Abstract
Background:
Fascia iliaca compartment block (FICB) provides an analgesic option for positioning before spinal anesthesia in patients suffering from a femur fracture. The evidence supporting FICB is still not well established. The aim of our study is to assess the efficacy and safety of FICB comparing with intravenous analgesic (IVA) on the quality for positioning before spinal anesthesia in participants with a femur fracture.
Methods:
PubMed, Embase, and Scopus databases were interrogated from their inceptions to September 2017. We included randomized controlled studies reported as full text, those published as abstracts only and unpublished data, if available. Data were independently extracted by 2 reviewers and synthesized using a random-effects model.
Main Results:
Three studies comprising 141 participants showed that FICB compared to IVA led to a significant between-group standard mean differences in quality during positioning within 30 minutes before spinal anesthesia (standardized mean difference (SMD) −2.02, 95% confidence interval (CI): −2.43 to −1.61, I2 = 0%) and time for spinal anesthesia (pooled mean difference (PMD) −2.86 minutes, 95% CI −3.70 to −2.01, I2 = 0%). Two studies with 101 participants suggested that FICB is superior to IVA on opioid requirements 24 hours postoperatively (pooled odds ratio (POR): 0.11, 95% CI: 0.03 to 0.35, I2 = 13%). There were no significant differences in complications or hemodynamic effects
Conclusions:
Comparing with IVA, FICB can provide significantly better quality during positioning of femur fracture patients for a spinal block and a shorter time for spinal anesthesia. FICB is safe method.
Keywords: analgesic, fascia iliaca compartment block, fracture
1. Introduction
A fracture of the femur is a well-known reason for surgical repair in patients of all ages. Locations include the neck, intertrochanteric, shaft, and distal fractures. Spinal anesthesia is the commonly used, preferred method for surgery and is associated with lower odds of mortality compared to general anesthesia.[1] Spinal anesthesia is administered in either a sitting or lateral decubitus position. Positioning patients with a fractured femur for spinal anesthesia is challenging since minimal movement of the overriding fracture ends can cause extreme pain.
Fractures of the femur are a particularly painful bone injury because the periosteum has the lowest pain threshold of the deep somatic structures.[2] As a result, the majority of patients with femoral fractures are in great pain. Failure to effectively control the pain before the procedure increases neurohormonal stress responses leading to potential risks of cardiovascular events during surgery in femur fracture patients. These patients are generally elderly with multiple comorbidities and have potential risks of undesirable effects of opioids including respiratory depression, confusion, and other side effects.[3] Therefore, proper management of pain is important. Current modalities used for analgesia are systematic nonsteroidal anti-inflammatory drugs and opioids, or peripheral nerve block, such as fascia iliac compartment nerve block (FICB).[4–7]
FICB is seen as a simple safe method which is easy to learn and use. The injection is performed via the landmark method or under ultrasound (US) guidance. FICB is also well described for acute pain management of femur fractures and was shown to decrease opioid requirements.[8] Moreover, FICB was reported as being used in poor-risk patients such as those with renal and respiratory compromise where opioids are to be avoided.[9] In addition, adequate pain control in these patients not only decreases the discomfort but was also shown to improve positioning for spinal anesthesia. Correct positioning during spinal anesthesia is also crucial for a successful procedure.
Currently, application of FICB in femur fractures for positioning for spinal anesthesia is seldom reported. The evidence supporting FICB is still not well established. Therefore, we performed a meta-analysis to assess the efficacy and safety of FICB to facilitate good positioning for spinal anesthesia in patients with a femur fracture.
2. Methods
We followed the preferred reporting items for systematic review and meta-analyses (PRISMA) guidelines for this meta-analysis.[10] We registered on PROSPERO (https://www.crd.york.ac.uk/prospero, PROSPERO ID: CRD42017079665). Ethical approval or patient consent was not required as the present study was a review of previously published articles.
2.1. Search strategy and study selection
A literature search was performed using PubMed, Scopus, and Embase using eligibility criteria with the following search terms: fascia iliaca compartment block, analgesic, and fracture. We also manually searched references of recently published relevant articles. The last literature search was performed in September 2017.
2.2. Inclusion and exclusion criteria
All published human randomized controlled trials (RCTs) in English were considered for inclusion. Participants received FICB comparing with IVA were included. Studies focusing on our outcomes of interest are included. Case reports, case series, prospective cohort studies, and retrospective cohort studies were excluded. In addition, we identified other studies using the reference sections of relevant papers and by corresponding with subject experts. Finally, unpublished studies were collected from the ClinicalTrials.gov registry (http://clinicaltrials.gov/). No language restrictions were applied.
2.3. Outcomes of interest
Our primary outcomes of interest were the quality during positioning before spinal anesthesia and time for spinal anesthesia. Secondary outcomes were anesthesiologists’ satisfaction with the quality of positioning for spinal anesthesia, time to the first opioid requirement, pain scores at 6 hours postoperatively, opioid requirement at 24 hours postoperatively, and participant acceptance.
2.4. Data extraction and management
Baseline and outcome data were independently abstracted by 2 reviewers (CC and YPH), and the study designs, study population characteristics, inclusion and exclusion criteria, method of intervention, complications, and post-treatment parameters were extracted. Decisions individually recorded by the reviewers were compared, and disagreements were resolved by a third reviewer (CHB). The authors of the studies were contacted for additional information if required.
2.5. Assessment of the risk of bias in the included studies
Two reviewers (CC and YPH) independently assessed the methodological quality of each study using the risk of bias method recommended by the Cochrane Collaboration.[11] Several domains were assessed, including the adequacy of randomization, allocation concealment, blinding of patients and outcome assessors, length of follow-up, information provided to patients regarding study withdrawal, whether an intention-to-treat analysis was performed, and freedom from other biases. In addition, any disagreements on data extraction and/or quality assessment were resolved through comprehensive discussions.
2.6. Statistical analysis
2.6.1. Measures of the treatment effect
We analyzed outcomes as continuous or dichotomous data using standard statistical techniques with a random-effects model up to the end of follow-up. For continuous outcomes, we used mean differences (MDs) and 95% confidence intervals (CIs). For dichotomous outcomes, we calculated the odds ratios (ORs) with 95% CIs. If some of the continuous data were given on different scales, we produced the results as the standardized mean difference (SMD) and 95% CI. For SMD, we considered 0.2 a small effect, 0.5 a medium effect, and 0.8 a large effect.[12] Analyses were conducted using Review Manager (RevMan) vers. 5.3 (Copenhagen, Denmark), and results are presented as forest plots in random-effects models. A 2-sided P value of < .05 was considered statistically significant.
2.6.2. Assessment of heterogeneity
We used the I2 statistic and χ2 test to measure heterogeneity among studies in each analysis. Heterogeneity was categorized as low (< 30%), moderate (30%–60%), or high (> 60%) by I2 values.[13] If we identified substantial heterogeneity, we reported this and explored possible causes by performing prespecified subgroup analyses (fracture location, technique or method of FICB, and approach for spinal anesthesia).
2.6.3. Assessment of reporting biases
Publication bias was assessed by detecting asymmetry in funnel plots if at least 10 studies were included. We used Egger's test to examine possible small study effects.
3. Results
3.1. Results of the search
A flowchart is presented to show the screening and selection processes of the trials (Fig. 1). Our initial search yielded 382 studies from PubMed and Embase and 144 studies from Scopus and by searching references. Records after duplicates were removed yielded 317 studies, of which 297 articles were deemed ineligible after the screening of titles and abstracts. Full-text articles were excluded with different intervention (n = 2), no relevant outcome measurement (n = 13) and no comparison (n = 1). Four RCTs were included for qualitative synthesis,[4–7] and one of these was excluded from further quantitative synthesis due to only the abstract being available[6] (n = 3).
Figure 1.
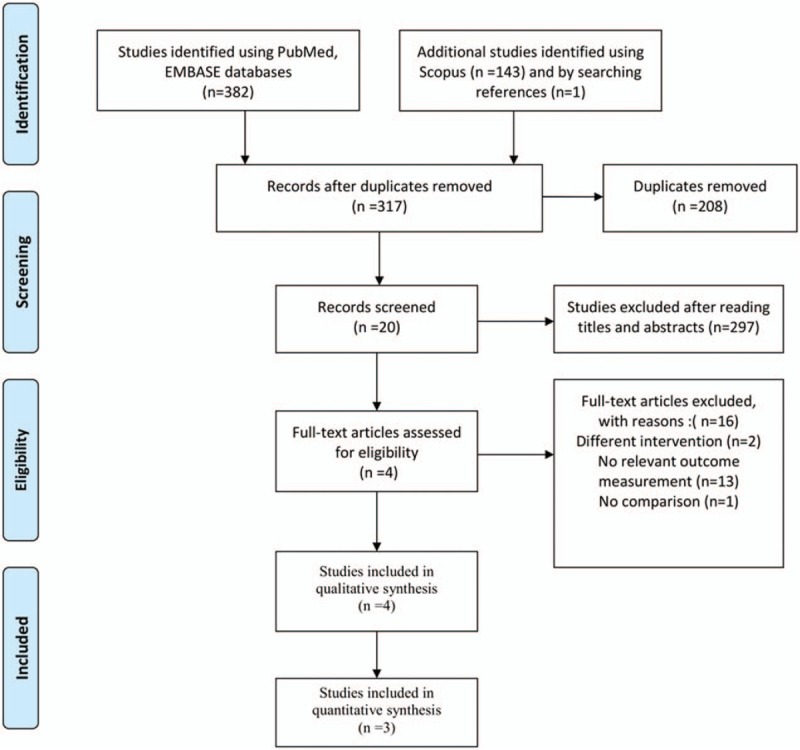
Flow diagram of the search process and search results.
3.2. Study characteristics
A complete overview of the included studies can be found in Table 1 of characteristics of the included studies. All of the included studies were RCTs. The study sample sizes ranged 40 to 60, with 181 in total. All of the studies were from single center. These studies were conducted in Iran,[6] Korea,[7] Greece,[4] and India.[5] The average age of participants ranged 58.8 to 80 years. Mosaffa et al[6] provided only an abstract without detailed methods and results. For the remaining 3 trials that provided detailed methods and results, 2[4,7] of the trials recruited more females than males, with the third trial recruiting a majority of males.[5] Two trials[4,7] reported the time from trauma to surgery, for which the average ranged 1.3 to 4.0 days. Inclusion criteria were similar for the included studies, but slight differences were found. Similarly, participants needed to have a femoral neck fracture or hip fracture and be ASA I to III. In contrast, Diakomi et al[4] and Madabushi et al[5] included participants aged 38 to 94 and 25 to 75 years, respectively, versus Yun et al[7] whose participants were aged 62 to 88 years. Exclusion criteria were similar for the included studies. All trials used the landmark method to perform FICB. As to approaches for spinal anesthesia, 3 trials used the lateral decubitus position,[4,6,7] and one trial used a sitting position.[5] There were variations in the dosage and type of anesthetic drugs for spinal anesthesia. Three trials used ropivacaine[4,5,7] and one used lidocaine for FICB.[6] Three trials[4–6] used fentanyl and one[7] used alfentanyl in the controls. There was also a variation in dosage of local anesthetic drugs and IVA. The time of follow-up was 24 hours in 3 trials.
Table 1.
Characteristics of the included studies.
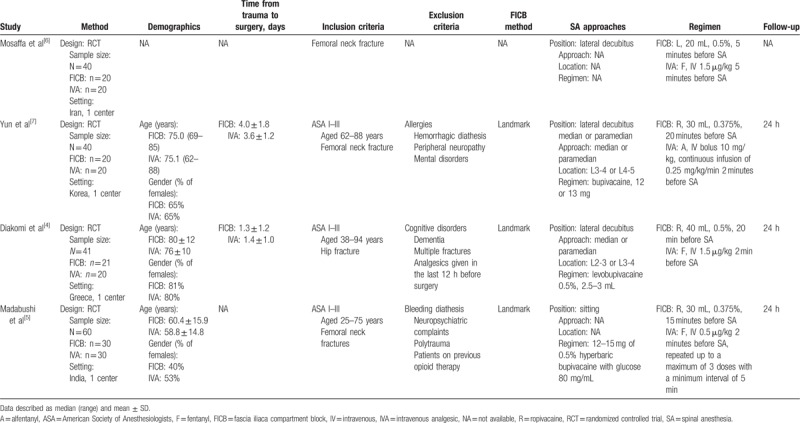
3.3. Outcome measurements
The majority of studies reported on the quality during positioning before spinal anesthesia (within 30 minutes) by visual analog scale (VAS) scores, but Madabushi et al[5] reported improvements in the sitting angle, which was objectively measured using a goniometer. Mosaffa et al[6] reported medians and ranges rather than mean values of VAS scores. The time for spinal anesthesia was mentioned in 3 studies. Anesthesiologists’ satisfaction with the quality of positioning for spinal anesthesia was demonstrated in 3 studies. Yun et al[7] only provided information on VAS scores at 6 hours. The time to the first opioid requirement was mentioned by 2 studies. Two studies assessed opioid requirements at 24 hours postoperatively. Participant acceptance was evaluated by 3 studies. Three studies provided limited information about outcomes of safety including adverse effects of the local anesthetic and IVA, hemodynamic effects, and complications.
3.4. Risk of bias in the included studies
The quality and risk of bias assessment of individual studies were performed using the Cochrane risk of bias assessment tool (Fig. 2). All 4[4–6,10] of the studies had a low risk for incomplete outcome data and selective reporting. However, all 4[4–6,10] of the studies had an unclear risk of the performance bias due to the intervention method used. The study by Mosaffa et al[6] had unclear risks of random sequence generation, allocation concealment, blinding of outcome assessments, and other biases. The study by Madabushi et al[5] had an unclear risk of allocation concealment. The study by Yun et al[7] had an unclear risk for blinding of outcome assessment. Publication bias and a sensitivity analysis were not performed due to the limited number of studies.
Figure 2.
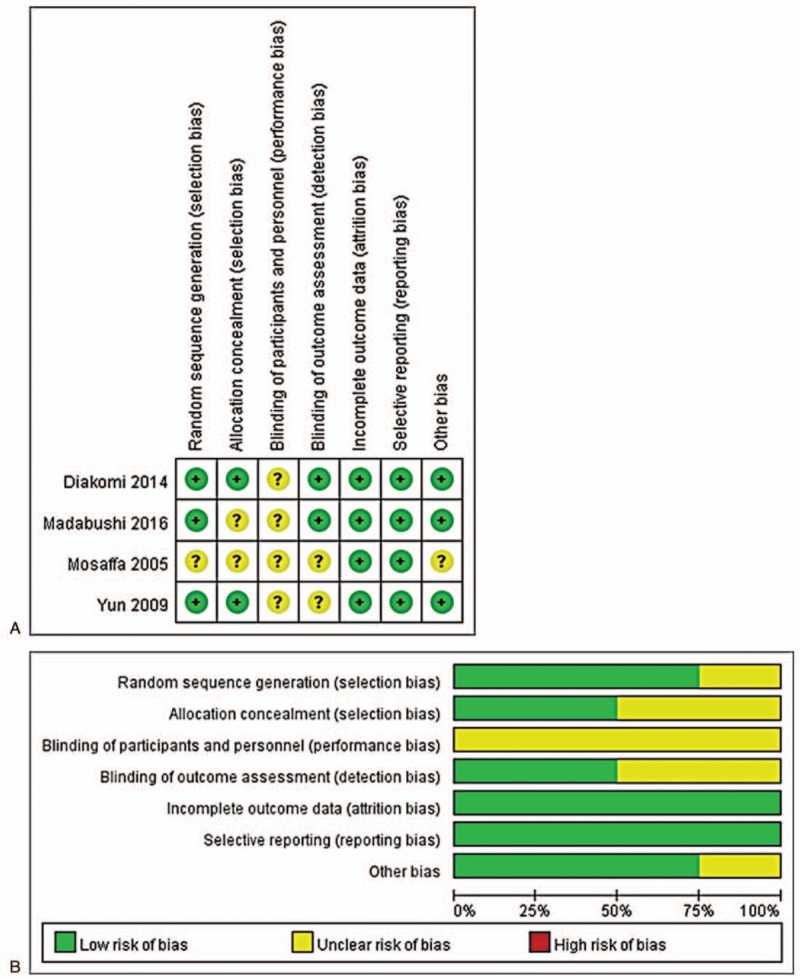
Methodological quality: (A) Risk of bias summary of the randomized controlled trials; (B) Risk of bias graph of the randomized controlled trials.
3.5. Results of individual studies
3.5.1. Primary outcomes
3.5.1.1. Quality during positioning before spinal anesthesia (within 30 minutes)
Three RCTs,[4,5,7] including 141 participants (71 in the FICB group and 70 in the IVA group), evaluated the quality during positioning before spinal anesthesia within 30 minutes (Fig. 3A). Two RCTs[4,7] that evaluated pain scores showed that pain scores were lower in the FICB group (standardized mean difference (SMD): −1.98, 95% CI: −2.52 to −1.44; I2 = 0%). One RCT[5] that evaluated the improvement in the sitting angle showed greater improvement in the FICB group (SMD: −2.08, 95% CI: −2.71 to −1.44; I2 = 0%). The pooled SMDs of the quality during positioning before spinal anesthesia within 30 minutes indicated a significant difference across treatment by FICB over IVA (SMD: −2.02, 95% CI: −2.43 to −1.61, I2 = 0%). Mosaffa et al[6] also reported that VAS scores (median and range) during positioning were significantly lower in the FICB group than the IVA group (0.5 [0–1] vs 4 [2–6], P > .001).
Figure 3.
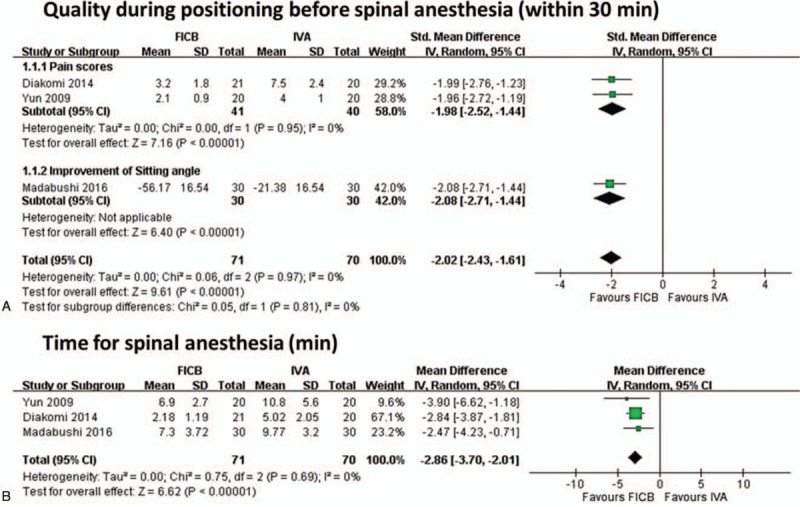
Meta-analysis for primary outcomes. (A) Forest plot of positioning before spinal anesthesia (within 30 minutes), (B) forest plot of time for spinal anesthesia (minutes).
3.5.1.2. Time for spinal anesthesia (minutes)
Three RCTs,[4,5,7] including 141 participants (71 in the FICB group and 70 in the control group), evaluated the time for spinal anesthesia (Fig. 3B). The result indicated a significant difference favoring the FICB group compared to the IVA group (PMD: −2.86 minutes, 95% CI: −3.70 to −2.01, I2 = 0%).
3.5.2. Secondary outcomes
3.5.2.1. Anesthesiologists’ satisfaction with the quality of positioning for spinal anesthesia
Two RCTs,[4,5] including 101 participants (51 in the FICB group and 50 in the IVA group), evaluated anesthesiologists’ satisfaction with the quality of positioning for spinal anesthesia (Fig. 4A). Satisfaction was evaluated as 0 = unsatisfactory, 1 = satisfactory, 2 = good, or 3 = optimal or very good. The SMD indicated a significant difference favoring FICB compared to IVA (SMD: 1.23, 95% CI: −0.65–1.81, I2 = 43%). Mosaffa et al[6] also reported that anesthesiologists were more satisfied in the FICB than the IVA group (median [range]:3 [2–3] vs 1.5 [1–3], P < .005).
Figure 4.
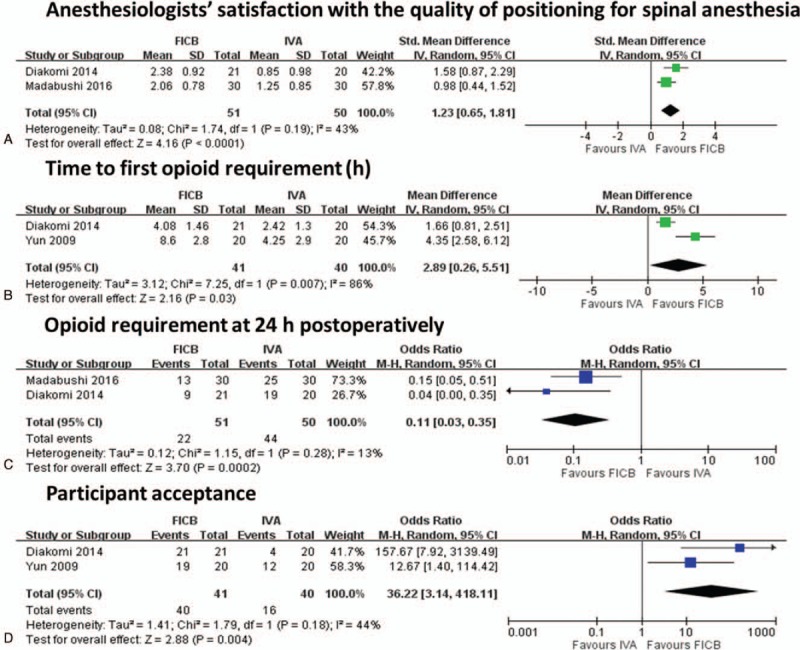
Meta-analysis for secondary outcomes. (A) Forest plot of anesthesiologists’ satisfaction with the quality for spinal anesthesia, (B) forest plot of time to first opioid requirement (hours), (C) forest plot of opioid requirement at 24 hours postoperatively, (D) forest plot of participant acceptance.
3.5.2.2. Time to first opioid requirement (hours)
Two RCTs,[4,7] including 81 participants (41 in the FICB group and 40 in the IVA group), evaluated the time (hours) to the first opioid requirement postoperatively (Fig. 4B). The PMD indicated a significant difference favoring FICB compared to IVA (PMD: 2.89, 95% CI: 0.26–5.51, I2 = 86%). However, the PMD revealed a wide 95% CI, meaning that caution needs to be taken when interpreting these results and an I2 test of > 60% indicated high heterogeneity.
3.5.2.3. Pain scores at 6 hours postoperatively
One among these RCTs evaluated the VAS at 6 hours postoperatively.[7] Yun et al[7] reported that mean VAS scores at 6 hours postoperatively were lower in the FICB group than in the IVA group (2.9 ± 1.3 vs 3.6 ± 3.0, P = .3444).
3.5.2.4. Opioid requirement at 24 hours postoperatively
Two RCTs,[4,14] including 101 participants (51 in the FICB group and 50 in the IVA group), evaluated opioid requirements at 24 hours postoperatively (Fig. 4C). The pooled odds ratio (POR) indicated a significant difference favoring FICB compared to IVA between treatments with FICB over IVA (POR: 0.11, 95% CI: 0.03–0.35, I2 = 13%). Yun et al[7] reported that the amounts of rescue analgesics at 24 hours did not statistically differ between the 2 groups, but the trend was for lower amounts in the FICB group (Demerol, 41 ± 48 mg in the FICB group vs 72 ± 69 mg in the IVA group). Diakomi et al[4] reported that the mean of the amounts of opioid required was lower in the FICB group than the IVA group (morphine, 4.11 ± 3.3 mg in the FICB group vs 7.42 ± 4.65 mg in the IVA group, P = .026).
3.5.2.5. Participant acceptance
Two RCTs,[4,7] including 81 participants (41 in the FICB group and 40 in the IVA group), evaluated participant acceptance at the end of follow-up (Fig. 4D). The POR showed a significant difference, indicating a higher number of participant acceptance for those treated with FICB compared to those treated with IVA (POR: 36.22, 95% CI: 3.14–418.11, I2 = 44).
3.5.3. Outcome of safety
3.5.3.1. Adverse effects of local anesthesia
Yun et al[7] reported that no adverse systemic toxicity of ropivacaine was noted, and neither vascular puncture nor paresthesia was elicited in the FICB group.
3.5.3.2. Adverse effects of IVA
Yun et al[7] reported that 12 participants in the IVA group experienced mild dizziness and mild drowsiness.
3.5.3.3. Hemodynamic effects
Yun et al[7] reported hypoventilation (a ventilatory rate of 6–8 times/min) or pulse oximetric desaturation (oxygen saturation of 88% or 89%) was encountered in 4 patients (20%) in the IVA group. Madabushi et al[5] reported that patients in the IVA group had a significantly reduced mean heart rate compared to patients in the FICB group (88.30 ± 15.92 vs 92.45 ± 17.31 beats per minute; P = .001). Diakomi et al[4] reported that there was no statistically significant difference between groups in overall mean systolic, diastolic, and mean arterial pressures, heart rate, hemoglobin saturation, or fluids and vasoconstrictors administered at any time point until study completion at 24 h after surgery. Madabushi et al[5] reported that hemodynamic parameters were compared and showed no major differences between the groups at various intervals.
3.5.3.4. Complications
None of the included trials reported any complications in either group within 24 hours after the operation.[4,5,7]
3.6. Risk of bias across studies and additional analysis
Publication bias was not ascertained due to the limited number of studies. Subgroup analysis was not performed due to homogeneity of the result.
4. Discussion
The evidence supporting application of FICB in femur fractures for positioning for spinal anesthesia is still not well established. In this systematic review and meta-analysis, we included 3 studies comprising 141 participants showing that FICB provided more effective analgesia to improve patient positioning and shorter the time to perform spinal anesthesia than IVA. In addition, 2 RCTs with 101 participants suggest that FICB is superior to IVA on opioid requirements 24 hours postoperatively. The other important finding of the meta-analysis is that FICB had greater anesthesiologist's satisfaction for positioning and patient acceptance than IVA. No complications and no significant effect on hemodynamic were identified by using FICB.
Fractures of the femur include the neck, intertrochanteric, shaft, and distal areas. In the present systematic review, 3 trials included femoral neck fracture and 1 included hip fracture. Application of these results to patients with femoral shaft and distal fractures should be done with caution. Somvanshi et al[15] also reported that pain scores significantly decreased, but the quality of analgesia did not change when patients underwent a radiological examination and traction application after femoral nerve block in patients with fracture of the femur shaft in the emergency ward.
The age of the patients of included trials ranged 25 to 94 years, so there was limited evidence of a pediatric population. Black et al[16] reported low-quality evidence for better pain management in the FICB group, low-quality evidence that FICB has a better safety profile than morphine, and did not report on pain during procedures or transfers in a pediatric population. Neubrand et al[17] reported that median postintervention pain scores in the FICB group were 1.5 points lower than those in the IVA group, and there was no difference in the total adverse events between the FICB and IVA groups in patients with a femur fracture. Also no report mentioned pain during the procedures or transfers in a pediatric population.[17]
All identified trials in the present systematic review used landmark-based FICB. Some authors reported that FICB performed under US guidance would have improved efficacy.[18] However, the use of a US machine requires a special needle, which adds to the cost. In addition, another anesthesiologist is needed to perform FICB. It may lead to an increase in the total operation time. In fact, landmark-based FICB is a simple and inexpensive low-skill technique with a high success rate and is easily performed by anesthesiologists familiar with the technique. Moreover, using landmark-based FICB is also supported by the present systematic review due to homogeneity across the identified trials and positive effect on primary outcomes.
In addition, spinal anesthesia can be performed in a sitting or lateral decubitus position. When approaching spinal anesthesia, the lateral decubitus position may have some impact on complications. Zorrilla-Vaca et al[19] conducted a meta-analysis which showed that the lateral decubitus position was associated with a significant reduction in the incidence of post-dural puncture headaches compared to the sitting position (risk ratio = 0.61, 95% CI: 0.44–0.86, I2 = 25%). Due to a limited number of included studies, a small number of participants, and a short duration of follow-up in present systemic review, we failed to address results of this issue and other complications related to spinal anesthesia, such as spinal hematoma.
Opioids are used and provide good analgesia in femur fracture patients. Opioid-related adverse effects, including nausea, vomiting, and respiratory depression, are well known.[3] Opioids also contribute to delirium in these patients.[20,21] It is crucial to minimize opioid consumption and avoid unnecessary complications.[9] In particular, the majorities of patients suffering from a fractured femur neck are typically elderly with multiple comorbidities and are thus more susceptible to serious adverse effects such as hypoventilation or apnea. Yun et al[7] reported more events of hypoventilation or desaturation in the IVA group, and Madabushi et al[5] reported significantly reduced mean heart rates that reveal the effect of IV opioid use in the control group which may lead to more hemodynamic events. On the other hand, the opioid-sparing effect of FICB, even a single shot in all identified trials, is of critical clinical importance and is suggested in the present systematic review by lower opioid requirements in the first 24 hours postoperatively than IVA. Besides, the amounts of opioid requirements were also lower, and the time to first analgesia was longer in the FICB than IVA. A similar effect of a single-shot peripheral nerve block providing good postoperative analgesia was also supported by other studies.[14,22]
Last, none of the included trials reported any complication noted in either group within 24 hours postoperatively. Most of the identified trials revealed no statistically significant difference between groups in terms of the hemodynamic effects, showing that FICB seems to be a safe technique with no complications.
The present meta-analysis has some limitations. Since the included trials did not involve a pediatric or femur shaft fracture patients, generalization of the present finding to that group should be done with great caution. Our analysis is based only on 4 RCTs. Although most of them are recently published RCTs, the number of included studies and patients were small. However, by combining studies, a meta-analysis increases the power of the study effects of interest. We failed to analyze functional outcomes due to insufficient data. Concomitant pain management regimes differed from one other, which may be an important confounding factor that has an influence on the pooling results. In addition, the active comparators in our study are fentanyl and alfentanil, so further studies are needed to examine comparison using alterative analgesics, e.g. ketamine. The short duration of follow-up may have resulted in our underestimating complications. A publication bias was not evaluated due to limited studies.
5. Conclusion
In conclusion, FICB can provide significantly lower pain scores to facilitate positioning of femur fracture patients for a spinal block, lower postoperative opioid requirements, and no significant complication rates. But the current available evidence was limited and the power may still be insufficient. Further research remains necessary.
Author contributions
Yuan-Pin Hsu and Chiehfeng Chen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acquisition, analysis, or interpretation of data: Yuan-Pin Hsu, Chin-Wang Hsu, Sheng-Wei Cheng, Chiehfeng Chen, and Chyi-Huey Bai
Concept and design: Yuan-Pin Hsu
Conceptualization: Yuan-Pin Hsu, Chiehfeng Chen.
Data curation: Yuan-Pin Hsu, Chin-Wang Hsu, Chyi-Huey Bai, Sheng-Wei Cheng, Chiehfeng Chen.
Drafting of the manuscript: Yuan-Pin Hsu and Chiehfeng Chen
Formal analysis: Yuan-Pin Hsu, Chin-Wang Hsu, Chyi-Huey Bai, Sheng-Wei Cheng, Chiehfeng Chen.
Funding acquisition: Yuan-Pin Hsu.
Investigation: Chin-Wang Hsu, Chyi-Huey Bai.
Methodology: Yuan-Pin Hsu, Chin-Wang Hsu, Sheng-Wei Cheng.
Project administration: Yuan-Pin Hsu, Sheng-Wei Cheng, Chiehfeng Chen.
Software: Yuan-Pin Hsu, Chin-Wang Hsu, Chyi-Huey Bai, Sheng-Wei Cheng.
Statistical analysis: Yuan-Pin Hsu, Chiehfeng Chen, and Chyi-Huey Bai
Study supervision: Chiehfeng Chen
Supervision: Chiehfeng Chen.
Validation: Chyi-Huey Bai.
Visualization: Chyi-Huey Bai.
Writing – original draft: Yuan-Pin Hsu.
Writing – review & editing: Yuan-Pin Hsu.
Yuan-Pin Hsu orcid: 0000-0001-8895-9133.
Footnotes
Abbreviation: CI = confidence interval, FICB = fascia iliaca compartment block, IVA = intravenous analgesic, MD = mean difference. OR = odds ratio, PMD = pooled mean difference, POR = pooled odds ratio, PRISMA = preferred reporting items for systematic review and meta-analyses, RCT = randomized controlled trial, SMD = standardized mean difference, US = ultrasound, VAS = visual analog scale.
The authors YPH, CWH, SWC, CC, and CHB are very grateful for financial support from project no. 106-eva-18 of Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan.
The authors have no conflicts of interest to disclose.
References
- [1].Neuman MD, Silber JH, Elkassabany NM, et al. Comparative effectiveness of regional versus general anesthesia for hip fracture surgery in adults. Anesthesiology 2012;117:72–92. [DOI] [PubMed] [Google Scholar]
- [2].Duc TA. Conroy JM, Dorman BH. Postoperative pain control. Anesthesia for Orthopedic Surgery. New York, NY: Raven Press; 1994. 355–65. [Google Scholar]
- [3].Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008;11(2 suppl):S105–20. [PubMed] [Google Scholar]
- [4].Diakomi M, Papaioannou M, Mela A, et al. Preoperative fascia iliaca compartment block for positioning patients with hip fractures for central nervous blockade: A randomized trial. Reg Anesth Pain Med 2014;39:394–8. [DOI] [PubMed] [Google Scholar]
- [5].Madabushi R, Rajappa GC, Thammanna PP, et al. Fascia iliaca block vs intravenous fentanyl as an analgesic technique before positioning for spinal anesthesia in patients undergoing surgery for femur fractures—a randomized trial. J Clin Anesth 2016;35:398–403. [DOI] [PubMed] [Google Scholar]
- [6].Mosaffa F, Esmaelijah A, Khoshnevis H. Analgesia before performing a spinal block in the lateral decubitus position in patients with femoral neck fracture: a comparison between fascia iliaca block and IV fentanyl. Reg Anesth Pain Med 2005;30:61–161. [Google Scholar]
- [7].Yun M, Han M, Park S, et al. Analgesia prior to spinal block in the lateral position in elderly patients with a femoral neck fracture: A comparison of fascia iliaca compartment block and intravenous alfentanil. Eur J Anaesthesiol 2009;26:111.19142083 [Google Scholar]
- [8].Zhang P, Li J, Song Y, et al. The efficiency and safety of fascia iliaca block for pain control after total joint arthroplasty: A meta-analysis. Medicine 2017;96:e6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology 2015;122:659–65. [DOI] [PubMed] [Google Scholar]
- [10].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pace NL. Research methods for meta-analyses. Best Pract Res Clin Anaesthesiol 2011;25:523–33. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [14].Wang C, Cai XZ, Yan SG. Comparison of periarticular multimodal drug injection and femoral nerve block for postoperative pain management in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2015;30:1281–6. [DOI] [PubMed] [Google Scholar]
- [15].Somvanshi M, Tripathi A, Meena N. Femoral nerve block for acute pain relief in fracture shaft femur in an emergency ward. Saudi J Anaesth 2015;9:439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Black KJ, Bevan CA, Murphy NG, et al. Nerve blocks for initial pain management of femoral fractures in children. Cochrane Database Syst Rev 2013. CD009587. [DOI] [PubMed] [Google Scholar]
- [17].Neubrand TL, Roswell K, Deakyne S, et al. Fascia iliaca compartment nerve block versus systemic pain control for acute femur fractures in the pediatric emergency department. Pediatr Emerg Care 2014;30:469–73. [DOI] [PubMed] [Google Scholar]
- [18].Dolan J, Williams A, Murney E, et al. Ultrasound guided fascia iliaca block: a comparison with the loss of resistance technique. Reg Anesth Pain Med 2008;33:526–31. [DOI] [PubMed] [Google Scholar]
- [19].Andres Zorrilla-Vaca MaJKM, MD DNB. Effectiveness of lateral decubitus position for preventing post-dural puncture headache: a meta-analysis. Pain Physician 2017;10:E521–9. [PubMed] [Google Scholar]
- [20].Petre BM, Roxbury CR, McCallum JR, et al. Pain reporting, opiate dosing, and the adverse effects of opiates after hip or knee replacement in patients 60 years old or older. Geriatr Orthop Surg Rehabil 2012;3:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zywiel MG, Prabhu A, Perruccio AV, et al. The influence of anesthesia and pain management on cognitive dysfunction after joint arthroplasty: a systematic review. Clin Orthop Relat Res 2014;472:1453–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guay J, Parker MJ, Griffiths R, et al. Peripheral nerve blocks for hip fractures. Cochrane Database Syst Rev 2017;5:CD001159. [DOI] [PMC free article] [PubMed] [Google Scholar]


