. Safety of higher dosages of Viscum album L. in animals and humans—systematic review of immune changes and safety parameters. BMC Complement Altern Med 2011;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tröger W, Galun D, Reif M, et al. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival. Eur J Cancer 2013;49:3788–97. [DOI] [PubMed] [Google Scholar]
- [11].Tröger W, Galun D, Reif M, et al. Quality of life of patients with advanced pancreatic cancer during treatment with mistletoe: a randomized controlled trial. Dtsch Arztebl Int 2014;111:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Karger Publishers, Galun D, Tröger W, Milicevic M. Zänker KS, Kaveri SV. Cancer Surgery and Supportive Mistletoe Therapy: From Scepticism to Randomised Clinical Trials. Mistletoe: From Mythology to Evidence-Based Medicine. Vol 4 2015;57–66. [Google Scholar]
- [13].Riley D, Barber MS, Kienle GS, et al. CARE 2013 explanations and elaborations: reporting guidelines for case reports. J Clin Epidemiol 2017;89:218–35. [DOI] [PubMed] [Google Scholar]
- [14].Zuzak T, Rist L, Viviani A, et al. Das mistelpräparat iscucin®— herstellung, analytik, wirkung in vitro. Merkurstab J Anthr Med 2004;57:467–73. [Google Scholar]
- [15].Le Scodan R, Mornex F, Partensky C, et al. Histopathological response to preoperative chemoradiation for resectable pancreatic adenocarcinoma: the French phase II FFCD 9704-SFRO trial. Am J Clin Oncol 2008;31:545–52. [DOI] [PubMed] [Google Scholar]
- [16].Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- [17].Hackert T, Sachsenmaier M, Hinz U, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg 2016;264:457–63. [DOI] [PubMed] [Google Scholar]
- [18].Nikolaou C, Matikas A, Papavasilopoulou M, et al. Prolonged complete response in a patient with metastatic pancreatic adenocarcinoma after FOLFIRINOX chemotherapy and maintenance with FOLFIRI. Case Rep Oncol Med 2015;2015:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gostimir M, Bennett S, Moyana T, et al. Complete pathological response following neoadjuvant FOLFIRINOX in borderline resectable pancreatic cancer—a case report and review. BMC Cancer 2016;16:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schneitler S. Metastasized pancreatic carcinoma with neoadjuvant FOLFIRINOX therapy and R0 resection. World J Gastroenterol 2015;21:6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Oh SY. Rare long-term survivors of pancreatic adenocarcinoma without curative resection. World J Gastroenterol 2015;21:13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bonucci M, Pastore C, Ferrera V, et al. Integrated cancer treatment in the course of metastatic pancreatic cancer: complete resolution in 2 cases. Integr Cancer Ther 2018;17:994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mabed M, El-Helw L, Shamaa S. Phase II study of viscum fraxini-2 in patients with advanced hepatocellular carcinoma. BrJCancer 2004;90:65–9. (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mahfouz M, Ghaleb H, Zawawy A. Significant Tumor Reduction, Improvement of Pain and Quality of Life and Normalization of Sleeping Patterns of Cancer Patients Treated with a High Dose of Mistletoe. In: Vol 9. 1998;Spuiboulevard, the Netherlands: Kluwer Academic Publisher, 129–129. [Google Scholar]
- [25].Werthmann PG, Hintze A, Kienle GS. Complete remission and long-term survival of a patient with melanoma metastases treated with high-dose fever-inducing Viscum album extract: a case report. Medicine (Baltimore) 2017;96:e8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kienle GS. Fever in cancer treatment: Coley's therapy and epidemiologic observations. Glob Adv Health Med 2012;1:92–100. http://www.gahmj.com/doi/abs/10.7453/gahmj.2012.1.1.016http://www.gahmj.com/doi/abs/10.7453/gahmj.2012.1.1.016. Accessed September 9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Weissenstein U, Kunz M, Urech K, et al. Interaction of standardized mistletoe (Viscum album) extracts with chemotherapeutic drugs regarding cytostatic and cytotoxic effects in vitro. BMC Complement Altern Med 2014;14:1http://www.biomedcentral.com/1472-6882/14/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Burger AM, Mengs U, Schüler JB, et al. Antiproliferative activity of an aqueous mistletoe extract in human tumor cell lines and xenografts in vitro. Arzneimittelforschung 2001;51:748–57. https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0031-1300110 [DOI] [PubMed] [Google Scholar]
- [29].Rostock M, Huber R, Greiner T, et al. Anticancer activity of a lectin-rich mistletoe extract injected intratumorally into human pancreatic cancer xenografts. Anticancer Res 2005;25:1969–75. [PubMed] [Google Scholar]
- [30].Matthes H, Schad F, Buchwald D, et al. Endoscopic ultrasound-guided fine-needle injection of Viscum album L. (mistletoe; Helixor (R) M) in the therapy of primary inoperable pancreas cancer: a pilot study. Gastroenterology 2005;128:A433–4. [Google Scholar]


