Abstract
This research aims to explore the accurate incidence, severity and outcomes of dysphagia and dysphonia after Hangman fractures.
A total of 93 patients were included in this study and clinical data were reviewed. The Bazaz grading system (0-None; 1-Mild; 2-Moderate; 3-Severe) was used for dysphagia evaluation and the Voice Handicap Index-10 used to evaluate dysphonia. In all of the patients, evaluation of dysphagia and dysphonia was performed preoperatively and at 1 week, 1 month, 3, 6, and 12 months after surgery. SPSS 22.0 software (SPSS Inc, Chicago, IL) was used for all statistical analyses.
Posttraumatic immediate dysphagia was found in 8 patients and posttraumatic immediate dysphonia was observed in 3 patients. The incidence of dysphagia was 22.6% at the 1st week, 16.1% at the 1st month, and 9.7% at the 3rd month of follow-up. The incidence of dysphonia was 24.5% at the 1st week, 11.3% at the 1st month, and 3.8% at the 3rd month of follow-up.
Posttraumatic immediate dysphagia and dysphonia occurred and the anterior surgical approach was associated with a higher incidence of dysphagia compared to posterior surgery and nonoperative patients. Most dysphagia and dysphonia were mild and gradually decreased during the subsequent 3 months after surgery. Future prospective, randomized studies with larger sample sizes are required to validate these data.
Keywords: dysphagia, dysphonia, Hangman fracture, swallowing dysfunction, traumatic spondylolisthesis of the axis
1. Introduction
Traumatic spondylolisthesis of the axis, termed “Hangman fractures”, was firstly described by Schneider in 1965.[1] Hangman fractures are characterized by a bilateral arch fracture of the C2 vertebrae with a variable degree of displacement of the C2 corpus on C3 vertebrae, and are the 2nd most common fracture of the axis vertebra, accounting for 4% to 7% of all cervical trauma injuries.[2,3] Extension force was regarded as the most common injury mechanism, however, flexion force has also been proven to cause such a fracture. Several classification systems have been introduced to describe Hangman fractures, whilst the 4 most accepted systems are the Effendi, Levine–Edward (which modified the Effendi classification), Josten and Francis classifications.[4–7] Hangman fractures have been explored and understood for several decades but treatment strategies still remain controversial.[8] Treatment methods included nonoperative treatments such as traction and various types of external immobilization, anterior cervical discectomy and fusion (ACDF) with or without anterior plating at C2--3, posterior fixation and fusion of C2-3, posterior fixation and fusion of C1--3, posterior percutaneous transpedicular screw fixation, and an anterior-posterior union approach.[9–13] Different studies have presented different concepts for the treatment of Hangman fractures with approximately 40 concepts in total according to previous reviews.[8,14]
Several previous studies have attempted to compare clinical outcomes for different treatment methods which have often focused on outcomes such as a visual analog scale for neck pain, operation time, blood loss, the American Spinal Injury Association Impairment scale, the clinical posttraumatic neck score, translation of C2, local kyphotic angle and fusion rates of C2-3.[11,15,16] However, dysphagia and dysphonia after Hangman fractures have not been specifically investigated.
Dysphagia, defined as difficulty in forming or moving an alimentary bolus safely from the mouth to the stomach, has been reported to be associated with increased morbidity, mortality and costs in anterior cervical fusion.[17,18] Dysphonia is also one of the most common complications following anterior cervical surgeries and it has been reported that even at 5 years after surgery, 8.9% of patients may still suffer from voice problems.[19,20] In order to further explore the accurate incidence, severity and outcomes of dysphagia and dysphonia after Hangman fractures, a retrospective study based on 93 patients was performed.
2. Materials and methods
This retrospective observational study was approved by the Medical Ethical Committee of West China Hospital. Informed consent was received from all patients to participate in the study.
2.1. Patient inclusion and exclusion criteria
All patients diagnosed with Hangman fractures between December 2010 and December 2015 in our hospital were included in this study if they meet the following inclusion and exclusion criteria.
The inclusion criteria were as follows: patients who diagnosed as having Hangman fractures in accordance with the results of a standard antero-posterior and lateral radiographs, computed tomography (CT) scans, ages over 18 years, completed at least 12 months follow-up time, presented with no symptoms of dysphagia and dysphonia before fracture.
The exclusion criteria were as follows: patients with dysphagia and dysphonia before fracture; patients with fractures combined with traumatic brain injury; patients who suffered from central nervous system disorders such as stroke patients with mental or psychological disorders such as hysteria, and unconscious patients who were not capable of being evaluated for dysphagia and dysphonia.
2.2. Patient evaluations
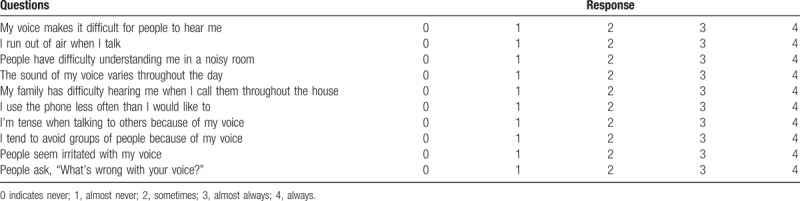
Preoperative standard antero-posterior and lateral cervical X-rays, cervical CT and cervical magnetic resonance imaging (MRI) were obtained. The Levine–Edward classification system was used to classify and describe the fractures.[7] The ASIA scale was used for grading of neurological function.[21] All of the patients were evaluated for dysphagia and dysphonia at preoperation, and at 1 week, 1, 3, 6, and 12 months after surgery. The Bazaz grading system was used to evaluate the severity of dysphagia (0-None; 1-Mild; 2-Moderate; 3-Severe; as listed in Table 1).[22] The Voice Handicap Index-10 (VHI-10), a frequently used patient self-reported dysphonia evaluation system, was used for dysphonia evaluation (Table 2).[23] Nonoperative treatments such as traction and external immobilization, ACDF, or posterior fixation and fusion of C2-3 were performed when appropriate.
Table 1.
The Bazaz grading system for dysphagia.

Table 2.
The Voice Handicap Index-10.
2.3. Surgical techniques
For ACDF, the patient was placed in the supine position with the neck slightly extended. After general anesthesia by endo-tracheal intubation, a standard horizontal incision was made in accordance with the dermatoglyph. After successful exposure of C2-3, the anterior longitudinal ligament was resected and the C2-3 discectomy completed. Preparation of the sub-chondral endplate of the vertebral body was completed using a high speed drill and curet. When the end-plate preparation was complete, the disc space was distracted, and a 3 cortical iliac bone of suitable size or a cage fulfilled with autologous or artificial bone was implanted in the disc space of C2-3. After confirmation of the size and position, an anterior plate was placed and screws were tightened. Before wound closure, a final imaging of the device implantation was performed and the wound then was closed with drainage.
For patients who underwent posterior fixation and fusion of C2-3, they were placed in a prone position with the help of a Mayfield Cranial Fixation device under the guidance of fluoroscopic images. After general anesthesia, a standard midline incision was made to achieve the exposure of C2-3, pedicle screws were used at C2 and lateral mass screws were used at C3 under guidance of fluoroscopic images. A rod of the appropriate length was placed and connected to the screws. The masses, facet joints and laminae were decorticated for bone grafting. After bone grafting, final fluoroscopy was performed to confirm the position of the implants. The wound was then routinely closed and drainage was removed 2 days after surgery.
2.4. Statistical analysis
All statistical analysis was performed using SPSS 22.0 software (SPSS Inc., Chicago, IL). The Chi-squared test and Student t test were used when appropriate. P < .05 were considered as significant.
3. Results
A total of 93 patients with an average age of 47 years (ranging from 18 to 73 years) were included in this study, and amongst them 68 were male and 25 were female patients. According to the Levine–Edward classification system, 10 patients were evaluated as type III, 11 patients evaluated as type IIA, 46 patients as type II, and 26 patients as type I. After careful evaluation, 53 patients underwent ACDF (anterior plate + cage or 3 cortical iliac bone), 10 patients received posterior surgery and 30 patients received nonoperative treatment. The overall average length of hospital stay was 12.2 days (ranging from 2 to 42 days), the average length of hospital stay for nonoperative patients was 8.4 days (ranging from 2 to 42 days), the average length of hospital stay for patients who underwent ACDF was 14.4 days (ranging from 4 to 27 days) and the average length of hospital stay for patients who received posterior surgery was 12.1 days (ranging from 6 to 19 days).
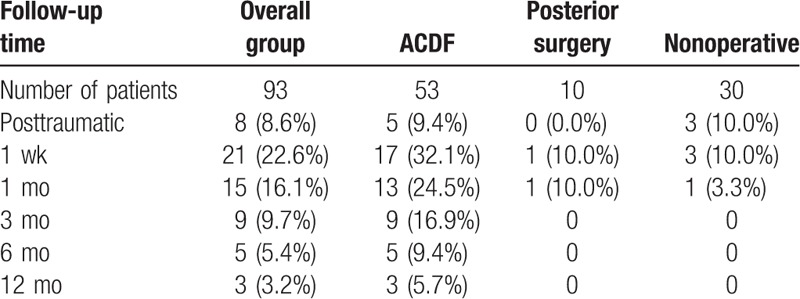
Posttraumatic immediate dysphagia was found in 8 patients (moderate in 2 patients and mild in 6 patients) and amongst them, 5 patients received ACDF, whilst 3 patients received nonoperative treatment in our department. In the posterior surgery group, there was only 1 (10.0%) patient who suffered from mild dysphagia which was symptomatic for 1 month following the surgery. In the nonoperative group, 3 patients suffered from mild dysphagia and the symptoms disappeared at the 3rd month of follow-up. In the ACDF group, there were 17 patients (32.1%) who suffered from dysphagia at the first week after surgery and amongst them 2 patients were severe, 5 patients were moderate and 10 patients mild. The incidence of dysphagia in the ACDF group decreased to 24.5% at the 1st month of follow-up, and decreased to 16.9% at the 3rd month of follow-up. There were only 5 and 3 patients who suffered from dysphagia at 6 and 12 months respectively after surgery in the ACDF group. The incidence of dysphagia after Hangman fractures is summarized in detail in Table 3.
Table 3.
Incidence of dysphagia after Hangman fractures at each of the follow-up times.
Posttraumatic immediate dysphonia was observed in 3 patients whose VHI-10 scores were 10, 14, and 16 respectively. All of the 3 patients received ACDF in our department and no patient suffered from dysphonia in the nonoperative and posterior groups. In the ACDF groups, 13 patients (24.5%) suffered from dysphonia at the first week after surgery and the number of patients suffering from dysphonia decreased to 6 patients (11.3%) at the 1st month of follow-up. During the following times, the incidence of dysphonia decreased to 3.8% at 3 months follow-up and 1.9% at the 6 months. The incidences of dysphonia after ACDF for the treatment of Hangman fractures are summarized in detail in Table 4. A 38-year-old female patient suffered from both dysphonia and dysphagia during the 12 months follow-up after surgery and the symptoms were relieved after the patient received rehabilitation therapy in our hospital (Fig. 1).
Table 4.
Characteristics of dysphonia after C2–3 ACDF for the treatment of Hangman fractures.
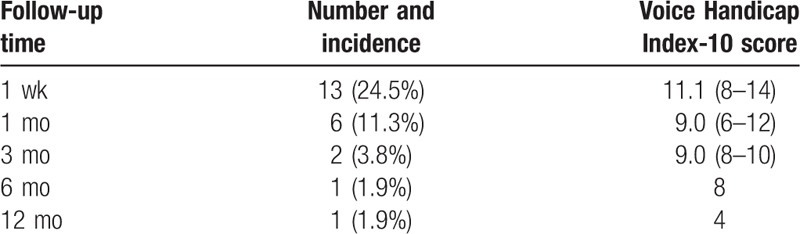
Figure 1.
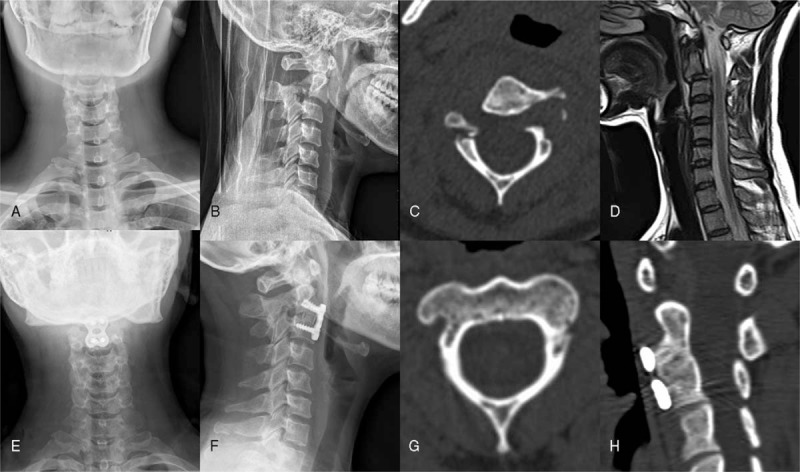
A 38-year old female patient suffered from both dysphonia and dysphagia during the 12 months follow-up after the operation. This patient presented to our hospital with a history of neck pain for 200ï¿ hours after being involving in a traffic accident. The physical examination of the patient showed no neurological compromises and she did not have any symptoms of dysphonia or dysphagia. The preoperative cervical X-ray (A and B) and computed tomography (CT) images (C) showed a bilateral arch fracture of C2 with a displacement of C2 corpus on C3 vertebrae. The magnetic resonance imaging (D) showed intervertebral disc injury of C2-3. The patient was diagnosed with a Hangman fracture and anterior surgery was performed. The 12 months postoperative X-ray and CT images (E–H) showed the favorable position of the plate, fusion of C2-3 and fusion of bilateral arch of C2. The patient's symptoms of dysphonia and dysphagia were relieved after rehabilitation therapy in our hospital.
4. Discussion
Previous studies focusing on Hangman fractures have attempted to compare clinical outcomes and fusion rates between different treatment methods but have ignored complications of dysphagia and dysphonia after fractures. The main purpose of this study was to determine the accurate incidence, severity and outcomes of dysphagia and dysphonia after Hangman fractures based on recruitment of 93 selected patients. Our results showed the incidence of self-reported dysphasia after Hangman fractures was 8.6% (8 of 93 patients) and the incidence of dysphonia was 3.2% (3 of 93 patients). It was shown that dysphagia and dysphonia after Hangman fractures occur and surgeons who deal with Hangman fractures should not only focus on neurological function and the fracture itself but should also take care of the swallowing and voice function of patients.
In our opinion, the main mechanism of dysphagia after Hangman fractures results from stretch injury of the esophagus, prevertebral soft tissue and related nerves when the fracture occurred. In addition, posttraumatic edema of the esophagus and the prevertebral soft tissue may also make a contribution to posttraumatic dysphagia in patients with Hangman fractures. In some cases the huge tear-drop fracture fragments of the C2 vertebra can cause direct esophagus compression consequently leading to dysphagia.[24] The posterior approach surgery is also not free from postoperative dysphagia in Hangman fracture patients. Our results demonstrate that the incidence of dysphagia after the posterior surgical approach in Hangman fracture patients was 10.0% (1 of 10 patients), which was similar to previous studies.[25] However, the patient's symptom was completely relived at 3 months follow-up, which was different from the previously reported approx. 12% incidence of long-term dysphagia after posterior cervical spine surgery.[26] Pain from posterior neck dissection, immobilization from a cervical collar and the tracheal intubation may be the possible reasons for dysphagia after posterior cervical surgery.[26]
For Hangman fracture patients who underwent C2-3 ACDF surgery, a higher incidence of dysphagia compared with posterior surgery and nonoperative treatment patients was observed in this study. The postoperative immediate incidence of dysphagia in the C2-3 ACDF surgery group was 32.1% (17 of 53 patients) which decreased to 16.9% (9 of 53 patients) at the 3rd month of follow-up, and further to 5.7% (3 of 53 patients) at the 12th month of follow-up. Previous studies have reported the following factors to be associated with higher possibility of dysphagia after anterior cervical surgery: female patients, older patients, C4-5 surgery, anterior plating, long operation time, multi-level surgery, and use of bone morphogenetic protein.[27–29] Theoretically anterior C2-3 surgery was expected to result in a higher incidence of postoperative dysphagia due to the special location and difficult exposure which often requires a much more powerful traction strength during surgery. The Hangman fracture patients often suffered from edema of the esophagus and prevertebral soft tissue which can contribute to the occurrence of dysphagia. In addition, all of the anterior surgical patients in this study experienced anterior plating which can cause mechanical irritation or impingement against the esophagus.[30,31] Shriver et al performed a systematic review and meta-analysis and found that the incidences of general anterior cervical surgeries at different follow-up times of <12, 12 to 24, and >24 months were 19.9% (6.0–33.7%), 7.0% (5.2–8.7%), and 7.6% (1.4–13.8%), respectively.[32] It was observed that the incidence of dysphagia after Hangman fractures in this study was similar to the incidence of general anterior cervical surgeries. However, a recent retrospective study demonstrated that upper cervical spine surgeries were one of the causes of dysphagia after operations for anterior cervical decompression and fusion.[33] Future, prospective randomized studies are required to further explore these data. Dysphonia is considered one of the most common neurologic complications after anterior cervical spine surgeries. Direct injury or surgical stretch of the recurrent laryngeal nerve during operations may contribute to the occurrence of dysphonia. Tew and Mayfield found that prolonged pressure on the nerve is the most likely cause of vocal cord paralysis in anterior cervical spine surgery.[34] Proper intubation, traction and avoidance of long time significant laryngeal displacement may decrease the incidence.[35]
5. Conclusion
Posttraumatic immediate dysphagia and dysphonia were observed in Hangman fracture patients, and anterior approach surgery was associated with a higher incidence of dysphagia. Most dysphagia and dysphonia were mild and gradually decreased during the subsequent 3 months following surgery. Future, prospective randomized studies with larger sample sizes were warranted to further validate the findings presented in this study.
Author contributions
Conceptualization: Yi Yang, Litai Ma.
Data curation: Yi Yang, Lijuan Dai, Litai Ma.
Formal analysis: Yi Yang, Lijuan Dai.
Funding acquisition: Hao Liu.
Investigation: Hao Liu.
Methodology: Yi Yang.
Project administration: Hao Liu.
Resources: Litai Ma.
Software: Lijuan Dai.
Supervision: Hao Liu.
Writing – original draft: Yi Yang, Litai Ma.
Writing – review & editing: Lijuan Dai, Litai Ma, Xinlin Gao, Hao Liu.
Footnotes
Abbreviations: ACDF = anterior cervical discectomy and fusion, CT = computed tomography, MRI = magnetic resonance imaging.
Funding: This study was supported by the Foundation for Science & Technology Department of Sichuan Provincial Government, China (grant no: 2014SZ0236).
The authors have no conflicts of interest to disclose.
References
- [1].Schneider RC, Livingston KE, Cave AJE, et al. Hangmans fracture of cervical spine. J Neurosurg 1965;22:141-+. [DOI] [PubMed] [Google Scholar]
- [2].Al-Mahfoudh R, Beagrie C, Woolley E, et al. Management of typical and atypical Hangman's fractures. Global Spine J 2016;6:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hadley MN, Dickman CA, Browner CM, et al. Acute axis fractures: a review of 229 cases. J Neurosurg 1989;71(5 Pt 1):642–7. [DOI] [PubMed] [Google Scholar]
- [4].Effendi B, Roy D, Cornish B, et al. Fractures of the ring of the axis - a classification based on the analysis of 131 cases. J Bone Joint Surg—Br Vol 1981;63:319–27. [DOI] [PubMed] [Google Scholar]
- [5].Francis WR, Fielding JW, Hawkins RJ, et al. Traumatic spondylolisthesis of the axis. J Bone Joint Surg—Br Vol 1981;63:313–8. [DOI] [PubMed] [Google Scholar]
- [6].Josten C. The traumatic spondylolisthesis of the axis. Der Orthopade 1999;28:394–400. [DOI] [PubMed] [Google Scholar]
- [7].Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am 1985;67:217–26. [PubMed] [Google Scholar]
- [8].Suchomel P, Hradil J, Barsa P, et al. Fractures of the ring of axis (Hangman type fractures). Acta chirurgiae orthopaedicae et traumatologiae Cechoslovaca 2006;73:321–8. [PubMed] [Google Scholar]
- [9].Wu Y-S, Lin Y, Zhang X-L, et al. Management of Hangman's fracture with percutaneous transpedicular screw fixation. Eur Spine J 2013;22:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ge C, Hao D, He B, et al. Anterior cervical discectomy and fusion versus posterior fixation and fusion of C2–3 for unstable Hangman's fracture. J Spinal Disord 2015;28:E61–6. [DOI] [PubMed] [Google Scholar]
- [11].Wei F, Pan X, Zhou Z, et al. Anterior-only stabilization using cage versus plating with bone autograft for the treatment of type II/IIA Hangman's fracture combined with intervertebral disc injury. J Orthop Surg Res 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Salunke P, Sahoo SK, Krishnan P, et al. Are C2 pars-pedicle screws alone for type II Hangman's fracture overrated? Clin Neurol Neurosurg 2016;141:7–12. [DOI] [PubMed] [Google Scholar]
- [13].Cao G, Meng C, Zhang W, et al. Operative strategy and clinical outcomes of ROI-C (TM) fusion device in the treatment of Hangman's fracture. Int J Clin Exp Med 2015;8:18665–72. [PMC free article] [PubMed] [Google Scholar]
- [14].Koller H, Kathrein A. Letter to the editor concerning: a systematic review of the management of Hangman's fractures by Xin-Feng Li et al. (2006). Eur Spine J 15:257–69.European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2006; 15(9):1415–1418; author reply 1419–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li Z, Li F, Hou S, et al. Anterior discectomy/corpectomy and fusion with internal fixation for the treatment of unstable Hangman's fractures: a retrospective study of 38 cases. J Neurosurg Spine 2015;22:387–93. [DOI] [PubMed] [Google Scholar]
- [16].Wei F, Wang L, Zhou Z, et al. Cervical cage without plating in management of type II/II A Hangman's fracture combined with intervertebral disc injury. BMC Musculoskelet Disord 2015;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Clave P, Shaker R. Dysphagia: current reality and scope of the problem. Nat Rev Gastroenterol Hepatol 2015;12:259–70. [DOI] [PubMed] [Google Scholar]
- [18].Joseph JR, Smith BW, Mummaneni PV, et al. Postoperative dysphagia correlates with increased morbidity, mortality, and costs in anterior cervical fusion. J Clin Neurosci 2016;31:172–5. [DOI] [PubMed] [Google Scholar]
- [19].Yue WM, Brodner W, Highland TR. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year follow-up study. Eur Spine J: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2005;14:677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zeng JH, Li XD, Deng L, et al. Lower cervical levels: Increased risk of early dysphonia following anterior cervical spine surgery. Clin Neurol Neurosurg 2016;149:118–21. [DOI] [PubMed] [Google Scholar]
- [21].El Masry WS, Tsubo M, Katoh S, et al. Validation of the American Spinal Injury Association (ASIA) motor score and the National Acute Spinal Cord Injury Study (NASCIS) motor score. Spine 1996;21:614–9. [DOI] [PubMed] [Google Scholar]
- [22].Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine 2002;27:2453–8. [DOI] [PubMed] [Google Scholar]
- [23].Rosen CA, Lee AS, Osborne J, et al. Development and validation of the voice handicap index-10. Laryngoscope 2004;114:1549–56. [DOI] [PubMed] [Google Scholar]
- [24].Ma L, Yang Y, Gong Q, et al. Anterior reduction, discectomy, and three cortical iliac bone grafting with instrumentation to treat a huge tear drop fracture of the axis: a case report and literature review. Medicine 2016;95:e3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smith-Hammond CA, New KC, Pietrobon R, et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine 2004;29:1441–6. [DOI] [PubMed] [Google Scholar]
- [26].Radcliff KE, Koyonos L, Clyde C, et al. What is the incidence of dysphagia after posterior cervical surgery? Spine 2013;38:1082–8. [DOI] [PubMed] [Google Scholar]
- [27].Yang Y, Ma L, Liu H, et al. A meta-analysis of the incidence of patient-reported dysphagia after anterior cervical decompression and fusion with the zero-profile implant system. Dysphagia 2016;31:134–45. [DOI] [PubMed] [Google Scholar]
- [28].Yang Y, Ma L, Liu H, et al. Comparison of the incidence of patient-reported post-operative dysphagia between ACDF with a traditional anterior plate and artificial cervical disc replacement. Clin Neurol Neurosurg 2016;148:72–8. [DOI] [PubMed] [Google Scholar]
- [29].Joaquim AF, Murar J, Savage JW, et al. Dysphagia after anterior cervical spine surgery: a systematic review of potential preventative measures. Spine J 2014;14:2246–60. [DOI] [PubMed] [Google Scholar]
- [30].Lee MJ, Bazaz R, Furey CG, et al. Influence of anterior cervical plate design on Dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord 2005;18:406–9. [DOI] [PubMed] [Google Scholar]
- [31].Xiao S, Liang Z, Wei W, et al. Zero-profile anchored cage reduces risk of postoperative dysphagia compared with cage with plate fixation after anterior cervical discectomy and fusion. Eur Spine J: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2017;26:975–84. [DOI] [PubMed] [Google Scholar]
- [32].Shriver MF, Lewis DJ, Kshettry VR, et al. Dysphagia rates after anterior cervical diskectomy and fusion: a systematic review and meta-analysis. Global Spine J 2017;7:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wu B, Song F, Zhu S. Reasons of Dysphagia after operation of anterior cervical decompression and fusion. Clin Spine Surg 2017;30:E554–e559. [DOI] [PubMed] [Google Scholar]
- [34].Tew JM, Jr, Mayfield FH. Complications of surgery of the anterior cervical spine. Clin Neurosurg 1976;23:424–34. [DOI] [PubMed] [Google Scholar]
- [35].Apfelbaum RI, Kriskovich MD, Haller JR. The incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine 2000;25:2906–12. [DOI] [PubMed] [Google Scholar]


